Abstract
Animal models are critical for the study of psychiatric disorders since they allow the use of invasive methods that cannot be used for ethical reasons in humans. Currently there are three general models of schizophrenia; (i) those produced with acute pharmacological intervention (i.e. MK-801, ketamine, PCP and amphetamine), (ii) genetic models (i.e. mutant DISC-1, D2-R over expression) and (iii) developmental disruption models (i.e. MAM, neonatal ventral hippocampal lesion, isolation rearing, maternal infection). Here we review evidence for the validity of gestational (day 17) MAM administration as a developmental disruption rodent model of schizophrenia. Offspring from MAM-treated dams are reported to display deficits consistent with those observed in schizophrenia patients, including anatomical changes, behavioral deficits and altered neuronal information processing. Thus gestational MAM administration has been demonstrated to induce a pathodevelopmental process leading to neuroanatomical and behavioral phenotypes consistent with that observed in schizophrenia in humans.
Keywords: MAM, animal model, schizophrenia, hippocampus, prefrontal cortex, dopamine, psychosis
Animal models are critical for the study of psychiatric disorders since they enable the use of invasive methods to examine the mechanisms underlying pathophysiology of disease states. For this reason, there have been numerous attempts to generate valid and reliable animal models for studying human behavior and disorders. This has led to the discovery of three general models of schizophrenia; (i) those produced with acute pharmacological intervention (i.e. MK-801, ketamine, PCP and amphetamine) [1-5], (ii) genetic models (i.e. mutant DISC-1, D2-R over expression) [6, 7] and (iii) developmental disruption models (i.e. MAM, neonatal ventral hippocampal lesion, isolation rearing, maternal infection) [8-11]. The finding that multiple and diverse methods can lead to a similar behavioral phenotype is consistent with the hypothesis that schizophrenia in humans arises from multiple unique etiologies, including both genetic and environmental factors that results in a similar pathophysiology in the adult [12-15].
Schizophrenia as a developmental disruption
Increasing evidence suggests that schizophrenia is a developmental disorder that results in the emergence of symptoms following adolescence [16-20]. Evidence for a neurodevelopmental disruption is based largely on follow-back, cohort, and population studies in which the pre-morbid history of schizophrenia patients indicates a prevalence of subtle prenatal perturbations such as famine, maternal infection, or hypoxia during the second trimester [21-23] that may interact with genetic predisposition to result in the schizophrenia phenotype. This finding is central to the development of animal models of psychosis which utilize perinatal insults to produce a behavioral phenotype in the adult that corresponds to some of the general features of schizophrenia in humans [10, 11, 24-26]. One such model employs the administration of a mitotoxin, methylazoxymethanol acetate (MAM), during gestation to induce a developmental disruption. This technique of targeted cellular impairment has been extensively investigated and it has been demonstrated that MAM administration induces anatomical and behavioral deficits that are intimately associated with the time during gestation that the mitotoxin is administered [27-28]. For example, MAM administration on gestational day 15 or earlier reliably produces a number of gross deficits including a decrease in total cortical mass, microcephaly, and profound cortical dysplasias [11]. However, such deficits are not routinely observed in human schizophrenia patients. More recently we have modified this developmental disruption paradigm to specifically target gestational day 17 [30], a period in development when neuronal proliferation has peaked in most cortical regions and is essentially completed or suspended in the majority of subcortical regions [11, 29-30]. At this specific stage of development (GD17), MAM exposure has a more selective effect on developing paralimbic, frontal, and temporal cortices, regions where deficits are typically observed in schizophrenia patients [11, 24-26, 30]. Indeed, MAM GD17 administration has been demonstrated to recapitulate a pathodevelopmental process leading to schizophrenia-like neuroanatomical and behavioral phenotypes in rodent offspring [11, 24-26, 30]. Here we review evidence for the validity of GD17 MAM administration as a developmental disruption rodent model of schizophrenia.
