Abstract
Glioblastoma is the most common and most malignant intrinsic human brain tumor, characterized by extensive invasion and proliferation of glial (astrocytic) tumor cells, frequent activation of tyrosine kinase receptor signaling pathways, relative resistance to chemotherapy and radiotherapy, and poor prognosis. Using the Gal4-UAS system, we have produced glioma models in Drosophila by overexpressing homologs of human tyrosine kinase receptors under control of the glia-specific promoter reversed polarity (repo). Glial overexpression of activated epidermal growth factor receptor (EGFR) resulted in enhanced proliferation and migration of larval glial cells with increased numbers in the eye imaginal disc, diffuse tumor-like enlargement of the optic stalk, and marked ectopic invasion of glial cells along the optic nerve. Glial overexpression of the downstream kinase PI3K showed similar pathology. Overexpression of activated pvr (platelet-derived growth factor receptor/vascular endothelial growth factor receptor homolog) led to migration of glial cells along the optic nerve, whereas expression of activated htl (fibroblast growth factor receptor 1 homolog) and INR (insulin receptor) showed markedly elevated numbers of glial cells in the optic stalk. The EGFR/phosphatidylinositol 3-phosphate kinase (PI3K) phenotype was partly reverted by the administration of the EGFR tyrosine kinase inhibitor gefitinib and completely rescued by the PI3K inhibitor wortmannin and the Akt inhibitor triciribine. We suggest that Drosophila models will be useful for deciphering signaling cascades underlying abnormal behavior of glioma cells for genetic screens to reveal interacting genes involved in gliomagenesis and for experimental therapy approaches.
Introduction
Glioblastomas represent the most common and most malignant intrinsic human brain tumors. These astrocytic neoplasms are characterized by a high proliferative activity, diffuse invasion of brain tissue, and relative resistance to conventional cytotoxic chemotherapy and radiotherapy, leading to poor prognosis with median survival of 12 to 18 months [1]. New types of “personalized” therapies targeting the underlying molecular alterations of individual glioblastomas have increasingly been considered and used in ongoing clinical studies. Promising molecular targets for therapeutic intervention have included the tyrosine kinase receptors epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR) and their downstream signaling cascades, the phosphatidylinositol 3-phosphate kinase (PI3K)/AKT and Ras/mitogen-activated protein kinase pathways [2,3].
Experimental in vivo models of glioblastoma are required for evaluating new types of experimental therapies as well as for better understanding the biology of these fatal neoplasms. Most models are based on transplantation of glioma cell lines into mouse or rat brains or on the use of transgenic mice developing gliomas [4–6]. Advantages of rodent models include their wide distribution among institutions with accumulated experience for decades, the existence of a blood-brain barrier, and the possibilities of applying therapeutic agents and studying the interaction of tumor and brain tissue. However, there are potential problems of rodent models, such as restrictions of host genotype (only syngenic or immunodeficient animals being susceptible to tumor growth), the artificial nature of tumorigenesis after transplantation, a high number of genetic changes in tumor cells, the long duration of experiments, high cost, and ethical considerations.
Drosophila melanogaster, as a model organism, offers several advantages, including easy handling, rapid generation time, low cost, and a wide armamentarium of genetic techniques [7]. Many molecular pathways are conserved between invertebrates and humans, such as tyrosine kinase receptor signaling cascades. Furthermore, Drosophila can be used in neuropharmacological experiments because this organism is amenable to external/food application, inhalation, or injection of substances in a large number of wild type or mutant animals [8–12]. Fly models of the hereditary tumor syndrome tuberous sclerosis as well as neurofibromatosis types 1 and 2 have been established [13–16]. Furthermore, several mutants interfering with asymmetric cell division of neuroblasts exhibit neuronal/neuroblastic tumors that are referred to as “hyperplastic” in case of preserved architecture such as malignant brain tumor (l(3)mbt) [17] or “neoplastic” with loss of architecture and invasion such as brain tumor (brat), raps, numb, pros, and mira [18].
