Abstract
The timing of ovulation is critically important to the success of reproduction. Current thinking attributes the timing of ovulation to LH secretion by the pituitary, itself timed by signals from the hypothalamus. The discovery of an internal circadian timer in the ovary raises the possibility that ovulation is in fact timed by an interaction between clocks in the hypothalamus/pituitary and those in the ovary. We asked whether ovarian clocks were influenced by signals from the brain and pituitary. Ovaries of Period1-luciferase transgenic rats display circadian rhythms in vitro. To determine whether the phase of these rhythms is set by neural or endocrine signals, we surgically denervated or heterotopically transplanted ovaries with or without encapsulation in dialysis membranes. Animals’ light-dark cycles were phase advanced or delayed 6 h, and the resetting of the ovarian clock was tracked by culturing ovaries at intervals over the next 12 d. Resetting trajectories of control, surgically denervated, and encapsulated ovaries were similar, demonstrating that endocrine signals are sufficient to transmit phase information to the ovary. We next evaluated LH and FSH as potential endocrine signals. Using the phase of Per1-luc expression in granulosa cell cultures, we demonstrated that both of these pituitary hormones caused large phase shifts when applied to the cultured cells. We hypothesize that the ovarian circadian clock is entrained by hormonal signals from the pituitary and that ovulation depends, in part, on the phase in the ovarian circadian cycle at which these signals occur.
The circadian clock in the ovary is synchronized to the central clock in the suprachiasmatic nucleus and entrained to the environment by humoral signals, which include the gonadotrophins.
In mammals, light signals received by photoreceptor cells in the retina are transmitted to the hypothalamic suprachiasmatic nucleus (SCN) where they entrain circadian oscillators. In addition, most peripheral tissues contain self-sustaining circadian oscillators that continue to show rhythms of gene expression in culture (1). When animals are entrained to a light-dark (LD) cycle, phase relationships between oscillators in the SCN, and individual peripheral oscillators are kept in tissue-specific temporal relationships (1,2); SCN lesions disrupt these phase relationships (3). These results reinforce the concept of the SCN as a dominant central oscillator that regulates phase in the periphery. Support for this idea also comes from phase-shifting experiments. After a 6-h LD cycle shift, the SCN entrains to the new LD cycle in a few days, whereas peripheral oscillators shift more slowly to the new phase (1,2). Interestingly, the rate at which these shifts occur varies among peripheral tissues.
Parabiosis between intact and SCN-lesioned mice suggests that nonneural signals are adequate to maintain circadian rhythms in some, but not all, peripheral tissues (4). Therefore, it is possible that each peripheral oscillator is synchronized by a unique signal, perhaps related to its physiological function. Transplanting SCN tissue into the brain of arrhythmic SCN-lesioned animals restores circadian rhythmicity in behavior (5,6,7), whereas endocrine outputs remain arrhythmic (8). Restoration of the activity rhythms is thought to be due to diffusible signals from the SCN transplant (9), such as prokineticin 2 (10,11) and TGF-α (12). On the other hand, little is known about the mechanisms by which the SCN controls the phase of peripheral oscillators. Although the circadian control of pituitary peptide hormone secretion has been thoroughly examined (13,14), it remains to be determined whether these hormones affect the timing of circadian clocks in target organs such as the ovary.
The clock genes (Per1, Per2, and Bmal1) and their transcripts are expressed rhythmically in rat ovary (15,16,17). Rhythmic expression was observed in both granulosa cells and thecal cells within follicles and in corpora lutea (16,17). The peak phases of Per2 and Bmal1 are in antiphase, indicating that the ovary contains a molecular clock similar to that in the SCN and other peripheral oscillators (16). In a survey of peripheral tissues from transgenic Per1-luciferase rats, we found that the ovary contains robust self-sustaining circadian oscillators. We hypothesize that the circadian oscillator in the ovary may be of critical importance to its physiological function.
The aim of the experiments reported here was to understand how the phase of the circadian oscillator in the ovary is regulated. To this end, we first evaluated the importance of neural and/or endocrine control. The rat ovary has two major sympathetic nerve inputs that can be surgically sectioned: the superior ovarian nerve and the ovarian plexus (18). If these sympathetic nerves are conduits for transmission of circadian timing cues to the ovary, sectioning them should eliminate or reduce the ovary’s ability to be synchronized by the SCN and slow or abolish its ability to adjust its phase after a shift of the LD cycle. We compared the resetting trajectories of circadian oscillators in intact and nerve-sectioned ovaries after LD cycle shifts. Because the surgical approach we employed eliminated most, but not all, sympathetic input, in a second experiment, we completely eliminated neural input to the ovary by transplanting it heterotopically and, additionally, encapsulating it in a dialysis membrane. The phase-resetting kinetics of the transplanted and encapsulated ovaries was analyzed to determine the importance of endocrine signals in synchronizing ovarian circadian oscillators. Finally, we attempted to identify the endocrine signals that control circadian phase in the ovary. We focused on the pituitary gonadotropins LH and FSH, which are known to have major effects on ovarian physiology.
Materials and Methods
Animals
Female Period1-luciferase (Per1-luc) transgenic rats (1) were bred in the animal facility at the University of Virginia under a 12-h light, 12-h dark cycle (lights on at 0400 h, off at 1600 h; intensity was approximately 100 lux at cage level). Animals were housed in light- and temperature-controlled boxes with food and water provided ad libitum. Adult rats (2–3 months old) were used in nerve sectioning and ovarian transplant experiments. Immature females (6–7 wk old) were used in ovarian cell culture experiments. All procedures were approved by the Animal Care and Use Committee at the University of Virginia.
