Abstract
Successful implantation necessitates modulation of the uterine environment by the embryo for a specific period of time during the menstrual cycle. Infusion of chorionic gonadotropin (CG) into the oviducts of baboons to mimic embryo transit induces a myriad of morphological, biochemical, and molecular changes in the endometrium. Endometrial epithelial cells from both baboons and humans when stimulated by CG in vitro, activates a cAMP-independent MAPK pathway leading to prostaglandin E2 (PGE2) synthesis. This study shows that in the human endometrial cell line, HES, CG, acting via its G-protein coupled receptor, phosphorylates protein kinase B, c-Raf, and ERK1/2 in a phosphatidylinositol 3-kinase (PI3K)-dependent manner. Furthermore, ERK1/2 phosphorylation is independent of the signaling paradigms of Gαs, GαI, and epidermal growth factor receptor (EGFR) transactivation, typical of gonadal cells, indicating an alternative signaling pattern in the endometrium. After phosphorylation by CG, ERK1/2 translocates to the nucleus in a time-dependent manner. Downstream of ERK1/2, CG activates the nuclear transcription factor, Elk1, also in a PI3K-MAPK-dependent manner. Lastly, we show that in HES cells, this pathway regulates the expression of the microsomal enzyme PGE2 synthase (mPTGES), a terminal prostanoid synthase responsible for PGE2 synthesis. CG regulates the mPTGES promoter and also induces mPTGES synthesis in HES cells via the PI3K-ERK1/2 pathway. We suggest that this alternative PI3K-ERK-Elk pathway activated by CG regulates prostaglandin production by the endometrial epithelium and serves as an early trigger to prepare the endometrium for implantation.
Chorionic gonadotropin regulates a PI3K-dependent MAPK pathway in a human endometrial epithelial cell line, leading to phosphorylation and nuclear translocation of ERK1/2, activation of Elk1, and induction of prostaglandin E synthase, presumably as a preliminary response for embryo implantation.
Embryo implantation is one of the most vital and elegant processes in human physiology. Despite the magnitude of its importance for the survival of species, the relative inefficiency of this process remains unexplained. Of the pregnancies that are lost, 50–75% represent a failure in implantation (1). Due to the intricate manner by which numerous factors regulate the process of implantation, our understanding of the mechanisms required for successful implantation is far from complete. A better insight into the molecular mechanisms responsible for implantation may improve the chances of treating infertility and early pregnancy loss.
The process of implantation requires a coordination of two simultaneous processes: development of the newly formed embryo and the maturation of the uterine endometrium. Successful implantation necessitates the embryo-uterine interaction to be restricted to a short period of time termed the window of uterine receptivity, when the endometrium becomes responsive to embryonic signals. One of the earliest embryonic signals in primates is chorionic gonadotropin (CG), a glycoprotein hormone synthesized primarily by the syncitiotrophoblast cells of the blastocyst (2) and detected in the maternal blood and urine as early as 6–8 d after fertilization (3). Clinical validation of the close association of CG and endometrial receptivity comes from the fact that the time for the rise in CG levels corresponds to the time of optimum implantation (4).
To study the molecular mechanisms of maternal-fetal communication during the window of uterine receptivity in vivo, we used the baboon as a nonhuman primate model. Infusion of CG into the oviduct of normal cycling animals, in a manner mimicking blastocyst transit, induced marked morphological, and biochemical changes in the endometrial epithelium and the stroma (5). CG was also capable of inducing changes in gene expression, which could enhance endometrial receptivity and support embryo implantation (6). Additionally, in pathological conditions associated with infertility, e.g. endometriosis, there is a significant attenuation in the endometrial response to CG (7).
Our laboratory is interested in investigating the early responses of the endometrial epithelium to stimulation by CG. The epithelium is the first site of contact between the embryo and the endometrium. It is vital in mediating selectivity of implantation sites, particularly during the preimplantation period, when the embryo is free floating in the uterine cavity (8). Prostaglandins are known to be critical players in regulation of embryo attachment to specific sites in the epithelium by promoting endometrial proliferation, differentiation, and vascularization, as evidenced by extensive studies predominantly in the rodent model (9).
To closely examine the molecular mechanisms of CG signaling on the endometrial epithelium and the regulation of prostaglandin production, we initiated an in vitro study using primary baboon endometrial epithelial cells and a human endometrial epithelial cell line, HES. Both these cell types respond in an identical manner to stimulation by CG (10). CG signals through the same G protein-coupled receptor as the LH, the LH-CG receptor (LHCGR) (11). The LHCGR plays diverse roles in various tissues of reproductive and nonreproductive origin (12,13,14). Mechanisms of LHCGR action include induction of multiple signal transduction effector systems, including adenylyl cyclase and inositol phospholipid-specific phospholipase C, leading to the activation of the MAPK pathway in various cell paradigms (15,16). In a previous in vitro study, we showed that CG induced phosphorylation of ERK1/2 independent of the adenylyl cyclase/protein kinase A (PKA) pathway in endometrial epithelial cells leading to prostaglandin E2 (PGE2) production (10).
Our current investigation used the HES cell line to elucidate the upstream and downstream factors regulating the activation of ERK1/2 in response to CG stimulation. Studies on CG signaling were carried out in parallel in a Chinese hamster ovarian cell line, stably transfected with the human LHCGR (CHO-LH). We describe here a phosphatidylinositol 3-kinase (PI3K)-dependent MAPK pathway in the endometrial epithelial cell line leading to activation of the nuclear transcription factor Elk1, member of ETS oncogene family of transcription factors. This pathway further regulates the expression of the microsomal enzyme, prostaglandin E synthase (mPTGES), a PGE2 precursor, in response to CG stimulation.
Materials and Methods
Reagents and chemicals
Purified recombinant human CG was obtained from EMD Serono (Rockland, MA). Pharmacological inhibitors and activators, cholera toxin (Ctx), pertussis toxin (Ptx), AG1478, LY29400, and PD98059, were purchased from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA). LHCGR antibody was made in male rabbits against a synthetic peptide, corresponding to amino acids 257-271 of the extracellular domain of the human LH/CG receptor (exon 9: amino acids 257-271; Gene Bank accession no. S57793), conjugated to keyhole lymphocyte hemaglutinin (17). Monoclonal antibodies against total (t-ERK1/2) and phosphorylated ERK1/2 (p-ERK1/2; Thr202/Tyr204), phosphorylated c-Raf (Ser338), phosphorylated Akt (Ser473), and phosphorylated Elk1 (Ser383) were obtained from Cell Signaling Technology (Beverly, MA). Polyclonal antibody against mPTGES was purchased from Cayman Chemicals (Ann Arbor, MI). Monoclonal anti-β-actin antibody was from Sigma Aldrich (St. Louis, MO), and monoclonal c-Myc antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Secondary antibodies against rabbit and mouse were obtained from Jackson Immunoresearch Laboratories, Inc. (West Grove, PA). Fluorescein isothiocyanate-labeled antirabbit secondary antibody and Vectashield hard-set mounting media with 4′,6-diamidino-2-phenylindole (DAPI) were from Vector Laboratories Inc. (Burlingame, CA). Enhanced chemiluminescence kits were from GE Amersham Life Sciences (Arlington Heights, IL). PTGES-pGL4 luciferase reporter plasmid was a generous gift from Dr. Jonna Frasor (University of Illinois at Chicago, Chicago, IL). The renilla luciferase plasmid, the pGl4 plasmid, and the dual-luciferase assay were from Promega (Madison, WI). Transfection reagents, Lipofectamine, Opti-MEM, certified fetal bovine serum (FBS), and cell culture media, were purchased from Invitrogen (Carlsbad, CA).