Neuroanatomical pathology in schizophrenia
Schizophrenia is a disease with no gross histopathology that is specific for this disorder. Nonetheless, post-mortem and human imaging studies have demonstrated subtle structural abnormalities in schizophrenia patients (for review see: [31]). Such studies reliably report moderate but consistent reductions in tissue volume or thickness throughout prefrontal cortical and temporal lobe regions [32, 33]. Interestingly, these reductions in cortical volume are not necessarily correlated with alterations in neocortical neuron number [34]. Given that, in the rat, cortical neurons are created between GD14 and GD20, with GD15 being the peak of neurogenesis [29], one may expect that prenatal MAM administration may induce marked neuronal loss and microcephaly. Indeed, when administered on gestational day 15 or earlier, total cortical mass is reduced by up to 67% and brain weight is reduced by almost 30% when compared to controls [11]. In contrast, by GD17, neuronal proliferation has peaked in most cortical regions and is essentially completed or suspended in the majority of subcortical regions [29]. Thus MAM GD17 administration induces more subtle pathologies that are similar to those observed in human patients. More specifically, post-mortem stereological analyses have demonstrated that MAM-treated rats display regionally selective reductions in cortical thickness throughout the medial prefrontal cortex, hippocampus and parahippocampal corticies [11, 26, 35]. Despite the fact that GD17 MAM treatment induces a significant reduction in cortical volume, no significant changes were observed in neocortical neuron number [11]. Therefore, similar to that reported post-mortem in human patients, MAM administration results in an increase in the density of neuronal packing that is prevalent throughout cortical regions, including mPFC [34].
In addition to the morphometric alterations in cortical volume and neuronal density, heterotopias resulting from altered neuronal migration have been reported in the hippocampus of MAM GD17 rats [11, 26, 35]. Thus, in the normal hippocampus, pyramidal neurons are tightly packed and aligned with their apical dendrite oriented towards the stratum radiatum [36]. In contrast, hippocampal pyramidal neurons in schizophrenia patients are more disorganized and display a greater variation in neuronal orientation [36]. These findings are consistent with the heterotopias and sporadic density of pyramidal neurons in the hippocampus of MAM-treated rats [11, 26]. Taken together, the morphological and histological deficits observed following GD17 MAM administration are subtle, and restricted to the later developing paralimbic and temporal cortices, a pattern that accurately reflects the histopathology observed in human patients.
Behavioral correlates of psychosis
A significant hindrance to the development of any animal model is the ability to evaluate the inherently human psychopathology recognized as schizophrenia. More specifically, it is impossible to directly measure symptoms such as delusion, hallucination and thought disorder in rodents. For this reason there is no one simple behavioral validation for animal models of schizophrenia; however, a number of behavioral paradigms have been considered to be valid translational models due to similar measures of assessment in rats and humans. Such behavioral paradigms include prepulse inhibition of startle [37], latent inhibition [38], working memory tasks [39] and hyper-responsivity to psychomotor stimulants [40]. Deficits in these and similar paradigms are consistently observed in human schizophrenia patients and animal models of psychosis.
Prepulse Inhibition of Startle
Prepulse inhibition of startle is a neurological phenomenon whereby a small amplitude sensory stimulus inhibits the behavioral response to a subsequent stimulus and has been suggested as a model of sensorimotor gating [37]. Deficits in prepulse inhibition of startle are suggested to reflect an inability to filter non-relevant sensory information and are present in a number of disease states including schizophrenia [37]. Studies in rodent models of schizophrenia consistently utilize the PPI model as a correlate for psychosis; however, deficits in PPI alone are not sufficient to define an animal model of this disorder. Nevertheless, PPI is a reproducible index of sensorimotor gating and it has been consistently demonstrated that MAM treated rats display robust deficits in PPI, which are not due to alterations in sensory processing or startle amplitude [11, 25, 26]. Moreover, decreases in PPI are only observed after puberty in MAM-treated rats, a finding that parallels the adult onset of the disease in humans [11].
Latent Inhibition
Most people are able to filter the constant stream of sensory information that inundates our everyday lives; however, those with schizophrenia cannot [38]. More specifically, it is believed that one of the more pertinent factors in the appearance of psychosis is a disturbance in attention process functioning [41, 42]. From this perspective, behavioral paradigms that help to evaluate normal attentional processing are extremely useful tools for understanding the mechanisms associated with aberrant filtering of sensory information. One such paradigm is the latent inhibition model, in which exposure to a non-salient stimulus prevents conditioned associations with that stimulus from being formed [38, 43]. Using such a paradigm, there is a significant literature demonstrating a reduction in latent inhibition in a number of animal models of schizophrenia including the MAM treated rat [44, 45]. More specifically, whereas control rats pre-exposed to a conditioned cue demonstrate pronounced latent inhibition, rats treated on GD17 with MAM show none [44, 45]. Thus the latent inhibition paradigm accurately models deficits in the filtering of sensory information processing suggested to underlie the positive symptoms of schizophrenia.
