Abstract
Mucus is a complex biological material that lubricates and protects the human lungs, gastrointestinal (GI) tract, vagina, eyes, and other moist mucosal surfaces. Mucus serves as a physical barrier against foreign particles, including toxins, pathogens, and environmental ultrafine particles, while allowing rapid passage of selected gases, ions, nutrients, and many proteins. Its selective barrier properties are precisely regulated at the biochemical level across vastly different length scales. At the macroscale, mucus behaves as a non-Newtonian gel, distinguished from classical solids and liquids by its response to shear rate and shear stress, while, at the nanoscale, it behaves as a low viscosity fluid. Advances in the rheological characterization of mucus from the macroscopic to nanoscopic levels have contributed critical understanding to mucus physiology, disease pathology, and the development of drug delivery systems designed for use at mucosal surfaces. This article reviews the biochemistry that governs mucus rheology, the macro- and microrheology of human and laboratory animal mucus, rheological techniques applied to mucus, and the importance of an improved understanding of the physical properties of mucus to advancing the field of drug and gene delivery.
1. Introduction
Mucus is a thick substance that lines the luminal surface of the gastrointestinal (GI), respiratory, urogenital, and eye tissues, as well as the peritoneal surface of intra-abdominal organs in humans and most animals. The function of mucus varies between different organs. At exposed surfaces, such as those of the gastrointestinal tract, airways, female reproductive tract, and eyes, mucus acts as the outermost line of protection against foreign pathogens [1–3], toxins [4], and environmental ultrafine particles [2, 5]. In the GI tract, mucus also aids the transport of chyme from the gut to the colon by serving as a lubricant during the peristaltic process while allowing rapid entry and exit of nutrients and waste [6, 7]. At the surfaces of internal organs, mucus serves as a lubricant to minimize friction between organs. In performing its numerous functions, mucus is continuously secreted, shed, and finally digested, recycled, or discarded.
At the chemical level, mucus is an integrated structure of biopolymers. Its physical behavior is complex (non-Newtonian), with highly variable properties that are between those of a viscous liquid and an elastic solid. Rheological measurements, including viscosity (resistance to flow) and elasticity (stiffness), are often used together to describe the consistency of mucus. The rheological properties of mucus vary as a function of shear stress, time scale (rate) of shearing, and length scale. Changes in the rheological properties of mucus may greatly affect its ability to function as a lubricant, selective barrier, and the body’s first line of defense against infection [8–10]. If mucus becomes too thick, for example in severe bronchitis [11] or cystic fibrosis [12–14] where the sputum viscosity can be more than 100,000 times that of water, patients experience great difficulty in mucus clearance, resulting in bacterial overgrowth. On the other hand, in women with bacterial vaginosis, the viscosity of vaginal fluids is significantly lower than in those with normal flora, which may be responsible for the increased risk of infection by HIV and Neisseria gonorrhoeae, as well as other adverse gynecological conditions [15].
We first discuss the distinction between macro- and microrheology of mucus and provide important background on the biochemistry of mucus, with an emphasis on the regulatory mechanisms that control its viscoelastic properties. We then discuss the microrheology of mucus, focusing on the rheology of mucus as encountered by micro- and nanoscopic entities, such as viruses, proteins, bacteria, and drug delivery particles. We specifically address the importance of understanding mucus microrheology to the design of therapeutic nanoparticle systems targeted to mucosal tissues. We then examine the macrorheological behavior of mucus and the impact of macrorheology on improved understanding of human physiology, disease pathology, and therapeutic strategies. In the last section, we review rheological techniques used to characterize mucus across vastly different length scales.
