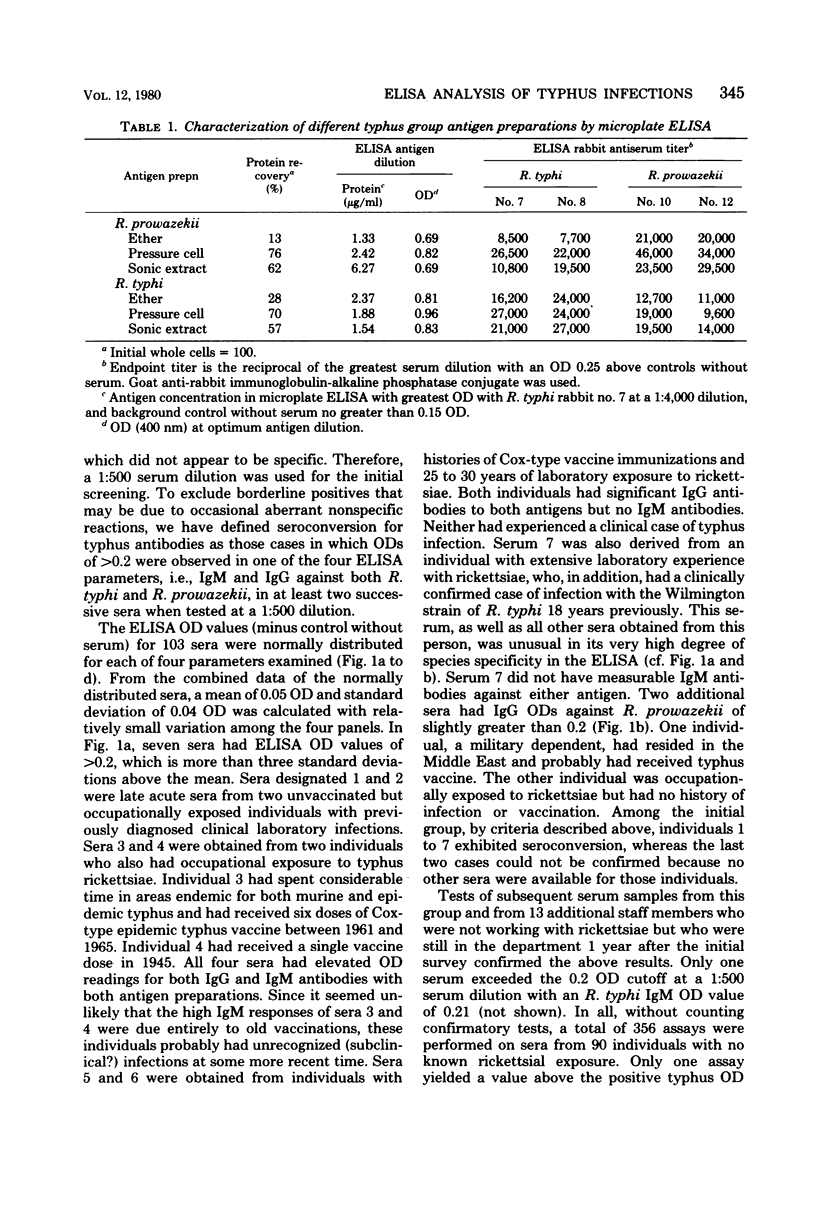
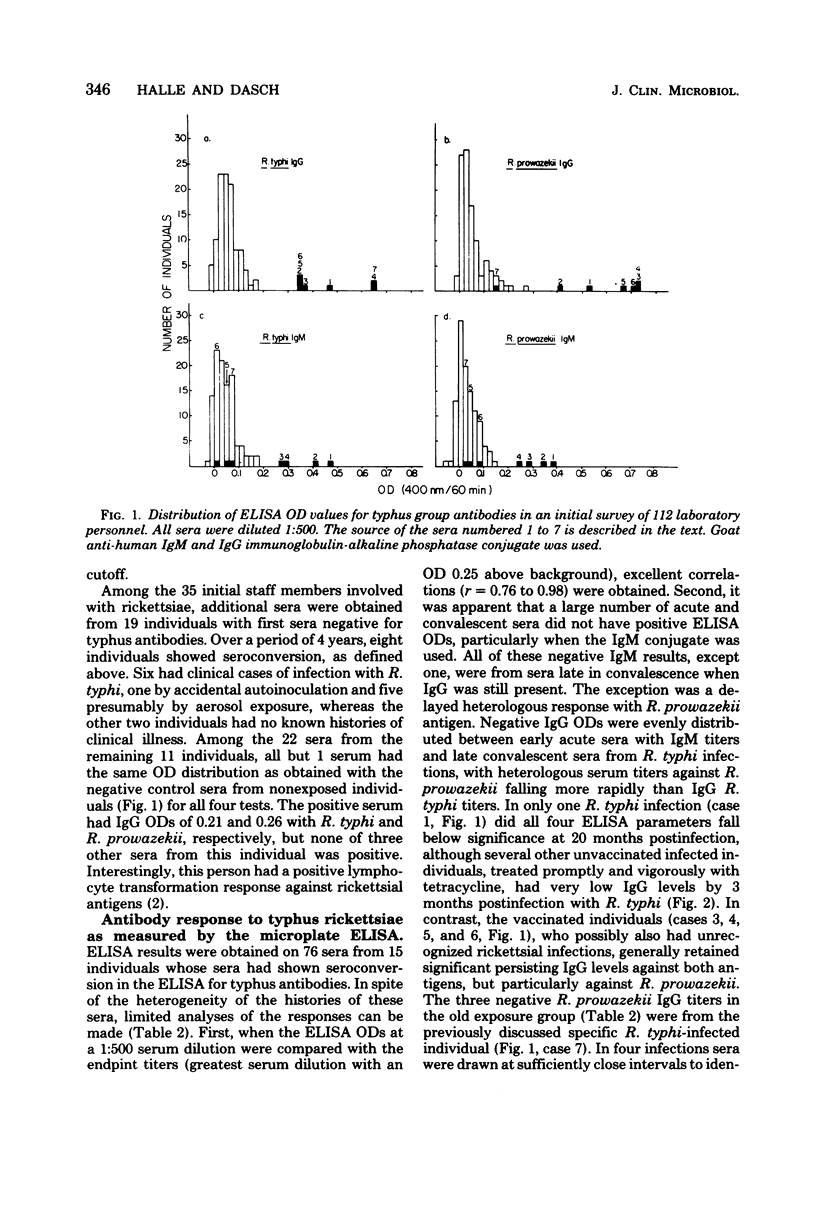
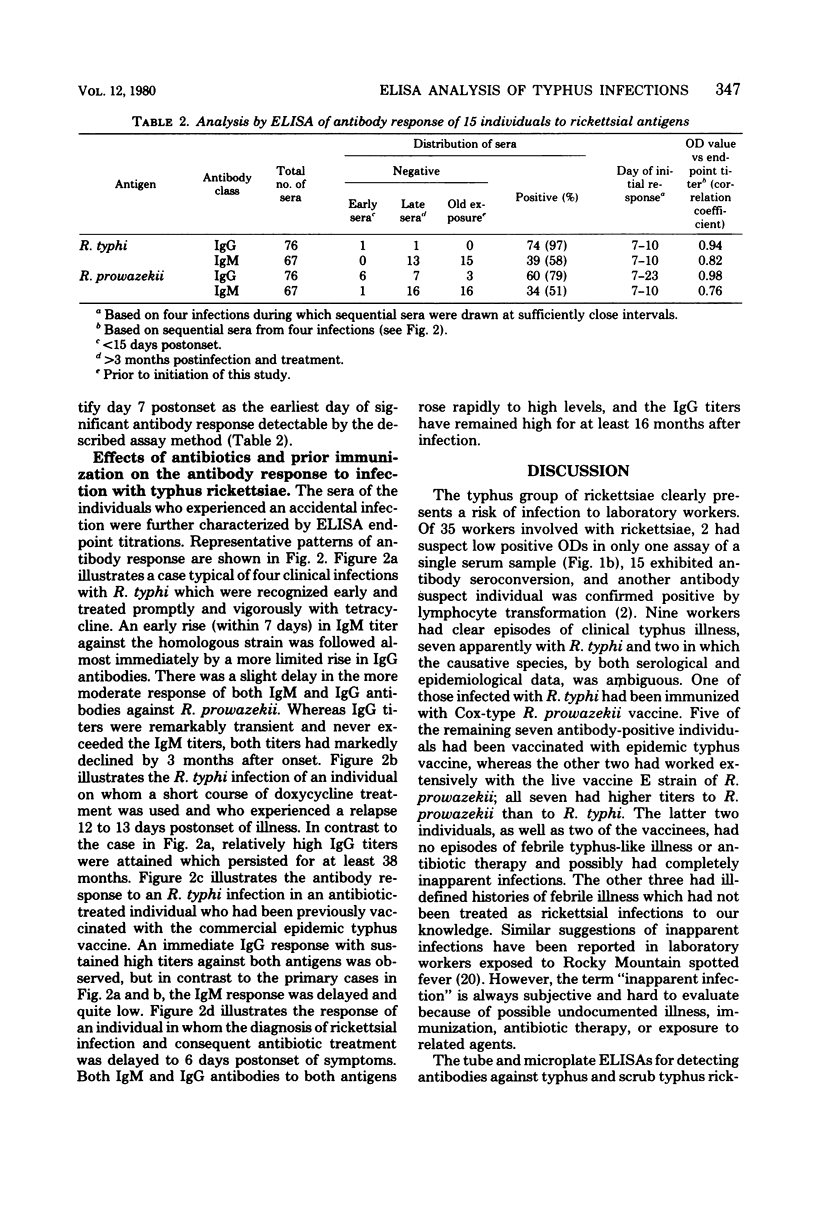
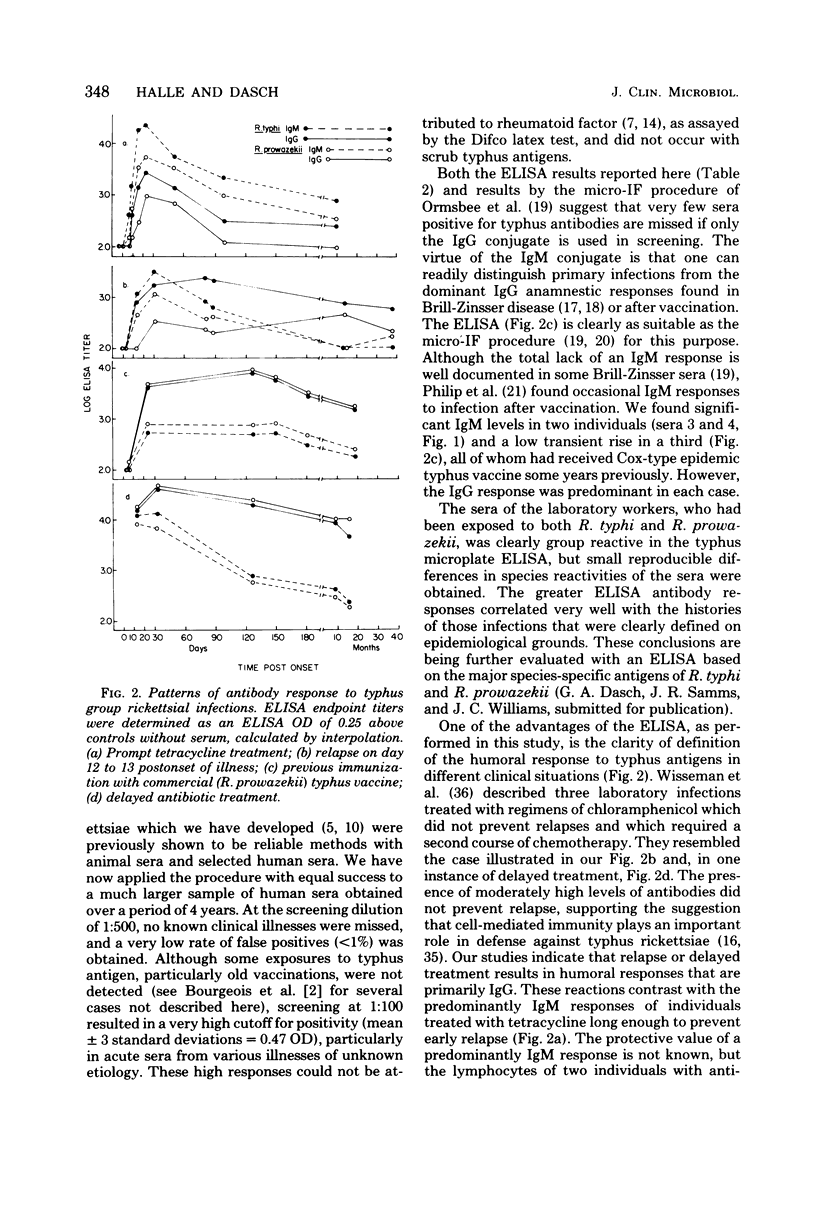
Abstract
A microplate enzyme-linked immunosorbent assay (ELISA), developed for the detection of antibodies to typhus group rickettsiae, was used to analyze human sera from individuals engaged directly or indirectly in rickettsial research. The earliest serum available from each of 112 individuals was tested for immunoglobulin M (IgM) and IgG antibodies against Rickettsia typhi and Rickettsia prowazekii by ELISA at a 1:500 dilution. In at least one assay, nine sera had ELISA optical densities of greater than 0.2, which were above the mean optical densities plus three standard deviations of the other 103 sera. Three of the positive sera were from individuals with known clinical cases of typhus infection. The other sera with predominantly IgG titers were from individuals with extended laboratory exposure to rickettsiae or histories of typhus vaccination, or both. During continued serological surveillance, eight additional people with repeated occupational exposure to typhus rickettsiae had seroconversions in the ELISA to optical densities of greater than 0.2. No apparent clinical illness occurred in two individuals, whereas six clinical cases of infection occurred in others subsequent to accidental laboratory autoinoculation (one) or aerosol exposures (five). In the clinical infections, antibodies were first detected at 7 days, but in subsequent sera, rises and declines in