Ovarian nerve-sectioning surgery
Approximately 2 mm of the superior ovarian nerve along with the ovarian ligament was removed directly above the left ovary. The left ovarian plexus and the ovarian artery were sectioned. No contact was made with the ovary during these procedures. The superior ovarian nerve and ovarian plexus on the right side were left intact, and the right ovary served as a control. In some animals, the left ovarian plexus was sectioned without damaging the ovarian artery. Results from those animals were combined with the results from the animals in which the ovarian artery was sectioned because there were no differences in Per1-luc rhythmicity as a result of these two approaches. After 1 wk recovery, animals were subjected to a shift of the LD cycle as described below.
Ovary transplant surgery
The ovary on one side was removed and sliced into four parts in saline solution. Two of the four slices were transplanted directly into a sc pocket in the femoral area (sc transplant; SQ). The other two slices were packed in a dialysis membrane (Spectra/Por 7, molecular cutoff 50,000 kDa; Spectrum, Rancho Dominguez, CA) before being transplanted to a sc pocket in the other side of the femoral area (encapsulated sc transplant; E-SQ). The other ovary was left intact to serve as a control. After 1 wk recovery, animals were subjected to a shift of the LD cycle.
LD cycle shifts
Six-hour light cycle phase shifts were performed as previously described (1,2). Briefly, the 6-h advance in the light schedule was accomplished by advancing the time of lights on, leading to a short 6-h night. The 6-h phase delay was accomplished by delaying the time of lights off, resulting in a long 18-h day. Animals were euthanized with CO2, and tissues were removed for culture 1 h before lights off on the day before the LD cycle shift (baseline) or 1, 6, or 12 d after the shift. We have found that killing within 1–2 h of lights off avoids the confounding effects of culture time on peak phase in vitro.
Ovarian tissue culture
Ovaries were quickly removed and chilled in Hanks’ balanced salt solution (HBSS) at 4 C. Follicles and corpora lutea were separated from the ovaries and cultured. Bioluminescence was measured as previously described (1,2,19). Briefly, tissues were placed in 35-mm culture dishes with 1.2 ml culture medium [DMEM (D2902; Sigma Chemical Co., St. Louis, MO), supplemented with B27 (Invitrogen, Carlsbad, CA), 10 mm HEPES, 352.5 μg/ml NaHCO3, 3.5 mg/ml d-glucose, 25 U/ml penicillin, 25 μg/ml streptomycin, and 0.1 mm beetle luciferin (Promega, Madison, WI)], sealed with sterile vacuum grease and a coverglass. Sealed cultures were maintained at 36 C in a light-tight incubator, and bioluminescence was continuously monitored with photomultiplier tubes (Hamamatsu, Bridgewater, NJ).
Granulosa cell culture
Immature rats were injected with pregnant mare serum gonadotropin (Calbiochem, San Diego, CA; 30 IU) at 1400 h (2 h before lights off) to induce follicular development. Forty-eight hours later, animals were euthanized with CO2, and the ovaries were quickly removed and placed in chilled HBSS. Ovaries were separated from peritoneal fat and oviducts, rinsed in clean HBSS, and placed in 0.6 ml chilled cell culture media. After the ovaries were transferred to chilled media, granulosa cells were recovered by manual puncture of follicles with a 23-gauge needle followed by slight pressure applied with a sterile spatula. Cells were dissociated by gentle trituration with a micropipetter. Six cultures were obtained from each rat (5–6.5 × 105 cells per culture; three cultures per ovary). Each 35-mm culture dish contained 1.2 ml cell suspension. Cultures were sealed and incubated at 36 C for 5 d. On d 5, the cultures were exposed to fresh media containing luciferin and placed inside a bioluminescence recording chamber. Bioluminescence recording was temporarily stopped after 80–108 h, and 10 μl sterile saline containing various concentrations of equine LH (L9773; Sigma) or porcine FSH (F2293; Sigma) were added to the medium (0, 1 × 10−6, 1 × 10−5, 1 × 10−4, 1 × 10−3, 1 × 10−2, 1 × 10−1 or 1.0 IU/1.2 ml culture medium). Bioluminescence recording was resumed and continued for a minimum of 3 d. This paradigm produced a steep, square-wave increase in hormone level that remained in the culture throughout the remainder of the recording.
Given the fact that exposure to fresh cell culture media has been shown to induce phase shifts in tissue explants, we developed a method to deliver shorter-duration LH or FSH treatments while avoiding the confounding response to a medium change. In a separate experiment, two equal groups of cultures were prepared as described above, and luminescence recording was initiated. Bioluminescence recording was temporarily stopped, and LH or FSH was added to one group of cultures (0, 1 × 10−6 or 1 × 10−4 IU/1.2 ml culture medium), whereas the duplicate cultures were left untreated. After 1.5 or 3 h exposure to gonadotropin or saline vehicle, culture medium containing hormone was removed and replaced with medium from the untreated duplicate cultures, which we expect to be depleted of nutrients to the same degree as the LH- or FSH-treated cultures (referred to as conditioned medium; see Fig. 5A for description). Bioluminescence recording was resumed and continued for a minimum of 3 d. Regardless of the method used for hormone treatment, the peak phase of Per1-luc expression before and after addition of LH or FSH was determined as described below.
Figure 5.
