Abstract
Heme cooling signals and diatomic ligand recombination kinetics are measured in strong magnetic fields (up to 10 Tesla). We examined diatomic ligand recombination to heme model compounds (NO and CO), myoglobin (NO and O2), and horseradish peroxidase (NO). No magnetic field induced rate changes in any of the samples were observed within the experimental detection limit. However, in the case of CO binding to heme in glycerol and O2 binding to myoglobin, we observe a small magnetic field dependent change in the early time amplitude of the optical response that is assigned to heme cooling. One possibility, consistent with this observation, is that there is a weak magnetic field dependence of the non-radiative branching ratio into the vibrationally hot electronic ground state during CO photolysis. Ancillary studies of the “spin-forbidden” CO binding reaction in a variety of heme compounds in the absence of magnetic field demonstrate a surprisingly wide range for the Arrhenius prefactor. We conclude that CO binding to heme is not always retarded by unfavorable spin selection rules involving a double spin-flip superexchange mechanism. In fact, it appears that the small prefactor (~109s−1) found for CO rebinding to Mb may be anomalous, rather than the general rule for heme-CO rebinding. These results point to unresolved fundamental issues that underlie the theory of heme-ligand photolysis and rebinding.
Keywords: Kinetics, spin-forbidden, heme proteins, Arrhenius prefactor
Introduction
Iron is a crucial trace metal that is involved in many physical and chemical activities of living cells and organisms. In one important example, it is chelated by protoporphyrin IX (PPIX) to form the heme group. Heme proteins play key roles in oxygen metabolism, both for oxygen and electron transport (e.g., hemoglobin, myoglobin, cytochrome c), and as the terminal oxidase in mitochondria (cytochrome oxidase). Recently, it has also become apparent that many heme proteins are also involved in important regulatory processes that utilize the binding of diatomic ligands as both catalytic and signaling agents 1–5. For this reason, it is important to understand the mechanism of diatomic ligand binding to heme systems and to determine, for example, if “spin selection” rules 6–10 or entropy production timescales 11 are important in regulating this process.
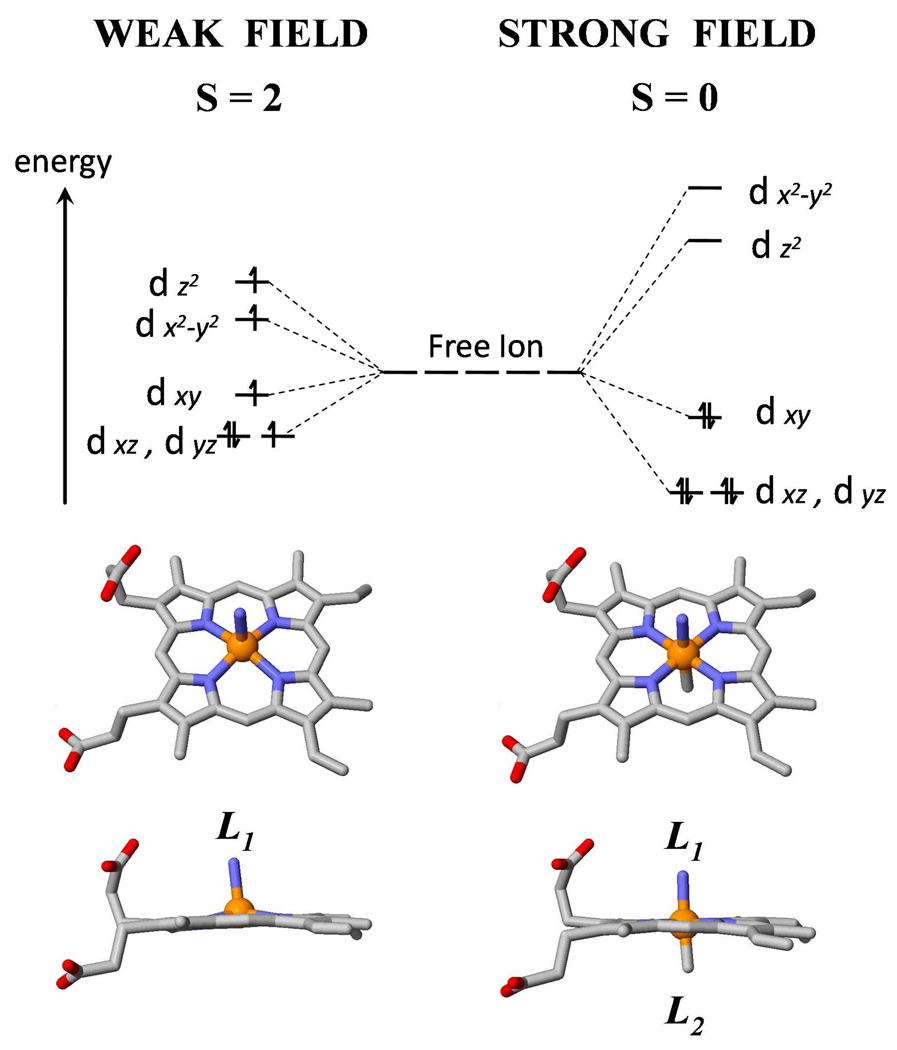
When ionized, the ferrous (Fe2+) iron atom has an outer shell electronic configuration of 3d6. In octahedral coordination complexes, such as the heme, the fivefold d-orbital degeneracy is lifted by the ligand field of the porphyrin nitrogens and the axial ligands (L1 and L2) as shown in Fig. 1. When both L1 and L2 are bound, the iron d-electrons are paired in a S=0 ground state. When L2 is absent, the weaker ligand field leads to a S=2 ground state 12–14. Thus, for a spin-zero ligand like CO, there is reason to believe that the ΔS=2 nature of the reaction may require binding via a double spin flip superexchange mechanism that slows the reaction rate and manifests itself in significantly reduced Arrhenius prefactors, as observed for MbCO6–8,15.
Figure 1.
The energy level scheme for ferrous (Fe2+) iron showing the occupation of the 3d orbitals for strong and weak ligand fields in a distorted octahedral geometry. On the left side, for a 5-coordinate iron, such as found for deoxy Mb where L1 is the proximal histidine, the electron pairing energy (P) is bigger than the cubic term (10Dq) of the ligand field splitting. Under this condition, electrons occupy the dx2–y2 and dz2 orbitals to avoid the electron pairing energy, resulting in the high spin (S=2) state of the ferrous iron atom. On the right side, when a sixth ligand (L2) binds to the iron atom, the large cubic term in the crystal field results in pairing of the 6 d electrons and the formation of a low-spin (S=0) ferrous state. A similar situation holds for ferric (Fe3+) heme complexes, leading to S=5/2 for high-spin and S=1/2 for low-spin.
Myoglobin (Mb) has been studied extensively both experimentally and theoretically over many decades, and is often taken as a prototype of heme proteins 16–20. The proximal histidine residue (His93) binds to the iron and forms the only covalent link between the Mb polypeptide chain and the heme group (L1 in Fig. 1). Small molecules (L2 in Fig. 1) such as oxygen (O2), carbon monoxide (CO), and nitric oxide (NO) can reversibly bind to and dissociate from the iron atom on the distal side of the iron-porphyrin plane.
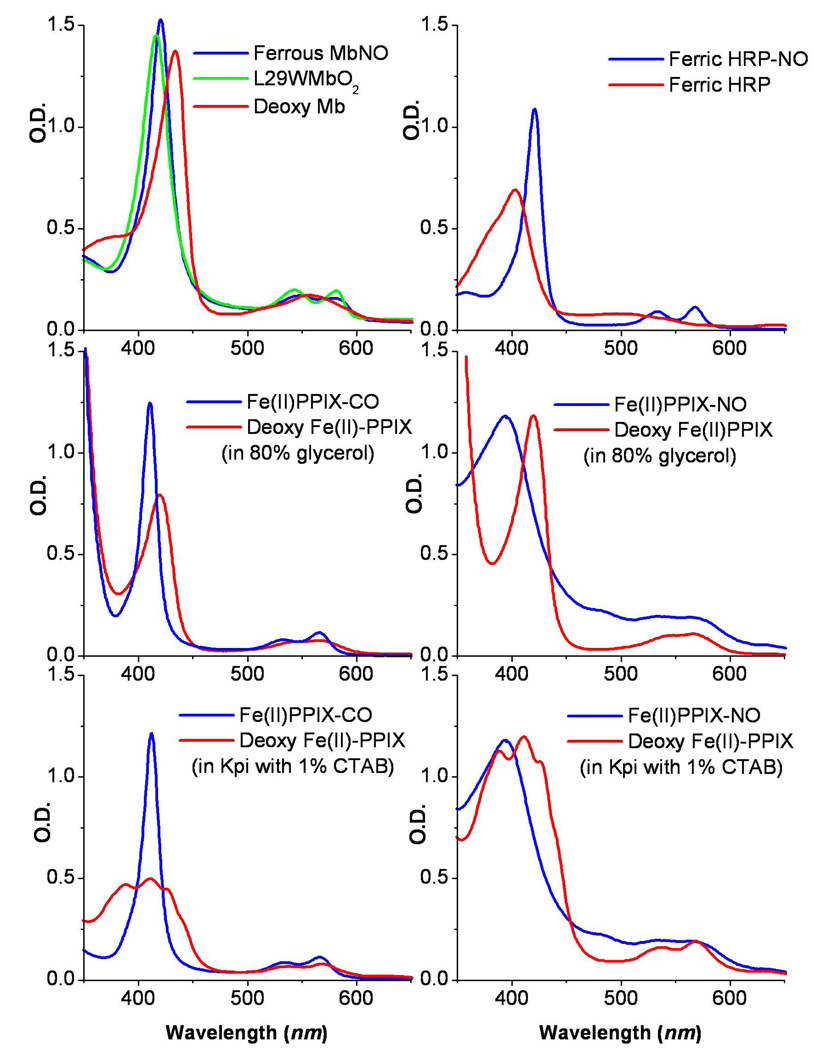
In this study we investigate the effects of large magnetic fields on the ligand binding kinetics and non-radiative relaxation of heme in glycerol solution as well as when bound to both Mb and horseradish peroxidase (HRP). HRP catalyzes the reduction of H2O2 to H2O in two serial one electron steps and oxidizes various aromatic substrates in the process. HRP can bind NO in either its ferrous or ferric state and the fast geminate kinetics of NO rebinding to this system have been reported under ambient conditions21. The search for magnetic field effects on diatomic ligand binding to heme in the absence of protein material focuses on ferrous iron protoporphyrin IX (FePPIX) in 80% glycerol solution, in the absence of 2-methylimidazole (2MeIm), so that water or hydroxide is the likely axial ligand, L1. The equilibrium absorption spectra of some of the L2-bound and unbound species investigated in this study are presented in Fig. 2.