Stimulant induced hyperlocomotion
The heightened sensitivity to amphetamine and phencyclidine in human schizophrenia patients has been suggested to reflect aberrant mesolimbic dopamine transmission thought to underlie the positive symptoms of the disease [46]. Thus, Abi-Dargham and Laruelle has shown that amphetamine will induce significantly greater dopamine release (measured by raclopride displacement) in the striatum of schizophrenia patients, and moreover the amplitude of the release correlates with the ability to exacerbate psychosis [40, 47]. Moreover, dopamine antagonists are particularly effective in treating psychosis [48], and dopamine agonists will mimic and/or exacerbate psychosis in humans [49]. The aberrant increase in dopamine transmission can be readily modeled in rodents by examining the dopamine-dependent locomotor response in freely moving animals. Thus in control rats, the systemic administration of amphetamine, MK-801, or phencyclidine (PCP) induces a significant increase in locomotor activity that is correlated with significant elevations in striatal dopamine levels [50-52]. Interestingly, in most animal models of schizophrenia, a significantly augmented locomotor response to these drugs above that observed in controls is observed [10, 11, 26, 35, 53]. Specifically, MAM treated rats display an enhanced locomotor response to amphetamine, MK-801 and PCP [11, 26, 53] that is correlated with an enhanced dopamine efflux in the nucleus accumbens [35]. This aberrant response to psychomotor stimulants in MAM treated rats is consistent with an increased sensitivity to dopamine agonists in human patients [40, 47]. Thus, low doses of amphetamine, which do not typically induce a robust response in the general population, can precipitate psychosis in schizophrenia patients [46]. Given that the prominent psychotic symptoms of schizophrenia usually are not observed until after puberty, a rodent model that displays a similar delayed onset is essential. Indeed, prior to puberty, MAM-treated rats show comparable increases in locomotor activity following amphetamine administration [11]. Thus an enhanced sensitivity to psychomotor stimulants that only occurs after puberty is a consistent finding in both human schizophrenia patients as well as the MAM model of psychosis.
Working memory
In addition to the positive symptoms of schizophrenia, patients consistently demonstrate deficits in cognitive properties such as working memory [41, 54-56]. Furthermore, in contrast to the dopamine-dependent psychosis, dysfunctions in cognition are more consistently associated with deficits in prefrontal cortical information processing [57-60]. Thus, schizophrenia patients typically perform poorly on working memory task that are correlated with an altered activation of the dorsolateral prefrontal cortex [57-60]. In addition to working memory deficits, frontal dysfunctions may also lead to distortions in planning and execution as well as to perseveration and rigid behavior [61]. More specifically, schizophrenia patients typically exhibit an inability to maintain behavioral flexibility in the face of changing schedules of reinforcement [57]. Working memory performance and perseverative behavior can be accurately assessed in rodents using traditional set-shifting or radial maze paradigms [3, 39, 62-65]. Thus, although MAM treated rats are not impaired in learning a simple discrimination task, they have been shown to display impairments in learning to reverse a previously acquired response in an attentional set shifting task (a rodent analog of the Wisconsin Card Sort Test) [11, 24, 26, 44]. In addition, deficits in reversal learning have been reported in MAM rats examining behavioral flexibility in a Y-maze paradigm [11]. It is important to note that MAM-treated rats do not show a deficit in the learning of a novel discrimination suggesting that forebrain circuits involved in Pavlovian conditioning are not significantly affected by gestational MAM administration [11]. In contrast, the perseverative behavior observed in MAM rats and human schizophrenia patients are likely attributable to frontal lobe, particularly prefrontal cortical, dysfunction.