Gonadotropin-induced phase shifts of the Per1-luc rhythm in cultured granulosa cells after transient exposure and conditioned medium replacement. A, Experimental protocol for giving LH or FSH treatments of short duration to granulosa cell cultures. At the start of the experiment, two equal groups of cultures were prepared. Hormone was added to one group of cultures, and 1.5 or 3 h later, the hormone-containing medium was removed and replaced with medium from the untreated group of cultures. B, Significant phase delays were produced by LH [1.5-h dose (5/5), top panel; 3-h dose (3/ 3), middle panel] or FSH (3 h; 5/5, bottom panel). Shaded areas indicate timing of LH or FSH treatments. C, Mean (±sem) phase delays induced by the gonadotropins (black bar, 1 × 10−6 IU/1.2 ml; white bars, 1 × 10−4 IU/1.2 ml). Both high (1 × 10−4 IU/1.2 ml) and low (1 × 10−6 IU/1.2 ml) doses induced significant delays when compared with vehicle treatment (t test: #, P < 0.05). The higher dose induced larger phase delays than the low dose (1 × 10−6 IU vs. 1 × 10−4 IU, t test: *, P < 0.02; **, P < 0.01).
Bioluminescence was also recorded from individual granulosa cells in monolayer culture. For single-cell imaging, granulosa cells were cultured on poly-l-lysine-coated 35-mm glass-bottom culture dishes (Mat-Tek, Ashland, MA) for 5 d as described above. After a medium change on d 5, cultures were placed within our light- and temperature-controlled bioluminescence imaging system. Cultures were imaged with a cooled ICCD camera (XR-Mega 10Z; Stanford Photonics, Palo Alto, CA) attached to an Olympus CKX-41 inverted compound microscope with a heated stage (35 C). Images were collected at 1-min intervals (16 bit at 1240 × 1024 resolution), further integrated offline into 3-min images and smoothed with a 3-h moving average. Images were then imported into ImageJ (National Institutes of Health, Bethesda, MD) as TIFF sequences at 30-min intervals (48 images every 24 h), and individual cells were identified as regions of interest and analyzed for pixel intensity over time.
Data analysis
Bioluminescence data sets were detrended and smoothed as previously described (2,19). Cultures that expressed more than two circadian cycles were considered rhythmic. The point of maximum bioluminescence in each cycle was considered the peak phase. The peak phase of the first full cycle in culture was graphed with a modified version of the Rayleigh plot. The peak phase for each culture was plotted as a unit of degrees (θ) on a circle. An average vector position was calculated based on the distribution of phase points around the unit circle where X = mean SINθ and Y = mean COSθ. The vector length, equal to the square root of the sum of squares for X and Y, was then calculated for each group. The angle of the vector corresponds to the mean phase for the group, and the length of the vector is inversely related to the variability in phase clustering, such that the lower the variability, the longer the vector identifying the mean phase. A Rayleigh test was used to verify significant phase clustering around the vector (20,21). Comparisons between Rayleigh vectors were made with the Watson-Williams f test. The magnitude of phase shifts between treatment groups was analyzed with one-factor ANOVA followed by a t test or a Tukey’s post hoc test. Rayleigh tests and Watson-Williams f test were conducted with Oriana software (Kovach Computer Services, Anglesey, Wales, UK). One-factor ANOVA and Student’s t test were performed with Origin Pro (Origin Labs, Northampton, MA). For all statistical analysis, results were considered significant at P < 0.05.
Results
Phase resetting of the ovarian circadian clock after ovarian nerve section
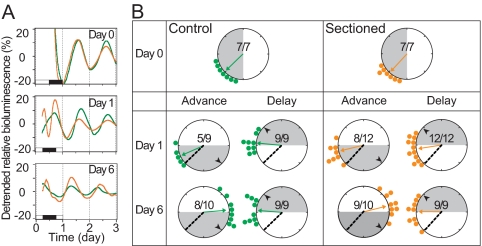
To evaluate the importance of sympathetic nerve input to the phase of the Per1-luc rhythm in the ovary, the phase resetting of the rhythm was compared between intact and sympathetic nerve-sectioned ovaries immediately before or 1 and 6 d after an advance or delay of the LD cycle. In each Rayleigh plot, regardless of surgical treatment or phase shift, we observed significant phase clustering around the mean (Rayleigh tests; P < 0.05). No significant difference was seen between control and nerve-sectioned ovarian cultures in the peak phase of Per1-luc expression before the LD cycle shifts (d 0 in Fig. 1, A and B; Watson-Williams f test, P > 0.05). Furthermore, there were no differences in the phase-shifting trajectories of intact and nerve-sectioned ovaries. Surprisingly, on the first day after the 6-h advance, cultures from nerve-sectioned, but not control, ovaries displayed a delay in the phase of peak Per1-luc expression (P < 0.05 compared with d 0). Cultures prepared from both control and nerve-sectioned ovaries failed to reach the target phase within 6 d of the advance (Fig. 1B). In addition, we observed a decrease in the number of rhythmic cultures after a 6-h advance, regardless of surgical manipulation. As seen in Table 1, we found that advanced cultures from control and nerve-sectioned ovaries were only 68 and 77% rhythmic, compared with 100% rhythmicity in cultures taken from d 0 or after a 6-h delay.
Figure 1.