The larval brain is composed of two hemispheres and the ventral ganglion where peripheral nerves originate (Figure 1A). In the Drosophila central nervous system, approximately 10% of cells are of glial nature, which are classified as either midline glia or lateral glia, the latter being positive for the glial marker reversed polarity (repo) [19]. During larval development, photoreceptor neurons are specified within the eye imaginal disc and project their axons along the basal surface of the disc into the optic stalk, bridging the eye imaginal disc with the optic lobes. Repo-positive glial cells that originate from the optic stalk populate the eye disc in a stereotyped manner, so that the number of glial cells is predictable at certain time points of development [20], and changes of proliferation or migration are easily detectable. The larval visual system is located adjacent to the eye imaginal disc and consists of a pair of 12 cells, the Bolwig organs. These send axonal projections (Bolwig nerves, BNs) that enter the brain through the optic stalks and are physiologically not accompanied by glial cells [21,22], enabling convenient detection of glial overmigration along these axonal projections.
Figure 1.
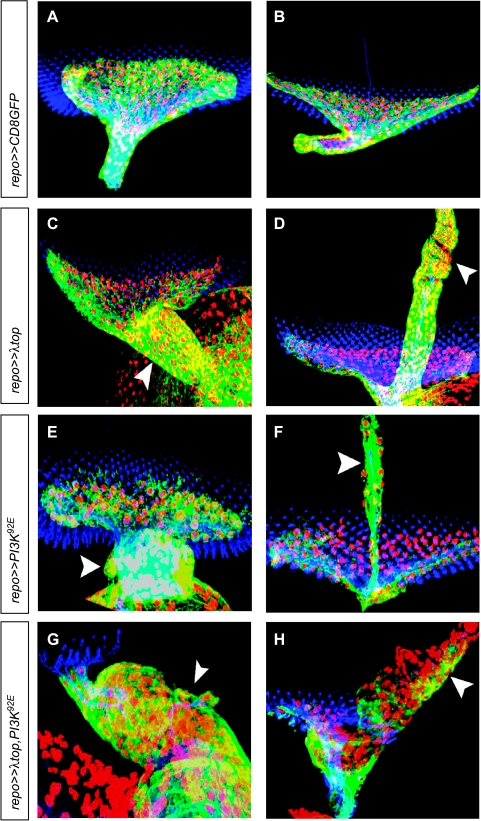
Eye imaginal discs of third instar larvae with overexpression of activated Egfr and/or wild-type PI3K in glial cells. Immunofluorescence staining of (A–H) eye imaginal discs of third instar larvae. Nuclei of glial cells are red (α-Repo), glial cytoplasms are green (α-GFP), and photoreceptor neurons are blue (α-HRP). Images are projections of confocal image stacks. (A) Wild-type eye imaginal disc with optic stalk. (C, E, G) Ectopic gene expression resulted in a thickened optic stalk due to increased numbers of glial cells (arrowheads). (B) Wild-type eye imaginal disc with BN. (D, F, H) Ectopic gene expression resulted in overmigration of glial cells along BN (arrowheads).
Only very recently, we and others have started to explore the usefulness of Drosophila in modeling gliomas by inducing EGFR/PI3K signaling in larval glia [23,24]. We here report that not only Drosophila lines overexpressing EGFR and/or PI3K but also lines overexpressing other tyrosine kinase receptors, including PDGFR/VEGFR, fibroblast growth factor receptor (FGFR), and insulin receptor homologs, show increased proliferation and/or overmigration of glial cells in larval eye structures, recapitulating histologic key features of human gliomas. Moreover, we demonstrate that these experimental gliomas can be reverted by drugs targeting the EGFR signaling pathway.
Materials and Methods
Fly strains and genetics
All crosses were performed on standard food at room temperature unless indicated otherwise. We used fly strains repo Gal4 (III), generated by a random Gal4 P-element insertion into the repo locus [25], UAS λtop, the activated form of torpedo/Egfr [26,27], UAS λpvr, the constitutively active form of pvr [28], as well as UAS λhtl [29]. UAS PI3K92E, generated by P-element insertion, UAS INR, and UAS CD8GFP, which targets green fluorescent protein to the plasma membrane, were obtained from Bloomington Stock Center (Bloomington, IN).
Crossings
Flies homozygous for UAS λtop, UAS PI3K92E, UAS CD8GFP, UAS λpvr, UAS λhtl, UAS INR and flies harboring UAS λtop as well as UAS PI3K92E were crossed against a stock carrying a repo Gal4 transgene.