Neuronal information processing in schizophrenia
The dopamine hypothesis of psychosis is one of the longest standing theories of schizophrenia and posits that enhanced mesolimbic dopamine transmission underlies the positive symptoms of the disease [46, 66, 67]. This hypothesis is based on several observations, including the ability of DA agonists to exacerbate psychosis, the efficacy of DA antagonists in treating schizophrenia, and imaging studies demonstrating increased amphetamine-induced DA release in schizophrenia [46, 66, 67]. Interestingly, recent electrophysiological studies in the MAM G17 rodent model of schizophrenia have demonstrated an enhanced baseline activity of the population of dopamine neurons in the VTA [53]. More specifically, MAM-treated rats display a significantly greater number of spontaneously active VTA dopamine neurons when compared to control rats [53]. The number of spontaneously active dopamine neurons (or ‘population activity’) is highly correlated with tonic levels of extrasynaptic dopamine in the nucleus accumbens and thus has been suggested to regulate the long-term responsivity of the dopamine system [68]. In addition to regulating tonic dopamine transmission, population activity also regulates the gain of the dopamine system by determining the proportion of neurons that can respond to phasic activation [69]. Thus in control animals there is a submaximal number of spontaneously active dopamine neurons that can be increased or decreased by afferent input [68-70]. In contrast, MAM-treated rats display essentially a maximal activation of the mesolimbic dopamine system [53]. Furthermore, activation of afferent structures known to enhance dopamine neuron population activity in control animals, has no significant effect in MAM-treated rats suggesting a loss of a process critical to normal dopamine system functioning [53]. Because of the increase in number of dopamine neurons active, the dopamine system is in a state of hyper-responsivity to a behaviorally salient stimulus that activates dopamine neuron phasic activity. Thus, the enhanced VTA neuron population activity observed in MAM rats is consistent with the hyper-responsive mesolimbic dopamine transmission purported to underlie psychosis in human patients. The one major caveat in the dopamine hypothesis is that there appears to be no primary pathology in the midbrain dopamine system of schizophrenia patients; rather, the DA system appears to be abnormally regulated [46, 71, 72]. Given structural and functional evidence for alterations in afferent inputs to the VTA, we propose that the alteration in dopamine neuron activity is secondary to aberrant afferent transmission [53]. Two such inputs are the medial prefrontal cortex (thought to be largely associated with cognitive deficits [73]) and the hippocampus, a temporal lobe structure principally associated with learning and memory [74, 75]. Alterations in hippocampal structure and function in schizophrenia are consistently demonstrated in postmortem and neuro-imaging studies [34, 76-79]. More specifically, a postmortem reduction in hippocampal volume is one of the more consistent structural abnormalities observed in schizophrenia patients [34, 76-79]. How this affects hippocampal information processing is not entirely known; however, there is increasing evidence for baseline hippocampal hyperactivity in human schizophrenia patients. Specifically, baseline hyperperfusion of the medial temporal lobe was reported almost 10 years ago using SPECT imaging in human schizophrenia patients [80]. More recently, techniques with higher spatial resolution have provided evidence for increased regional cerebral blood flow and volume in the more limbic, anterior hippocampus of unmedicated human schizophrenia patients [81, 82]. Consistent with this, in vivo extracellular recordings throughout the ventral extent of the hippocampus (the rodent analogue of the human anterior hippocampus) have demonstrated baseline hyperactivity in MAM-treated rats [53]. More specifically, MAM rats display a significantly greater average firing rate of presumed glutamatergic, pyramidal neurons when compared to control rats. [53] Interestingly it seems that this aberrant increase in hippocampal activity appears to be the driving force behind the dopamine hyperfunction [53].
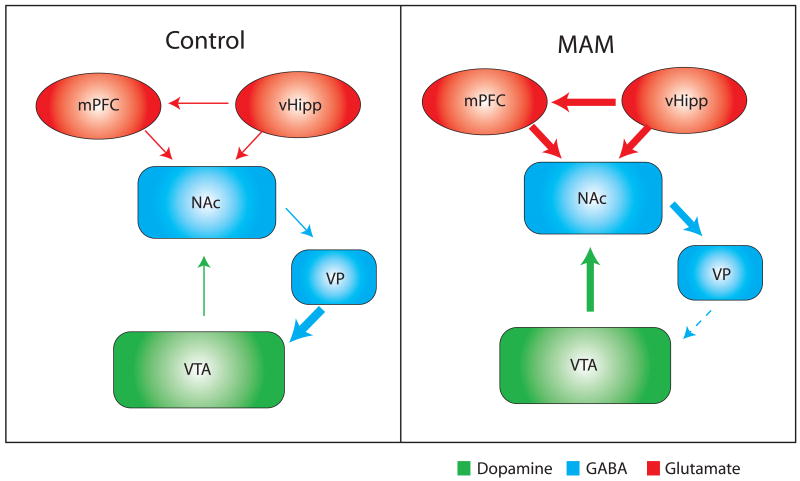
An association between hippocampal function and dopamine neuron activity has been demonstrated previously [68, 69, 83]. More specifically, the ventral subiculum of the hippocampus potently and selectively modulates dopamine neuron population activity which is correlated with an altered dopamine efflux in the nucleus accumbens [68]. Given that the positive symptoms of schizophrenia are correlated with dopamine hyperfunction combined with evidence for hyperactivity within limbic regions of the hippocampus, we suggest that aberrant hippocampal transmission may underlie the augmented DA transmission. This has been investigated recently in the MAM model of schizophrenia. Thus, tetrodotoxin (TTX) inactivation of the ventral hippocampus has been shown to normalize the aberrant increase in dopamine neuron population activity in MAM-treated rats, without significantly altering dopamine neuron activity in control rats [53]. Moreover, inactivation of the ventral hippocampus was also able to reverse the behavioral hyperresponsivity to amphetamine administration that is consistently demonstrated in rodent models of schizophrenia, including MAM rats [53]. Thus, we propose that the augmented dopamine transmission observed in MAM animals is attributable to a pathologically enhanced hippocampal transmission and further suggest this is consistent with observations of hippocampal hyperperfusion in human schizophrenia patients (Figure 1).