Cell lines and plasmids
HES cells (a generous gift from Dr. Douglas Kniss, Ohio State University, Columbus, OH) were cultured, passaged, and maintained as previously described (10). For the present set of experiments, HES cells were seeded in 100-mm tissue culture dishes, six- or 24-well plates, or chamber slides and maintained until the required confluence was attained. For comparative studies, CHO-LH cells were cultured in 100-mm tissue culture dishes in αMEM with phenol-red, 0.1 mm sodium pyruvate, 1× penicillin/streptomycin, geneticin (G418 sulfate, 600 mg/ml), and 10% charcoal-stripped FBS up to the same level of confluence. The small interfering RNA (siRNA) duplexes targeting LHCGR was designed using the siDESIGN Center web site from Dharmacon (http://www.dharmacon.com/designcenter/designcenterpage. aspx). The siRNA sequence for the LHCGR (5′-ATTGCCACGTCATCCTATT-3′) targets the 814-834 region of the open reading frame of the human LHCGR mRNA. Scrambled siRNA sequence (5′-TAAGGCTATGAAGAGATAC-3′) from siCONTROL nontargeting siRNA pool (D-001206-13) was used as a control. These synthetic oligonucleotide duplexes were purchased from Invitrogen. They were further cloned into the circular mammalian expression plasmid pSUPER, purchased from OligoEngine (Seattle, WA) as previously described (18) to generate the final short hairpin RNA (shRNA) cassette.
Transfection of siRNA sequences
HES cells were seeded in six-well dishes at 50,000 cells/well in culture medium. Lipofectamine 2000 (Invitrogen) was used to transfect the cells with the siRNA and scrambled sequence expression vectors. The plasmids were dissolved in Lipofectamine 2000 (Invitrogen) diluted in serum and antibiotic-free OptiMEM, and the cells were incubated in this cocktail for 8 h. A GFP plasmid was used to measure and confirm transfection efficiency. After this incubation, the wells were washed and incubated in fresh DMEM with serum and antibiotics. After 24 h, the media were aspirated and total cell lysates were extracted as described below.
Treatment paradigms and total cell lysate extraction
Doses for CG and the pharmacological inhibitors have been previously described (10) or were empirically determined in preliminary experiments. For these studies, 24 h before the initiation of treatment, the cells (at 60% confluence) were made quiescent by two washes with 1× PBS to remove traces of FBS and then incubated in serum-free media. After overnight starvation, they were subjected to the appropriate treatment paradigms as indicated in each figure legend in fresh serum-free media. After treatment, each 100-mm dish was rinsed with ice-cold PBS and the cells lysed on ice with 250 μl lysis buffer (0.5 m EGTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 25 mm Tris, 25 mm NaCl, 100 mm sodium pyrophosphate, and 10 mm NaF, with protease inhibitor cocktail). Cells were scraped and incubated in the lysis buffer cocktail on ice for 20 min, followed by centrifugation at 14,000 rpm for 15 min. The supernatant was frozen at −20 C until use. The protein concentration was estimated using the Bradford assay from Bio-Rad (Hercules, CA).
Cell fractionation: cytoplasmic, nuclear extracts
After CG treatment, the cells were rinsed three times with ice-cold PBS, trypsinized, pelleted, and resuspended in PBS. The washes were repeated three times to remove all traces of trypsin. The pellet was then suspended in buffer A (10 mm HEPES, 1.5 mm MgCl2, 10 mm KCl, 1 mm dithiothreitol, protease inhibitor cocktail) and incubated on ice for 10 min, after which the solution was repeatedly passed through a P-1000 pipette tip to remove all clumps. The solution was spun at 100,000 × g for 10 min at 4 C and the supernatant, the cytoplasmic fraction, was stored at −20 C until further use. The remaining pellet was resuspended in buffer B [20 mm HEPES, 330 mm NaCl, 1.5 mm MgCl2, 25% (vol/vol) glycerol, 1 mm dithiothreitol, protease inhibitor cocktail] and homogenized by pipetting, avoiding foaming to the greatest extent possible. The solution was centrifuged at 100,000 × g for 10 min at 4 C, after which the supernatant, the nuclear extract, was removed and stored at −20 C.
Immunoblotting
Equal amounts of proteins from cell lysates were separated on a precast Tris HCL 10% gel (Invitrogen) using SDS-PAGE and transferred onto polyvinyledene difluoride membranes. The membranes were blocked for 1 h at room temperature in Tris-buffered saline and 0.1% Tween 20, containing 5% (wt/vol) nonfat milk and then incubated overnight with primary antibodies against LHCGR (1:20,000), p- or t-ERK1/2 (1:1000), phospho-Elk1 (1:1000), phospho-c-Raf (1:2000), phospho-Akt (1:1000) or β-actin (1:5000) in Tris-buffered saline and 0.1% Tween 20 containing 5% (wt/vol) nonfat milk or 5% BSA at 4 C. Incubation in primary antibody was followed by incubation in the respective secondary antibodies labeled with horseradish peroxidase at a 1:20,000 dilution for 1 h at room temperature. Immunocomplexes were visualized by enhanced chemiluminiscence (GE Amersham, Piscataway, NJ). All membranes were reprobed with antibodies against t-ERK1/2, β-actin, or β-tubulin, which served as loading controls.
Immunocytochemistry
For immunocytochemical staining, HES cells were plated in four-well chamber slides and grown to 50% confluency. Media were changed to serum-free DMEM overnight before treatment with inhibitors as specified followed by 10 IU/ml CG for the time period indicated. After each treatment time point, the cells were washed three times with PBS and fixed in 4% paraformaldehyde for 20 min at 4 C and rinsed with PBS. The cells were permeabilized with 0.1% saponin in Tris-buffered saline at 37 C for 10mins and then blocked in 5% normal goat serum in 0.1% saponin Tris-buffered saline for 30 min at room temperature. They were then incubated with antibodies against p-ERK1/2 (1:350 dilution) or mPTGES (1:250 dilution) overnight at 4 C in a humid chamber. Nonspecific rabbit IgG was used as the procedural control. After washing three times in PBS, they were subjected to fluorescein isothiocyanate-labeled antirabbit secondary antibody (1:200 dilution) at room temperature in the dark for 1 h, washed three times in PBS and mounted using Vectashield hard-set mounting media with DAPI (Vector Laboratories). The images were visualized with a Nikon Eclipse E400 series fluorescent microscope (Fryer Co., Huntley, IL) and captured using a digital Spot Camera and the Image-Pro Plus software package (Media Cybernetics, San Diego, CA).
