Abstract
Negative energy balance during lactation is reflected by low levels of insulin and leptin and is associated with chronic hyperphagia and suppressed GnRH/LH activity. We studied whether restoration of insulin and/or leptin to physiological levels would reverse the lactation-associated hyperphagia, changes in hypothalamic neuropeptide expression [increased neuropeptide Y (NPY) and agouti-related protein (AGRP) and decreased proopiomelanocortin (POMC), kisspeptin (Kiss1), and neurokinin B (NKB)] and suppression of LH. Ovariectomized lactating rats (eight pups) were treated for 48 h with sc minipumps containing saline, human insulin, or rat leptin. The arcuate nucleus (ARH) was analyzed for NPY, AGRP, POMC, Kiss1, and NKB mRNA expression; the dorsal medial hypothalamus (DMH) was analyzed for NPY mRNA. Insulin replacement reversed the increase in ARH NPY/AGRP mRNAs, partially recovered POMC, but had no effect on recovering Kiss1/NKB. Leptin replacement only affected POMC, which was fully recovered. Insulin/leptin dual replacement had similar effects as insulin replacement alone but with a slight increase in Kiss1/NKB. The lactation-induced increase in DMH NPY was unchanged after treatments. Restoration of insulin and/or leptin had no effect on food intake, body weight, serum glucose or serum LH. These results suggest that the negative energy balance of lactation is not required for the hyperphagic drive, although it is involved in the orexigenic changes in the ARH. The chronic hyperphagia of lactation is most likely sustained by the induction of NPY in the DMH. The negative energy balance also does not appear to be a necessary prerequisite for the suppression of GnRH/LH activity.
The low levels of insulin and leptin, reflecting negative energy balance, are not a necessary prerequisite of the large sustained increase in food intake or the suppression of GnRH/LH secretion during lactation.
Chronic hyperphagia, suppression of GnRH/LH, and negative energy balance due to the energy drain from milk production are hallmarks of lactation (1). The mechanisms responsible for the sustained hyperphagia and suppression of cyclic reproductive function remain unclear but are thought to involve metabolic factors associated with the negative energy balance and the neural impulses arising from the suckling stimulus (1,2).
The energy demands of milk production in the rat are met by a 3- to 4-fold increase in food intake (1,3). Associated with the hyperphagia of lactation are changes in the arcuate nucleus (ARH) that include increases in the orexigenic signals, neuropeptide Y (NPY) and agouti-related protein (AGRP) (4,5,6,7) and decreases in the anorexigenic signal, promelanocortin (POMC) (4). In addition to changes in the ARH, lactation induces NPY expression in the dorsal medial hypothalamus (DMH) (5,8), a region also associated with the regulation of food intake (9,10,11). Taken together, the changes in orexigenic and anorexigenic signals within the ARH and DMH would promote the chronic increase in food intake during lactation.
GnRH neuronal activity is greatly suppressed during lactation as reflected by the inhibition of pulsatile LH secretion in both ovarian intact and ovariectomized lactators (1,2). A likely factor that plays a role in the suppression of GnRH is NPY, which is a known modulator of reproductive function (12,13). Although NPY has been shown to have both stimulatory and inhibitory effects on GnRH/LH, its inhibitory effects likely predominate during lactation because of the chronic increase in NPY and low levels of estradiol (14,15). Recent studies from our laboratory used electrophysiological techniques to record from GnRH-green fluorescent protein neurons in the rat. We found that basal spontaneous GnRH neuronal activity is suppressed, and there is increased inhibitory NPY tone acting directly on the GnRH cell body, through the postsynaptic NPY Y5 receptor (16), to inhibit GnRH activity during lactation (17).
Other factors that may contribute to the suppression of GnRH/LH are the dramatic decrease in kisspeptin and neurokinin B (NKB) expression in the ARH during lactation (18,19). Kisspeptin has recently been identified as the primary signal in the neural control of GnRH neurons (20,21,22,23). Kisspeptin neurons in the ARH are thought to be involved in generating GnRH pulses and mediating negative feedback actions of estrogen on GnRH release (20,22). NKB has been shown to stimulate LH release under conditions of low estrogen, likely through modulation of GnRH release (24,25). Thus, Kisspeptin and NKB in the ARH could also be key factors in the suppression of GnRH/LH during lactation.
The negative energy balance during lactation, which is characterized by low levels of insulin and leptin (26,27), could be a primary peripheral signal for the chronic hyperphagia and the suppression of cyclic reproductive function. The ARH serves to integrate insulin and leptin signaling through direct actions via their respective receptors on NPY/AGRP and POMC neurons (28,29,30,31). The modulatory role of leptin in reproductive function is also well established (32,33) and is thought to involve its actions on ARH neurons. In particular, leptin likely plays a pivotal role in the control of the Kisspeptin system because the neurons express leptin receptors (34).
The purpose of this study was to investigate the importance of the low insulin and leptin levels associated with lactation as signals for the hyperphagia and suppression of GnRH/LH secretion. For these studies, insulin and/or leptin was restored to normal physiological levels in lactating rats and effects on food intake, hypothalamic neuropeptide activity, and LH levels were examined.