Figure 1.
Enhanced activity within the ventral hippocampus results in an increased activation of the nucleus accumbens (NAc). The subsequent increase in NAc output inhibits the ventral pallidum (VP) resulting in the disinhibition of VTA dopamine neurons. This enhanced mesolimbic dopamine neuron population activity is suggested to underlie the augmented response to amphetamine in MAM-treated rats and psychosis in human schizophrenia patients.
The mechanisms underlying the pathological enhancement of hippocampal output are not currently known; however, we propose that a reduction in local inhibitory transmission may underlie the augmented hippocampal output. Evidence for an alteration in cortical and hippocampal inhibitory transmission is abundant based largely on post-mortem studies in human schizophrenia patients [84-89]. Thus a significant reduction in cortical glutamic acid decarboxylase, an enzyme critical for GABA biosynthesis, is consistently reported in post mortem studies [90-92]. Such a decrease in inhibitory transmission in the cortex and hippocampus would likely result in a decrease in intrinsic inhibition. Consistent with this suggestion, there is significant evidence for an upregulation of post-synaptic GABA-A receptors and decrease in GABA uptake sites in patients which may reflect compensatory changes that are acting to increase the diminished intrinsic GABAergic functioning [93-97]. More recent work by Lewis and colleagues have provided significant evidence that the GABA deficits in schizophrenia may be largely restricted to a specific class of GABAergic interneurons; specifically those containing the calcium binding protein parvalbumin [85]. Interestingly, a decrease in parvalbumin-containing interneurons is also a consistent observation in a diverse variety of animal models of schizophrenia, including the MAM model [45, 98-101]. The strong perisomatic innervations of these neurons suggest that parvalbumin interneurons are capable of potently regulating pyramidal cell output, and thus a decreased functionality of these neurons would likely result in both an increase in tonic pyramidal cell activity as well as a decrease in interneuron-generated co-ordinated activity. Indeed, recent data from the MAM G17 model have demonstrated deficits in the activation of cortical and hippocampal assemblies during a latent inhibition paradigm [45]. More specifically, the presentation of a conditioned tone did not significantly alter high-frequency oscillatory activity in MAM-treated rats and was therefore significantly diminished compared to responses observed in saline-treated rats. Furthermore, the deficit in coordinated activity in the MAM-treated rats was task-independent and observed in both the mPFC and vHipp; regions that exhibited significant reductions in PV neuron density [45]. Taken together we suggest that that the deficits in parvalbumin interneuron functionality throughout the ventral hippocampus may be the cause of the baseline hyperactivity and corresponding increase in mesolimbic dopamine transmission.
Conclusions
There is substantial support for gestational MAM administration as a developmental disruption model of schizophrenia. This is based on anatomical, behavioral and neurophysiological deficits that are consistent with those observed in human patients. More specifically, the morphological and histological deficits observed following GD17 MAM administration are subtle, and restricted to the later developing paralimbic and temporal cortices, a pattern that accurately reflects the histopathology observed in human patients. Furthermore, using a number of distinct behavioral paradigms, it has been demonstrated that the MAM-treated rat displays behavioral deficits that are consistent with the positive, negative and cognitive symptoms of schizophrenia in humans. Finally, there is increasing evidence that the neurophysiological deficits underlying these aberrant behaviors are associated with aberrant hippocampal and prefrontal cortical functioning. Thus, the MAM-G17 model recapitulates a pathodevelopmental process leading to a pattern of changes consistent with schizophrenia in rodent offspring. It should be mentioned that gestational MAM administration does not necessarily reflect the etiology of schizophrenia in humans. Nonetheless, the MAM model provides a valid disruption in key neuronal systems that, when placed in the context of complex human brain and behavioral patterns, results in the complex psychopathology that is schizophrenia. The ability to examine the functional interactions among these systems and how disruption within these circuits affects information processing is central to gaining a better understanding of disease and the generation of more effective pharmacotherapies.