Transient transfection and luciferase assay
HES cells were seeded in 24-well dishes at 30,000 cells/well in normal culture medium. They were cotransfected with the mPTGES-pGL4 luciferase reporter plasmid, the control pGl4 plasmid, and Renilla luciferase as previously described (19). Briefly, Lipofectamine 2000 (Invitrogen) diluted in serum-free, antibiotic-free OptiMEM was used to transfect the cells with the plasmids. Six hours after transfection, media were changed to media without serum along with the specific treatment paradigm. A GFP plasmid was used to assess efficiency. After treatment, reporter activity was measured using a dual-luciferase assay from Promega according to the manufacturer’s directions. The data shown represent a mean ± sem from at least three independent determinations.
Statistical analysis
One-way ANOVA was used to test the null hypothesis of group differences, followed by the Student t test for pairwise comparison. Each experiment was repeated three times in triplicate and a P < 0.05 was considered significant.
Results
Detection and silencing of LHCGR and ERK1/2 signaling in HES cells
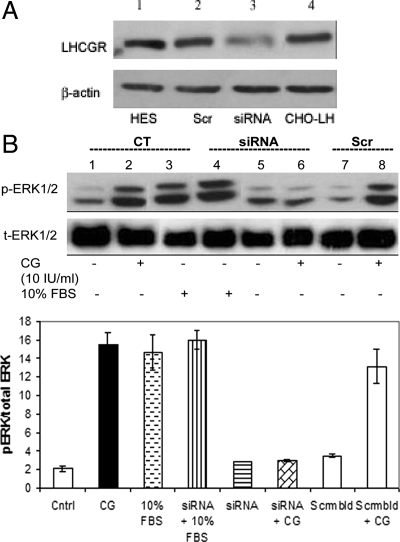
The presence of the 80-kDa protein for the LHCGR was confirmed in the HES cells by Western blot analysis in HES and CHO-LH cells (Fig. 1A, lanes 1 and 4). Transfection of HES cells with a specific siRNA oligonucleotide targeted against the LHCGR (Fig. 1A, lane 3) significantly decreased the level of endogenous LHCGR compared with control or cells transfected with scrambled siRNA control (Fig. 1A, lane 2). Treatment with 10 IU/ml CG induced activation of the MAPK pathway by phosphorylation of ERK1/2 (Fig. 1B, lane 2). Silencing the LHCGR using siRNA inhibited CG-induced ERK1/2 phosphorylation (Fig. 1B, lane 6), confirming that the ERK1/2 activation is specific to CG stimulation via the LHCGR. Figure 1B, lane 4, in shows the specificity of the siRNA to the LHCGR and indicates that ERK1/2 phosphorylation induced by FBS (Fig. 1B, lane 3) is not inhibited by the siRNA against LHCGR (Fig. 1B, lane 4). A scrambled sequence was used as a control for the siRNA sequence (Fig. 1B, lanes 7 and 8).
Figure 1.
Detection and silencing of LHCGR- and CG-induced signaling in HES cells. A, HES and CHO-LH cells were plated on six-well plates at a visual confluence of 80%. HES cells were transfected with the shRNA cassettes targeted against the LHCGR or scrambled siRNA controls. Cells were then lysed and total protein was extracted from the cells after transfection. Western blot analysis was carried out to detect levels of the 80-kDa LHCGR protein. Note the decrease in LHCGR expression upon transfection with siRNA cassette (lane 3). B, HES cells were plated on six-well plates at a visual confluence of 80%. Cells were transfected with shRNA cassettes or the scrambled sequence as a control. Thirty-six hours after transfection, they were treated with or without 10 IU/ml of CG or 10% FBS for 10 min. The immunoblot shows p- and t-ERK1/2 levels detected in 30 μg of lysate from the cells separated by SDS-PAGE. The histograms represent the normalization to t-ERK1/2. Note that ERK1/2 phosphorylation by CG (and not by FBS) is significantly reduced upon silencing the LHCGR with the siRNA sequence. Data are expressed as the mean sem of three different experiments done in triplicate. *, P < 0.05. Cntrl, Control; CT, control; Scr, scrambled (Scrmbld) siRNA sequence; siRNA, siRNA specific against LHCGR.
ERK1/2 phosphorylation by CG is independent of Gαs/Gαi/EGFR activation in the endometrial epithelial cell line
Gαs
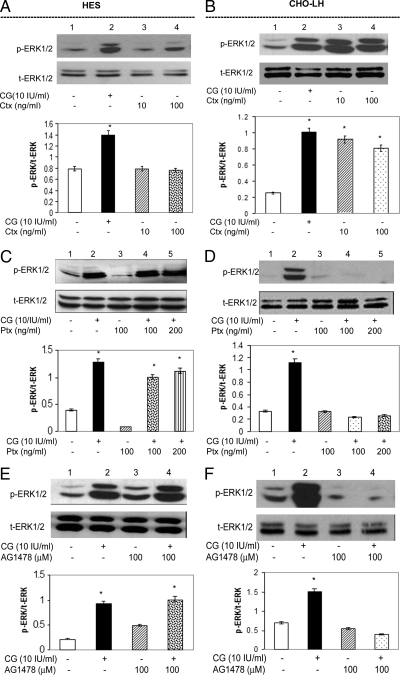
The absence of the cAMP/PKA pathway in HES cells in contrast to the CHO-LH cells (10) prompted us to investigate whether activating the Gαs subunit was capable of enabling ERK1/2 phosphorylation in HES cells. The CHO-LH cells were used as a comparative control in this study. HES and CHO-LH cells were treated with 10 and 100 ng/ml Ctx for 1 h. However, this treatment had no effect on ERK1/2 phosphorylation in HES cells (Fig. 2A, lanes 3 and 4). In CHO-LH cells, on the other hand, stimulation with Ctx lead to ERK1/2 phosphorylation (Fig. 2B, lanes 3 and 4). This, in addition to the absence of cAMP induction, indicated that the Gαs subunit is unlikely to be involved in MAPK activation in the HES cells in contrast to the CHO-LH cells.
Figure 2.