Materials and Methods
Experimental animals
Adult virgin and d 18–19 pregnant Wistar rats (Simonsen Laboratories, Inc., Gilroy, CA) were housed individually and maintained under a 12-h light, 12-h dark cycle (lights on at 0700 h) and constant temperature (23 ± 2 C). Purina Lab Chow (Ralston Purina Co., St. Louis, MO) and water were provided ad libitum. The pregnant rats were checked for the birth of the pups; the day of delivery was considered d 0 postpartum. The lactating rats were ovariectomized and litters adjusted to eight pups on d 2–3 postpartum (for experimental paradigm see supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) and were assigned to lactation, replacement, and pup-removal groups (n = 8/group). Virgin control rats (n = 8) were ovariectomized during random days of the estrous cycle and used 6 d later, mimicking the postsurgical time for the ovariectomized lactators (supplemental Fig. S1). Ovariectomized animals were used so as to eliminate the variable of differing ovarian steroid levels among the groups. Ovariectomy also greatly enhanced the differences in LH levels and ARH kisspeptin (Kiss1) and NKB mRNA expression between the control and lactating animals, thus making it possible to detect effects of treatment. Food intake and body weight measurements were taken daily at approximately 1100 h. The trunk blood and brains were collected at approximately 1300 h. All protocols were approved by the Oregon National Primate Research Center Animal Care and Use Committee and conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experiment 1: insulin replacement
Alzet microosmotic pumps (model 1003D, 1.0 μl/h; Durect Corp., Cupertino, CA) were implanted sc between the scapulae under isoflurane (Hospira, Inc., Lake Forest, IL) anesthesia into the lactating (d 8–9 postpartum) and control (6 d after ovariectomy) rats (supplemental Fig. S1). The insulin replacement lactation group received Alzet minipumps containing 500 ng/μl of human insulin (Sigma-Aldrich, St. Louis, MO). Preliminary studies tested the dose response for insulin replacement and found that 500 ng/μl · h of human insulin treatment restored serum insulin to control, physiological levels and did not cause hypoglycemia. Control, lactation, and pup-removal groups received minipumps filled with saline vehicle. The pups were removed from the pup-removal group at the time of minipump implantation. The pup-removal groups were included to provide a measure of recovery of the various parameters during a 48-h period in the absence of the suckling stimulus. Food intake and body weight were measured daily from the day of ovariectomy to the time the animals were killed. The rats were decapitated 48 h after pump implantation (supplemental Fig. S1) and the trunk blood was collected. The ARH was microdissected in Krebs solution [composition in millimoles: NaCl 126, KCl 2.5, CaCl2 2.4, NaH2PO4 1.2, MgCl2 1.2, NaHCO3 21.4, and glucose 11.1 (pH 7.4), osmolarity 310 osmol/liter] from the brain (600 μm from the bottom of the hypothalamus) on a microtome (Leica VT1000S; GMI, Inc., Ramsey, MN) and stored at −80 C until processed for RNA extraction. All chemicals and reagents were purchased from Sigma-Aldrich. The dorsal portion of the brain containing DMH was embedded in the optimal cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA) and stored at −80 C for in situ hybridization.
Experiment 2: leptin replacement
The same treatment groups and conditions were repeated as described for experiment 1 (supplemental Fig. S1). The leptin replacement rats received Alzet minipumps containing 500 ng/μl of rat leptin (Peprotech Inc., Rocky Hill, NJ). Preliminary studies indicated that 500 ng/μl · h of leptin treatment restored serum leptin to physiological levels.
Experiment 3: insulin + leptin replacement
The same treatment groups and experimental protocol were repeated in experiment 3 (supplemental Fig. S1). The dual-replacement rats each were implanted with two Alzet minipumps containing 500 ng/μl of human insulin or rat leptin, respectively. The pup-removal group was not repeated in experiment 3.
Blood glucose measurements and serum hormone assays
Blood glucose concentration was measured using an ACCU-CHEK Advantage blood glucose meter (Roche U.S. Diagnostics, Indianapolis, IN) immediately after the trunk blood was taken. The blood was then centrifuged and the serum was stored for subsequent insulin and leptin RIAs using the rat insulin RIA kit (detects 100% of rat and human insulin) and the rat leptin RIA kit (Linco Research, Inc., St. Charles, MO). The assays were performed by the Endocrine Services Laboratory at the Oregon National Primate Research Center. The rat LH sandwich assay was performed by the Ligand Assay and Analysis Core Laboratory at University of Virginia, according to methods described previously (35). Samples from the groups in all three experiments were run in one assay.
RNA extraction and real-time PCR analysis
RNA extraction and PCR analysis were performed as previously described (19). Dissected ARHs were homogenized in 1 ml Trizol reagent (Invitrogen Corp., Carlsbad, CA), and total cellular RNA was isolated. RNA samples (1 μg) were reverse transcribed using random hexamer primers (Promega Corp., Madison, WI). The reaction mixture (10 μl) consisted of 5 μl of TaqMan universal PCR master mix, 300 nm inventoried primers and probes, 80 nm 18S rRNA primers, 250 nm 18S rRNA probe (Applied Biosystems, Foster City, CA), and 2 μl cDNA. Amplification was performed using the ABI/Prism 7700 sequences detector system (Applied Biosystems) with 2 min at 50 C, 10 min at 95 C, and then 45 cycles each at 95 C for 15 sec and 60 C for 60 sec. The target genes were NPY (Rn01410146_m1), AGRP (Rn01431703_g1), POMC (Rn00595020_m1), NKB (Rn00569758_m1), Kiss1 (Rn00710914_m1), and prodynorphin (PDYN) (Rn00571351_m1) (Applied Biosystems). RNA samples from each experiment were run in a separate PCR analysis.