Effects of surgical denervation on the resetting trajectory of the ovarian clock. A, Representative Per1-luc expression rhythms of cultured ovaries from animals subjected to a 6-h advance of the LD cycle (green, control; orange, nerve-sectioned). Black and white bars at the bottom of each panel on d 1 indicate the LD cycles to which the animals were exposed before euthanasia and tissue collection. The cultures were prepared before the LD cycle shift (d 0, top panel), on d 1 (middle panel), and on d 6 (bottom panel) of the shifted LD cycle. When data from all the cultures were analyzed, there was no significant difference in phase or amplitude of the rhythms between control and nerve-sectioned ovaries. B, Resetting trajectories of Per1-luc expression rhythms in the control and nerve-sectioned ovaries. Time is indicated by position on the circle. Light conditions at the time of killing are shown by shading. The peak phase of each culture is represented by small circles (green, control ovary; orange, nerve-sectioned ovary). Arrows inside the large circles indicate the average phase of each group. The degree of synchrony within each group is directly related to the length of the arrow. Black arrowheads within the large circles show the point of complete phase shift, or target (i.e. 6 h advanced or delayed from the phase on d 0). The dotted line within the large circle indicates the mean phase before the shift (baseline). Numbers in the large circles indicate the number of rhythmic cultures over the number of total cultures. Note that this ratio is always 1 except for cultures from advanced animals.
Table 1.
Effects of phase shifts on the degree of rhythmicity in ovarian explant cultures
| Experimental group | Rhythmic culture (%) | Rhythmic/total |
|---|---|---|
| Nerve sectioned | ||
| Control | ||
| d 0 | 100 | 7/7 |
| Delay | 100 | 18/18 |
| Advance | 68 | 13/19 |
| Sectioned | ||
| d 0 | 100 | 7/7 |
| Delay | 100 | 21/21 |
| Advance | 77 | 17/22 |
| SQ and E-SQ | ||
| Control | ||
| d 0 | 92 | 11/12 |
| Delay | 97 | 30/31 |
| Advance | 86 | 24/28 |
| Transplants | ||
| d 0 | 83 | 19/23 |
| Delay | 93 | 55/59 |
| Advance | 59 | 35/59 |
On the first day after the delay LD cycle shift, the peak phases of the Per1-luc rhythms in the control and nerve-sectioned ovaries were delayed more than 2 h (P < 0.05 for both when compared with d 0). No further delay was seen in the cultures prepared on d 6 (Fig. 1B). Therefore, like cultures from animals in an advanced LD cycle, these tissues did not reach their target phase within 6 d of the shift. We did not observe a significant difference in phase between control and nerve-sectioned ovaries 6 d after either a phase advance or delay. These data show a lack of significant differences in rate of phase shifting between the control and nerve-sectioned ovaries, suggesting that sympathetic input is not necessary for controlling the phase of circadian oscillators in the ovary.
Phase resetting of the ovarian circadian clock after heterotopic ovarian transplant
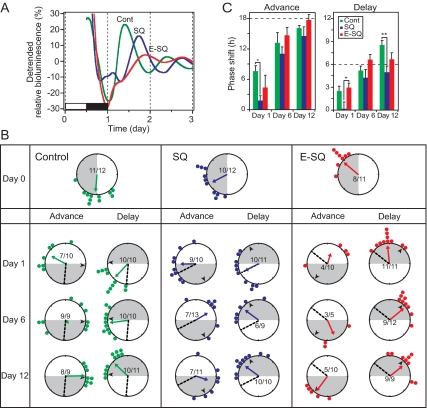
To eliminate the influence of neural input to the ovary, fragments of ovarian tissue were removed and transplanted into a sc pocket. Half of these fragments were encapsulated in a dialysis membrane before being transplanted to the sc space. Cultures of control (intact), sc (SQ) and encapsulated (E-SQ) transplants all showed rhythmic Per1-luc expression (Fig. 2A). Peak phases of the rhythms in these three groups were significantly different from each other before the LD cycle shifts, with control cultures peaking 3–9 h before SQ and E-SQ cultures (Fig. 2, A and B, Watson-Williams f test P < 0.01). As in the nerve section experiment, all cultures delayed their Per1-luc rhythms after a 6-h advance of the LD cycle. On the first day after the 6-h advance, SQ transplants showed a slightly delayed peak phase of the Per1-luc rhythm. However, the amount of the shift was smaller than that in E-SQ transplants (Fig. 2B). Peak phase variability was significantly increased in control ovaries on d 6 after the advance, in SQ ovaries on d 6 and 12, and in E-SQ cultures on d 1 and 6 (Rayleigh test, P > 0.05; Fig. 2B). These data are not surprising and are supported by the data shown on Table 1. As in the nerve section experiment, we observed a decline in the number of rhythmic cultures from control ovaries after a 6-h advance (86 vs. 92% on d 0 and 97% after delays; Table 1). This effect was even more pronounced in transplanted ovaries (advance 59 vs. 83% on d 0 and 93% after delays; Table 1). Entrainment of Per1-luc rhythms to the advanced LD cycles in control ovary and E-SQ transplants were accomplished by d 12 (i.e. they reached their target phases), by which time SQ transplants were not completely entrained (Fig. 2B).
Figure 2.
Phase resetting of the ovarian clock after heterotopic transplant with or without isolation in dialysis membranes. A, Representative Per1-luc expression rhythms from cultured ovaries prepared before a 6-h LD cycle shift (Cont, control ovary). The black and white bar at the bottom of the panel on d 1 indicates the LD cycle to which the animals had been entrained before euthanasia and tissue collection. Encapsulated cultures consistently showed lower-amplitude rhythms. B, Resetting trajectories of Per1-luc expression rhythms in control and transplanted ovaries. Control ovary, SQ, and E-SQ transplants were cultured 0, 1, 6, and 12 d after the light cycle shifts (details are as in Fig. 1). Advance and delay shifts were completed by d 12 and 6, respectively, in the control ovaries and the E-SQ transplants, whereas SQ transplants did not reach to the target phase by d 12 after either advance or delay shifts. The dotted line within the large circle indicates the mean phase before the shift (baseline). C, Amplitude (mean ± sem) of phase shifts in Per1-luc expression rhythms in control and transplanted ovaries after a 6-h phase advance (left panel) or delay (right panel) of the LD cycle. The dashed lines in both panels of C represent the target phase (marked with a black arrowhead in B), and a positive value on the y-axis indicates a phase delay. *, P < 0.05; **, P < 0.01, ANOVA followed by Tukey post hoc test).