Immunohistochemistry
Fluorescent immunostaining was performed on third instar larva brains and eye imaginal discs. Specimens were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Anti-repo antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Rabbit and mouse anti-GFP (both 1:500; Invitrogen, Eugene, OR) and goat anti-HRPCy5 (1:200; Dianova, Hamburg, Germany) antisera were used according to the manufacturer's instructions. Fixation and treatment of tissues for immunohistochemistry were performed according to standard procedures.
Cy2, Cy3, or Cy5 (1:200; Dianova GmbH) and Alexa 488, 568, or 647 (1:500; Molecular Probes, Carlsbad, CA) were used as secondary antibodies.
Pharmacological Inhibition of Ectopically Expressed Genes
Substances used were gefitinib (Biomol, Hamburg, Germany), wortmannin (Biaffin, Kassel, Germany), and triciribine (Biaffin). One gram of Drosophila medium (Carolina Biological Supply, Burlington, NC) was mixed with 5 ml of H2O. The inhibitors were made soluble in 100 mM DMSO. Flies harboring double insertions UAS PI3K92E and UAS λtop were set for 24 hours on normal food (at 25°C) and then on prepared food for 24 hours (at 25°C). Effects of inhibition were analyzed by immunohistochemistry.
Confocal Laser Scanning Microscopy and Cell Counting
Images were taken with a confocal laser scanning microscope (Axiovert 200M or Axio Imager Z.1 with LSM 510 META scanning module; Zeiss, Oberkochen, Germany). Numbers of repo-expressing glial cells were scored in different developmental stages. Cell counts were performed on confocal image stacks with sections taken at 0.6-µm distance (resolution, 512 x 512 pixels; pinhole, 100 mm; scan speed, 8). Glial nuclei were counted using a three-dimensional image processing program (Volocity 4.0; Improvision, Waltham, MA). For each age group, 15 imaginal discs were investigated. Volocity quantitation was set with an intensity range of 60 to 255 and a minimal volume of 25 µm3. Only complete confocal stacks were counted. White/black balance was performed using Photoshop 7.0 (Adobe, Dublin, Ireland).
Statistical Analysis
Quantitative data were expressed as arithmetic means ± SEM. One-way analysis of variance was performed followed by Scheffé procedure; P < .05 was considered significant. Statistical analysis was performed using SPSS version 15.0 (SPSS, Inc, Chicago, IL).
Results
Ectopic Expression of Activated Egfr and PI3K92E in Eye Imaginal Discs of Third Instar Larva
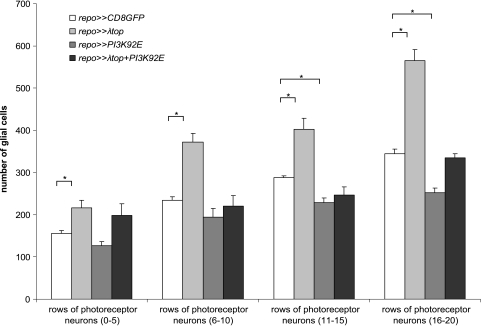
Activated Egfr as well as activated Egfr in combination with wild-type PI3K was expressed in Drosophila glial cells using the Gal4/UAS system and the repo promoter, directing expression to lateral glial cells. Expression of λtop, the activated form of torpedo/Egfr (top/Egfr) led to increased numbers of glial cells in the optic stalk (Figure 1C). Overmigration of glial cells along BNs of the third instar larva was also observed (Figure 1D). Similarly, expression of PI3K92E resulted in enlargement of the optic stalk and overmigration of glial cells along BN (Figure 1, E and F). Ectopic coexpression of λtop and PI3K92E resulted in thickening of the optic stalk (Figure 1G) and in massive ectopic migration along BN (Figure 1H), being more pronounced than in the single transgenic lines. Quantitative analysis revealed that glial overexpression of λtop significantly increased glial cell number in the eye imaginal disc in all stages in comparison to wild type (Figure 2). However, overexpression of PI3K92E resulted in reduced glial cell numbers in later stages of development (Figure 2), presumably because most glial cells were misplaced in optic stalk and BN. Consequently, no differences with the wild type were established after activation of both λtop and PI3K92E with respect to the number of glial cells in the eye disc.