In situ hybridization
The dorsal portion of the brains were sectioned (20 μm) on a cryostat (MICROM HM500OM; Carl Zeiss IMT Corp., Maple Grove, MN) in a one-in-three coronal series through the entire extent of the DMH. NPY mRNA levels were determined in one series of tissue sections, as previously described (8,36). The plasmid (obtained from Dr. S. L. Sabol, National Institutes of Health, Bethesda, MD) contained 511 bp NPY cDNA, and a cRNA probe was transcribed using T3 RNA polymerase (Promega) in which 25% of the uridine 5-triphosphate was 35S labeled (PerkinElmer, Wellesley, MA). Brain sections were fixed, dehydrated, delipidated, rehydrated, and then air dried, as previously described (8,36). The sections were exposed to the labeled NPY probe (specific activity 5–6 × 108 dpm/μg; saturating concentration 0.3 μg/ml · kb) overnight in a humidified chamber at 55 C. After incubation, the slides were washed, dehydrated, and dried. For quantification of the DMH NPY mRNA levels, slides were dipped in nitroblue tetrazolium salt emulsion (Eastman Kodak, Rochester, NY) diluted 1:1 in 600 mm ammonium acetate (Fisher Scientific, Fair Lawn, NJ), placed in light-tight boxes containing desiccant, and stored at 4 C for 5 d.
Methods for analyzing DMH NPY mRNA have been previously described (36). Images of silver grains were captured under dark-field illumination using a CoolSNAP charge-coupled device camera (Photometrics, Tucson, AZ) and analyzed using the MetaMorph Imaging system (Universal Imaging Corp., West Chester, PA). Silver grains were analyzed using a sampling box that encompassed the entire region of interest and measured as the integrated intensity. Background labeling, determined using the same sampling box over an adjacent region that contained no NPY mRNA expression, was subtracted from this measurement. For the DMH, the sampling box encompassed both the central compact zone (DMHc) and the surrounding scattered neurons of the noncompact zone (DMHnc). To distinguish between these two regions, a second region of interest was drawn to outline only the DMHc, and this measurement (minus its corresponding background measurement) was subtracted from the entire DMH measurement to produce a measure of the DMHnc. Measurements were taken bilaterally through the complete rostrocaudal extent of the DMH. The mean value per section per animal was determined and used for statistical analysis. Three separate in situ hybridization assays were run, one for each experiment.
Statistical analysis
Statistical evaluation of mean differences in daily measurements within each group, blood glucose and serum hormone concentrations, and ARH neuropeptide mRNA levels among the groups was performed by one-way ANOVA, with a significance level set at 0.05. To identify significant differences, the Student-Newman-Keuls post hoc test was used for pairwise multiple comparisons. A Student’s t test was used to compare DMH NPY mRNA expression between lactation and replacement groups. Data are presented as mean ± sem.
Results
Serum insulin and leptin concentrations
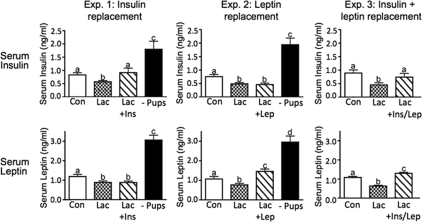
Lactating rats displayed significantly reduced serum concentrations of insulin and leptin, as measured by RIA, compared with controls in all three experiments (Fig. 1), in agreement with previous results (26). In response to insulin infusion via the minipumps, serum insulin concentrations were restored to control levels, whereas serum leptin remained unaffected (Fig. 1, experiment 1). In response to leptin replacement, serum leptin concentrations somewhat exceeded the levels in controls but were still in the normal physiological range, whereas serum insulin remained unchanged (Fig. 1, experiment 2). Dual replacement (Fig. 1, experiment 3) restored both insulin and leptin to normal physiological levels. Pup removal for 48 h caused a rebound of both serum insulin and leptin to levels that were significantly higher than controls in both experiments 1 and 2 (Fig. 1), as reported previously by our group (26).
Figure 1.
Serum insulin and leptin concentrations for experiments 1–3 as determined by RIA. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins, lactation + insulin replacement; Lac + Lep, lactation + leptin replacement; Lac + Ins/Lep, lactation + insulin and leptin replacement; − Pups, 48 h pup removal. Columns with different letters are significantly different (P < 0.05).
Blood glucose concentrations
The lactating rats in experiments 1 and 3 had slightly, but significantly, lower blood glucose concentrations than those of controls (supplemental Fig. S2), a phenomenon that has been observed occasionally in previous experiments. However, blood glucose concentrations were still in the normal physiological range and did not decrease further after insulin, leptin, or dual replacement (supplemental Fig. S2). Blood glucose concentrations did not change after 48 h pup removal (supplemental Fig. S2, experiments 1 and 2), even though the animals were hyperinsulinemic (Fig. 1).