Figure 2.
The absorption spectra of some of the samples investigated in this study.
Flash photolysis is an effective way to initialize ligand dissociation and study the protein and ligand responses over many timescales 9,16,17,22–26. During photolysis, the iron-ligand bond is broken nearly instantaneously (probably within a fraction of the ~60fs Fe-L2 vibrational period 27–29). In Mb, the Fe2+ ion moves towards the His93 and out of the porphyrin plane by about 0.4 Å. Subsequently, the photodissociated ligand, L2, first located inside the distal pocket, can rebind to the iron (geminate rebinding) or explore other pockets inside the protein 30 and ultimately escape to the solvent through protein fluctuations that involve the distal histidine 31–33. The diatomic ligands (O2, CO and NO) undergo very different dynamical processes when rebinding to the heme in Mb 9,34–36. Using ultrafast pump-probe spectroscopy, it is now possible to measure the photolysis quantum yields (QY) for these ligands 34 as well as to follow the optical response over a very large dynamic range in time 9,11,22,25,26,29,35–38. For heme systems, the optical response between 400–450nm includes both the ligand rebinding kinetics as well as signals due to the spectral diffusion of the hot Soret band lineshape as it undergoes vibrational relaxation back to thermal equilibrium39–43.
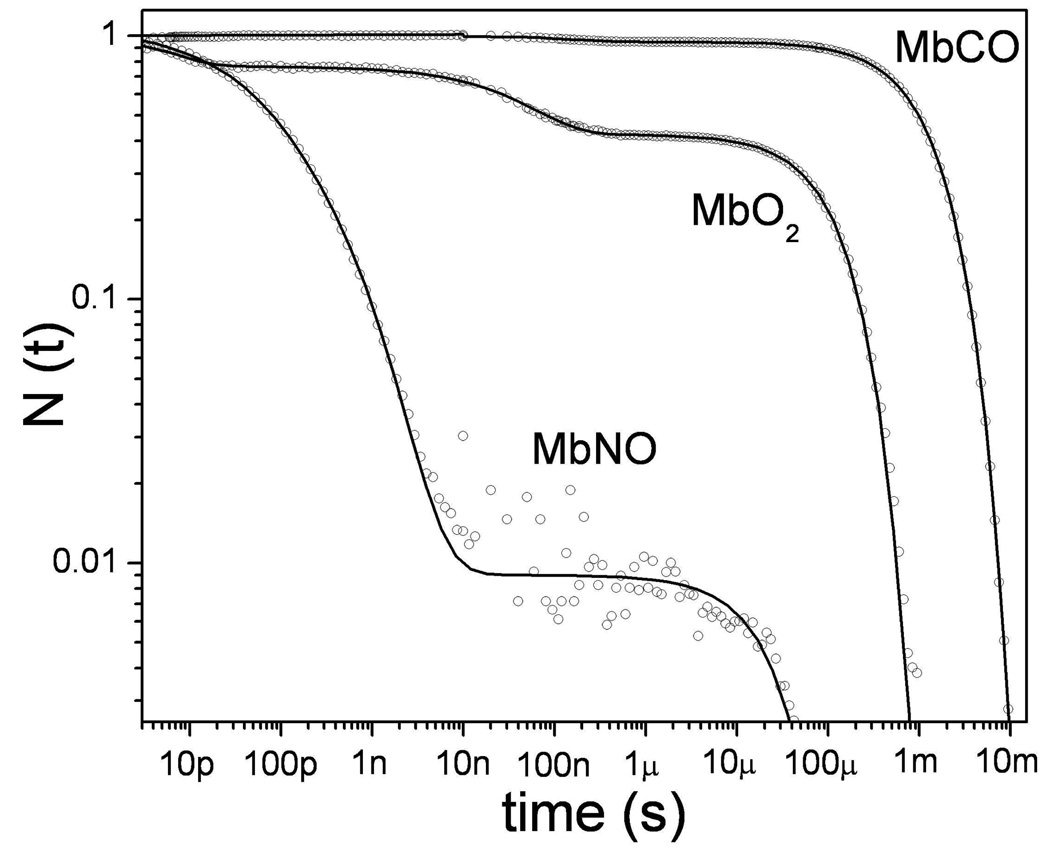
Figure 3 shows a log-log plot of the “survival fraction”, N(t), of the deoxy Mb photoproduct, following photolysis of NO, O2, and CO, over the time range 3ps-10ms. One striking observation is that the three ligands have vastly different geminate rebinding yields. At room temperature, only about 4% of the CO molecules find their way back to the iron and rebind geminately 35,44,45. Thus, most of the CO molecules escape from Mb following dissociation. In contrast, NO rebinds almost completely via ~10–100ps geminate processes that have been analyzed intensively in several studies 9,25,46,47. The dramatic difference in kinetics for CO and NO binding has been discussed by Ionascu et al.46 and the temperature dependence of the kinetics was used to show that both the Arrhenius prefactors and the enthalpic heme binding barriers are very different for the two ligands. For example, the main rebinding channel for NO geminate recombination is temperature independent and therefore barrierless. Other recent studies, involving O2 rebinding to Mb, reveal two well-separated geminate phases having time-constants of ~6ps and ~40ns, each with amplitudes of ~30% 22.
Figure 3.
The rebinding kinetics of diatomic ligands to ferrous myoglobin, following photolysis at room temperature in aqueous solution, are displayed on a logarithmic scale. The survival probablility, N(t), measures the population of the deoxyMb photoproduct as ligand rebinding proceeds. The decay times for the three ligands span approximately 9 decades in time. In the case of CO, approximately 96% of the photodissociated CO molecules escape from the heme pocket into the solvent so that rebinding primarily involves a relatively slow bimolecular process. In contrast, 99% of the photolyzed NO molecules rebind geminately in two separable phases with time constants of 15ps and 170 ps. In the case of O2 there are again two geminate phases, but the second phase (~50ns) is well separated from the first (~6ps). The bimolecular yield for oxygen is in the range 40–50%.
For many years it has been thought that spin selection rules are an important underlying reason for the different dynamical behavior of the three ligands 6,7,10,48,49. The ligand fields of heme are such that the ferrous iron lies near to the spin crossover point. Thus, a perturbation due to the presence or absence of the diatomic axial ligand causes the spin-state to change. When L2 is not bound, the ferrous heme iron is five coordinate and has a Hund’s rule high spin (S= 2) ground state. When the diatomic ligand binds, the d-electrons of the ferrous iron pair to a low spin state. The spin states of ligated myoglobin are: MbCO (S=0), MbNO (S= ½), and MbO2 (S=0), where the MbO2 complex is thought to involve Fe3+O2− antiferromagnetic coupling 50 or triplet state spin paring in a three center π-bond 51. In the recombination process from the initial unbound state to the final bound state, several system spin states can potentially participate. Using angular momentum addition, we see that Mb-CO presents only a single initial (S= 2) and final (S=0) spin state. However, for ferrous Mb-NO, the initial total spin angular momentum could be either 5/2 or 3/2, while the final state is S =1/2. In the case of Mb-O2, the possible initial spin states include S=3, S=2 and S=1, while the spin state is S =0 in the final ligated form.
In theoretical considerations by Harvey 6,52 and Franzen7, an imidazole (Im) ligated iron-porphine (FeP) system was used as a model, and the iron spin-state energy surfaces for the binding of different diatomic ligands (Im-FeP-XO where X=C, N, O) were computed. Franzen 7 used density functional theory (DFT), to calculate the optimized ground state potential energy surfaces for each of the possible spin states as L2 approached at three different proximal heme doming distances (0.0Ǻ, 0.2Ǻ and 0.4Ǻ, respectively). The fastest geminate recombination rate was assigned to the transition from the lowest initial spin state to the final correlated spin state (eg. Si =1→Sf =0 for MbO2 and Si =3/2→Sf =1/2 for MbNO). The slower recombination rates were explicitly linked to the higher initial spin states as they transformed sequentially into the final spin states (eg. MbO2: 2→1→0 and MbNO: 5/2→3/2→1/2). However, recent studies of MbNO recombination as a function of temperature and distal pocket mutation 46 have definitively shown that the slower (~200ps) geminate phase for NO binding is due to docking in the distal pocket, rather than to a spin selection channel inherent to the NO ligand.
Strickland and Harvey6 have calibrated their DFT calculations at the level of CCSD(T) (i.e., coupled cluster single and double substitutions and triple excitations). They have also compared both pure and hybrid DFT functionals in their calculations and focused on the binding of CO and H2O to ferrous heme. Their NO binding calculations6 suffer from the appearance of a local minimum in the quartet state (S=3/2) that yields a barrier of 1.6kcal/mol that must be surmounted in order to reach the final NO bound state doublet (S=1/2) surface. This barrier is roughly half that calculated for MbCO rebinding using the same methodology 6,52. Such a barrier for NO binding to heme is inconsistent with the temperature-independent rates experimentally observed for MbNO and PPIXNO rebinding 11,46. Thus, at this stage, it is probably premature to use the calculated transition-state Fe-NO bond-lengths as evidence against 6 the “harpoon” 46 and the “product-like” 53 transition state models for NO binding that have been put forward previously.
For CO binding, the direct quintet-singlet transition is spin forbidden so a superexchange process, or sequential rebinding involving an intermediate S=1 triplet spin-state, is usually invoked. Several groups 6,7,49 have suggested that the superexchange (double spin flip) process is the likely CO binding channel and that this accounts for the slower CO rebinding reaction observed in Mb 35,44,45,54 compared to the other diatomics. This possibility has also been explicitly discussed by Hopfield et al. using a spin-orbit coupling model, operating at second order, that couples the S=0 and S=2 states through the superexchange mechanism involving the S=1 state 10,15.