ERK1/2 phosphorylation by CG is independent of Gαs/Gαi/EGFR activation in HES cells. HES cells (A, C, and E) and CHO-LH cells (B, D, and F) were grown to 60% of visual confluence. HES (A) and CHO-LH (B) cells were treated with 10 IU/ml CG for 10 min or 10 or 100 ng/ml Ctx for 1 h. HES (C) and CHO-LH (D) cells were treated for 10 min with 10 IU/ml CG after pretreatment with 100 or 200 ng/ml Ptx-Gi inhibitor for 1 h. HES cells (E) and CHO-LH cells (F) were treated for 10 min with 10 IU/ml CG after pretreatment with 100 nm AG1478, an EGFR inhibitor, for 1 h. Thirty micrograms of the total cell lysates from each experiment were isolated and analyzed using Western blot analysis for levels of p-ERK1/2. T-ERK1/2 was used as a loading control, and the corresponding histograms represent the normalization t-ERK1/2. Data are expressed as the mean ± sem of three different experiments done in triplicate. *, P < 0.05. Note that treatment with Ctx failed to induce ERK1/2 phosphorylation in the HES cells in contrast to the CHO-LH cells. Additionally, both Ptx and AG1478 failed to inhibit CG-induced ERK1/2 phosphorylation in HES cells but significantly decreased ERK1/2 phosphorylation in CHO-LH cells.
Gαi
Agonist-mediated G protein signaling pathways have frequently demonstrated the involvement of the inhibitory subunit of G-proteins, Gαi (20,21). We evaluated the association of the Gαi subunit of the LHCGR in the activation of ERK1/2 in HES cells and in CHO-LH cells in parallel. Both cells were subjected to pretreatment with 100 and 200 ng/ml of the Gαi inhibitor, Ptx, for 1 h before stimulation by 10 IU/ml CG. Western blot analysis demonstrated that ERK1/2 phosphorylation by CG was unaffected by Ptx in HES cells (Fig. 2C, lanes 4 and 5) in contrast to CHO-LH cells in which Ptx significantly decreased CG-induced ERK1/2 phosphorylation (Fig. 2D, lanes 4 and 5). This indicated that the MAPK activation by CG in HES was independent of the Gαi subunit in HES cells but not CHO-LH cells.
EGFR
Transient transactivation of receptor tyrosine kinases, particularly the epidermal growth factor receptor (EGFR) is a commonly exhibited phenomenon in G protein-coupled receptors like the LHCGR (22,23). To assess whether ERK1/2 signaling by CG is mediated via the transactivation of the EGFR tyrosine kinase, HES and CHO-LH cells were pretreated with the EGFR inhibitor, AG1478 (100 nm), for 1 h before 10 IU/ml CG treatment for 10 min. The treatment with AG1478 also had no effect on phosphorylation of ERK1/2 by CG in HES cells (Fig. 2E, lane 4), suggesting the MAPK pathway is independent of EGFR transactivation. However, the same dose of the inhibitor significantly inhibited ERK1/2 phosphorylation by CG in the CHO-LH cells (Fig. 2F, lane 4), showing that this pathway was activated by CG in this cell type.
PI3K-dependent activation of ERK1/2, Akt, and c-Raf by CG in HES cells
Because CG signaling in the endometrial epithelial cell line was independent of the canonical signaling pathways activated in gonadal cells, the possibility of alternative signaling pathways for the LHCGR was explored in the HES cells. The association of PI3K in ERK1/2 activation by gonadotropins has been elucidated in many studies (24,25,26,27). We investigated the role of the inositol phosphate pathway in activation of ERK1/2 by CG. HES and CHO-LH cells were pretreated with a specific inhibitor against PI3K, LY29400 (1 and 20 μm), for 1 h and then stimulated with 10 IU/ml CG for 10 min. Levels of CG-induced p-ERK1/2 were significantly decreased in both cell lines on inhibition of PI3K (Fig. 3, A and B, lanes 4 and 5, HES and CHO-LH, respectively), indicating that this MAPK phosphorylation is mediated via PI3K. CG also induced phosphorylation of Akt or protein kinase B, a key downstream effector of PI3K pathway at Ser473 (Fig. 3, C and D, lane 2). Phosphorylation of Akt by CG was also inhibited by pretreatment with the PI3K inhibitor LY29400 for 1 h (Fig. 3, C and D, lanes 4 and 5). Thus, both cell lines showed the activation of the PI3K-Akt pathway in response to stimulation with CG.
Figure 3.
PI3K-dependent activation of ERK1/2, Akt, and c-Raf by CG in HES cells. Sixty percent visually confluent HES cells (A and C) and CHO-LH cells (B and D) were treated for 10 min with 10 IU/ml CG after pretreatment with 1 or 20 μm LY29400 for 1 h. Thirty micrograms of proteins were separated by SDS-PAGE and probed using p-ERK1/2 antibody (A and B) or phosphorylated Akt (p-Akt) Ser473 antibody (C and D). E, Sixty percent visually confluent HES cells were treated for 10min with 10 IU/ml CG with or without pretreatment with 1 or 20 μm LY29400 for 1 h. Thirty micrograms of proteins were separated by SDS-PAGE and probed using phosphorylated c-Raf antibody. The blots were reprobed with t-ERK1/2 or β-actin as loading control, and the histograms represent the normalization to β-actin or t-ERK1/2. Note the PI3K-dependent phosphorylation of ERK1/2, Akt in both cell types, and of c-Raf in HES cells. Values are expressed as the mean ± sem (n = 3). *, P < 0.05.
The established upstream modulator in the hierarchy of the MAPK/ERK (MEK) pathway is the intracellular signaling molecule Raf. Raf is a MAPK kinase and phosphorylates MEK, the MAPK kinase, which in turn activates ERK, the MAPK (28). HES cells were treated for 10 min with 10 IU/ml CG, and phosphorylated c-Raf was measured by immunoblot analysis. CG led to a significant increase in phosphorylated c-Raf, which was significantly decreased upon inhibition of PI3K, indicating that PI3K plays a role in mediating the Raf-ERK pathway (Fig. 3E).
CG-mediated nuclear translocation of p-ERK1/2
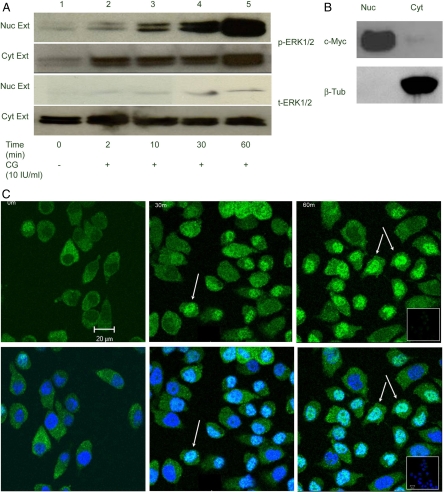
To trace the path of CG-induced p-ERK1/2 in HES cells, we analyzed nuclear and cytoplasmic extracts of HES cells that were treated with 10IU/ml CG. As shown in Fig. 4A, p-ERK1/2 translocated into the nucleus after treatment with CG from 2 to 60 min. The levels of p-ERK1/2 in the cytoplasm also showed a modest increase after CG stimulation. This can probably be attributed to the very high levels of ERK1/2 that are present and are phosphorylated in these cells in response to CG. The amount of t-ERK1/2 was preferentially found in the cytoplasm, and only very small levels of t-ERK1/2 were detected in the nuclear fraction (Fig. 4A). The purity of the fractionations was verified by Western blot analysis using c-Myc for nuclear extracts and β-tubulin for cytoplasmic extracts (Fig. 4B). These data indicate that nuclear translocation was limited only to the activated or phosphorylated form of ERK1/2. Additionally, using a monoclonal antibody against p-ERK1/2, we visualized the movement of CG-induced p-ERK1/2 in HES cells by fluorescent immunocytochemistry. At the two most prominent time points of nuclear translocation, 30 and 60 min, fluorescent staining for p-ERK1/2 was detected in the nucleus, in contrast to primary cytoplasmic location at the 0 min time point (Fig. 4C). The IgG control for the rabbit antibody is shown (Fig. 4C, inset).