titers were quite variable and were influenced by vaccination, relapse, and time and extent of antibiotic therapy. In primary infections the sera of several individuals who received immediate antibiotic therapy had brief strong IgM responses without pronounced increases in IgG. In contrast, much higher IgG levels were attained in three cases in which relapse occurred, the individual had previously been immunized, or treatment had been delayed. The microplate ELISA proved to be a highly sensitive and reliable test for detection of the human serological response to typhus antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine H. M., Marx R., Barre A. Considerations cliniques et immunologiques à propos d'un cas de typhus murin (contamination de laboratoire) Bull Mens Soc Med Mil Fr. 1965 Jul;59(7):419–423. [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. HLA-D restriction of the macrophage-dependent response of immune human T lymphocytes to PPD in vitro: inhibition by anti-HLA-DR antisera. Scand J Immunol. 1978;8(1):63–73. doi: 10.1111/j.1365-3083.1978.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois A. L., Dasch G. A., Strong D. M. In vitro stimulation of human peripheral blood lymphocytes by soluble and membrane fractions of renografin-purified typhus group rickettsiae. Infect Immun. 1980 Feb;27(2):483–491. doi: 10.1128/iai.27.2.483-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calia F. M., Bartelloni P. J., McKinney R. W. Rocky Mountain spotted fever. Laboratory infection in a vaccinated individual. JAMA. 1970 Mar 23;211(12):2012–2014. [PubMed] [Google Scholar]