Table 1.
Comparison of reported deficits in MAM GD17 rats with human schizophrenia patients.
| Human Schizophrenia | Refs | MAM Rodent Model | Refs |
|---|---|---|---|
| Decrease in medial temporal lobe volume | [34, 76, 78, 79] | Reduced hippocampal volume | [11, 26, 35, 102] |
| Increased neuronal density in prefrontal areas 9 and 46 | [34] | Selective increase in neuronal density in mPFC | [11] |
| Hippocampal neuronal disarray | [36] | Heterotopias and sporadic neuronal packing in hippocampus | [11, 26, 35] |
| Sensorimotor gating deficits | [37, 103-105] | Deficit in prepulse inhibition of startle | [11, 26] |
| Perseveration | [57, 61]. | Impaired reversal learning | [11, 44] |
| Impaired attentional processing | [38, 41, 42]. | Deficits in latent inhibition | [44, 45] |
| Hypersensitivity to drugs that exacerbate positive symptoms | [40, 47] | Enhanced locomotor response to phencyclidine & amphetamine | [11, 35, 53, 99] |
| Dopamine hyperfunction | [40, 47] | Increased dopamine neuron population activity | [53] |
| Decreased PV expression in dlPFC and hippocampus | [85] | Decreased PV interneuron number in mPFC and vHipp | [45, 99] |
| Hypofunctionality of dlPFC and hippocampus by functional imaging studies | [57] | Inability to activate mPFC and vHipp assemblies during a latent inhibition paradigm | [45] |
| Hippocampal hyperperfusion at rest | [80, 82] | Increased average firing rate of vHipp neurons | [53] |
| Increased sensitivity to stress | [106] | Stress-induced disruption in mPFC neuronal plasticity | [107] |
| Working memory deficits | [41, 54-56] | Impaired performance on radial and Y- maze tasks | [11, 26, 44] |
| Social withdrawal | [108-110] | Decreased social interaction | [26, 35] |
| Symptoms appear after puberty | [16-20] | Most behavioral deficits emerge post puberty | [11, 26] |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harvard Review of Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 3.Moghaddam B, et al. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal of Neuroscience. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 5.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Research Reviews. 1986;11(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 6.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Ozeki Y, et al. Disrupted-in-Schizophrenia-1 (DISC-1): Mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortier ME, et al. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. Journal of Psychiatric Research. 2004;38(3):335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Geyer MA, et al. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34(6):361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- 10.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9(1):67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 11.Moore H, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–64. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreasen NC, et al. Symptoms of schizophrenia: Methods, meanings, and mechanisms. Archives of General Psychiatry. 1995;52(5):341–351. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen NC, Carpenter WT., Jr Diagnosis and classification of schizophrenia. Schizophrenia Bulletin. 1993;19(2):199–214. doi: 10.1093/schbul/19.2.199. [DOI] [PubMed] [Google Scholar]
- 14.Tsuang MT, Gilbertson MW, Faraone SV. The genetics of schizophrenia. Current knowledge and future directions. Schizophrenia Research. 1991;4(2):157–171. doi: 10.1016/0920-9964(91)90031-l. [DOI] [PubMed] [Google Scholar]
- 15.Pearlson GD. Neurobiology of schizophrenia. Annals of Neurology. 2000;48(4):556–566. [PubMed] [Google Scholar]
- 16.Jones P, et al. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 17.Raedler TJ, Knable MB, Weinberger DR. Schizophrenia as a developmental disorder of the cerebral cortex. Current Opinion in Neurobiology. 1998;8(1):157–161. doi: 10.1016/s0959-4388(98)80019-6. [DOI] [PubMed] [Google Scholar]
- 18.Niemi LT, et al. Childhood developmental abnormalities in schizophrenia: Evidence from high-risk studies. Schizophrenia Research. 2003;60(23):239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 19.Neumann CS, et al. Developmental pathways to schizophrenia: Behavioral subtypes. Journal of Abnormal Psychology. 1995;104(4):558–566. doi: 10.1037//0021-843x.104.4.558. [DOI] [PubMed] [Google Scholar]
- 20.DeLisi LE. The significance of age of onset for schizophrenia. Schizophrenia Bulletin. 1992;18(2):209–215. doi: 10.1093/schbul/18.2.209. [DOI] [PubMed] [Google Scholar]
- 21.McDonald C, Murray RM. Early and late environmental risk factors for schizophrenia. Brain Res Brain Res Rev. 2000;31(23):130–7. doi: 10.1016/s0165-0173(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 22.Pilowsky LS, Kerwin RW, Murray RM. Schizophrenia: A neurodevelopmental perspective. Neuropsychopharmacology. 1993;9(1):83–91. doi: 10.1038/npp.1993.46. [DOI] [PubMed] [Google Scholar]
- 23.Susser E, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53(1):25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 24.Gourevitch R, et al. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behav Pharmacol. 2004;15(4):287–92. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- 25.Talamini LM, et al. Impaired sensory gating and attention in rats with developmental abnormalities of the mesocortex. Implications for schizophrenia. Ann N Y Acad Sci. 2000;911:486–94. doi: 10.1111/j.1749-6632.2000.tb06751.x. [DOI] [PubMed] [Google Scholar]