Figure 2.
Number of glial cells in different genotypes in eye imaginal discs of different age. Cell numbers expressing the glial marker repo were morphometrically determined in different developmental stages. Age of imaginal discs was grouped according to rows of photo-receptor neurons (0–5, 6–10, 11–15, 16–20 rows). In all investigated developmental stages, overexpression of λtop (activated Egfr) resulted in increased glial cell numbers. In later stages, overexpression of PI3K92E led to a significant decrease of cell numbers (*P < .05).
Pharmacologically Induced Inhibition of Ectopically Activated Genes
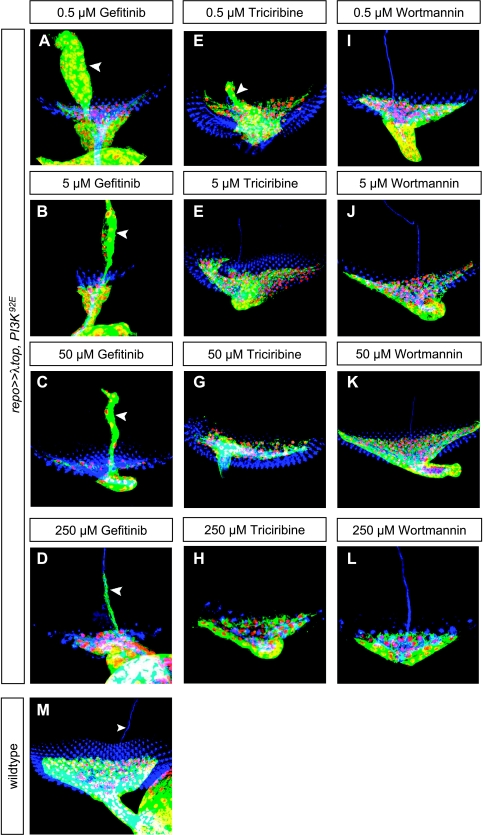
To determine whether the effects of transgene overexpression can be rescued by pharmacological inhibitors, we used flies simultaneously overexpressing UAS λtop and UAS PI3K92E. First, the effect of gefitinib (an EGFR-specific tyrosine kinase inhibitor) on the larval eye imaginal disc was investigated. After applying the lowest concentration (0.5 µM) of gefitinib in Drosophila food, the optic stalk was still thickened, and large numbers of glial cells were migrating along BN. Using the highest concentration of gefitinib (250 µM), the number of glial cells in the optic stalk remained high, whereas the number of glial cells abnormally migrating along BN was considerably reduced (Figure 3D).
Figure 3.
Effects of EGFR inhibitor gefitinib, Akt inhibitor triciribine, and PI3K inhibitor wortmannin on proliferation and migration induced by ectopic expression of λtop and PI3K92E. Effects of gefitinib on eye imaginal disc of larvae were tested using genotype repo ≫ λtop, PI3K92E. Increasing concentrations of gefitinib reduced ectopic migration along BN (arrowheads; B, C, D), although the wild-type phenotype was not reached. At low concentrations, the Akt inhibitor triciribine decreased glial migration along BN only partially (E, arrowhead), whereas higher concentrations resulted in complete rescue (F–H). With increasing concentrations of the EGFR inhibitor wortmannin, optic stalks showed complete rescue, corresponding to wild type. No overmigration along BN was seen even with the lowest concentration of wortmannin (I–L). M shows a wild-type eye imaginal disc.
Even at low concentrations (0.5 µM), the Akt inhibitor triciribine markedly decreased glial overmigration along BN (Figure 3E), whereas higher concentrations showed complete rescue (Figure 3, F–H).
Low concentrations of 0.5 µM wortmannin (a specific PI3K inhibitor) were not sufficient to reduce optic stalk mass (Figure 3I), whereas higher concentrations resulted in wild-type morphology optic stalks (Figure 3, K and L). In contrast, BN (Figure 3, I–L) showed wild-type configuration with all applied concentrations, indicating that even the lowest concentration of wortmannin was sufficient to rescue abnormal migration along BN.