Figure 4.
A, Nuclear translocation of p-ERK1/2. CG induced nuclear translocation of p-ERK1/2 in HES cells: A, Fractionation of cellular components was carried out as indicated in Materials and Methods. Western blot analysis of p- and t-ERK1/2 in 50 μg nuclear (Nuc Ext) and 40 μg of cytoplasmic extracts (Cyt Ext) of HES cells treated with 10 IU/ml CG for the times indicated are shown. Lanes 1–5 represent increasing treatment times of 0, 2, 10, 30, and 60 min. B, Fifty micrograms of nuclear and 40 μg of cytoplasmic extracts were analyzed by Western blot using antibodies against c-Myc and β-tubulin (β-Tub) to verify for purity of the cellular fractions. C, Immunofluorescent imaging of p-ERK1/2 upon CG stimulation: 90% confluent HES cells were treated with 10 IU/ml CG for 0, 30, and 60 min. They were analyzed using an immunocytochemistry-compatible p-ERK1/2 antibody and visualized using confocal microscopy. The green indicates p-ERK1/2 staining in the upper panels, and the lower panels in blue show an overlay with nuclear staining by DAPI. The nuclear localization of p-ERK1/2 after treatment with CG at 30 and 60 min are indicated by the arrows. All images are at the same magnification; the bar (A), 20 μm. The inset represents the IgG control. The experiments have been performed in triplicate and the images are the best representation of the data.
CG induced a PI3K-ERK-mediated activation of the nuclear transcription factor, Elk1
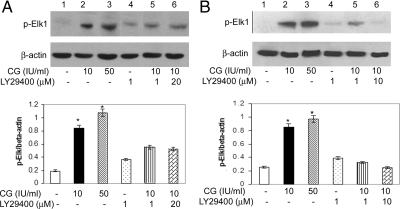
Elk1 is a well-established nuclear target for the Raf-MAPK signaling cascade. ERK1/2 has been shown to recruit and activate Elk1, a member of the ternary complex factor subfamily, responsible for activating promoters of early genes (29,30). We show that treatment of HES cells with 10 and 50 IU/ml CG for 10 min increases phosphorylation of Elk1 in a dose-dependent manner (Fig. 5, lanes 2 and 3). Phosphorylation was inhibited by both PI3K and MEK inhibitors, 1 and 20 μm LY29400 (Fig. 5A, lanes 5 and 6) and 1 and 10 μm PD98059 (Fig. 5B, lanes 5 and 6), respectively. These data suggest that the PI3K-mediated ERK pathway was the upstream effector of the nuclear transcription factor Elk1.
Figure 5.
Activation of the nuclear transcription factor Elk1 by CG. Sixty percent visually confluent HES cells were stimulated by 10 and 50 IU/ml of CG (lanes 2 and 3) for 10 min. In addition, cells were pretreated for 1 h with 1 or 20 μm PI3K inhibitor LY29400 (lanes 4, 5, 6) (A), or 1 or 10 μm MEK inhibitor, PD98059 (lanes 4, 5, 6) (B) before stimulation with 10 IU/ml CG for 10 min. After treatment, 30 μg of total cell lysate were analyzed by Western blot using an antibody against phosphorylated Elk1 (p-Elk1). The blots were reprobed with β-actin to confirm equal protein loading. The histograms represent the normalization to β-actin, and data are expressed as the mean sem of three different experiments done in triplicate. *, P < 0.05. Note the dose-dependent increase in Elk1 phosphorylation by CG mediated by the PI3K-ERK1/2 pathway.
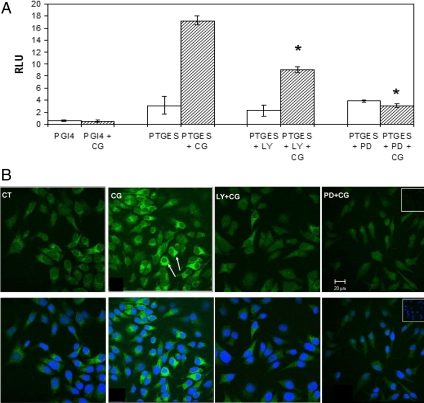
Induction of mPTGES promoter activity and protein expression by CG
In the eicosanoid synthesis pathway, the prostanoid E synthase (PTGES) catalyzes the terminal step converting PGH2 to PGE2, downstream of cyclooxygenases (COXs). Although the nuclear transcription factor Elk1 has been shown to directly up-regulate COX-2 promoter activity (31), a direct regulation of PGE2 synthesis via the MAPK pathway has not been described. Using an mPTGES luciferase reporter plasmid, we show that a 24-h treatment with 10 IU/ml CG significantly up-regulated mPTGES promoter activity (Fig. 6A). In addition, this induction was significantly inhibited upon pretreatment with 10 μm of the MEK inhibitor PD98059 or 20 μm of the PI3K inhibitor LY29400 (Fig. 6A).
Figure 6.
mPTGES induction in response to CG stimulation. A, HES cells were cotransfected with either PTGES-pGl4 luciferase reporter plasmid and the Renilla luciferase or the pGl4 vector alone with the Renilla luciferase. Reporter activity was measured after the cells were subjected to stimulation by 10 IU/ml CG for 24 h, with or without a 1-h preincubation with 20 μm PI3K inhibitor LY29400 (LY) or 10 μm MEK inhibitor PD98059 (PD). Activity is represented as relative luciferase units (RLU) derived on normalization with renilla luciferase units. Data are expressed as the mean sem of three different experiments done in triplicate. *, P < 0.05. B, Immunofluorescent imaging of HES cells using an antibody against mPTGES: near confluent HES cells, grown on chamber slides, were treated with 10 IU/ml CG for 24 h, with or without a 1-h pretreatment with 20 μm LY29400 or a 1-h pretreatment with 10 μm PD98059 before CG treatment. The IgG control is shown in the inset. The cells were incubated and analyzed using a polyclonal antibody against mPTGES and visualized using confocal microscopy. The green color represents mPTGES and the bottom panels depict an overlay with DAPI, representing nuclear staining. The arrows indicate positive staining for mPTGES. All images are at the same magnification. Bar, 20 μm. CT, Control.