These theoretical studies suggested that strong external magnetic fields (~10 Tesla) might compete with the spin-orbit coupling to mix the iron spin-states enough to influence the CO recombination process, particularly at low temperatures 10. The external magnetic field influences the quantization axis for the iron spin. Spin-orbit interactions and the internal ligand fields at the iron are dominant, but because of the competition for the spin quantization axis, the magnitude of the (spin-orbit) coupling matrix elements between the different spin configurations can be altered as the eigenstates are mixed by the magnetic field. As a result, the transitions between spin-states can be affected, leading to a potentially observable change in the ligand binding rate.
The theoretical considerations 6,7,10 suggest that the diatomic heme ligands (CO, NO, and O2) will have differences in their ligand binding and spin transition rates that should be revealed in the Arrhenius rate expression
| (1) |
through differences in the prefactor, k0. In Eq. 1, kBA represents the overall temperature dependent rate of ligand rebinding from the initially photolyzed state, “B”, to the ligand bound state, “A”, where HBA is the enthalpic barrier for this process and kB is the Boltzman constant. Thus, comparative studies of the magnetic field dependence of the rebinding kinetics of these three ligands have the potential to reveal spin-dependent effects on the rebinding rate. When such studies are carried out with extremely good signal-to-noise, there is the possibility to reveal even relatively small effects. Such studies can yield new insights into the underlying fundamental mechanisms associated with the important life process of diatomic ligand binding in heme proteins.
The experiments presented here document our measurements of heme relaxation and diatomic ligand recombination kinetics under high magnetic fields. We are aware of only one other experimental study of this nature 15, which monitored a weak magnetic field induced heme polarization anisotropy on much longer time scales and at very low temperatures. Recent improvements in the signal-to-noise of pump-probe experiments that are used to detect coherent oscillations in heme proteins 55,56 offer the possibility to observe relatively small magnetic field dependent kinetic effects. Although small systematic changes in the amplitude of the short time (<10ps) optical response due to vibrational relaxation were observed in some cases, no magnetic field dependent differences in the ligand binding rates were observed beyond the noise level of the system. Based on the absence of magnetic field dependent ligand binding rates, and on the measurement of a wide range of Arrhenius prefactors for CO binding in several heme systems, we suggest that either the energy gaps between the pure spin states are larger than previously thought or that a significant mixing of the these states is already taking place in the absence of the magnetic field.
Experimental Methods
Magnet and Laser System
The closed cycle helium-cooled superconducting magnet (CFM-14T-50) used in this work is manufactured by Cryogenic Limited (London, United Kingdom). The maximum possible central field is 14.3 T with effective field homogeneity of 0.1% in a 10mm dsv (diameter sphere volume). An upper limit of 10T was used in these experiments. The magnet has a room temperature clear diameter of 52mm and the region of highly homogeneous field strength is centered over a 2mm long region at the middle of the magnet bore. The sample interaction volume is off the bore axis by 11mm, which leads to less than a ~1% change in the magnetic field strength compared to the bore centerline. The magnetic field direction can be switched by changing the direction of the superconducting current. The magnet needs ~24 hours to cool down, prior to energizing the superconducting current. In operation, the actual temperature near the center of the bore was 2–3 °C degree lower than ambient room temperature.
The kinetic data were obtained using several different pump-probe laser systems. For the magnetic field studies, an electronically synchronized pair of Ti:sapphire lasers was used to continuously scan over the range from 3ps to 16 ns. Longer time data could be extracted in discrete 26ns steps out to ~5µs. A detailed description of the electronically delayed pump-probe fs/ps ultrafast laser system used in the magnetic field studies can be found elsewhere 22. It operates at 190kHz with two colors and a collinear optical geometry, allowing the ~200fs pump pulse near 403nm to be blocked with a suitable filter and the electronically delayed (and tunable 410–450nm) probe pulse (~3ps) to be detected (after passing through the sample)using fiber optics and a lock-in amplifier. An additional kinetic system was used, in the absence of magnetic fields, to extend the full dynamic range shown in Fig. 3. This system involves a pulsed Nd:YAG laser and covers timescales from 10 nanoseconds to 10 milliseconds45. A third optical pump-probe system with 100fs time resolution was also used for some of the zero-field kinetic experiments 57.
All samples in the magnetic field studies were probed at 435nm with the exception of the ferric HRPNO, which was probed at 420nm. The pump pulse average power was 1mW, with the exception of the L29W MbO2 sample, which was pumped at 0.4mW. The average probe power was 0.2mW with the exception of the L29W MbO2 sample, which was probed with 0.1mW.
Sample Geometry
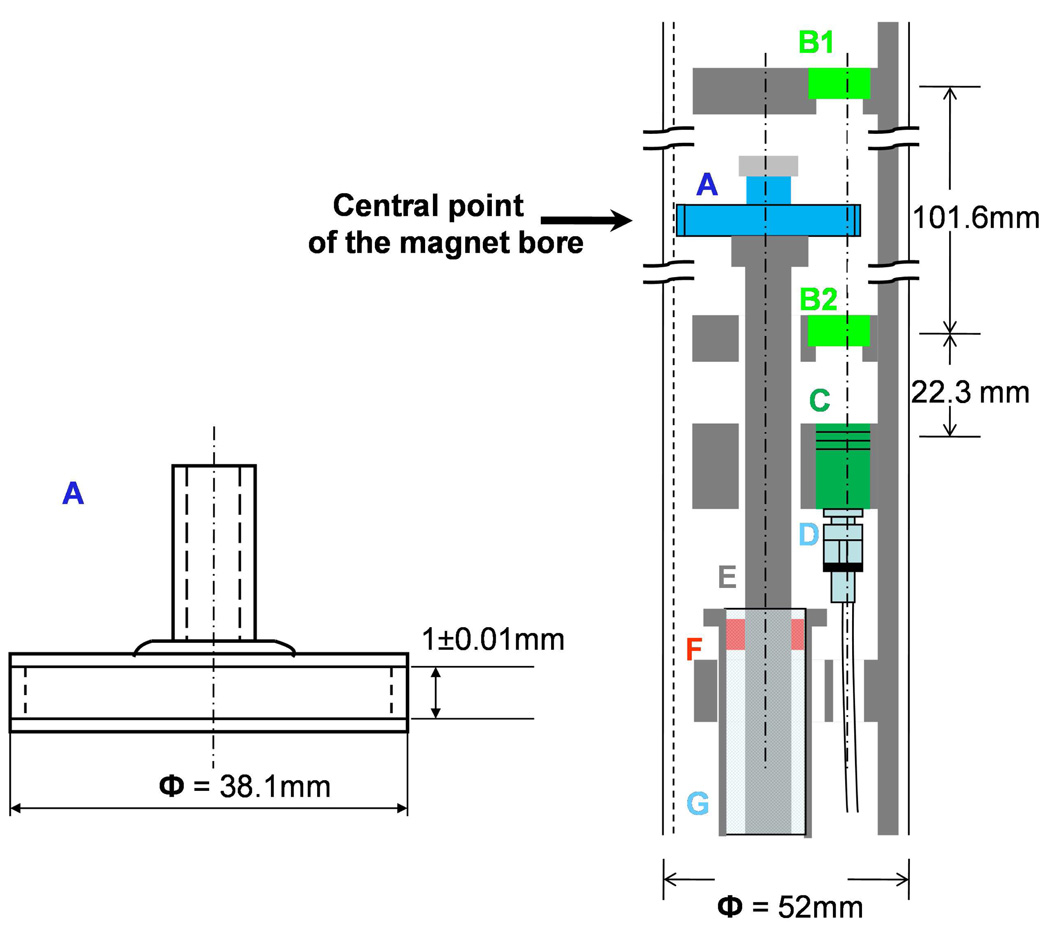
The quartz sample cell was custom-made (NSG Precision Cells, Inc. New York, NY) to the specifications shown in Fig. 4A. The sample cell was carefully centered and glued onto an adapter which is locked onto a central shaft that spins at 3500 rpm. In order to minimize field-induced changes in optical geometry, all the components inside the magnet bore were designed using non-magnetic materials. For example, ceramic bearings were supported inside an aluminum tube and used to define the position of a high-speed spinning shaft made from hard-coat anodized aluminum. This assembly was connected to a 42V DC motor (Maxon Precison Motors, Inc. Fall River, MA) that was secured to the floor below the magnet. The assembly holding the lenses was also made from aluminum and it was supported by the optical table and suspended into the magnet bore from above. Figure 4B illustrates the geometry inside the magnet bore. In this arrangement, the optical and mechanical parts are totally decoupled to prevent mechanical vibrations from being transferred to the optical part of the system. The spinning shaft could be released from its attachment to the motor below and the entire apparatus could be pulled out of the magnet from above, in order to disconnect the optical cell and change samples. The focal sample volume is 11.1mm away from the central axis of the magnet bore. At such a position, the strength and homogeneity of the magnetic field are better than 99% of their values on axis.
Figure 4.
(A) The sample cell is made from quartz and the open end is typically fitted with a rubber septum cap, which is sealed with parafilm following the preparation of the sample. (B) The optical arrangement is maintained using a cut-out aluminum tube (grey) that is inserted and coaxially aligned in the 52mm magnet bore. Lenses (B1, B2) each have a 50mm focal length and are set to focus (B1) and collect (B2) the laser light and transfer it to the detector lens assembly (C). The sample cell (S) is glued onto a spinning shaft (E) that is connected to a motor below the magnet. The shaft is supported and confined by two ceramic bearings (F) on each end of a hollow aluminum cylinder (G). The larger aluminum tube insert assembly holding the lenses is fixed to a XYZ translation stage that rests on an aluminum optical table extension above the magnet. The translation stage is adjusted so that the sample resides at a point that is equidistant between the lenses B1 and B2. The laser pulses enter from the top, and are focused by B1 into the sample and then re-collimated by B2 so that they reach the detector collecting lens C. The laser pulses are then transported by a fiber-optic cable (D) to a photodiode that is external to the system. A long wavelength-pass filter is inserted in front of the detector to extinguish the 403nm pump pulse. There is only 3.2mm of clearance within the hole of the lens holding plates (B2 and C) and the spinning shaft. This is designed to be smaller than the distance between the outer edge of the sample cell and the magnet bore wall. This helps to protect the rapidly spinning sample cell from touching any surface and breaking.