Ectopic Expression of Other Receptor Tyrosine Kinases in Eye Imaginal Discs of Third Instar Larva
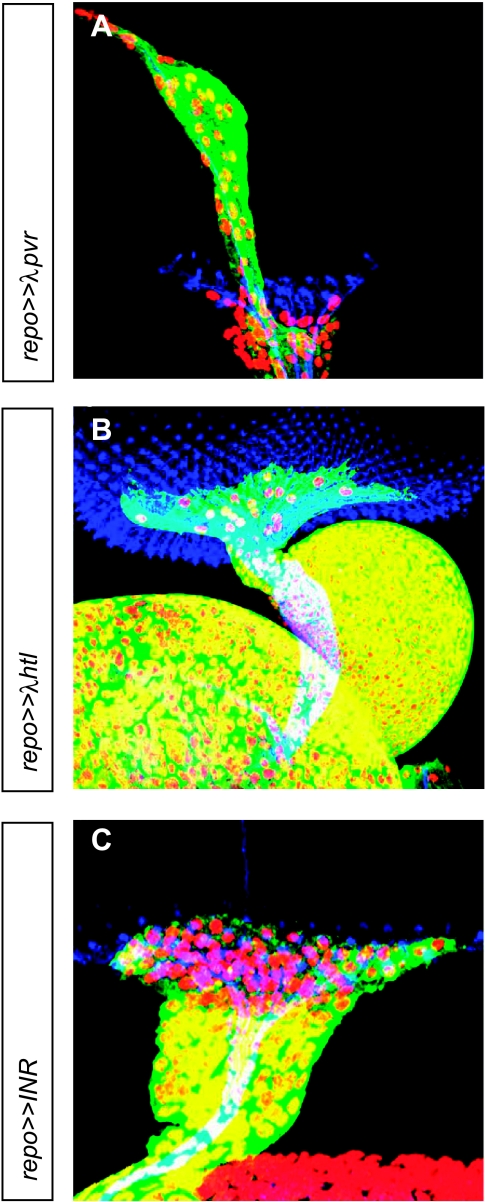
To see whether the overexpression of other receptor tyrosine kinases (RTKs) involved in gliomagenesis leads to similar results, activated pvr, the Drosophila homolog of PDGFR/VEGFR, activated htl, the Drosophila homolog of FGFR1, as well as INR, the Drosophila insulin receptor, were expressed in Drosophila lateral glial cells under repo control. Overexpression of λpvr led to abnormal migration of glial cells along BN (Figure 4A). Expression of λhtl and INR showed extensively elevated numbers of glial cells in the optic stalk, whereas no or only little overmigration along BN was observed (Figure 4, B and C).
Figure 4.
Eye imaginal discs of third instar larvae with overexpression of other tyrosine kinase receptors in glial cells. Immunofluorescence staining of (A–C) eye imaginal discs. Nuclei of glial cells are red (α-Repo), glial cytoplasms are green (α-GFP), and photoreceptor neurons are blue (α-HRP). Images are projections of confocal image stacks. (A) Ectopic gene expression of activated pvr, the Drosophila homolog of PDGFR/VEGFR, resulted in overmigration of glial cells along BN. (B and C) Ectopic gene expression of λhtl (homolog of activated FGFR1) and INR (insulin receptor) resulted in a thickened optic stalk due to increased numbers of glial cells.
Discussion
We here describe invertebrate glioma models that are based on the overexpression of tyrosine kinase receptors in Drosophila glia. Morphologically, key features of human gliomas are recapitulated, such as diffuse enlargement of brain structures due to single cell infiltration. Invasion of brain structures along nerve tracts, which is a common feature in human gliomas [30,31], is modeled in Drosophila larvae by migration along optic nerves (BNs), which, in the wild type, is devoid of glial cells, thereby facilitating detection of abnormal migration because all glial cells along BN are invasive. Furthermore, invasion can be easily quantitated by counting the number of glial cells in the BN and by determining their distance from the eye imaginal disc. Because of natural limitations of the species, including absence of blood vessels and an adaptive immune system, other features of malignant gliomas could not be reproduced, such as activated microglial cells and presence of immune cells, vascular proliferation, and necrotic areas, the latter being closely related to dysfunctional blood vessels in human gliomas.