- 26.Le Pen G, et al. Peri-pubertal maturation after developmental disturbance: A model for psychosis onset in the rat. Neuroscience. 2006;143(2):395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Balduini W, et al. Treatment with methylazoxymethanol at different gestational days: Physical, reflex development and spontaneous activity in the offspring. NeuroToxicology. 1991;12(2):179–188. [PubMed] [Google Scholar]
- 28.Cattabeni F, Di Luca M. Developmental models of brain dysfunctions induced by targeted cellular ablations with methylazoxymethanol. Physiol Rev. 1997;77(1):199–215. doi: 10.1152/physrev.1997.77.1.199. [DOI] [PubMed] [Google Scholar]
- 29.Bayer SA, Altman J. Neurogenesis and neuronal migration. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 1041–1078. [Google Scholar]
- 30.Grace AA, Moore H. Regulation of information flow in the nucleus accumbens: A model for the pathophysiology of schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. American Psychological Association Press; Washington D.C.: 1998. pp. 123–157. [Google Scholar]
- 31.Shenton ME, et al. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49(12):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honea R, et al. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 33.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 34.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 35.Flagstad P, et al. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29(11):2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- 36.Kovelman JA, Scheibel AB. A neurohistological correlate of schizophrenia. Biological Psychiatry. 1984;19(12):1601–1621. [PubMed] [Google Scholar]
- 37.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: Human and animal model studies. Archives of General Psychiatry. 1990;47(2):181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 38.Lubow RE, Gewirtz JC. Latent inhibition in humans: Data, theory, and implications for Schizophrenia. Psychological Bulletin. 1995;117(1):87–103. doi: 10.1037/0033-2909.117.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 40.Laruelle M, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Science. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophrenia Bulletin. 1993;19(2):233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 42.Gray JA. Integrating schizophrenia. Schizophrenia Bulletin. 1998;24(2):249–266. doi: 10.1093/oxfordjournals.schbul.a033324. [DOI] [PubMed] [Google Scholar]
- 43.Weiner I. Neural substrates of latent inhibition: The switching model. Psychological Bulletin. 1990;108(3):442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- 44.Flagstad P, Glenthoj BY, Didriksen M. Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology. 2005;30(2):250–60. doi: 10.1038/sj.npp.1300625. [DOI] [PubMed] [Google Scholar]
- 45.Lodge D, Behrens M, Grace A. A loss of parvalbumin containing interneurons is associated with diminished gamma oscillatory activity in an animal model of schizophrenia. Schizophrenia Research. 2008;102(13 Supplement 2):112–112. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7 Suppl 1:S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 47.Abi-Dargham A, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Science. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeman P, et al. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophrenia Bulletin. 1990;16(1):97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- 50.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(26):12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. Journal of Neurochemistry. 1997;68(5):2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 52.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: A microdialysis study in behaving rats. Synapse. 1995;19(1):56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green MF, et al. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 55.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 56.Gray JA, et al. The neuropsychology of schizophrenia. Behavioral and Brain Sciences. 1991;14(1):1–84. [Google Scholar]
- 57.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 59.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 60.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biological Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 61.Ridley RM. The psychology of perseverative and stereotyped behaviour. Progress in Neurobiology. 1994;44(2):221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 62.Murphy BL, et al. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(3):1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. Journal of Neurophysiology. 2000;83(3):1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- 64.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 65.De Bruin JPC, et al. A behavioural analysis of rats with damage to the medial prefrontal cortex using the morris water maze: Evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Research. 1994;652(2):323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 66.Carlsson A, et al. Network interactions in schizophrenia - therapeutic implications. Brain Res Brain Res Rev. 2000;31(23):342–9. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 67.Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 68.Floresco SB, et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 69.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 70.Grace AA, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 72.Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Rev. 2000;31(23):330–41. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 73.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 74.Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Research Bulletin. 2002;58(5):517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- 75.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 76.Heckers S. The hippocampus in schizophrenia. American Journal of Psychiatry. 2004;161(11):2138–2139. doi: 10.1176/appi.ajp.161.11.2138-a. [DOI] [PubMed] [Google Scholar]