- Curet L. B., Paust J. C. Transmission of Q fever from experimental sheep to laboratory personnel. Am J Obstet Gynecol. 1972 Oct 15;114(4):566–568. doi: 10.1016/0002-9378(72)90222-0. [DOI] [PubMed] [Google Scholar]
- Dasch G. A., Halle S., Bourgeois A. L. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J Clin Microbiol. 1979 Jan;9(1):38–48. doi: 10.1128/jcm.9.1.38-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Samms J. R., Weiss E. Biochemical characteristics of typhus group rickettsiae with special attention to the Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1978 Feb;19(2):676–685. doi: 10.1128/iai.19.2.676-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duermeyer W., van der Veen J. Specific detection of IgM-antibodies by ELISA, applied in hepatitis-A. Lancet. 1978 Sep 23;2(8091):684–685. doi: 10.1016/s0140-6736(78)92802-7. [DOI] [PubMed] [Google Scholar]
- FLECK L., PORAT S., EVENCHIK Z., KLINGBERG M. A. The renal excretion of specific microbial substances during the course of infection with murine typhus rickettsiae. Am J Hyg. 1960 Nov;72:351–361. doi: 10.1093/oxfordjournals.aje.a120158. [DOI] [PubMed] [Google Scholar]
- GOLDWASSER R. A., SHEPARD C. C. Fluorescent antibody methods in the differentiation of murine and epidemic typhus sera; specificity changes resulting from previous immunization. J Immunol. 1959 Apr;82(4):373–380. [PubMed] [Google Scholar]