The incident light (pump and probe pulses) come from above and pass through normal focusing optics (B1) to minimize pulse distortion and deterioration of the time resolution. Fiber optics were used to collect the light after it passed through the sample, since the time resolved transmission has already been established at that point. The collection lenses and fiber optic components were supplied by Thorlabs, Inc. (Newton, NJ). The fiber is optimized at blue wavelengths and the probe laser light (435nm, 3ps, 190 KHz) is reduced in power to 80% of its original value after 2m of fiber. Fiber optic collimation/coupling packages (Thorlabs, Inc. Newton, NJ) are used on both ends of the fiber. The package (collecting lenses and mounts) were aligned in the lab and glued in place to optimize the collimation and recovery of the ~435nm probe wavelength.
Sample preparation
Horse heart Mb and HRP (type VI-A) were purchased from Sigma Chemical Co. (St. Louis, MO), Hemin (Ferriprotoporphyrin IX Chloride) was purchased from Porphyrin Products Inc. (Logan, UT), and these samples were used without further purification. The Mb mutant L29W was kindly provided by Professor John Olson, Rice University.
In all cases, the sample concentration was adjusted to ~1 O.D. /mm at the Soret peak using an appropriate buffer: potassium phosphate (pH 7.0, 100mM) for Mb, HRP and PPIX; borate (pH 8.3, 100mM) for L29W MbO2. Hemin powder needs to be dissolved in 1M NaOH in advance and only a tiny volume of the highly concentrated solution is used to make the final samples when diluted into either a 80% glycerol/water mixture or a potassium phosphate buffer with 1% CTAB. The sample in 1% CTAB is adjusted to pH 7 by titrating with monobasic sodium phosphate solution.
In order to form the reduced species, the ferric solution was sealed in a glass vial using a septum cap and parafilm. A rough vacuum followed by flushing with moisturized argon gas was repeated three times, the last flush lasts 15 minutes. The same buffer was used to prepare 1M sodium dithionite (Na2S2O4) and 1M sodium nitrite (NaNO2) solutions, following the procedure described above. Sodium dithionite was added to the protein samples in the ratio of 1:150 (v/v) to generate the reduced species. For the NO bound species, the volume of sodium dithionite needs to be doubled because 1 unit of sodium dithionite is used up by its reaction with 1 unit of sodium nitrite to generate the NO that binds to the reduced protein (The equilibrated NO solution concentration is ~60µM for the ultrafast studies when prepared using NaNO2 under argon atmosphere, and ~1.6mM for the bi-molecular kinetics, where NO gas was bubbled into the solution). For the CO bound species, CO gas (Med-Tech, Medford, MA) was flushed slowly over the surface of the reduced sample for about 40 minutes so that the CO concentration is saturated (~1mM). Oxygen bound Mb and its mutant L29W were prepared by adding a small amount of ascorbic acid powder into the sample solution and exposing it to the atmosphere for ~2 hours (O2 concentration ~280µM).
NO bound ferric horseradish peroxidase (NO-HRP (III)) was made by flushing the NO gas (99.0% purity, Med-tech, Medford, MA) through degassed ferric HRP solution for about 20 seconds (saturated concentration ~1.6mM). The NO gas needs to be purified by passing through a NaOH solution and then through a degassed buffer solution. A spectrophotometer (Hitachi U-3410) was used during the preparation processes to monitor the sample evolution. The absorption spectra were recorded before and after each experiment.
For all the samples studied, the absorption spectra inside the magnet were also taken using a portable CCD array UV-Vis Spectrophotometer (S.I. Photonics, Inc. Tucson, Arizona). Before taking the absorption spectra, the sample was placed in the experimental position for ~1 hour to reach thermal equilibrium with the cooler environment of the magnet bore. No difference in absorption spectra was observed with/without the application of the magnetic field. In typical experiments, the magnetic field intensity was changed in 2T steps. After each change in magnetic field, time was taken for mechanical re-equilibration (~30minutes) and, if necessary, the optics were readjusted to optimize the signal.
Data analysis
There are several significant advantages to performing the pump-probe experiments with an electronically delayed laser system. However, because it is a two-color laser system, we used the experimental signal itself to define the temporal crossing point of the two pulses, which is necessary to define time-zero in the pump-probe experiments. In our situation, the 200fs pulse was used as the pump pulse, and because the probe pulse is ~3ps in duration, we assume that the instrument function reacts to the pump as an effective δ-function excitation. Figure S1 in the supporting materials displays convolutions of exponential decay with a 3ps Gaussian that simulates the probe pulse component of the instrument function. Exponential fits using t=0 located at the half-height of the initial signal rise agree quite well with the input time-constants (τ), so long as τ is longer than ~10ps. When τ=5ps, the method overestimates the true time-constant by ~20%. This systematic deviation for short time constants is not important for comparative studies of magnetic field effects, because it affects the field-off and field-on measurements in the same manner.
The kinetic data were analyzed using both multi-exponential fitting and a maximum entropy method (MEM) 58. Statistical error analysis using sets of 10 runs, each with 99 logarithmic sample points, reveals the entire kinetics in roughly 30 minutes. The standard deviation of each data point along the N(t) axis is on the order of ~1%, while the laser jitter along the time axis is less than 800fs. As shown below, the application of strong magnetic fields to heme protein samples leads to no detectable effect on the kinetic rates. The peak of the kinetic data near 3ps represents the maximum value of the signal and, as demonstrated in Fig. S1, this is the time point after which the kinetics are fit in order to generate the MEM rate distributions. By comparing kinetics, where the underlying rate is systematically varied we find that the experimental data can be used to set a limit on magnetic field-induced changes of the observed geminate kinetic rate constant, kg. After including the possibility of systematic errors due to optical geometry changes and fitting procedures, this limit is found to be Δkg/kg ~ ±5×10−2. As discussed above and in the supplement, the error in the absolute time constant will be larger than the error in the magnetic field induced change when it approaches the instrument time resolution.
Experimental results
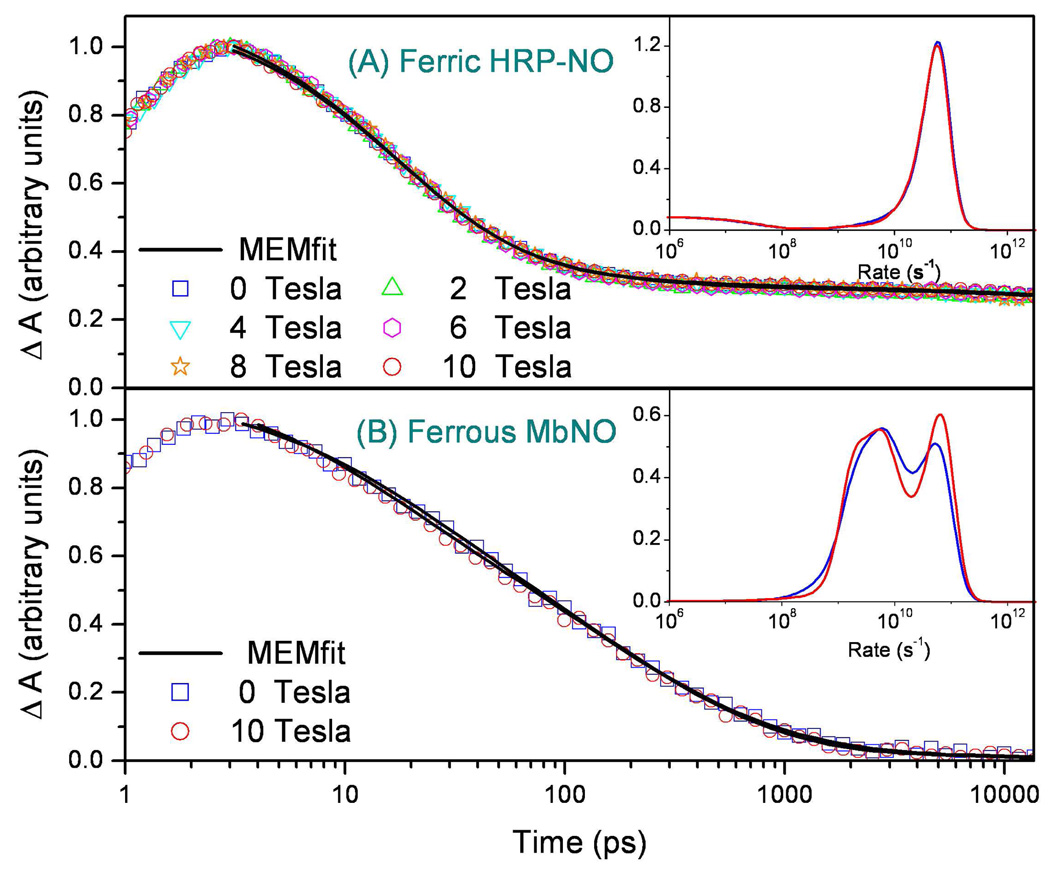
The NO recombination kinetics to ferric horseradish peroxidase (HRP) at a series of magnetic fields are shown in Figure 5A. A maximum entropy method (MEM) was used to fit the data 58 and the rate distributions that fit the observed kinetics are presented as an insert in the figure. It can be seen that the rate distributions obtained at the different field strengths agree quite well. Figure 5B shows a similar experiment conducted on ferrous MbNO. The invariance of both the raw kinetic data and the MEM analysis points to the absence of a measureable magnetic field effect on the NO binding reaction in ferric HRP and ferrous Mb. Ferrous HRPNO was also studied and no magnetic field effects were observed.
Figure 5.
(A) Recombination kinetics of ferric HRP-NO as a function of magnetic field. The sample was stabilized for 30 minutes after each field intensity change. The pump and probe wavelengths were 403nm and 420nm, respectively. Black solid lines are the MEM fits and the insert shows the MEM rate distributions as a function of magnetic field. (B) Comparison of the ferrous MbNO rebinding kinetics with (H=10 T) and without application of a magnetic field. No difference outside of experimental error is observed. The inset shows the MEM rate distributions, which contain differences due to experimental noise and finite data truncation effects (see ref. 58).
Figure 6 shows the geminate kinetics and the magnetic field response of the NO complex of (Fe2+)PPIX under two solvent conditions (80% glycerol and 1% CTAB). The data again clearly demonstrate that the applied magnetic field leads to no measurable effect on the heme-NO rebinding.