Food intake and body weight
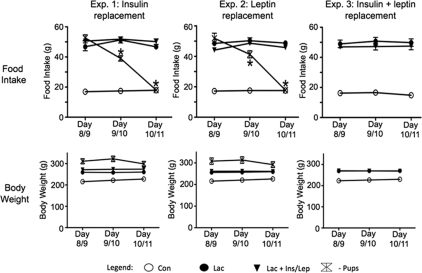
Food intake and body weight were measured to determine whether the insulin and/or leptin treatment affected the hyperphagia during lactation. At the beginning of treatment, food intake during lactation was 3- to 4-fold higher compared with controls in all three experiments (Fig. 2). There was no change in food intake after saline treatment of the controls or after insulin, leptin, or dual replacement treatment of the lactation groups (Fig. 2). After pup removal, the increased food intake during lactation was completely reversed by 48 h in both experiments 1 and 2 (Fig. 2), as reported previously (26).
Figure 2.
Food intake and body weight measurements at the beginning and during 48 h of treatment in experiments 1–3. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins/Lep, lactation + insulin and leptin replacement; −Pups, 48 h pup removal. The lactating rats had significantly greater food intake and body weight compared with controls in all three experiments. *, Significant difference in food intake within the − Pups group during the 48 h (P < 0.05). There were no significant differences in food intake or body weight within any other groups after treatment.
The lactating rats had higher body weights (∼20%) compared with controls in all three experiments (Fig. 3). Replacement of insulin, leptin, or insulin and leptin had no effect on body weight (Fig. 2). There was a small but insignificant decrease in body weight after pup removal in experiments 1 and 2 (Fig. 2). The pup-removal groups had slightly higher initial body weights in both experiments 1 and 2 (Fig. 2). At the initiation of treatment of the lactating rats, the heaviest animals were placed in the pup-removal groups, which greatly reduced the variation in initial body weights for the lactation control and lactation plus replacement groups.
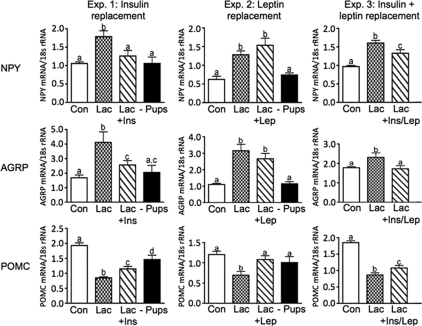
Figure 3.
ARH appetite-regulating neuropeptide mRNA expression analyzed by real-time PCR for NPY, AGRP, and POMC in experiments 1–3. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins, − lactation + insulin replacement; Lac + Lep; lactation + leptin replacement; Lac + Ins/Lep, lactation + insulin and leptin replacement; − Pups, 48 h pup removal. Columns with different letters are significantly different (P < 0.05).
ARH appetite-regulating neuropeptide mRNA expression
ARH NPY, AGRP, and POMC mRNA were analyzed by quantitative real-time PCR. Compared with controls, ARH NPY and AGRP mRNA expression were significantly increased, whereas POMC mRNA expression was significantly decreased in lactating rats in all three experiments (Fig. 3). Insulin replacement decreased NPY mRNA expression to control levels and also decreased AGRP but not completely to control levels (Fig. 3, experiment 1). Similarly, POMC mRNA levels were partially increased to control levels (Fig. 3, experiment 1). Leptin replacement completely restored ARH POMC mRNA expression to control levels without altering ARH NPY and AGRP mRNA expression (Fig. 3, experiment 2). Replacement of both insulin and leptin had similar effects as insulin replacement alone and decreased NPY and AGRP mRNA, although to different degrees (Fig. 3, experiment 3). Surprisingly, POMC was partially restored only to control levels (Fig. 3, experiment 3), in contrast to the effects of leptin treatment alone (Fig. 3, experiment 2). Pup removal for 48 h generally reversed the changes in NPY, AGRP, and POMC mRNA expression to control levels (Fig. 3, experiments 1 and 2).
DMH NPY mRNA expression
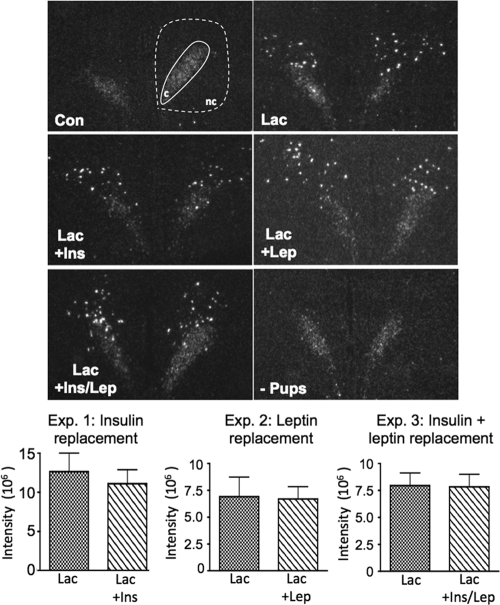
NPY mRNA levels in the DMH were assessed by in situ hybridization in separate assays for each experiment; thus, there was variability in the relative amounts of NPY mRNA among the three experiments. Representative examples of DMH NPY mRNA expression are shown for the various groups in Fig. 4. NPY mRNA expression in the DMHc was similar in all treatment groups through all three experiments. NPY mRNA expression was induced in the DMHnc during lactation, as previously reported (5,8,36), and was not altered after insulin, leptin, or dual replacement. The expression of NPY mRNA in the DMHnc was completely gone after 48 h pup removal (Fig. 4). The quantitative analysis of NPY mRNA expression for each of the experiments is shown in the bar graphs at the bottom of Fig. 4. There were no differences in NPY mRNA expression in the DMHnc between lactation and replacement groups in the three experiments.