Compared with most xenotransplantation models, our model is more similar to human glioma specimens with respect to the typical single-cell invasion pattern. Xenotransplanted glioma cell lines are often cohesive and look like metastatic sarcomas, and despite common claims, their invasion pattern is usually different from that of human gliomas. The cells in our model show diffuse invasion, and they are clearly glial so that the invasion pattern is more “human” than that of many xenotransplants. However, although adhesion molecules of glia and blood-brain barrier show considerable homologies between Drosophila and vertebrates, almost nothing is known about the cerebral extracellular matrix in Drosophila, which makes analysis of cell-matrix interactions more difficult in our model.
EGFR is the most frequently amplified gene in glioblastoma. It occurs in approximately 40% of primary glioblastomas and is associated with EGFR overexpression. Amplification of the EGFR gene is often associated with structural alterations, the most common being variant III (EGFRvIII), which is present in 20% to 50% of glioblastomas with EGFR amplification and constitutively activated in a legend independent manner, leading to cell proliferation through PI3K, RAS, and mitogen-activated protein kinase signaling pathways [1]. The PI3K signaling cascade may also be activated by PTEN mutations in 15% to 40% of primary glioblastomas and more infrequently by mutations of the PI3K components PIK3CA [32] and PIK3R1 [33]. Because more than 80% of glioblastomas show robust Akt activation, the PI3K pathway is central in molecular pathogenesis. In fact, large-scale multidimensional genomic characterization of 91 glioblastomas revealed somatic alterations in RTK/PI3K pathways in 88% of tumors, the genes most commonly involved being EGFR (45%), PTEN (36%), and components of the PI3K complex (15%) [33]. In vitro evidence suggests that activation of EGFR inhibits apoptosis and promotes invasion of glioma cells [34,35].
Our data show that overexpression of activated EGFR or PI3K in Drosophila is sufficient to produce tumor-like overgrowth and invasion of glial cells. Furthermore, our findings demonstrate that in addition to EGFR/PI3K, overexpressing other RTK receptors involved in gliomagenesis leads to glioma-like lesions in Drosophila. Because the number of glial cells in the eye imaginal disc was increased after EGFR expression, but decreased after PI3K expression and unchanged after coexpressing EGFR and PI3K, our findings suggest that there are additional signaling pathways upstream of PI3K regulating directed migrating of glial cells. They also suggest that mutations in the same signaling cascade are not necessarily associated with identical consequences on cell biology, such as migration. It might be interesting to compare the invasion patterns of human glioblastomas with EGFR amplification to those with mutations of PI3K components.
New types of molecular therapies targeting EGFR or PI3K have recently evolved as promising experimental and clinical approaches in treating glioblastomas. Response of glioblastoma patients to the EGFR inhibitor erlotinib was associated with EGFR expression/amplification and coexpression of EGFRvIII and PTEN in biopsy specimens [36,37]. However, only 10% to 20% of patients benefit from EGFR inhibition [38], pointing to confounding factors such as coactivation of multiple RTKs, thereby limiting the effects of therapies targeting single RTK. In fact, combined treatment with several RTK inhibitors was more successful [39]. Furthermore, glioblastomas harbor a variety of additional genetic changes that may interact with RTK targeting. Drosophila lines showing several coactivated RTK and inactivated tumor suppressor genes in glial cells can be produced to better model genetic changes typically encountered in glioblastomas. These invertebrate models can then be used for large-scale genetic screens in detecting interacting genes and for novel molecular-based experimental approaches.
Acknowledgments
Statistical analysis was performed by Joachim Gerss, Institute of Medical Informatics and Biomathematics, University Hospital Münster.