Figure 6.
Comparison of NO rebinding kinetics to Fe2+-protoporphyrin IX with and without an applied magnetic field. The pump is 403nm and the probe is 435nm nm. The upper panel (A) is for a CTAB solution and the lower panel (B) is for a glycerol solution. No difference outside of experimental error is observed.
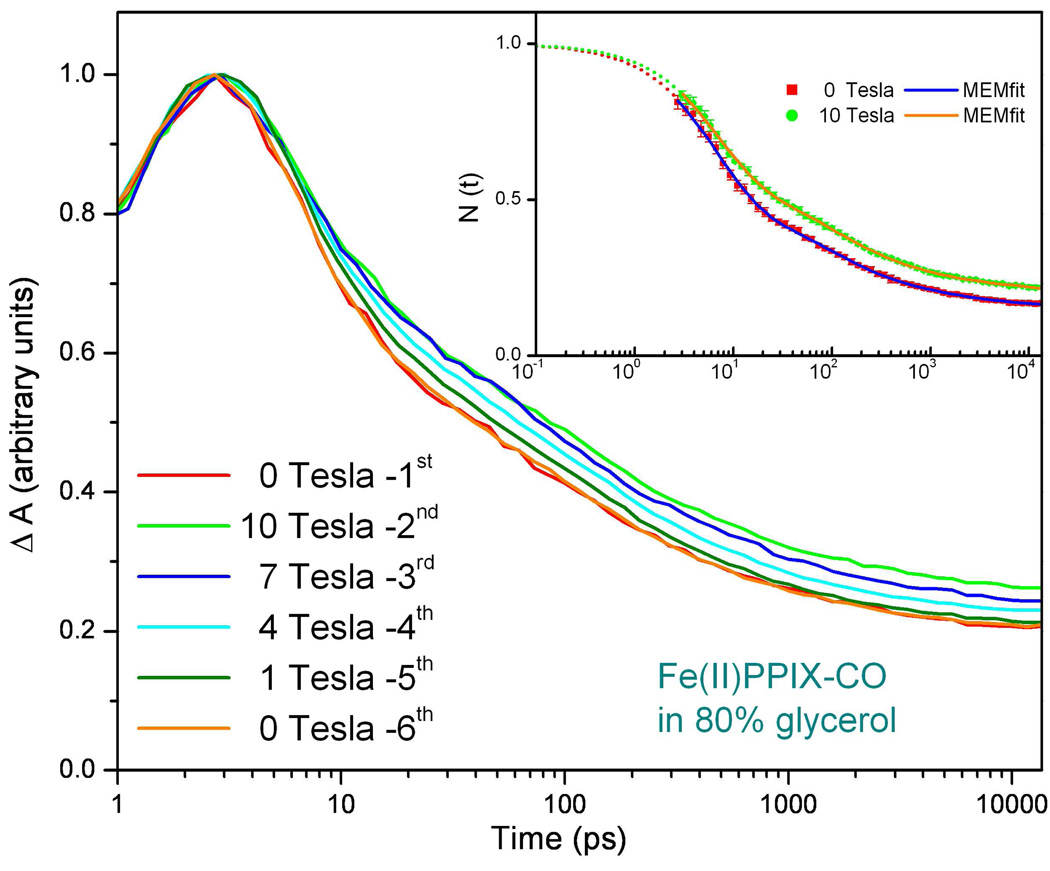
Figure 7 and Figure 8 present the effects of strong magnetic fields on the binding of CO to FePPIX. The CO complex of FePPIX in the absence of 2MeIm was chosen for study because the CO binding is much faster when water (or OH−) is in place as the heme axial ligand, L1 57. The high repetition rate lasers used in these studies generate excellent signal to noise, but do not allow the study of the slower rebinding systems such as MbCO and 2MeIm-FePPIX-CO because these samples do not fully reset to equilibrium within the arrival time (5.3µs) of the pump-probe pulse pairs. The FePPIX-CO sample is studied in 80% glycerol because the spin and optical properties of FePPIX and FePPIX-CO under this condition mimic those of deoxy Mb with surprising accuracy, especially when 2MeIm is bound as L1. In the absence of 2MeIm, the optical transitions are blue shifted by ~10nm, but, as can be seen in the insert to Fig. 9, there is a clear isosbestic point as the CO rebinds under these conditions 57.
Figure 7.
Comparison of the CO rebinding kinetics to Fe2+-protoporphyrin IX in 80% glycerol with and without the application of a magnetic field. The sample is pumped at 403nm and probed at 435nm. The black solid lines are the MEM fits of data and the rate distributions are displayed in the inserts. The maximum in the data trace is arbitrarily normalized to unity and represents the cut-off time beyond which data are analyzed. The time zero is taken at the half height of the rising signal as discussed in the Supporting Materials Fig. S1.
Figure 8.
Detailed measurements of the magnetic field dependence of FePPIX-CO in 80% glycerol pumped at 403nm and probed at 435nm. The measured rate distributions are the same as shown in Fig. 7. The amplitude of the 5ps process appears to vary systematically with applied magnetic field. The insert shows two of the kinetic traces renormalized to one at time zero using an exponential function to fit the ~5ps component. This allows a better visualization of that the amplitude of the fast response is changing rather than the rate constant(s). The error bars for the measurement are barely discernable in the figure.
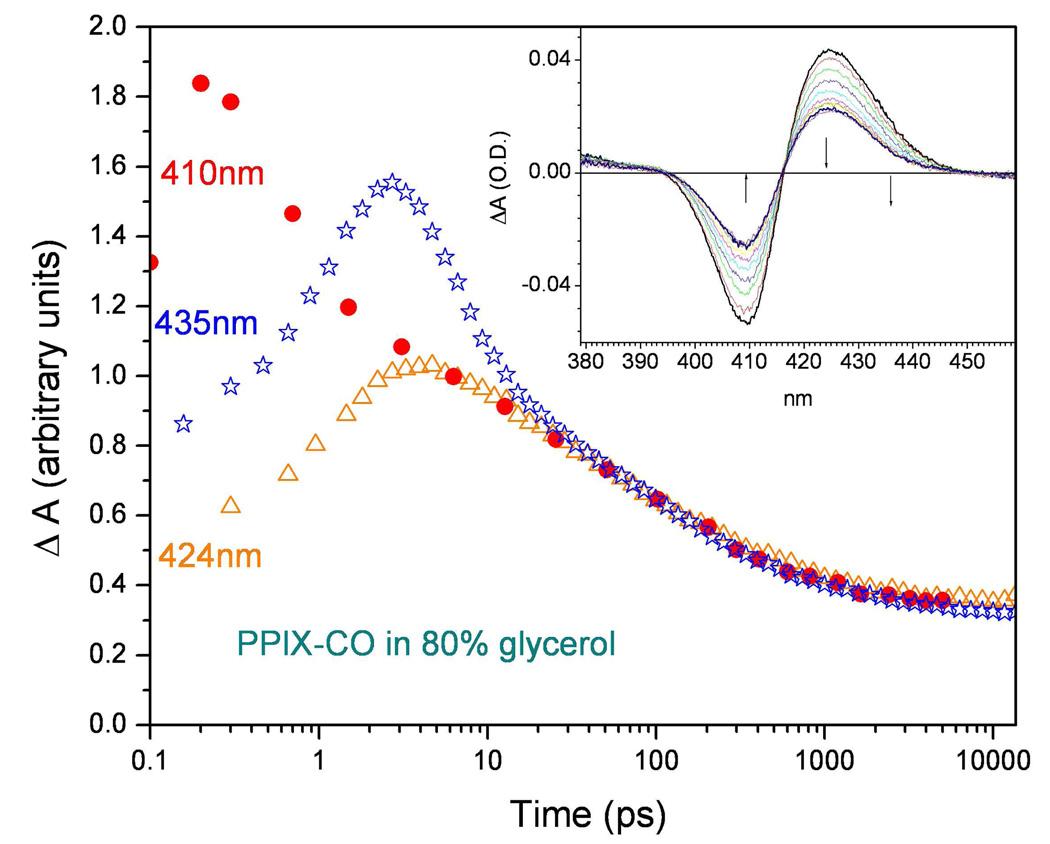
Figure 9.
The CO binding kinetics of FePPIX (pumped at 403nm and probed at 435nm) taken with the fs/ps pump-probe laser system (stars) are compared with the kinetics taken with a 100fs time resolved system (fs/fs) probed at 410nm (−ΔA410 is plotted as dots). Additional data taken with the fs/ps system pumped at 403nm and probed at 424nm are shown as the triangles. The data are scaled to equal values at long times in order to reveal the short time deviations arising from the instrument function (see supporting Fig. S3) when it convolves with a fast (0–5ps) thermal response and/or rebinding signal. The insert shows the spectral evolution of this sample at room temperature when probed using 100fs continuum pulses at time delays of: 10, 20, 40, 80, 160, 300, 500, 1000, 2000, 4000 ps. The small solid arrows show the probe wavelengths for the data in the main figure.
As shown in Fig. 7, following photolysis of FePPIX-CO, a magnetic field effect can be discerned in the amplitude of the early time (<10ps) optical response. Figure 8 explores this effect in more detail and indicates that it is systematic. The insert in Fig. 8 shows the zero field and 10T kinetics re-normalized by fitting the “fast” ~6ps phase of the response with an exponential function and then extrapolating back to time zero as discussed in the supporting materials, Fig. S1. The change in the overall kinetics is well described by a simple change in this “fast” phase amplitude from roughly ~55% at 0T to ~45% at 10T. The details involved in fitting of these data using a prior model 11,59 can be found in Fig.S2 of the supporting materials.
We want to stress at this point that the “fast” optical response, observed for the FePPIX-CO sample in Fig 7 and Fig 8, certainly involves a rapid (~0.3–5ps) spectral diffusion (cooling) response of the hot deoxy FePPIX photoproduct 39 , but it may also include an underlying fast component of CO rebinding, which has been previously observed at low temperature and is sometimes referred to as process I* 60,61. We note that the observed signal at 435nm between 1–10ps also involves a convolution of the instrument function.