- Halle S., Dasch G. A., Weiss E. Sensitive enzyme-linked immunosorbent assay for detection of antibodies against typhus rickettsiae, Rickettsia prowazekii and Rickettsia typhi. J Clin Microbiol. 1977 Aug;6(2):101–110. doi: 10.1128/jcm.6.2.101-110.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., 3rd, Kadull P. J. Rocky Mountain spotted fever acquired in a laboratory. N Engl J Med. 1967 Oct 19;277(16):842–847. doi: 10.1056/NEJM196710192771603. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Kadull P. J. Laboratory-acquired Q fever. A report of fifty cases. Am J Med. 1966 Sep;41(3):391–403. doi: 10.1016/0002-9343(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Kosina F., Kolouch Z. Laboratorní nákaza horeckou Q. Cas Lek Cesk. 1975 Jan 31;114(5):134–136. [PubMed] [Google Scholar]
- Leinikki P. O., Shekarchi I., Dorsett P., Sever J. L. Determination of virus-specific IgM antibodies by using ELISA: elimination of false-positive results with protein A-Sepharose absorption and subsequent IgM antibody assay. J Lab Clin Med. 1978 Dec;92(6):849–857. [PubMed] [Google Scholar]
- MURRAY E. S., O'CONNOR J. M., GAON J. A. DIFFERENTIATION OF 19S AND 7S COMPLEMENT FIXING ANTIBODIES IN PRIMARY VERSUS RECRUDESCENT TYPHUS BY EITHER ETHANETHIOL OR HEAT. Proc Soc Exp Biol Med. 1965 May;119:291–297. doi: 10.3181/00379727-119-30161. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S., O'CONNOR J. M., GAON J. A. SEROLOGIC STUDIES OF PRIMARY EPIDEMIC TYPHUS AND RECRUDESCENT TYPHUS (BRILL-ZINSSER DISEASE). II. DIFFERENCES IN IMMUNOELECTROPHORETIC PATTERNS, RESPONSE TO 2-MERCAPTOETHANOL AND RELATIONSHIPS TO 19 S AND 7 S ANTIBODIES. J Immunol. 1965 May;94:734–740. [PubMed] [Google Scholar]