2. Macrorheology vs. microrheology
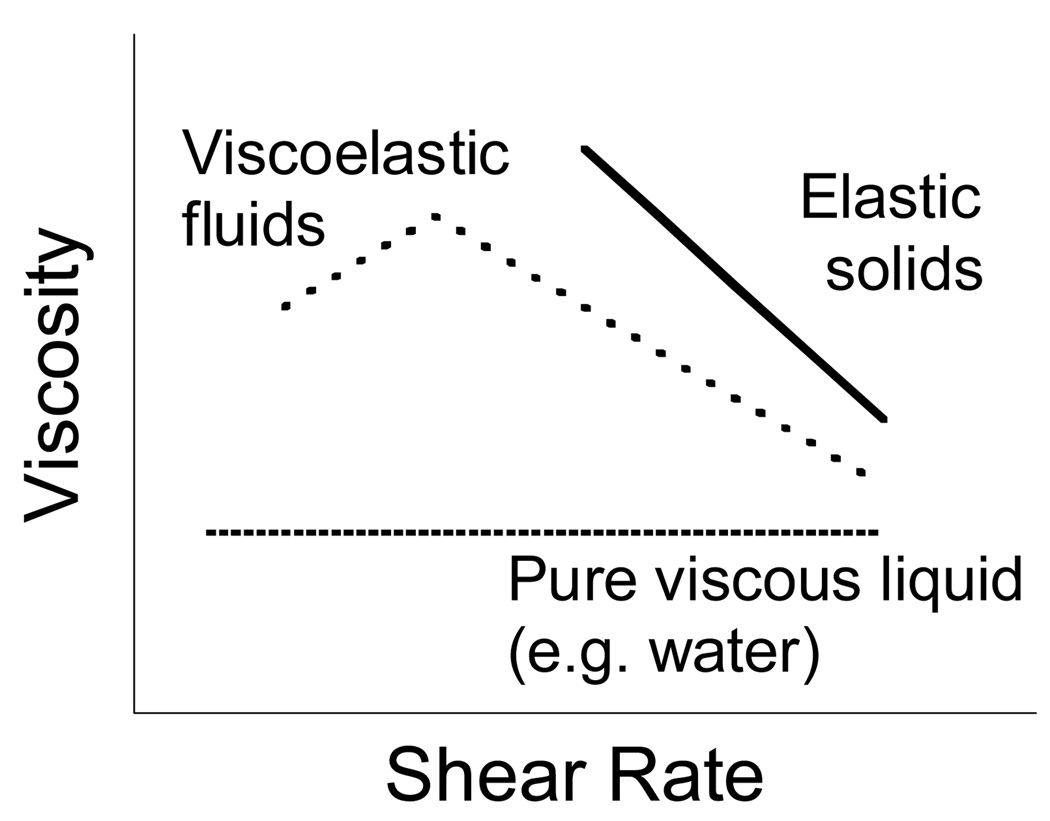
Characterization of the physical properties of mucus largely focuses on two properties: (i) viscosity or loss modulus (G″), which is the extent to which the gel resists the tendency to flow, and (ii) elasticity or storage modulus (G′), which measures the tendency for the gel to recover its original shape following stress-induced deformation. Together, these properties describe the rheology of complex biological fluids. An illustration of the steady state viscosity of a purely viscous fluid, an elastic solid and a viscoelastic gel is shown in Figure 1. The phase angle or loss tangent value δ, calculated from the inverse tangent of G″/G′, is also a common parameter for characterizing mucus (δ = 0° for a Hookean solid; δ = 90° for a viscous liquid; δ < 45° for a viscoelastic solid and δ > 45° for a viscoelastic liquid). A number of additional physical properties are also used to describe mucus. For example, creep, measured by applying a constant stress and measuring the strain or deformation created as a function of time, quantifies the tendency of a gel to deform permanently. Spinnability (or Spinnbarkeit), which measures the capacity of fluids to be drawn into threads, represents an indirect measurement of the adhesive and elastic properties of mucus.
Figure 1.
Illustration of the steady state viscosity vs. shear rate profiles of liquids, solids, and viscoelastic substances. The viscosity of a liquid is constant, while the viscosity of a yielding solid decreases with time. However, the viscosity of a viscoelastic material is more complex. In the above example of a thixotropic fluid, the steady state viscosity first increases at low shear rates (shear thickening), then progressively decreases at larger shear rates (shear thinning).
At the macro (bulk fluid) scale, mucus is commonly referred to as a viscoelastic gel because it possesses both flow (viscosity) and deformation (elasticity) properties. In particular, the bulk rheology of mucus is characterized by a non-Newtonian viscosity that is non-linear with shear rate, posing strong resistance to deformation at low shear rates and weak resistance at high shear rates. The bulk rheology is critical for the proper macroscale function of mucus, including mucus clearance and lubrication. However, the bulk viscosity of typical mucus secretions (∼2000-fold or more viscous than water at low shear) would seemingly preclude the diffusion of particles or even small proteins at appreciable rates. Since mucus affords rapid passage of select proteins and particles, bulk-fluid macrorheological characterization is inadequate for understanding the barrier properties of mucus, especially at length scales relevant to pathogens, toxins, and foreign particles.