Because of the potential for interference between the rebinding and cooling signals, we decided to examine the kinetics at other wavelengths. Figure 9 shows a comparison of the 0T data in Fig. 7 probed at 435nm (blue stars) to that (red dots) obtained at 410nm (i.e., the peak of the transient bleaching signal) using another pump-probe instrument with much better (~100fs) time resolution 57. We also include another 0T kinetic trace taken at 424nm (i.e., the peak of the transient anti-bleaching signal) using the fs/ps magnet-based system (orange triangles).
Independent examination of the spectral response of the photoexcited deoxy heme in glycerol solution reveals a bleaching signal near 424nm (unpublished). This observation helps to explain why the transient absorption signals observed at 424nm for t<10ps are so much weaker than those observed at 435nm. The CO rebinding signals at 424nm on this timescale have an opposite sign (i.e., increased transient absorption) compared to the deoxy heme transient cooling signal (decreased transient absorption). Thus, at 424nm, any fast (<10ps) CO rebinding signal would tend to be cancelled by the oppositely signed cooling signal of deoxy heme whereas at 435nm the cooling and rebinding signals are additive.
In an attempt to remove some of the ambiguity introduced by heme cooling signals and the fs/ps instrument response, we also carried out room temperature experiments using the FeCO infrared transition at 1954 cm−1 (to be published). It is clear from these experiments that approximately 10% of the CO photolyzed population rebinds in the first 10ps. However, the analysis of the t < 10ps IR data is consistent with a straightforward extension of the distributed coupling model that has been previously applied 11 to fit the kinetics for t> 10ps, so it does not appear that a separate ultrafast exponential CO rebinding phase (attributable to the I* process) is present. This, along with fits to the data at 435nm shown in the supporting materials (Fig. S2), indicates that the fast exponential phase (time constant ~5–6ps) is due to cooling of the deoxy heme photoproduct.
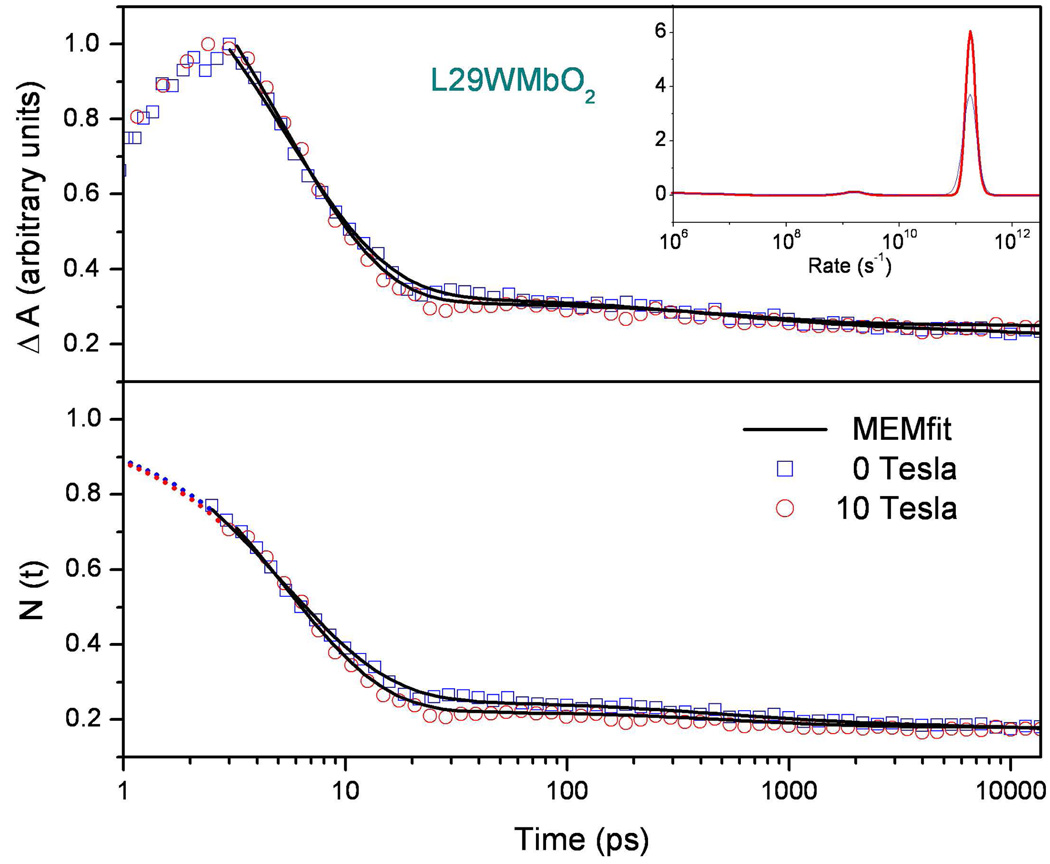
Figure 10 displays measurements of geminate recombination for the oxygenated L29W Mb mutant (L29WMbO2). This mutant is used because of its enhanced geminate rebinding amplitude, which is sufficient to allow reset to equilibrium between the pump-probe pulse pairs. There is a barely perceptible difference in the data trace when the 10T magnetic field is applied, but the MEM derived kinetic rate distributions shown in the insert do not show a significant variation. Thus, the small changes perceived in the O2 kinetic data do not arise from rate changes, but rather from a possible small change in the geminate amplitude. The bottom panel of the figure shows the data renormalized to unity by use of an exponential fit to the data that is extrapolated to time zero. Here the potential for a small change in the geminate amplitude is more easily visualized.
Figure 10.
Comparison of L29WMbO2 rebinding kinetics with (H=10 T) and without application of a magnetic field pumped at 403nm and probed at 435nm. The MEM rate distribution shown in the insert reveals an exponential geminate process that is independent of the magnetic field. The upper panel shows the MEM fits when the data are normalized to one at the maximum data point, which is the cut-off time beyond which data are analyzed. The time zero is taken at the half height of the rising signal as discussed in supporting Fig. S1. The lower panel shows the data and fits extrapolated back to unity at time zero by using an exponential fitting function.
Discussion
Under the assumption that the spin of the iron-ligand system plays an important role in determining the reaction rate, we can introduce a very simple model to estimate the perturbative effect of the applied magnetic field on the Arrhenius prefactor (k0 in Eq. 1). The prefactor incorporates all of the non-enthalpic factors (such as entropic barriers, attempt frequencies, frictional effects, and spin tunneling matrix elements) that affect the reaction rate in addition to the enthalpic barrier. Here we use a simple three level system that involves a (ligand bound) ground state as well as two “excited” unbound states, one with “allowed” (a), and one with “forbidden” (f ), propensity for making the spin transition to the ligand-bound ground state. In addition to spin-orbit coupling the magnetic field can perturb and alter the mixing of these states so that the amount of allowed transition amplitude from state a mixed into state f can be altered by the magnetic perturbation. Quantum admixtures of spin states differing by ΔS=1 have been treated previously by Maltempo62.
As a specific example, we can imagine the ground state to be the CO bound singlet and the “allowed” and “forbidden” states to be the deoxy triplet and quintet states, respectively. We take the allowed triplet state to be of higher energy as CO approaches the deoxy heme. The energy gap, Ea–Ef, between the triplet and quintet states of the equilibrium deoxy heme, denoted as ΔEaf, is thought to be relatively small 6,7,63. Both “excited” states (a and f) are assumed to be far removed (i.e., by the CO binding energy) from the L2 bound ground state when the nuclei are in equilibrium. The perturbation of the spin-dependent Arrhenius prefactor can be calculated as a quantum mechanical transition rate, where the applied magnetic field mixes the allowed and forbidden states. We take δB to be a measure of the magnetic field induced mixing that couples the allowed (a) and forbidden (f) states. The magnitude of the perturbative mixing term, δB/ΔEaf, can then be estimated based on the experimental detection limits. To do this, we take CO binding in Mb to be an experimental limiting case of a “spin-forbidden” prefactor (k0f ~109s−1) and NO binding as a limiting case for a “spin-allowed” prefactor (k0a~1011s−1). Application of simple perturbation theory within the subspace of the allowed and forbidden states then leads to mixing of the states and an approximate expression for the square of the perturbed probability amplitude (i.e., the perturbed prefactor, ).Takingas the difference between the perturbed and unperturbed prefactors for the “forbidden” CO binding reaction, we find
| (2) |
The leading term in the magnetic field perturbation carries a phase factor, cosΔφ, because it involves the interference term in the Golden Rule expression for the decay rate of the magnetically perturbed initial state |f〉 + (δB / ΔEaf)|a 〉 as it evolves into the ligand bound singlet ground state. The phases of the complex matrix elements involved in coupling the ground state with the “allowed” and “forbidden” states are unknown and Δφ is simply the difference of these two phases. Taking 5×10−2 as the detection limit for fractional change in the observed rate, and a maximum value for the phase factor (cosΔφ=±1) leads to |δB/ΔEaf|< 2.5×10−3. A similar calculation for the minimum phase (cosΔφ=0), using the second order term, leads to |δB/ΔEaf| < 2.2×10−2. Thus, a typical magnetic field induced mixing perturbation of δB~2µB10T~10cm−1, leads immediately to ΔEaf ≳ 500cm−1 for the minimum phase, while ΔEaf ≳ 4000cm−1 if the phase factors are such that they maximize the prefactor change upon application of a magnetic field. Thus, insofar as spin selection rules between “pure” spin states play a role in these reactions, the experiments reported here can be used to set approximate limits on the average energy separation between the forbidden and allowed spin levels as the transition state is approached along the Fe-L2 and heme doming coordinate11,59 . Prior calculations indicate a low lying spin S=1 triplet state within 300cm−1 of the ground state in order to account for the Mossbauer spectra of the deoxy heme system 64. More recent DFT calculations actually suggest that the triplet state energy can sometimes fall below the quintet state 6. Thus, the size of the energy gap between the triplet and quintet states that is needed to eliminate detectable magnetic field effects is surprisingly large.
On the other hand, it remains possible that spin admixtures are present at room temperature, even in the absence of the applied magnetic field. The presence of such admixtures is consistent with early far infrared magnetic resonance measurements on high-spin ferrous heme in Mb and Hb, where a simple S=2 spin Hamiltonian was found to be insufficient to account for the data 63. Magnetic susceptibility measurements have been interpreted to indicate that a “pure” S=2 spin is the likely 290K ground state for ferrous heme in Mb and Hb. However, uncertainty in the magnitude of the orbital component that generates the observed µeff =5.5µB 12–14 makes unequivocal assignment of a pure S=2 ground state difficult.