One day after the 6-h phase delay, Per1-luc rhythms in intact ovaries and E-SQ transplants were phase shifted (P < 0.05 vs. d 0), whereas SQ transplants did not shift at all (Fig. 2B). By d 6 of the delayed LD cycle, Per1-luc rhythms in the control ovary and E-SQ transplants reached their target phases (the control ovaries overshot the target phase by d 12). Peak phases of SQ transplants did not quite reach their target phases by d 6, and no further phase delay was seen on d 12.
It is possible that inter-ovarian signals between heterotopic transplants and contralateral control ovaries could account for phase variability between groups in the unshifted baseline cultures. To address this question, we conducted an additional experiment in which animals were divided into three groups: 1) unilateral ovariectomy (OVX), 2) bilateral OVX followed by sc heterotopic transplant of two ovarian fragments (SQ), and 3) bilateral OVX followed by sc heterotopic transplant of two ovarian fragments within a dialysis membrane (E-SQ). After 7 d, animals were euthanized, and ovarian tissue was cultured as described in Materials and Methods. There was not a significant difference between the mean baseline phase of Per1-luc expression in ovaries taken from animals with multiple transplants (see Fig. 2) and animals with a single untouched control ovary, a single SQ transplant or a single E-SQ ovarian transplant (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). These data suggest that the presence of multiple ovarian transplants, along with untouched control tissues, does not contribute to the phase of Per1-luc expression.
Gonadotropins induce phase shifts of Per1-luc rhythms in granulosa cell cultures
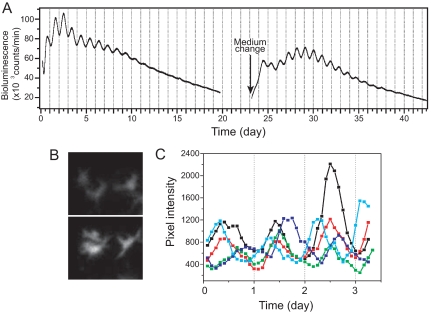
Granulosa cell cultures showed stable Per1-luc expression rhythms and could be kept for more than a month when the culture medium was changed regularly (Fig. 3A). Although the amplitude of the rhythm and total bioluminescence counts gradually decreased, they could be restored by changing the medium. Bioluminescence recording using our ICCD camera revealed variability in peak phase among individual cells (Fig. 3C).
Figure 3.
Per1-luc expression in primary granulosa cell cultures. A, Per1-luc expression rhythm recorded by photomultiplier tube from a granulosa cell culture. Although rhythmicity gradually damped, it was restored by a medium change. B, Bioluminescence image of cultured granulosa cells recorded with an ICCD camera. A set of three cells are shown at the times of low (upper panel) and high (12 h later, lower panel) Per1-luc expression. C, Superimposed plots of Per1-luc rhythms from five individual granulosa cells in a culture recorded with an ICCD camera. Individual cells showed different peak phases. Cells were imaged after 5 d in vitro immediately after a medium change.
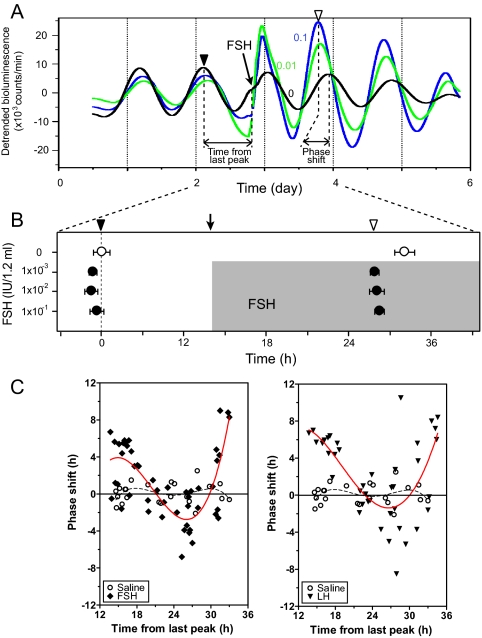
Nerve sectioning and transplant experiments indicated that endocrine signals have the ability to resynchronize ovarian circadian oscillators to shifted LD cycles. Gonadotropins are plausible candidates for such endocrine signals because they are periodically secreted under tight regulation by the SCN. We investigated the effects of FSH and LH on Per1-luc rhythms in granulosa cell cultures. When FSH was added to granulosa cell cultures, we observed phase shifts of Per1-luc expression on the following day (Fig. 4, A and B). Vehicle (saline) did not induce significant phase shifts. Similar phase shifts were seen when LH was added to granulosa cell cultures (Fig. 5A). Interestingly, the amount and direction of the shift depended on the time at which FSH or LH was added to the cultures, i.e. there was a phase-response curve (PRC) to both hormone treatments (Fig. 4C), but no dose dependency was observed (Fig. 4B). Both FSH and LH increased the amplitude of the Per1-luc rhythm in granulosa cells (Figs. 4A and 5A).
Figure 4.