To assess regulation of protein expression, we used immunofluorescence and demonstrated that treatment of HES cells with 10 IU/ml CG for 24 h led to a significant induction mPTGES, compared with control (Fig. 6B). Induction of mPTGES was also blocked with inhibition of the PI3K-ERK1/2 pathway using 20 μm LY29400 or 10 μm PD98059 (Fig. 6B). The correction for background signal due to secondary antibody was achieved by using a rabbit IgG as an internal control in the inset. Thus, CG-induced mPTGES expression in the endometrial epithelial cells via a PI3K-dependent MAPK pathway.
Discussion
Diversity of signaling by the LHCGR has been extensively described in both gonadal (32,33,34,35) and nongonadal tissues and cells (36,37,38). Our current findings elucidate for the first time a direct PI3K-ERK pathway activated by CG in an endometrial epithelial cell line that regulates prostaglandin synthesis. This pathway represents a deviation from the mechanisms of MAPK activation by the LHCGR demonstrated typically in gonadal tissues. Although primary cells are a very good platform to study endometrial epithelial responses, they have their limitations because they cannot be passaged or maintained over a long period of time. Additionally, the low epithelial to stromal cell ratio yielded from hysterectomies or endometrial scrapings makes the use of primary epithelial cells impractical for extensive in vitro signaling studies. The HES cell line was originally isolated from a proliferative, noncancerous endometrium at hysterectomy and spontaneously immortalized in culture (39). These cells have been used extensively to model the endometrial epithelium and have been shown to respond to various ligands, e.g. CG (10), TNFα (40), TGF-β, and GnRH (41). Furthermore, they respond to stimulation by CG in a manner identical with primary baboon epithelial cells (10), making them an ideal cell line to model the endometrial epithelium and study signal transduction by the LHCGR in vitro.
We show that the MAPK pathway induced by CG in the HES cell line is mediated exclusively by the PI3K signaling pathway. PI3K plays a crucial role in modulating a broad range of cellular functions in response to extracellular signals. Akt is a key downstream effector of PI3K and is a powerful promoter of cell survival, activated by growth factors (42). The PI3K-Akt pathway antagonizes various components of the apoptotic cascade (43) and supports processes like cell proliferation, cell migration (44), and angiogenesis (45). Critical physiological responses occurring in the endometrium during embryo implantation, such as regulation of trophoblast invasion, immune surveillance, and prevention of mense-induced apoptosis use these processes. We suggest that CG activates the PI3K-Akt pathway in the endometrial epithelium, thus promoting cell survival and growth during the initial phase of embryo implantation. This hypothesis is further supported by the fact that treatment with CG in vivo or in vitro significantly inhibits endometrial apoptosis (46,47). Although Akt activation leads to cell survival and transformation (48), it is important to note that MAPK activation by PI3K may occur independent of Akt activation (49). These may involve activation of protein kinase C, or GTP binding proteins and similar regulatory kinases (50,51). Our current investigation ascertains the activation of the PI3K-Akt pathway by CG and also the involvement of PI3K in activating the ERK1/2 cascade. We acknowledge, however, that the PI3K pathway might have multiple possible ways of activating the ERK1/2 signaling cascade.
The absence of the cAMP induction by CG in the HES cells (10) initially indicated that the endometrial epithelial response to CG differs from that observed in the gonads (16,52). LH/CG signaling independent of cAMP-PKA activation has been shown in many models (53,54); however, their presence in the endometrium has not yet been elaborated, except in endometrial cancer cell lines (55). In the HES cells, we failed to detect the classical pathways typically activated after CG stimulation in gonadal cells, including the Gαs, GαI, and transactivation of the EGFR (16). In parallel, the activation of these pathways by CG was analyzed in the CHO-LH cell line, which represents a classical gonadal cell type and resulted in the ubiquitous activation of the Gαs, Gαi, EGFR, and PI3K in response to CG stimulation and leading to ERK1/2 phosphorylation. In parallel experiments, we also sequenced the LHCGR from both the HES and CHO-LH cells and compared it against the published sequence. The sequences showed a 97 and 98% homology with the published human LHCGR sequence, respectively (accession no. NM_000233). This indicated that the same receptor was present in both cells but responded differently to stimulation by CG. The specificity of the PI3K signaling in the endometrial epithelial cells is a notable contrast to the diversity of signaling in the CHO-LH cells and presumably represents the unique endometrial response to specific embryonic signals.
Absence of the canonical signaling pathways can be due to various reasons. For example, in these studies, activating Gαs using Ctx (which ADP-ribosylates Gαs) failed to induce ERK1/2 signaling. This could imply either a defective Gαs subunit or failure of the Gαs subunit to couple with the receptor in HES cells. G protein uncoupling from the receptor can be attributed to receptor desensitization in presence of high agonist levels. Termination of the Gαs-mediated signaling due to this phenomenon has been described both in vivo and in vitro in different cell types (56,57). Additionally, arrestins have been demonstrated to play a role in G protein-coupled receptor desensitization and activate alternate pathways that lead to MAPK signaling (58,59). In the presence of the embryo, the uterine endometrium is subjected to high levels of CG. However, it is critical for the uterus to remain functional and responsive to the embryo throughout the process of implantation. Hence, we hypothesize that the endometrium maintains its functional responsiveness to CG by silencing the canonical Gαs and Gαi pathways and maintaining signaling through the PI3K-Akt pathway, thus preventing receptor desensitization and internalization.
Prostaglandins have been shown to regulate the attachment of the embryo to the epithelium by playing a role in promoting endometrial proliferation, differentiation, and vascular permeability (9,60,61). Murine models have shown that the absence of prostaglandins leads to repression of decidual growth (62) as well as multiple reproductive failures, particularly due to implantation defects (63), thus demonstrating the significance of this pathway in establishing a successful pregnancy. In the final step of prostaglandin synthesis, mPTGES acts downstream of COX-2 to enzymatically convert the COX-2 product prostaglandin H2 to PGE2 (64). Preliminary microarray studies in our laboratory on HES cells indicated that there was a significant increase in the induction of mPTGES on CG treatment (data not shown), prompting the investigation of mPTGES regulation by CG. mPTGES expression has also been characterized in the endometrium of the Rhesus monkey (65) and in the human (66). COX-2 gene or protein expression has been mapped during the implantation window in the human (67) and throughout pregnancy in the baboon (68) and mouse (69,70,71) uteri during pregnancy. In addition, COX-2 induction by CG has been demonstrated in vitro in human endometrial glandular epithelial cells (72) and adenocarcinoma cells (73,74). Furthermore, the transcription factor Elk1, regulated by CG (Fig. 5) in the endometrial epithelium, has been shown to stimulate COX-2 promoter activity (31). We show here that in the endometrial epithelial cell model, HES, CG induces mPTGES gene expression directly downstream of a PI3K-ERK-Elk pathway. This pathway presumably serves as a mitotic trigger for the endometrium to continue its proliferation and differentiation processes that may modulate critical changes mediated by prostaglandins during the process of implantation.