In the process of this investigation we studied samples with a variety of possible spin channels (see supporting materials Table S3). For example, the binding of NO to ferrous heme involves two potential spin transitions; one is an “allowed” first order transition (ΔS =1 with an “initial” spin, Si=3/2, and a “final” spin, Sf=1/2) and one is a “forbidden” transition (ΔS=2, with Si=5/2 and Sf=1/2) that can proceed via either a “sequential” or a second order “superexchange” process. In fact, it has been suggested 7 that the “fast” (~10ps) and “slow” (~200ps) geminate recombination phases observed 36,46,47 for MbNO binding are due to the allowed and forbidden (sequential) spin channels, respectively.
As direct evidence to the contrary, we note that there is a single ~10ps temperature independent 46 exponential geminate phase observed in Mb mutants that have the xenon pocket blocked. This demonstrates that spin selection is not responsible for the slower geminate rebinding phase of MbNO. Moreover, the temperature dependent measurement of the rates in native MbNO demonstrates that the prefactor for both the “slow” and the “fast” phase is ~1011 s−1 46. Thus, as suggested previously, we believe that the slower geminate phase results from a small enthalpic barrier involving the return of the NO ligand from a distal docking site near the heme, probably involving the Xe4 pocket 46. The model invoking a distal pocket docking site also explains why only the relative amplitudes, but not the rates, of MbNO binding are affected by external perturbations such as glycerol containing solvents 47, pumping wavelengths 46, or mutations 46.
An analogous situation is documented in Fig. 7 and Fig.8, where it can be seen that the application of the magnetic field affects the amplitude, but not the rates, of the fast optical response for PPIXCO binding. However, we must recognize that the sub-10ps optical response at 435nm contains a significant contribution from the cooling of the transient deoxy heme state as well as from any underlying CO rebinding signal that might be present. Our fits to the room temperature PPIXCO rebinding kinetics, using the IR data to eliminate the heme cooling signals, demonstrates that a separate sub-10ps exponential CO rebinding phase is not present. Thus, the exponential response observed at 435nm is assigned to transient cooling of the deoxy heme photoproduct.
When we fit the data in Fig. 7, using a superposition of the SRC rebinding model 11,59 along with a separate exponential phase to account for the short time heme cooling, we find the exponential component to have a time constant of 5.5ps and an amplitude that decreases by ~10% in going from 0 and 10T (see figure S2 and Table S2 in supporting materials). Using the same fitting protocols, we find that the exponential phase has a vanishing amplitude when applied to a data set that is composed of a superposition of the IR response t<40ps and the optical response t>40ps. From this we conclude that the amplitude of the 5.5ps exponential heme cooling response changes as a function of applied magnetic field. Independent studies of the deoxy heme cooling using improved 100fs time resolution reveal that the cooling response at 435nm has a time constant of ~4ps (unpublished), which is in good agreement with the present results, given that the instrument response of the fs/ps laser system increases the time constants in this range by ~20% (see Fig. S1).
One possibility that would account for the observation of a magnetic field dependent cooling amplitude involves a hypothesis where the amount of vibrationally hot electronic ground state heme is slightly reduced as the applied magnetic field is increased. This would lead to a smaller deoxy heme cooling signal and it would therefore be consistent with the observed reduction in the sub-10ps exponential amplitude at higher magnetic field strength. This is an unexpected result, and additional studies are necessary in order to further evaluate this possibility. If magnetic field effects alter the ultrafast non-radiative decay pathways associated with CO photolysis and the deoxy heme cooling process, one might generally expect that both the rates and amplitudes might be affected. However, the branching ratio for a prompt non-radiative transition into the vibrationally hot electronic ground state (following CO photolysis) could be less than unity9. If this is the case, and/or the branching ratio is further reduced by the applied magnetic field, the observed vibrational cooling rates in the ground electronic state should remain fixed but the amplitude of this signal would be decreased. Another obvious hypothesis involves a magnetic field dependent reduction in the CO quantum yield and the cooling of a residual hot six-coordinate CO bound species. However, this hypothesis predicts an increase, rather than a decrease, in the amplitude of the sub-10ps response and we therefore exclude this possibility.
A very weak magnetic field dependence of the O2 binding reaction in Mb (L29W) may also be present. Figure 10 shows that, although there are indications of a very small magnetic field induced change in the geminate amplitude, the MEM analysis reveals no statistically significant change in the rebinding rate distribution. In contrast to NO binding, the preliminary temperature dependent kinetic measurements of the O2 binding reaction in wild type Mb (unpublished) indicate that the prefactors for the two geminate phases seen in Fig. 3 are quite different 22. The ~5ps geminate phase has a prefactor near 1011s−1, while the ~40ns geminate phase has a prefactor near 109s−1. This could indicate the presence of either a spin selection mechanism, or an entropy production timescale 11 for the photoproduct that falls in the range between 10ps and 10ns and leads to a larger entropic barrier for the slower geminate phase.
Finally, we display in Table 1 the geminate rebinding rates and Arrhenius prefactors for CO binding to several different heme systems. The significant differences observed for the CO geminate rate demonstrates that there is not a universal spin selection rule for CO binding that acts as a consistent limiting factor in these reactions. The net spin change upon ligand binding in these systems is the same for each of the equilibrium species (ΔS=2), yet the rates are very different. We do not believe that this variation is due to non-equilibrium photoproduct species involving triplet states because the transient absorption spectra (e.g., see insert to Fig. 9) indicate that a genuine ΔS=2 transition is taking place over a wide range of timescales for the different samples. For example, a detailed analysis 11 of the temperature dependence of CO binding to the FePPIX model complex reveals a prefactor for CO binding near 1011s−1, which is similar to the “spin-allowed” prefactor for NO binding. Other heme systems, such as microperoxidase65 , carboxymethylated cytochrome c66, mutants of cytochrome c67 , and CooA68,69 all have CO geminate rebinding rates that are faster than 1010s−1 (see Table 1), which sets a lower limit for their Arrhenius prefactors.
Table 1.
Kinetics of CO binding to selected heme proteins
| Protein | kg (109 s−1) | Ig | kBAa(109 s−1) | k0 (109 s−1) | reference |
|---|---|---|---|---|---|
| WT Mbb | 0.0006 | 4% | 0.00002 | 1 | 35 |
| Mb (V68W)b | 0.0006 | 76% | 0.0005 | 0.88 | Unpublished |
| PPIX+2MeIm(95%Gly)c | 0.83 | 20% | 0.17 | > 0.17 | 57 |
| NP4 (pH=7.5)c | 0.15 | 60% | 0.09 | > 0.64 | Unpublished |
| HRP+BHAb | 2 | 90% | 1.8 | > 1.8 | 21,70 |
| PPIX (95% Gly)c | 20 | 95% | 19 | 150 | 57 |
| MP-11 (aggregated)c | 16 | 78% | 13 | > 13 | 65,71 |
| CooAd | 13 | 60% | 8 | > 8 | 69 |
| Cm Cyt Ce | 63 | 27% | 17 | > 17 | 66 |
To extract the kBA from these data we used a simple three state model57.
Fitting methods for CO geminate recombination :
Single exponential fit.
Stretched exponential fit.
Two exponential fit, only the fast component is presented here and used to estimate the value of the kBA.
Three exponential fit, only the fast component is presented here and used to estimate the value of the kBA
Additional temperature dependent studies are needed to establish the precise magnitude of these prefactors, but it is clear from the rapid rebinding kinetics of various heme systems, that CO binding is not generally being retarded by an unfavorable (ΔS=2) spin selection rule. In fact, it appears that the unusually small prefactor (~109s−1) found for CO rebinding to Mb may be anomalous, rather than the general rule for heme-CO rebinding. The possibility that these “fast” CO binding reactions take place through genuine population of a S=1 transition state (i.e., via a sequential rather than a superexchange mechanism) or that entropy production timescales and/or ligand confinement are involved in determining the prefactor will be the topic of future work.
Supplementary Material
Acknowledgements
This work was supported by NSF 0211816 and NIH DK35090. We thank Don Heiman for use of the magnet system. The infrared kinetic studies were done in collaboration with Jason Amsden and L.D. Ziegler and will be published elsewhere.