Figure 4.
NPY mRNA expression in DMH analyzed by in situ hybridization for experiments 1–3. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins, − lactation + insulin replacement; Lac + Lep; lactation + leptin replacement; Lac + Ins/Lep, lactation + insulin and leptin replacement; − Pups, 48 h pup removal. NPY mRNA expression in the DMHc was consistent in virgin controls during lactation and treatments. NPY mRNA expression is highly induced in the DMHnc during lactation and remained the same after insulin and/or leptin replacements. There was no statistically significant difference in DMHnc NPY mRNA expression between lactation and replacement groups in experiments 1–3. c, DMHc; nc, DMHnc.
ARH reproduction-regulating neuropeptide mRNA expression
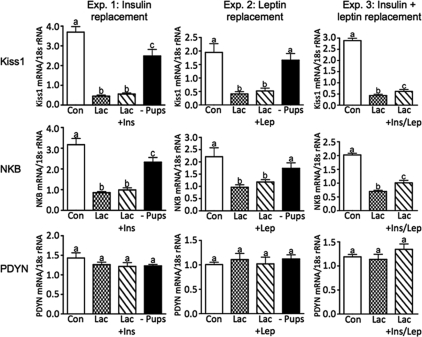
Quantitative real-time PCR was used to analyze mRNA expression of Kiss1, NKB, and PDYN. ARH Kiss1 and NKB mRNA expression were significantly reduced during lactation compared with controls (Fig. 5, experiments 1–3) and were not affected by insulin (Fig. 5, Experiment 1) or leptin (Fig. 5, experiment 2) replacement alone. Dual replacement of insulin and leptin increased Kiss1 and NKB mRNA expression slightly, but the levels were still significantly suppressed compared with the control levels (Fig. 5, experiment 3). Pup removal restored Kiss1 and NKB mRNA expression to or near control levels (Fig. 5, experiments 1 and 2). ARH PDYN mRNA expression did not differ among the various groups investigated through all three experiments (Fig. 5, experiments 1–3).
Figure 5.
ARH reproduction-regulating neuropeptide mRNA expression analyzed by real-time PCR for Kiss1, NKB, and PDYN in experiments 1–3. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins, − lactation + insulin replacement; Lac + Lep; lactation + leptin replacement; Lac + Ins/Lep, lactation + insulin and leptin replacement; − Pups, 48 h pup removal. Columns with different letters are significantly different (P < 0.05).
Serum LH concentrations
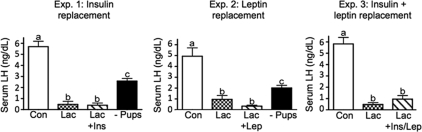
Serum LH concentrations, as measured by RIA, were significantly suppressed during lactation compared with controls in all three experiments (Fig. 6). Insulin, leptin, or dual replacement did not alter serum LH concentrations (Fig. 6, experiments 1–3). Pup removal significantly increased serum LH levels compared with the lactating rats, but the levels did not reach control values (Fig. 6, experiments 1 and 2).
Figure 6.
Serum LH concentrations analyzed by RIA for experiments 1–3. Con, Virgin control; Lac, lactation + eight pups; Lac + Ins/Lep, lactation + insulin and leptin replacement; −Pubs, 48 h pup removal. Columns with different letters are significantly different (P < 0.05). It should be noted that LH levels after 48 h pup removal do not catch up to control values due to the shorter time for the postcastration hypersecretion of LH (2 vs. 8 d, respectively).
Discussion
The present studies demonstrate that hypoinsulinemia and hypoleptinemia during lactation provide important signals that regulate neuropeptide mRNA expression in the ARH. Restoration of insulin alone to physiological levels reversed much of the orexigenic drive provided by the ARH by decreasing NPY and AGRP and increasing POMC mRNA expression, yet food intake was not affected because the lactating rats remained extremely hyperphagic, in general agreement with a previous study (37). Restoration of insulin or leptin also had little effect to restore the suppressed levels of kisspeptin and NKB or of serum LH concentrations. These results suggest that factors other than negative energy balance are acting during this midphase of lactation to maintain the chronic hyperphagia and the suppression of GnRH/LH, in agreement with others (38). It is possible that during later stages of lactation, the role of negative energy balance may become more important (2).
The protocol used to administer exogenous insulin and/or leptin to lactating rats was successful in restoring circulating concentrations of these hormones to physiological control levels. Administration of insulin or leptin alone had no effect on the other hormone. Others have reported that administration of bovine insulin to intact lactating rats restored both insulin and leptin to control levels (37). Possible explanations for the discrepancy between the two studies are the type of insulin used (human vs. bovine), the RIA assays used (whether they measured 100% of the potencies of the two forms of insulin), and the absence or presence of estrogen (ovariectomized vs. intact animals). In our study, restoring insulin and leptin to physiological levels also had no effect on blood glucose levels. It is interesting to note that even in the pup-removal groups, in which insulin levels were rebounded above normal levels, there was no associated hypoglycemia. In the absence of the suckling stimulus, these animals appear to be insulin insensitive, perhaps reflecting their increased body weight and adiposity.