Gonadotropin-induced phase shifts of the Per1-luc rhythm in cultured granulosa cells. A, Representative bioluminescence rhythm from three granulosa cell cultures. Culture medium was changed at the beginning of the record. Vehicle (black) or FSH at two concentrations (0.1 IU/1.2 ml, dark blue; 1.0 IU/1.2 ml, green) was added to the cultures at the time indicated by the vertical arrow. Black and white arrowheads indicate peak phases of cultures on the day before and after the addition of FSH, respectively. B, Mean peak phases (±sem) of cultures before and after addition of FSH to culture [rhythmic cultures/total cultures: controls 0 IU/1.2 ml (3/3); FSH 1 × 10−3 IU /1.2 ml (4/4), 1 × 10−2 IU /1.2 ml (4/4), 1 × 10−1 IU /1.2 ml (4/4)]. The peak phase of control culture is plotted as 0 h. The shaded area indicates the presence of FSH in the cultures. Cultures treated with FSH (•) are phase advanced compared with control cultures (○, saline treated); however, no dose dependency was seen within the range of concentrations tested (1 × 10−6 to 1.0 IU/ 1.2 ml). C, PRCs of Per1-luc rhythms in response to several concentrations of FSH and LH (1 × 10−6, 1 × 10−5, 1 × 10−4, 1 × 10−3, 1 × 10−2, 1 × 10−1 IU/1.2 ml) added to cultures of granulosa cells at different circadian times. The amount of the phase shift is plotted against the duration of time since the last peak of Per1-luc expression. The amount and direction of the shift depend on the time of FSH or LH application to the cultures but not on the concentration of hormone. The effect of vehicle addition is indicated by open circles; the effect of addition of hormone is indicated by filled diamonds (FSH) or triangles (LH). Data from the various concentrations of hormone are not indicated because we did not observe a dose-dependent response. In C, the solid red lines represent nonlinear best-fit lines for the shifted data from both LH- and FSH-treated cultures, whereas the dashed black lines are fitted to the data from saline-treated cultures.
The previous experiment represented a first attempt at replicating the in vivo response of the circadian clock in the ovary to gonadotropins. However, the continuous presence of the hormone in the culture medium did not mimic the in vivo condition. Secretion of LH from the pituitary is normally pulsatile (22). Patterns of FSH secretion throughout the estrous cycle are pulsatile as well (23). Therefore, the experiment that generated the PRC in Fig. 4 (adding hormone to the culture medium without a subsequent medium change) is not comparable to the in vivo condition. In those experiments, the gonadotropins were not removed to avoid the phase-shifting effect that we and others have seen in response to a medium change. To avoid this potential confound, we used conditioned medium to replace the hormone-carrying medium after 1.5 or 3 h (as described in Materials and Methods; see also Fig. 5A). Phase shifts of the Per1-luc rhythm were seen after either 1.5 or 3 h exposure to LH or FSH (for both times and gonadotropins, P < 0.05 vs. vehicle control), whereas there were no phase shifts in control cultures (Fig. 5, B and C). In this experiment, we observed significant dose-dependent effects of both LH and FSH (P < 0.05; Fig. 5C).
Discussion
The phases of circadian oscillators in peripheral tissues are synchronized in large part by signals from the central oscillator in the SCN (3). However, the identity of these signals is largely unknown. We have attempted to distinguish between neural and/or endocrine cues as carriers of synchronizing and entraining information to the ovary.
We first isolated the ovary from neural signals by ipsilateral sectioning of the two major sympathetic inputs, the superior ovarian nerve and the ovarian plexus (18). Signals carried by the superior ovarian nerve modulate spontaneous ovulation (24,25) but the role of the ovarian plexus is poorly understood. We unilaterally sectioned both nerves and measured the effect on the rhythm of Per1-luc expression in the ovary and its resetting trajectory to phase shifts of the LD cycle. This surgical intervention had no effect on the peak phase of Per1-luc expression. Furthermore, the peak phase that we measured [zeitgeber time (ZT) 15.0 ± 0.51 in Fig. 1B; ZT12.4 ± 0.69 in Fig. 2B] was consistent with the peak phase measured ex vivo using quantitative PCR (ZT12–14) (15). Nerve section did not change the trajectory of resetting after a 6-h phase advance or delay of the LD cycle (Fig. 1). There are at least two possible interpretations of this result. Nerves that were not sectioned may carry synchronizing signals to the ovary from the SCN. For example, it has been suggested that there is neural communication from one ovary to the other (26). Therefore, it is possible that signals from the control ovary synchronized the nerve-sectioned ovary. Alternatively, the synchronizing signal may be humoral.
To test the possibility of endocrine control, we used the heterotopic transplant technique (27). Heterotopically transplanted ovaries survive for months and continue hormone production and ovulation without normal circulatory or neural connections (27). Because transplanted ovaries in sc pockets are eventually reinnervated (28) and revascularized (29), half of our transplants were encapsulated in dialysis membrane so that only endocrine signals could reach them. The transplantation procedure itself influenced the phase of Per1-luc rhythmicity. That is, the phases of intact ovaries, SQ, and E-SQ transplants on d 0 were significantly different (Fig. 2B). There are several possible explanations for this result. The SQ transplants may have been reinnervated, whereas the E-SQ transplants were not. Isolation in a dialysis membrane may have restricted the transplants access to oxygen or nutrients, thus influencing the phase of the E-SQ ovaries. It is also possible that the surgical procedure itself produced this effect. However, we did not see this response in animals with unilateral nerve sectioning, suggesting that the differences observed in SQ and E-SQ ovaries are due to the environment the tissue was exposed to after the procedure. After the phase shifts, Per1-luc rhythms of E-SQ transplants shifted with very similar trajectories to those of the control ovaries, whereas the SQ transplants shifted more slowly (Fig. 2, B and C). The increased variability in the phase of peak Per1-luc expression in the control cultures on d 1 after the advance may reflect a potential competition between nervous and humoral cues. These cues may also be redundant or hierarchically organized, as in the case of circadian signals to the salivary glands (30). As with the unshifted cultures (d 0), the nonencapsulated ovarian transplants (SQ) could be affected by either reinnervation or some other aspect of the milieu that did not affect the E-SQ transplants because it was excluded by the dialysis membrane. The SQ transplants contained follicles and corpora lutea and showed signs of ovulation as long as a month after the transplant surgery, whereas the E-SQ transplants do not. Arrhythmic cultures with low bioluminescence occurred more often in E-SQ transplants than in control ovary or SQ transplants, particularly after an advance of the LD cycle. Among rhythmic E-SQ cultures, some showed low-amplitude rhythms (Fig. 2A), which may have been due to limited amounts of oxygen, nutrients, and hormones reaching the E-SQ transplants. Nevertheless, despite operating under less than ideal conditions, the circadian oscillators in the E-SQ transplants shifted with the same trajectory as those in the intact ovary. Taken together, these data provide novel evidence to support endocrine-mediated entrainment of a peripheral circadian clock in the absence of direct innervation.