- Miller M. B., Blankenship R., Bratton J. L., Lohr D. C., Hunt J., Reynolds R. D. Murine typhus in Vietnam. Mil Med. 1974 Mar;139(3):184–186. [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. G., Jr, Fiset P. Mechanisms of immunity in typhus infection: adoptive transfer of immunity to Rickettsia mooseri. Infect Immun. 1979 May;24(2):387–393. doi: 10.1128/iai.24.2.387-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormsbee R., Peacock M., Philip R., Casper E., Plorde J., Gabre-Kidan T., Wright L. Serologic diagnosis of epidemic typhus fever. Am J Epidemiol. 1977 Mar;105(3):261–271. doi: 10.1093/oxfordjournals.aje.a112382. [DOI] [PubMed] [Google Scholar]
- Oster C. N., Burke D. S., Kenyon R. H., Ascher M. S., Harber P., Pedersen C. E., Jr Laboratory-acquired Rocky Mountain spotted fever. The hazard of aerosol transmission. N Engl J Med. 1977 Oct 20;297(16):859–863. doi: 10.1056/NEJM197710202971604. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike R. M. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab Sci. 1976 Apr;13(2):105–114. [PubMed] [Google Scholar]
- Pultar J. Onemochnení Q-horeckou pri laboratorní epidemii. Cas Lek Cesk. 1966 Sep 9;105(36):1006–1008. [PubMed] [Google Scholar]
- Saunders G. C., Clinard E. H., Bartlett M. L., Sanders W. M. Application of the indirect enzyme-labeled antibody microtest to the detection and surveillance of animal diseases. J Infect Dis. 1977 Oct;136 (Suppl):S258–S266. doi: 10.1093/infdis/136.supplement_2.s258. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Van Weemen B. K. Enzyme-immunoassay. Clin Chim Acta. 1977 Nov 15;81(1):1–40. doi: 10.1016/0009-8981(77)90410-7. [DOI] [PubMed] [Google Scholar]