- 77.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of Neural Transmission. 2002;109(5):891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson MD, et al. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Archives of General Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 79.Shenton ME, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(12):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malaspina D, et al. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46(1):89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- 81.Malaspina D, et al. Hippocampal dysfunction in ca1 is associatedwith schizophrenia. Schizophrenia Research. 2008;102(13 Suppl 2):19–20. [Google Scholar]
- 82.Medoff DR, et al. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–50. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 83.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benes FM, et al. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 86.Reynolds GP, Beasley CL, Zhang ZJ. Understanding the neurotransmitter pathology of schizophrenia: Selective deficits of subtypes of cortical GABAergic neurons. Journal of Neural Transmission. 2002;109(56):881–889. doi: 10.1007/s007020200072. [DOI] [PubMed] [Google Scholar]
- 87.Heckers S, et al. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Archives of General Psychiatry. 2002;59(6):521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- 88.Benes FM. Is the GABA cell a final common pathway for the etiology and treatment of schizophrenia and bipolar disorder? Current Opinion in Psychiatry. 2002;15(3):277–278. [Google Scholar]
- 89.Benes FM, Berretta S. GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 90.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52(4):258–278. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 91.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volk DW, et al. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical ?-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 93.Benes FM, et al. Increased GABA(A) receptor binding in superficial layers of cingulate cortex in schizophrenics. Journal of Neuroscience. 1992;12(3):924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benes FM, et al. Up-regulation of GABA(A) receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75(4):1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 95.Reynolds GP, Czudek C, Andrews HB. Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biological Psychiatry. 1990;27(9):1038–1044. doi: 10.1016/0006-3223(90)90039-5. [DOI] [PubMed] [Google Scholar]
- 96.Simpson MDC, et al. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neuroscience Letters. 1989;107(13):211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- 97.Simpson MDC, et al. Regionally selective deficits in uptake sites for glutamate and gamma-aminobutyric acid in the basal ganglia in schizophrenia. Psychiatry Research. 1992;42(3):273–282. doi: 10.1016/0165-1781(92)90119-n. [DOI] [PubMed] [Google Scholar]
- 98.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–7. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 99.Penschuck S, et al. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. European Journal of Neuroscience. 2006;23(1):279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- 100.Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. Journal of Psychopharmacology. 2007;21(2):198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- 101.Berretta S, et al. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14(7):876–94. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- 102.Featherstone RE, et al. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: Parallels to schizophrenia. Neuropsychopharmacology. 2007;32(2):483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- 103.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 104.Swerdlow NR, et al. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51(2):139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 105.Geyer MA, et al. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Research Bulletin. 1990;25(3):485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 106.Thompson KN, et al. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. Journal of Psychiatric Research. 2007;41(7):561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry. 2006;60(11):1259–67. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 108.Lieberman JA, et al. The early stages of schizophrenia: Speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biological Psychiatry. 2001;50(11):884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 109.Olin SCS, Mednick SA. Risk factors of psychosis: Identifying vulnerable populations premorbidly. Schizophrenia Bulletin. 1996;22(2):223–240. doi: 10.1093/schbul/22.2.223. [DOI] [PubMed] [Google Scholar]
- 110.Mishlove M, Chapman LJ. Social anhedonia in the prediction of psychosis proneness. Journal of Abnormal Psychology. 1985;94(3):384–396. doi: 10.1037//0021-843x.94.3.384. [DOI] [PubMed] [Google Scholar]