The results from replacement of insulin and/or leptin were somewhat surprising because they showed differential effects on ARH neuropeptide mRNA expression. We recognize that changes in mRNA expression may not totally reflect changes in neuropeptide expression. In experiment 1, insulin replacement alone decreased both ARH NPY and AGRP mRNA expression and increased POMC mRNA expression. Others have shown that ARH NPY/AGRP and POMC neurons express insulin receptors (39,40). Third ventricular administration of insulin decreases expression of NPY in the ARH, and downstream melanocortin signaling is involved in the resultant decrease in food intake (29). The inability of insulin replacement to decrease food intake in the lactating rats, even though it removed of much of the orexigenic drive from the ARH, suggests that another brain area is acting to sustain the hyperphagia.
In contrast to the effects of insulin replacement, leptin replacement alone (experiment 2) only affected POMC neurons, in which POMC mRNA expression was increased to control levels, whereas ARH NPY and AGRP mRNA expression were unaffected. These results differ from a previous study in which a similar dose of leptin administered to lactating rats had no effect on POMC or NPY mRNA but caused a decrease in AGRP mRNA (37). However, the previous study did not observe the characteristic lactation-induced increase in NPY mRNA and decrease in POMC mRNA, as reported by others (1,3), and shown in experiments 1–3 in the current study. Thus, questions can be raised about the previous study regarding the analysis of mRNA expression (37). Our results are consistent with studies showing that leptin receptors are expressed on POMC neurons and leptin causes an increase in POMC signaling (31). However, NPY/AGRP neurons also express leptin receptors (41), and higher doses of leptin can clearly inhibit NPY and AGRP mRNA expression (31,42,43). The lack of effect of leptin on NPY and AGRP observed in our studies suggests that under normal physiological levels of leptin, and in the presence of low levels of insulin, leptin’s primary effect may be only on POMC neurons.
The results from experiment 3 were unexpected in that dual replacement of insulin and leptin did not increase POMC mRNA expression to control levels, as was observed with leptin treatment alone. Similar effects were observed after 48 h pup removal (Fig. 3), in which very high serum insulin and leptin levels are present (Fig. 1, experiment 1). These results suggest some possible interaction between insulin and leptin. Others have suggested that when administered together, the catabolic effect is subadditive and may reflect that leptin and insulin use common regulatory pathways such that the efficacy of one peptide is reduced by addition of the second peptide (44). It has been shown that similar to insulin, leptin also signals through the insulin receptor substrate/phosphatidylinositol 3-kinase pathway in neurons involved in regulation of food intake (45). The degree of additivity may also reflect the levels of insulin and leptin and the sensitivity to their effects. The exact mechanisms involved in the combined signaling of insulin plus leptin remain to be resolved.
The inability of insulin alone or insulin plus leptin replacement to decrease food intake, in the absence of much of the orexigenic drive provided by the ARH, suggests there are other hypothalamic areas involved in the hyperphagia of lactation. Studies from our group suggest that NPY mRNA induction in the DMH is critical for persistent hyperphagia during lactation (36). Other rodent models of obesity and chronic hyperphagia, such as diet-induced obesity (10) or those in which melanocortin signaling is inhibited (11) or cholecystokinin receptors are lacking (46), are also characterized by an induction of NPY expression in the DMH. The DMH NPY neurons project to the paraventricular nucleus of the hypothalamus, which provides neuroanatomical evidence for their potential role in the regulation of food intake (5). The mechanisms involved in the induction of NPY in the DMH during lactation appear to involve the suckling-induced increase in prolactin (6) and a decrease in melanocortin signaling through increased ARH AGRP and decreased POMC (36). Studies from our group showed that melanocortin receptor signaling within the DMH appears to play a critical role in induction of NPY and the hyperphagia of lactation (36). However, insulin or insulin plus leptin replacement reversed much of the decrease in melanocortin signaling coming from the ARH, at least based on mRNA levels, but the induction of the NPY in the DMH remained unchanged (Fig. 4). Therefore, the factors that are responsible for DMH NPY induction remain unclear but could include prolactin (6), whose central actions have been shown to contribute to the hyperphagia of lactation (38). Our results suggest that factors associated with the negative energy balance of lactation are not required for induction of DMH NPY.
Restoration of insulin and/or leptin was unable to restore GnRH/LH secretion in the lactating rats (Fig. 6). Therefore, factors associated with negative energy balance do not appear to be a necessary prerequisite for the suppression of GnRH/LH (38). We speculate that the continued increase in DMH NPY and the inhibition of Kisspeptin and NKB after insulin and/or leptin replacement are likely to play key roles in this suppression. Our hypothesis is supported by results from the pup removal group in which the near normalization of NPY, Kiss1, and NKB mRNA levels is associated with significant stimulation of LH. It should be noted that LH levels do not fully recover in the pup removal group because of insufficient time for stimulated LH secretion, i.e. 2 d after pup removal compared with 8 d in the controls.