- Sexton D. J., Gallis H. A., McRae J. R., Cate T. R. Letter: Possible needle-associated Rocky Mountain spotted fever. N Engl J Med. 1975 Mar 20;292(12):645–645. doi: 10.1056/nejm197503202921217. [DOI] [PubMed] [Google Scholar]
- Van Peenen P. F., Koesharjono C., See R., Bourgeois A. L., Irving G. S. Antibodies against murine typhyus in sera from Indonesians. Trans R Soc Trop Med Hyg. 1977;71(4):297–299. doi: 10.1016/0035-9203(77)90103-1. [DOI] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, WOOD W. H., Jr, NORIEGA A. R., JORDAN M. E., RILL D. J. Antibodies and clinical relapse of murine typhus fever following early chemotherapy. Ann Intern Med. 1962 Nov;57:743–754. doi: 10.7326/0003-4819-57-5-743. [DOI] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom G. B. Enzyme-immunoassay. Clin Chem. 1976 Aug;22(8):1243–1255. [PubMed] [Google Scholar]
- Woodward T. E., Pedersen C. E., Jr, Oster C. N., Bagley L. R., Romberger J., Snyder M. J. Prompt confirmation of Rocky Mountain spotted fever: identification of rickettsiae in skin tissues. J Infect Dis. 1976 Sep;134(3):297–301. doi: 10.1093/infdis/134.3.297. [DOI] [PubMed] [Google Scholar]
- Wright L. J., Barker L. F., Mickenberg I. D., Wolff S. M. Laboratory-acquired typhus fevers. Ann Intern Med. 1968 Oct;69(4):731–738. doi: 10.7326/0003-4819-69-4-731. [DOI] [PubMed] [Google Scholar]