In summary, our studies indicate that the embryonic signal CG modulates the endometrial epithelial cells directly and activates a Gαs/Gαi/EGFR-independent and PI3K-Akt-dependent Raf-ERK-Elk pathway, leading to mPTGES production. We believe that this alternative pathway represents a rapid initial response of CG, which permits the endometrium to continue to be responsive to CG at high ligand concentrations that are present in utero during early pregnancy.
Acknowledgments
We thank Dr. Douglas Kniss (Ohio State University, Columbus, OH) for his generous gift of the HES cells and Dr. Jonna Frasor (Department of Physiology and Biophysics, University of Illinois at Chicago) for the mPTGES-luciferase construct. We also thank Dr. Nirupama Mulherkar and Dr. Bellur Prabhakar (Department of Microbiology and Immunology, University of Illinois at Chicago) in assisting with the siRNA design and experimental approach.
Footnotes
Disclosure Summary: The authors have nothing to declare.
First Published Online June 25, 2009
Abbreviations: CG, Chorionic gonadotropin; COX, cyclooxygenase; Ctx, cholera toxin; DAPI, 4′,6-diamidino-2-phenylindole; EGFR, epidermal growth factor receptor; FBS, fetal bovine serum; LHCGR, LH-CG receptor; MEK, MAPK/ERK; mPTGES, microsomal enzyme PGE2 synthase; P-ERK1/2, phosphorylated ERK1/2; PGE2, prostaglandin E2; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PTGES, prostanoid E synthase; Ptx, pertussis toxin; shRNA, short hairpin RNA; siRNA, small interfering RNA; t-ERK1/2, total ERK1/2.
References
- Norwitz ER, Schust DJ, Fisher SJ 2001 Implantation and the survival of early pregnancy. N Engl J Med 345:1400–1408 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW 2004 Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol 2:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohstroh PN, Overstreet JW, Stewart DR, Nakajima ST, Cragun JR, Boyers SP, Lasley BL 2005 Secretion and excretion of human chorionic gonadotropin during early pregnancy. Fertil Steril 83:1000–1011 [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR 1999 Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 340:1796–1799 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB 1999 Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 96:2543–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT 2007 Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 148:618–626 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE 2003 Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril 80(Suppl 2):820–827 [DOI] [PubMed] [Google Scholar]
- Aplin JD 2006 Embryo implantation: the molecular mechanism remains elusive. Reprod Biomed Online 13:833–839 [DOI] [PubMed] [Google Scholar]
- Kennedy TG, Gillio-Meina C, Phang SH 2007 Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction 134:635–643 [DOI] [PubMed] [Google Scholar]
- Srisuparp S, Strakova Z, Brudney A, Mukherjee S, Reierstad S, Hunzicker-Dunn M, Fazleabas AT 2003 Signal transduction pathways activated by chorionic gonadotropin in the primate endometrial epithelial cells. Biol Reprod 68:457–464 [DOI] [PubMed] [Google Scholar]
- McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, Seeburg PH 1989 Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science (New York, NY) 245:494–499 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Ascoli M 1993 The lutropin/choriogonadotropin receptor. 4 years later. Endocr Rev 14:324–347 [DOI] [PubMed] [Google Scholar]
- Apaja PM, Aatsinki JT, Rajaniemi HJ, Petäjä-Repo UE 2005 Expression of the mature luteinizing hormone receptor in rodent urogenital and adrenal tissues is developmentally regulated at a posttranslational level. Endocrinology 146:3224–3232 [DOI] [PubMed] [Google Scholar]
- Pakarainen T, Ahtiainen P, Zhang FP, Rulli S, Poutanen M, Huhtaniemi I 2007 Extragonadal LH/hCG action—not yet time to rewrite textbooks. Mol Cell Endocrinol 269:9–16 [DOI] [PubMed] [Google Scholar]
- Ryu KS, Gilchrist RL, Koo YB, Ji I, Ji TH 1998 Gene, interaction, signal generation, signal divergence and signal transduction of the LH/CG receptor. Int J Gynaecol Obstet 60(Suppl 1):S9–S20 [DOI] [PubMed] [Google Scholar]
- Leung PC, Steele GL 1992 Intracellular signaling in the gonads. Endocr Rev 13:476–498 [DOI] [PubMed] [Google Scholar]
- Cameo P, Szmidt M, Strakova Z, Mavrogianis P, Sharpe-Timms KL, Fazleabas AT 2006 Decidualization regulates the expression of the endometrial chorionic gonadotrophin receptor in the primate. Biol Reprod 75:681–689 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R 2002 A system for stable expression of short interfering RNAs in mammalian cells. Science (New York, NY) 296:550–553 [DOI] [PubMed] [Google Scholar]
- Frasor J, Weaver AE, Pradhan M, Mehta K 2008 Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17β-estradiol and proinflammatory cytokines. Endocrinology 149:6272–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden PH, Clerk A 1997 Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cell Signal 9:337–351 [DOI] [PubMed] [Google Scholar]
- Rajagopalan-Gupta RM, Mukherjee S, Zhu X, Ho YK, Hamm H, Birnbaumer M, Birnbaumer L, Hunzicker-Dunn M 1999 Roles of Gi and Gq/11 in mediating desensitization of the luteinizing hormone/choriogonadotropin receptor in porcine ovarian follicular membranes. Endocrinology 140:1612–1621 [DOI] [PubMed] [Google Scholar]
- Evaul K, Hammes SR 2008 Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem 283:27525–27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R 2002 G protein pathways. Science (New York, NY) 296:1636–1639 [DOI] [PubMed] [Google Scholar]
- Choi JH, Choi KC, Auersperg N, Leung PC 2006 Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res 66:3912–3920 [DOI] [PubMed] [Google Scholar]
- Donadeu FX, Ascoli M 2005 The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology 146:3907–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon YL, Wong AS 2006 Gonadotropin-induced apoptosis in human ovarian surface epithelial cells is associated with cyclooxygenase-2 up-regulation via the β-catenin/T-cell factor signaling pathway. Mol Endocrinol 20:3336–3350 [DOI] [PubMed] [Google Scholar]
- Tai P, Shiraishi K, Ascoli M 30 April 2009 Activation of the lutropin/choriogonadotropin receptor (LHR) inhibits apoptosis of immature Leydig cells in primary culture. Endocrinology 10.1210/en.2009–0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W 2000 Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351(Pt 2):289–305 [PMC free article] [PubMed] [Google Scholar]
- Shaw PE, Saxton J 2003 Ternary complex factors: prime nuclear targets for mitogen-activated protein kinases. Int J Biochem Cell Biol 35:1210–1226 [DOI] [PubMed] [Google Scholar]
- Zhang HM, Li L, Papadopoulou N, Hodgson G, Evans E, Galbraith M, Dear M, Vougier S, Saxton J, Shaw PE 2008 Mitogen-induced recruitment of ERK and MSK to SRE promoter complexes by ternary complex factor Elk-1. Nucleic Acids Res 36:2594–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Yang X, Han X 2007 Several transcription factors regulate COX-2 gene expression in pancreatic β-cells. Mol Biol Rep 34:199–206 [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ascoli M 2007 Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology 148:3214–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T, Ascoli M 2003 The lutropin/choriogonadotropin receptor-induced phosphorylation of the extracellular signal-regulated kinases in Leydig cells is mediated by a protein kinase a-dependent activation of ras. Mol Endocrinol 17:2189–2200 [DOI] [PubMed] [Google Scholar]