LH and/or FSH target the ovary and are therefore strong candidates as humoral entraining and synchronizing agents. When either LH or FSH was added to granulosa cell cultures, they induced phase shifts of the Per1-luc rhythm (Figs. 4 and 5). As with the commonly used behavioral phase shifts to light, the phase shifts induced by LH and FSH were phase and intensity (concentration) dependent. The phasic response, especially the shape of the PRCs and their concentration dependency, suggest that LH and/or FSH signals may synchronize the ovarian clock with the central pacemaker in the SCN.
SCN control of rodent estrous cycles has been studied thoroughly (13,14,31). SCN lesion (32,33) or knife cuts severing the SCN efferents (34,35) abolish the estrous cycle, indicating that neural output from the SCN is required to maintain both the estrous cycle and the LH surge. In the absence of periodic synchronizing signals from the SCN (as in our long-term culture without medium change, Fig. 3A) the amplitude of the Per1-luc rhythm in granulosa cell cultures gradually declines. Because the cells have different peak phases (Fig. 3C), the decrease in rhythm amplitude may be due, in part, to desynchronization among cells in dispersal culture. Even in vivo, the ovary may require periodic hormonal entraining signals to keep its cellular oscillators in synchrony. These results suggest a complex pathway in which the light cycle entrains the SCN, the SCN controls the timing of LH and FSH secretion from the pituitary through neural pathways, and these hormonal signals then entrain the circadian oscillators in the ovary. Entraining cues such as light generally act on a daily basis to synchronize the circadian clock in the SCN to the environment. How then do signals arriving once every 4 d, such as the LH and FSH surges, entrain the ovarian clock? There is considerable evidence to suggest that environmental cues with periods that are multiples of 24 h (e.g. one light pulse every few days) can act to entrain circadian clocks (33,36,37,38). Both LH and FSH can induce large phase shifts in vitro, which suggests that they may be able to entrain the ovarian clock in vivo. However, our experiments do not exclude the possible participation of neural signals and/or other peptide and steroid hormones.
In agreement with the results reported by Fahrenkrug et al. (15) using in situ hybridization histochemistry, we found that preovulatory, antral, and preantral follicles isolated from Per1-luc rat ovaries show rhythmic Per1-luc expression in culture, whereas expression in smaller (i.e. less mature) follicles is arrhythmic or of low amplitude (data not shown). This suggests a correlation between responsiveness to gonadotropin and rhythmic Per1-luc expression that may be causal. Recent data suggest that both immature and mature granulosa cells isolated from Per2-dluc rats fail to display a strong circadian rhythm of Per2-dLuc expression before in vitro gonadotropin treatment (17). In contrast, our data show that granulosa cells from gonadotropin-primed (pregnant mare serum gonadotropin-treated) immature rats display robust circadian rhythms of Per1-luc expression in dispersal culture. Perhaps LH and/or FSH is (are) necessary to initiate and sustain rhythmic Per1-luc expression in ovarian follicles. This possibility is supported by our data on the effects of LH and FSH on the amplitude of Per1-luc rhythmicity (Figs. 4 and 5) and results reported by Karman and Tischkau (16). These authors showed that human chorionic gonadotropin treatment induced cyclic expression of Bmal1 and Per2 in the ovaries of hypophysectomized immature rats primed with equine chorionic gonadotropin. Comparable results have been reported in quail; only the largest preovulatory follicles show rhythmic Per2 expression, whereas smaller follicles show constant levels of Per2 expression (39). In the same paper, Nakao and colleagues (39) report that Japanese quail ovaries express circadian rhythms of steroid hormone synthetic enzymes, including the sterol carrier protein, steroid acute regulatory protein (StAR). These data suggest that the circadian clock in the ovary may regulate steroid hormone synthesis. Despite these intriguing correlations, the physiological significance of rhythmic clock gene expression in ovarian follicles remains unknown. One reasonable (and exciting) possibility is that such rhythms influence the phase of the circadian rhythm of ovulation and perhaps, as a consequence, alter the timing and amplitude of steroid hormone secretion. Our data strongly support the hypothesis that humoral cues, such as the gonadotropins, are the major signals synchronizing the ovarian clock to the master oscillator in the SCN, thus performing the vital function of synchronizing events in the ovary with the external environment.
Supplementary Material
Acknowledgments
We gratefully acknowledge the excellent technical assistance of undergraduate students Evagelia Papadimas, Susan Cha, and Aileen Deng. We thank Dr. Jennifer Mohawk for discussions and Naomi Ihara, Denise Holmes, and Jeffrey Hager for skillful technical assistance.