The term microrheology has been used to describe techniques that measure the macroviscoelasticity of minute volumes of mucus. Another common definition of microrheology, and the one we adopt, is related to the characterization of the viscoelasticity that is encountered by micro- and nanoscale entities. In contrast to bulk rheology, which provides averaged measurements of physical properties, microrheology can measure heterogeneity in a sample’s physical properties with high spatial resolution. In essence, microrheology affords detailed characterization of the viscosity and elasticity of biological fluids, accounting for both contributions from the fluid within the biopolymer network as well as the network mesh itself. Thus, microrheological studies are important for characterizing the local mechanical properties of biological fluids that are overlooked by bulk rheological techniques.
Mucus, similar to other complex biological fluids, has microscopic domains between entangled fibers that are filled by a low viscosity fluid. The dynamics of nano- or microscale entities diffusing in the nanoscopically heterogeneous mucus are thus controlled by their local environment (i.e. microrheology) rather than the bulk biophysical properties of the mucus gel. It is important to note that this does not reflect a breakdown in the Stokes-Einstein continuum, as was suggested for cadmium selenide nanoparticles in a polymeric fluid [16]. Instead, for complex biological fluids, the apparent viscosity governing the Stokes-Einstein relation is a function of length scale features reflecting the structural architecture of the medium. The dynamic motions of entities that do not interact with mucus elements and that are small with respect to fluid microdomains remain governed by the Stokes-Einstein relation for diffusion.
3. Biochemical factors governing the viscoelastic properties of mucus
3.1 Introduction
At the macroscopic level, mucus is a non-Newtonian, thixotropic gel distinguished from classical solids and liquids by its response to shear stress. Under low shear, mucus behaves like an elastic solid and regains shape over time; under high shear, mucus behaves like a viscous liquid and eventually deforms irreversibly [6]. The dynamic viscoelastic properties of mucus are closely regulated biochemically to ensure efficient clearance, while also maintaining sufficient adhesive and elastic strength to be retained on the epithelial surface despite shearing forces due to swallowing, peristalsis, blinking, gravity, and copulation. Mucus consists of mucins, DNA, lipids, ions, proteins, cells and cellular debris, and water [6, 17, 18]. The biochemical regulation of these various constituents is complex and highly interdependent, and failure in any one component can adversely affect the physical properties of mucus and greatly contribute to disease conditions.
3.2 Mucins
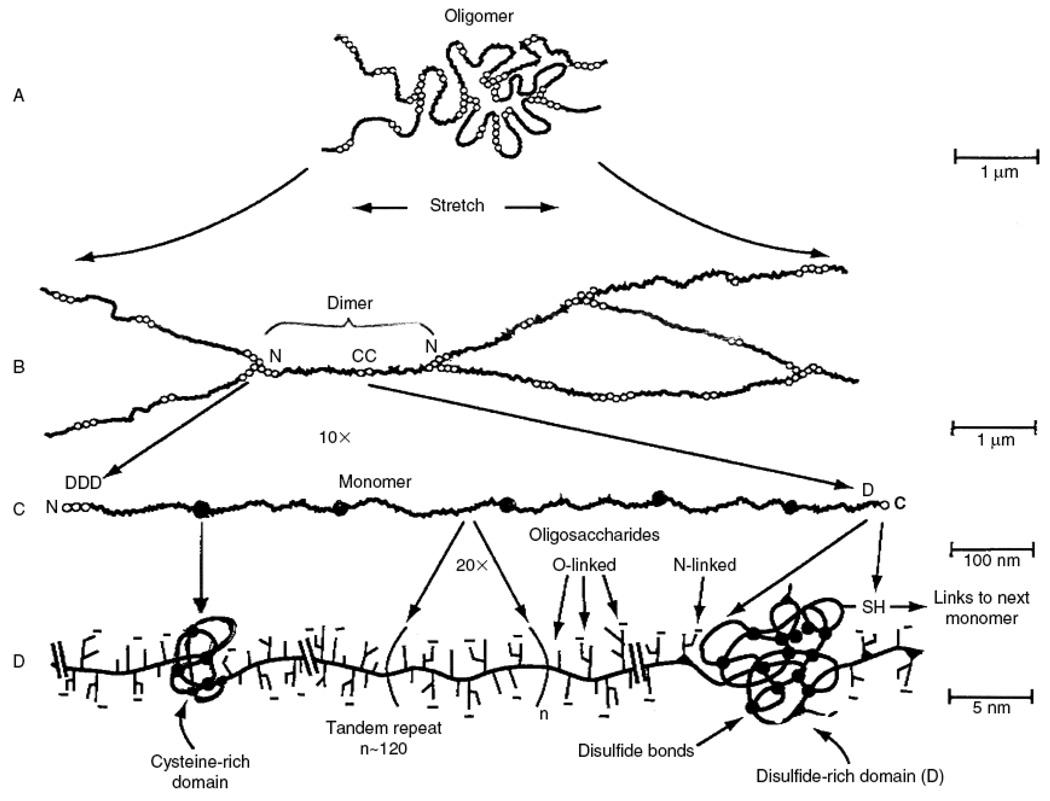
Mucus is composed primarily of crosslinked, bundled, and entangled mucin fibers secreted by both goblet cells and the seromucinous glands of the lamina propria at the apical epithelium [17, 18] (Figure 2). Mucin fibers, typically 10–40 MDa in size and 3–10 nm in diameter [19], are proteins glycosylated via proline, threonine, and/or serine residues by O-linked N-acetyl galactosamine as well as N-linked sulfate-bearing glycans [20]. Glycan coverage of mucins is dense, with 25–30 carbohydrate chains per 100 amino acid residues [21], and contributes up to 80% of the dry weight of mucus [22]. Most mucin glycoproteins have a high sialic acid and sulfate content, which leads to a strongly negative surface that increases the rigidity of the polymer via charge repulsion [19]. Sialomucin content is suggested to be highly correlated to mucus viscosity and elasticity [23].