Footnotes
This research was funded by Deutsche Forschungsgemeinschaft (grant Pa 328/7) and Innovative Medizinische Forschung, Medical Faculty of Münster (grant JE 210704).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colman H, Aldape K. Molecular predictors in glioblastoma: toward personalized therapy. Arch Neurol. 2008;65:877–883. doi: 10.1001/archneur.65.7.877. [DOI] [PubMed] [Google Scholar]
- 3.Mason WP, Cairncross JG. Invited article: the expanding impact of molecular biology on the diagnosis and treatment of gliomas. Neurology. 2008;71:365–373. doi: 10.1212/01.wnl.0000319721.98502.1b. [DOI] [PubMed] [Google Scholar]
- 4.Reilly KM, Rubin JB, Gilbertson RJ, Garbow JR, Roussel MF, Gutmann DH. Rethinking brain tumors: the fourth Mouse Models of Human Cancers Consortium Nervous System Tumors Workshop. Cancer Res. 2008;68:5508–5511. doi: 10.1158/0008-5472.CAN-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddison K, Clarke AR. New approaches for modelling cancer mechanisms in the mouse. J Pathol. 2005;205:181–193. doi: 10.1002/path.1698. [DOI] [PubMed] [Google Scholar]
- 6.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 7.Jeibmann A, Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci. 2009;10:407–440. doi: 10.3390/ijms10020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manev H, Dimitrijevic N, Dzitoyeva S. Techniques: fruit flies as models for neuropharmacological research. Trends Pharmacol Sci. 2003;24:41–43. doi: 10.1016/s0165-6147(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 9.Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dzitoyeva S, Dimitrijevic N, Manev H. Intra-abdominal injection of double-stranded RNA into anesthetized adult Drosophila triggers RNA interference in the central nervous system. Mol Psychiatry. 2001;6:665–670. doi: 10.1038/sj.mp.4000955. [DOI] [PubMed] [Google Scholar]
- 11.Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Manev H, Dimitrijevic N. Drosophila model for in vivo pharmacological analgesia research. Eur J Pharmacol. 2004;491:207–208. doi: 10.1016/j.ejphar.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Ito N, Rubin GM. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell. 1999;96:529–539. doi: 10.1016/s0092-8674(00)80657-1. [DOI] [PubMed] [Google Scholar]
- 14.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 15.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 16.The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 17.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–639. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 18.Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Klambt C, Hummel T, Granderath S, Schimmelpfeng K. Glial cell development in Drosophila. Int J Dev Neurosci. 2001;19:373–378. doi: 10.1016/s0736-5748(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 20.Silies M, Yuva Y, Engelen D, Aho A, Stork T, Klambt C. Glial cell migration in the eye disc. J Neurosci. 2007;27:13130–13139. doi: 10.1523/JNEUROSCI.3583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green P, Hartenstein AY, Hartenstein V. The embryonic development of the Drosophila visual system. Cell Tissue Res. 1993;273:583–598. doi: 10.1007/BF00333712. [DOI] [PubMed] [Google Scholar]
- 22.Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extraretinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443–1453. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- 23.Paulus W, Witte H, Jeibmann A, Klämbt C. Modelling glioma growth and invasion in Drosophila. J Neuropathol Exp Neurol. 2008;67:496–497. [Google Scholar]
- 24.Read RD, Cavenee WK, Furnari FB, Thomas JB. A Drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 2009;5:e1000374. doi: 10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev Biol. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- 26.Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- 27.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 28.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 29.Michelson AM, Gisselbrecht S, Zhou Y, Baek KH, Buff EM. Dual functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev Genet. 1998;22:212–229. doi: 10.1002/(SICI)1520-6408(1998)22:3<212::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oellers P, Schroer U, Senner V, Paulus W, Thanos S. Rocks are expressed in brain tumors and are required for glioma-cell migration on myelinated axons. Glia. 2009;57:499–509. doi: 10.1002/glia.20777. [DOI] [PubMed] [Google Scholar]
- 32.Kita D, Yonekawa Y, Weller M, Ohgaki H. PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 2007;113:295–302. doi: 10.1007/s00401-006-0186-1. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research Network, author. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappl A, Piontek G, Schlegel J. EGFR-dependent migration of glial cells is mediated by reorganisation of N-cadherin. J Cell Sci. 2008;121:4089–4097. doi: 10.1242/jcs.027995. [DOI] [PubMed] [Google Scholar]
- 35.Yamoutpour F, Bodempudi V, Park SE, Pan W, Mauzy MJ, Kratzke RA, Dudek A, Potter DA, Woo RA, O'Rourke DM, et al. Gene silencing for epidermal growth factor receptor variant III induces cell-specific cytotoxicity. Mol Cancer Ther. 2008;7:3586–3597. doi: 10.1158/1535-7163.MCT-08-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, Baumber R, Lamborn KR, Kapadia A, Malec M, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 37.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 38.Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 39.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]