Footnotes
Supporting Information Available: Instrument function convolutions, fitting of time-resolved optical signal with ligand rebinding model and exponential cooling response, possible spin-state changes and measured Arrhenius prefactors for selected heme-ligand binding. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ortiz de Montellano PR. CYTOCHROME P450 : Structure, Mechanism and Biochemistry. 3rd Edition. New York: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- 2.Bredt DS, Snyder SH. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 3.Gilles-Gonzalez MA, Ditta GS, Helinski DR. Nature. 1991;350:170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- 4.Aono S, Nakajima H. Coord. Chem. Rev. 1999;192:267–282. [Google Scholar]
- 5.Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- 6.Strickland N, Harvey JN. J. Phys. Chem. B. 2007;111:841–852. doi: 10.1021/jp064091j. [DOI] [PubMed] [Google Scholar]
- 7.Franzen S. Proc. Natl. Acad. Sci. USA. 2002;99:16754–16759. doi: 10.1073/pnas.252590999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frauenfelder H, Wolynes PG. Science. 1985;229:337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- 9.Petrich JW, Poyart C, Martin JL. Biochemistry. 1988;27:4049–4060. doi: 10.1021/bi00411a022. [DOI] [PubMed] [Google Scholar]
- 10.Redi MH, Gerstman BS, Hopfield JJ. Biophys. J. 1981;35:471–484. doi: 10.1016/S0006-3495(81)84803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Ionascu D, Gruia F, Yu A, Benabbas A, Champion PM. Proc. Natl. Acad. Sci. USA. 2007;104:14682–14687. doi: 10.1073/pnas.0702622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertini I, Luchinat C, Turano P, Battaini G, Casella L. Chemistry. 2003;9:2316–2322. doi: 10.1002/chem.200204562. [DOI] [PubMed] [Google Scholar]
- 13.Pauling L, Coryell CD. Proc. Natl. Acad. Sci. USA. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roder H, Berendzen J, Bowne SF, Frauenfelder H, Sauke TB, Shyamsunder E, Weissman MB. Proc. Nati. Acad. Sci. USA. 1984;81:2359–2363. doi: 10.1073/pnas.81.8.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstman B, Austin RH, Hopfield JJ. Phys. Rev. Lett. 1981;47:1636–1639. [Google Scholar]
- 16.Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam-London, The Netherlands: North-Holland Publishing Co; 1971. [Google Scholar]
- 17.Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Biochemistry. 1975;14:5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- 18.Springer BA, Sligar SG, Olson JS, Phillips JGN. Chem. Rev. 1994;94:699–714. [Google Scholar]
- 19.Sage JT, Champion PM, Suslick KS. Comprehensive Supramolecular Chemistry. Oxford, U. K.: Pergamon; 1996. pp. 171–218. [Google Scholar]
- 20.Srajer V, Teng TY, Ursby T, Pradervand C, Ren Z, Adachi S, Schildkamp W, Bourgeois D, Wulff M, Moffat K. Science. 1996;274:1726–1729. doi: 10.1126/science.274.5293.1726. [DOI] [PubMed] [Google Scholar]
- 21.Ye X, Yu AC, Champion PM. J. Am. Chem. Soc. 2006;128:1444–1445. doi: 10.1021/ja057172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu A, Ye X, Ionascu D, Cao W, Champion PM. Rev. Sci. Instr. 2005;75:114301. [Google Scholar]
- 23.Anfinrud PA, Han C, Hochstrasser RM. Proc. Natl. Acad. Sci. USA. 1989;86:8387–8391. doi: 10.1073/pnas.86.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller LM, Chance MR. J. Am. Chem. Soc. 1994;116:9662–9669. [Google Scholar]
- 25.Vos MH. Biochim. Biophys. Acta. 2008;1777:15–31. doi: 10.1016/j.bbabio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Kruglik SG, Jasaitis A, Hola K, Yamashita T, Liebl U, Martin JL, Vos MH. Proc. Natl. Acad. Sci. USA. 2007;104:7408–7413. doi: 10.1073/pnas.0700445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu LY, Sage JT, Champion PM. Science. 1994;266:629–632. doi: 10.1126/science.7939716. [DOI] [PubMed] [Google Scholar]
- 28.Rosca F, Kumar ATN, Ye X, Sjodin T, Demidov AA, Champion PM. J. Phys. Chem. A. 2000;104:4280–4290. [Google Scholar]
- 29.Liebl U, Lipowski G, Negrerie M, Lambry JC, Martin JL, Vos MH. Nature. 1999;401:181–184. doi: 10.1038/43699. [DOI] [PubMed] [Google Scholar]
- 30.Srajer V, Ren Z, Teng TY, Schmidt M, Ursby T, Bourgeois D, Pradervand C, Schildkamp W, Wulff M, Moffat K. Biochemistry. 2001;40:13802–13815. doi: 10.1021/bi010715u. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Sage JT, Rigos AA, Morikis D, Champion PM. J. Mol. Biol. 1992;224:207–215. doi: 10.1016/0022-2836(92)90584-7. [DOI] [PubMed] [Google Scholar]
- 32.Tian WD, Sage JT, Champion PM, Chien E, Sligar SG. Biochemistry. 1996;35:3487–3502. doi: 10.1021/bi952474u. [DOI] [PubMed] [Google Scholar]
- 33.Yang F, Phillips GN., Jr J. Mol. Biol. 1996;256:762–774. doi: 10.1006/jmbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, Demidov AA, Champion PM. J. Am. Chem. Soc. 2002;124:5914–5924. doi: 10.1021/ja017359n. [DOI] [PubMed] [Google Scholar]
- 35.Henry ER, Sommer JH, Hofrichter J, Eaton WA. J. Mol. Biol. 1983;166:443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- 36.Walda KN, Liu XY, Sharma VS, Magde D. Biochemistry. 1994;33:2198–2209. doi: 10.1021/bi00174a029. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong MR, Ogilvie JP, Cowan ML, Nagy AM, Miller RJD. Proc. Natl. Acad. Sci. USA. 2003;100:4990–4994. doi: 10.1073/pnas.0936507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dadusc G, Ogilvie JP, Schulenberg P, Marvet U, Miller RJD. Proc. Natl. Acad. Sci. USA. 2001;98:6110–6115. doi: 10.1073/pnas.101130298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye XO, Demidov A, Rosca F, Wang W, Kumar A, Ionascu D, Zhu LY, Barrick D, Wharton D, Champion PM. J. Phys. Chem. A. 2003;107:8156–8165. [Google Scholar]
- 40.Zhang Y, Fujisaki H, Straub JE. J. Chem. Phys. 2009;130 doi: 10.1063/1.3055277. [DOI] [PubMed] [Google Scholar]
- 41.Henry ER, Eaton WA, Hochstrasser RM. Proc. Natl. Acad. Sci. USA. 1986;83:8982–8986. doi: 10.1073/pnas.83.23.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizutani Y, Kitagawa T. Science. 1997;278:443–446. doi: 10.1126/science.278.5337.443. [DOI] [PubMed] [Google Scholar]
- 43.Sagnella DE, Straub JE. J. Phys. Chem. B. 2001;105:7057–7063. [Google Scholar]
- 44.Balasubramanian S, Lambright DG, Marden MC, Boxer SG. Biochemistry. 1993;32:2202–2212. doi: 10.1021/bi00060a011. [DOI] [PubMed] [Google Scholar]
- 45.Tian WD, Sage JT, Srajer V, Champion PM. Phys. Rev. Lett. 1992;68:408–411. doi: 10.1103/PhysRevLett.68.408. [DOI] [PubMed] [Google Scholar]
- 46.Ionascu D, Gruia F, Ye X, Yu AC, Rosca F, Beck C, Demidov A, Olson JS, Champion PM. J. Am. Chem. Soc. 2005;127:16921–16934. doi: 10.1021/ja054249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shreve AP, Franzen S, Simpson MC, Dyer RB. J. Phys. Chem. B. 1999;37:7969–7975. [Google Scholar]
- 48.Harvey JN. Farad. Disc. 2004;127:165–177. doi: 10.1039/b314768a. [DOI] [PubMed] [Google Scholar]
- 49.Greene BI, Hochstrasser RM, Weisman RB, Eaton WA. Proc. Natl. Acad. Sci. USA. 1978;75:5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss JJ. Nature. 1964;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- 51.Goddard WA, 3rd, Olafson BD. Proc. Natl. Acad. Sci. USA. 1975;72:2335–2339. doi: 10.1073/pnas.72.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey JN. J. Am. Chem. Soc. 2000;122:12401–12402. [Google Scholar]
- 53.Szabo A. Proc. Natl. Acad. Sci. USA. 1978;75:2108–2111. doi: 10.1073/pnas.75.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao W, Ye X, Sjodin T, Christian JF, Demidov AA, Berezhna S, Wang W, Barrick D, Sage JT, Champion PM. Biochemistry. 2004;43:11109–11117. doi: 10.1021/bi049077g. [DOI] [PubMed] [Google Scholar]
- 55.Gruia F, Kubo M, Ye X, Lonascu D, Lu C, Poole RK, Yeh SR, Champion PM. J. Am. Chem. Soc. 2008;130:5231–5244. doi: 10.1021/ja7104027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosca F, Kumar ATN, Ionascu D, Ye X, Demidov AA, Sjodin T, Wharton D, Barrick D, Sligar SG, Yonetani T, Champion PM. J. Phys. Chem. A. 2002;106:3540–3552. [Google Scholar]
- 57.Ye X, Yu AC, Georgiev GY, Gruia F, Ionascu D, Cao WX, Sage JT, Champion PM. J. Am. Chem. Soc. 2005;127:5854–5861. doi: 10.1021/ja042365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar ATN, Zhu L, Christian JF, Demidov AA, Champion PM. J. Phys. Chem. B. 2001;105:7847–7856. [Google Scholar]
- 59.Srajer V, Reinish L, Champion PM. J. Am. Chem. Soc. 1988;110:6656–6670. [Google Scholar]
- 60.Postlewaite JC, Miers JB, Dlott DD. J. Am. Chem. Soc. 1989;111:1248–1255. [Google Scholar]
- 61.Martin JL, Migus A, Poyart C, Lecarpentier Y, Astier R, Antonetti A. Proc. Natl. Acad. Sci. USA. 1983;80:173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maltempo MM. J. Chem. Phys. 1974;61:2540–2547. [Google Scholar]
- 63.Champion PM, Sievers AJ. J. Chem. Phys. 1980;72:1569–1582. [Google Scholar]
- 64.Huynh BH, Papaefthymoiu GC, Yen CS, Groves JL, Wu CS. J. Chem. Phys. 1974;61:3750–3758. [Google Scholar]
- 65.Cao W, Ye X, Georgiev GY, Berezhna S, Sjodin T, Demidov AA, Wang W, Sage JT, Champion PM. Biochemistry. 2004;43:7017–7027. doi: 10.1021/bi0497291. [DOI] [PubMed] [Google Scholar]
- 66.Silkstone G, Jasaitis A, Vos MH, Wilson MT. Dalton Trans. 2005:3489–3494. doi: 10.1039/b508183c. [DOI] [PubMed] [Google Scholar]
- 67.Silkstone G, Jasaitis A, Wilson MT, Vos MH. J. Biol. Chem. 2007;282:1638–1649. doi: 10.1074/jbc.M605760200. [DOI] [PubMed] [Google Scholar]
- 68.Rubtsov IV, Zhang TQ, Nakajima H, Aono S, Rubtsov GI, Kumazaki S, Yoshihara K. J. Am. Chem. Soc. 2001;123:10056–10062. doi: 10.1021/ja011023w. [DOI] [PubMed] [Google Scholar]
- 69.Kumazaki S, Nakajima H, Sakaguchi T, Nakagawa E, Shinohara H, Yoshihara K, Aono S. J. Biol. Chem. 2000;275:38378–38383. doi: 10.1074/jbc.M005533200. [DOI] [PubMed] [Google Scholar]
- 70.Berinstain AB, English AM, Hill BC, Sharma D. J. Am. Chem. Soc. 1990;112:9649–9651. [Google Scholar]
- 71.Lim M, Jackson TA, Anfinrud PA. J. Biol. Inor. Chem. 1997;2:531–536. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.