A role for an inhibitory effect of increased NPY on GnRH neurons is supported by studies from our group showing that there is increased inhibitory NPY tone acting directly on GnRH cell bodies during lactation (17) and that NPY fibers from the ARH make close appositions on GnRH cell bodies (47). Even though insulin replacement reversed the increase in ARH NPY mRNA, the high level of NPY expression in the DMH was unaffected by any treatments so that it could still provide an inhibitory influence because neurons from the DMH have also been shown to project to areas containing GnRH cell bodies (48).
Another important factor in the suppression of GnRH/LH after insulin and/or leptin replacement is the continued inhibition of ARH Kiss1 and NKB mRNA expression (Fig. 5) (33,34). Kisspeptin is thought to be the primary upstream regulator of GnRH neurons, and the ARH population of Kisspeptin neurons appears to be involved in regulation of basal GnRH/LH secretion and the negative feedback effects of estrogen (20,34). A key role for NKB in regulation of reproduction is less well established, although a recent report of hypogonadotropic hypogonadism in patients with mutations in the genes for NKB and the neurokinin 3 receptor (49) suggests that NKB may also play a primary role. Others have shown that administration of Kisspeptin to lactating rats can induce LH responses, presumably through stimulation of GnRH (19). Thus, the suppression of ARH Kisspeptin neurons may be a primary factor in the suppression of GnRH secretion.
It has recently been established that Kisspeptin, NKB, and dynorphin (DYN) are colocalized in the same neurons in the ARH in the sheep (50). We confirmed this observation in the rat (our unpublished data). The lack of change in PDYN mRNA levels in any of the treatment groups in this study demonstrates that these three neuropeptides are differentially regulated in the same neuron. It is unclear what role DYN may be playing in the regulation of GnRH in rodents. The inability of leptin replacement to increase Kiss1 and NKB mRNA levels was surprising because these neurons express leptin receptors and leptin administration can increase Kiss1 mRNA expression in the ARH (34). In addition, the restoration of NPY, AGRP, and POMC to near normal levels by insulin replacement also had no effect to increase Kiss1 and NKB mRNA levels, suggesting that these ARH neuropeptides are not involved in the inhibition of the Kisspeptin/NKB/DYN neurons. At this point, it is unclear whether there are special factors associated with lactation that are responsible for the suppression of Kisspeptin and NKB or whether similar factors are involved in their suppression under all conditions of negative energy balance. Leptin administration during a 48-h fast has been reported to prevent the fasting-induced suppression of LH secretion. However, the doses of leptin used were much higher than those used in this study and would have resulted in pharmacological levels of leptin (51). Therefore, it is unknown whether normal physiological levels of leptin would be able to restore Kisspeptin, GnRH, and LH during the fasted or chronically underfed state. Future studies will explore these possibilities.
In summary, replacement of physiological levels of insulin and/or leptin removes much of the hyperphagic drive provided by the ARH but has no effect on food intake or body weight, suggesting that the induction of NPY in the DMH may be critical for the chronic hyperphagia during lactation. These replacement studies also revealed differential effects of the hormones, in that leptin alone acted only on POMC neurons and not on NPY/AGRP, whereas insulin acted on both POMC and NPY/AGRP neurons. Restoration of insulin and/or leptin is not sufficient to recover Kiss1 or NKB mRNA expression or GnRH/LH secretion. This study strongly supports the conclusion that negative energy balance is not required for the hyperphagic drive or the suppression of GnRH/LH during lactation. This insulin/leptin replacement regimen may provide a critical model to identify new potent factors or systems outside the ARH that underlie hyperphagia or regulation of reproductive function.
Supplementary Material
Acknowledgments
We are grateful to members of the Division of Animal Resources and the Endocrine Services Laboratory at the Oregon National Primate Research Center for their technical assistance. We also thank the Ligand Assay and Analysis Core Laboratory at the University of Virginia for performing the LH assays.
Footnotes
This work was supported by National Institutes of Health Grants HD14643, DK60685, DK62202, and U54 HD18185 (through a cooperative agreement as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research), and the Oregon National Primate Research Center Base Grant RR00163.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 21, 2009
Abbreviations: AGRP, Agouti-related protein; ARH, arcuate nucleus; DMH, dorsal medial hypothalamus; DMHc, DMH compact zone; DMHnc, DMH noncompact zone; DYN, dynorphin; NKB, neurokinin B; NPY, neuropeptide Y; PDYN, prodynorphin; POMC, promelanocortin.