- Flynn MP, Maizels ET, Karlsson AB, McAvoy T, Ahn JH, Nairn AC, Hunzicker-Dunn M 2008 Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A-dependent dephosphorylation of microtubule-associated protein 2D. Mol Endocrinol 22:1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA 2006 Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol 299:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao ChV, Zhou XL, Lei ZM 2004 Functional luteinizing hormone/chorionic gonadotropin receptors in human adrenal cortical H295R cells. Biol Reprod 71:579–587 [DOI] [PubMed] [Google Scholar]
- Stepien A, Ziecik AJ 2002 Second messenger systems in the action of LH and oxytocin on porcine endometrial cells in vitro. Theriogenology 57:2217–2227 [DOI] [PubMed] [Google Scholar]
- Meng XL, Rennert OM, Chan WY 2007 Human chorionic gonadotropin induces neuronal differentiation of PC12 cells through activation of stably expressed lutropin/choriogonadotropin receptor. Endocrinology 148:5865–5873 [DOI] [PubMed] [Google Scholar]
- Desai NN, Kennard EA, Kniss DA, Friedman CI 1994 Novel human endometrial cell line promotes blastocyst development. Fertil Steril 61:760–766 [PubMed] [Google Scholar]
- Thathiah A, Brayman M, Dharmaraj N, Julian JJ, Lagow EL, Carson DD 2004 Tumor necrosis factor α stimulates MUC1 synthesis and ectodomain release in a human uterine epithelial cell line. Endocrinology 145:4192–4203 [DOI] [PubMed] [Google Scholar]
- Luo X, Ding L, Chegini N 2004 Gonadotropin-releasing hormone and TGF-β activate MAP kinase and differentially regulate fibronectin expression in endometrial epithelial and stromal cells. Am J Physiol Endocrinol Metab 287:E991–E1001 [DOI] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ 1997 Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol 17:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR 1998 Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335(Pt 1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini D, Busacca M, Di Francesco S, Vignali M, Viganò P, Di Blasio AM 2007 PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17β-estradiol and growth factors. Mol Hum Reprod 13:317–322 [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K 2002 Role of Akt signaling in vascular homeostasis and angiogenesis. Circulation Res 90:1243–1250 [DOI] [PubMed] [Google Scholar]
- Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA 2005 Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab 90:2351–2356 [DOI] [PubMed] [Google Scholar]
- Jasinska A, Strakova Z, Szmidt M, Fazleabas AT 2006 Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology 147:4112–4121 [DOI] [PubMed] [Google Scholar]
- Guzeloglu Kayisli O, Kayisli UA, Luleci G, Arici A 2004 In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol Reprod 71:714–721 [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL 2002 The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A 2005 Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation 111:1652–1659 [DOI] [PubMed] [Google Scholar]
- Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC 2007 Membrane translocation of P-Rex1 is mediated by G protein βγ subunits and phosphoinositide 3-kinase. J Biol Chem 282:29967–29976 [DOI] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, Jo Y, Stocco DM 2006 cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37:81–95 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000 Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- Viswanath G, Chatterjee S, Roy P 2007 Assessment of luteinizing hormone receptor function in an endometrial cancer cell line, Ishikawa cells in response to human chorionic gonadotrophin (hCG). Mol Cell Endocrinol 272:14–21 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley Jr WF, Plant TM 2006 Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET 2006 FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ 2001 Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2:727–733 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ 1999 β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science (New York, NY) 283:655–661 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK 2005 Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat 77:84–102 [DOI] [PubMed] [Google Scholar]
- Dey SK 2005 Reproductive biology: fatty link to fertility. Nature 435:34–35 [DOI] [PubMed] [Google Scholar]
- Cheng JG, Stewart CL 2003 Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol Reprod 68:401–404 [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK 1997 Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB 2006 Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119:229–240 [DOI] [PubMed] [Google Scholar]
- Sun T, Li SJ, Diao HL, Teng CB, Wang HB, Yang ZM 2004 Cyclooxygenases and prostaglandin E synthases in the endometrium of the rhesus monkey during the menstrual cycle. Reproduction 127:465–473 [DOI] [PubMed] [Google Scholar]
- Milne SA, Perchick GB, Boddy SC, Jabbour HN 2001 Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab 86:4453–4459 [DOI] [PubMed] [Google Scholar]
- Marions L, Danielsson KG 1999 Expression of cyclo-oxygenase in human endometrium during the implantation period. Mol Hum Reprod 5:961–965 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Wang J, Bambra C, Das SK, Dey SK, Fazleabas AT 1999 Expression of cyclooxygenase-1 and -2 in the baboon endometrium during the menstrual cycle and pregnancy. Endocrinology 140:2672–2678 [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Das SK, Wang J, Dey SK 1996 Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol 16:107–122 [DOI] [PubMed] [Google Scholar]
- Ni H, Sun T, Ding NZ, Ma XH, Yang ZM 2002 Differential expression of microsomal prostaglandin E synthase at implantation sites and in decidual cells of mouse uterus. Biol Reprod 67:351–358 [DOI] [PubMed] [Google Scholar]
- Ni H, Sun T, Ma XH, Yang ZM 2003 Expression and regulation of cytosolic prostaglandin E synthase in mouse uterus during the peri-implantation period. Biol Reprod 68:744–750 [DOI] [PubMed] [Google Scholar]
- Zhou XL, Lei ZM, Rao CV 1999 Treatment of human endometrial gland epithelial cells with chorionic gonadotropin/luteinizing hormone increases the expression of the cyclooxygenase-2 gene. J Clin Endocrinol Metab 84:3364–3377 [DOI] [PubMed] [Google Scholar]
- Munir I, Fukunaga K, Miyazaki K, Okamura H, Miyamoto E 1999 Mitogen-activated protein kinase activation and regulation of cyclooxygenase 2 expression by platelet-activating factor and hCG in human endometrial adenocarcinoma cell line HEC-1B. J Reprod Fertil 117:49–59 [DOI] [PubMed] [Google Scholar]
- Tsai EM, Chan TF, Chen YH, Hsu SC, Chuang CY, Lee JN 2008 Mifepristone attenuates human chorionic gonadotropin-induced extracellular signal-regulated kinase 1/2 phosphorylation, cyclooxygenase-2, and prostaglandin E2 production in human granulosa luteal cells. Fertil Steril 89:1522–1529 [DOI] [PubMed] [Google Scholar]