Footnotes
Present address for T.Y.: Department of Chronomedicine, Hokkaido University Graduate School of Medicine, Sapporo 060-8638, Japan.
Disclosure Summary: T.Y., M.S., and M.M. have an National Institute of Mental Health (NIMH) grant pending associated with this research. P.P. was funded for more than $10,000 by the NIMH during the collection of these data.
This work was supported by National Institutes of Health Grant MH56647 and National Space Biomedical Research Institute Grant NCC 9-58-HPF 00406 (to M.M.). T.Y. was supported by a fellowship from the Japan Society for the Promotion of Science for Young Scientists. M.S. is supported by a fellowship from the Center for Reproduction Research at the University of Virginia.
First Published Online June 11, 2009
Abbreviations: E-SQ, Encapsulated sc transplant; HBSS, Hanks’ balanced salt solution; LD, light-dark; OVX, ovariectomy; PRC, phase-response curve; SCN, suprachiasmatic nucleus; SQ, sc transplant; ZT, zeitberger time.
References
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H 2000 Resetting central and peripheral circadian oscillators in transgenic rats. Science 288:682–685 [DOI] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD 2002 Circadian rhythms in isolated brain regions. J Neurosci 22:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS 2004 PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101:5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL 2005 Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA 102:3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL 1987 Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7:1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Lehman MN, Gibson M, Gladstone WR, Bittman EL 1990 Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res 525:45–58 [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M 1990 Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978 [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL 1999 Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN 1996 A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382:810–813 [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY 2002 Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410 [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY 2005 Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci 6:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ 2001 Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294:2511–2515 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R 2006 The regulation of neuroendocrine function: timing is everything. Horm Behav 49:557–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ 2006 Timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153 [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gräs S 2006 Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147:3769–3776 [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA 2006 Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod 75:624–632 [DOI] [PubMed] [Google Scholar]
- He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA 2007 Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem 302:111–118 [DOI] [PubMed] [Google Scholar]
- Lawrence Jr IE, Burden HW 1980 The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec 196:51–59 [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Yamazaki S, Menaker M 2005 Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms 20:500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulivarthy SR, Tanaka N, Welsh DK, De Haro L, Verma IM, Panda S 2007 Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci USA 104:20356–20361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet E 1981 Circular statistics in biology. London: Academic Press [Google Scholar]
- Gallo RV 1980 Neuroendocrine regulation of the pulsatile release of LH. Res Reprod 12:2 [PubMed] [Google Scholar]
- Freeman ME 2006 Neuroendocrine control of the ovarian cycle of the rat. In: Neill J, ed. Physiology of reproduction. St. Louis: Elsevier Academic Press; 2327–2388 [Google Scholar]
- Chavez RA, Carrizosa L, Dominguez R 1991 Effects of superior ovarian nerve section on spontaneous and induced ovulation in adult rats. Med Sci Res 19:41–42 [Google Scholar]
- Morales L, Chávez R, Ayala ME, Domínguez R 1998 Effects of unilateral or bilateral superior ovarian nerve section in prepubertal rats on the ovulatory response to gonadotrophin administration. J Endocrinol 158:213–219 [DOI] [PubMed] [Google Scholar]
- Morales L, Chavez R, Dominguez R 1993 Participation of the superior ovarian nerve in the regulation of ovulation in the prepubertal rat: differential effects of unilateral and bilateral section of the nerve. Med Sci Res 21:15–17 [Google Scholar]
- Callejo J, Jáuregui MT, Valls C, Fernandez ME, Cabré S, Lailla JM 1999 Heterotopic ovarian transplantation without vascular pedicle in syngeneic Lewis rats: six-month control of estradiol and follicle-stimulating hormone concentrations after intraperitoneal and subcutaneous implants. Fertil Steril 72:513–517 [DOI] [PubMed] [Google Scholar]
- Lara HE, Dees WL, Hiney JK, Dissen GA, Rivier C, Ojeda SR 1991 Functional recovery of the developing rat ovary after transplantation: contribution of the extrinsic innervation. Endocrinology 129:1849–1860 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR 1994 Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology 134:1146–1154 [DOI] [PubMed] [Google Scholar]
- Vujovic N, Davidson AJ, Menaker M 2008 Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol 295:R355–R360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacka-Surowiak G, Surowiak J, Stokłosowa S 2003 The involvement of suprachiasmatic nuclei in the regulation of estrous cycles in rodents. Reprod Biol 3:99–129 [PubMed] [Google Scholar]
- Everett JW, Sawyer CH 1950 A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47:198–218 [DOI] [PubMed] [Google Scholar]
- Goldman BD 1999 The circadian timing system and reproduction in mammals. Steroids 64:679–685 [DOI] [PubMed] [Google Scholar]
- Hakim H, DeBernardo AP, Silver R 1991 Circadian locomotor rhythms, but not photoperiodic responses, survive surgical isolation of the SCN in hamsters. J Biol Rhythms 6:97–113 [DOI] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK 1977 The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol 20:224–234 [DOI] [PubMed] [Google Scholar]
- Lincoln GA 2006 Melatonin entrainment of circannual rhythms. Chronobiol Int 23:301–306 [DOI] [PubMed] [Google Scholar]
- Yoshimura T 2006 Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A Mol Integr Physiol 144:345–350 [DOI] [PubMed] [Google Scholar]
- DeCoursey PJ 1986 Light-sampling behavior in photoentrainment of a rodent circadian rhythm. J Comp Physiol A 159:161–169 [DOI] [PubMed] [Google Scholar]
- Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T 2007 Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology 148:3031–3038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.