References
- Smith MS, Grove KL 2002 Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol 23:225–256 [DOI] [PubMed] [Google Scholar]
- Tsukamura H, Maeda K 2001 Non-metabolic and metabolic factors causing lactational anestrus: rat models uncovering the neuroendocrine mechanism underlying the suckling-induced changes in the mother. Prog Brain Res 133:187–205 [DOI] [PubMed] [Google Scholar]
- Malabu UH, Kilpatrick A, Ware M, Vernon RG, Williams G 1994 Increased neuropeptide Y concentrations in specific hypothalamic regions of lactating rats: possible relationship to hyperphagia and adaptive changes in energy balance. Peptides 15:83–87 [DOI] [PubMed] [Google Scholar]
- Smith MS 1993 Lactation alters neuropeptide-Y and proopiomelanocortin gene expression in the arcuate nucleus of the rat. Endocrinology 133:1258–1265 [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS 1998a Neuropeptide Y (NPY) neurons in the arcuate nucleus (ARH) and dorsomedial nucleus (DMH), areas activated during lactation, project to the paraventricular nucleus of the hypothalamus (PVH). Regul Pept 75–76:93–100 [DOI] [PubMed] [Google Scholar]
- Chen P, Smith MS 2004 Regulation of hypothalamic neuropeptide Y messenger ribonucleic acid expression during lactation: role of prolactin. Endocrinology 145:823–829 [DOI] [PubMed] [Google Scholar]
- Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS 1999 Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology 140:2645–2650 [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS 1998 The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology 139:1645–1652 [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL 2002 The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav 76:431–442 [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H 1998 Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport 9:3415–3419 [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD 1997 Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the Agouti obesity syndrome. Mol Endocrinol 11:630–637 [DOI] [PubMed] [Google Scholar]
- Kalra SP, Crowley WR 1992 Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol 13:1–46 [PubMed] [Google Scholar]
- Kalra SP, Kalra PS 1996 Nutritional infertility: the role of the interconnected hypothalamic neuropeptide Y-galanin-opioid network. Front Neuroendocrinol 17:371–401 [DOI] [PubMed] [Google Scholar]
- Catzeflis C, Pierroz DD, Rohner-Jeanrenaud F, Rivier JE, Sizonenko PC, Aubert ML 1993 Neuropeptide Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology 132:224–234 [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Broqua P, Pierroz DD, Hayward A, Dumont Y, Quirion R, Junien JL, Aubert ML 1999 Evidence that the inhibition of luteinizing hormone secretion exerted by central administration of neuropeptide Y (NPY) in the rat is predominantly mediated by the NPY-Y5 receptor subtype. Endocrinology 140:4046–4055 [DOI] [PubMed] [Google Scholar]
- Campbell RE, ffrench-Mullen JM, Cowley MA, Smith MS, Grove KL 2001 Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology 74:106–119 [DOI] [PubMed] [Google Scholar]
- Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS 2009 Suppression of basal spontaneous GnRH neuronal activity during lactation: role of inhibitory effects of NPY. Endocrinology 150:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H 2007 Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 148:2226–2232 [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Grove KL, Lau SY, McWeeney S, Smith MS 2005 Deoxyribonucleic acid microarray analysis of gene expression pattern in the arcuate nucleus/ventromedial nucleus of hypothalamus during lactation. Endocrinology 146:4391–4398 [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA 2008 The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol 70:213–238 [DOI] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H 2007 Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocrinol Metab Disord 8:21–29 [DOI] [PubMed] [Google Scholar]
- Colledge WH 2008 GPR54 and kisspeptins. Results Probl Cell Differ 46:117–143 [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I 2004 Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- Brogan RS, Mitchell SE, Trayhurn P, Smith MS 1999 Suppression of leptin during lactation: contribution of the sucking stimulus versus milk production. Endocrinology 140:2621–2627 [DOI] [PubMed] [Google Scholar]
- Crowley WR, Ramoz G, Torto R, Keefe KA, Wang JJ, Kalra SP 2007 Neuroendocrine actions and regulation of hypothalamic neuropeptide Y during lactation. Peptides 28:447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW 2001 Brain pathways controlling food intake and body weight. Exp Biol Med 226:978–981 [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Seeley RJ, Woods SC 2004 Insulin and leptin as adiposity signals. Recent Prog Horm Res 59:267–285 [DOI] [PubMed] [Google Scholar]
- Cowley MA 2003 Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol 480:3–11 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M 2009 KISS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides 30:139–145 [DOI] [PubMed] [Google Scholar]
- Hill J, Elmquist JK Elias CF 2008 Hypothalamic pathways linking energy balance and reproduction. Am J Physiol 294:E827–E832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA 2006 KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18:298–303 [DOI] [PubMed] [Google Scholar]
- Fallest PC, Trader GL, Darrow JM, Shupnik MA 1995 Regulation of rat luteinizing hormone β gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod 53:103–109 [DOI] [PubMed] [Google Scholar]
- Chen P, Williams SM, Grove KL, Smith MS 2004 Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. J Neurosci 24:5091–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley WR, Ramoz G, Torto R, Kalra SP 2004 Role of leptin in orexigenic neuropeptide expression during lactation in rats. J Neuroendocrinol 16:637–644 [DOI] [PubMed] [Google Scholar]
- Woodside B 2007 Prolactin and the hyperphagia of lactation. Physiol Behav 91:375–382 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC 2002 The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22:9048–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hâkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B 1998 Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW 2005 Leptin inhibits hypothalamic NPY and AGRP gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289:E1051–E1057 [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua Jr SC, Leibel RL, Wardlaw SL 2001 Leptin regulation of AGRP and NPY mRNA in the rat hypothalamus. J Neuroendocrinol 13:959–966 [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC 2002 Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology 143:2449–2452 [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK 2008 Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118:1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH 2008 Unraveling the obesity of OLETF rats. Physiol Behav 94:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS 1999 Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology 140:5382–5390 [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW 1996 Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol 376:143–173 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek S, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL 1998 Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67:370–376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.