Figure 2.
Major biochemical features of gel-forming mucins. (A) Several mucin monomers are shown linked together in an oligomeric gel. (B) Mucin monomers are crosslinked end-to-end via disulfide bonds between disulfide-rich domains (labeled “D”) near the amino- and carboxyl-termini [45, 196, 197]. (C) Interspersed along each fiber are “naked” globular protein regions, with small exposed hydrophobic patches [198]. These regions are stabilized by multiple disulfide bonds. (D) Individual mucin fibers are densely glycosylated with O- and N-linked glycans, most of which are negatively charged with sialic acids or sulfate groups [45]. Figure is obtained from [6].
Mucus rheology changes based on the composition of mucins and their glycosylation, both of which vary with age, the host’s diet, and the presence and activity of specific antigens, commensals, and pathogens. For example, in response to infection with Helicobacter pylori, the viscoelasticity of gastric mucus increases, perhaps to help prevent infectious entry of motile pathogens [24]. Smoking, which causes an increase in sulfomucin compared to sialomucin content [25], leads to a decrease in viscosity [26]. In women with bacterial vaginosis, the overgrowth of anaerobic gram-negative bacteria that produce sialidase, glycosidases and other mucin-degrading enzymes causes a breakdown in the barrier properties of cervicovaginal mucus [27, 28].
Mucin content, governed by mucin secretion rates as well as the degree of mucus hydration, is a major determinant of mucus rheology. With the exception of specific disease states, mucin content usually ranges between 2–5% by weight for gastrointestinal, cervical, ocular, nasal, and lung mucus despite significant differences in glycosylation [29–33]. Likewise, water content is highly similar across various mucosal surfaces and usually within the 90–98% range [32–36]. Nonetheless, small differences in the concentrations of mucins may be sufficient to cause significant changes in the mucus viscoelasticity. For example, nonovulatory cervicovaginal mucus is roughly 100-fold more viscous than ovulatory mucus at low shear rates due to a moderately greater mucin concentration (2–4 fold). At constant shear rates, the viscosity of cervical mucus was estimated to increase roughly with the second or third power of mucin concentration [37]. In disease conditions, such as cystic fibrosis, where the mucin to water ratio is significantly increased to ∼5–10 times greater than normal [38], mucus viscoelasticity can approach that of rubber, with typical viscosities 104–105 fold higher than water at low shear rates. Mucus derived from the trachea is also rheologically distinct from mucus found in the small airways, and may be related to variations in mucin concentration due to differing rates of water transport from the airway lumen. It is typically believed that the movement of water across the airway epithelium follows the transfer of ions, which is related to the transepithelial electric potential difference (PD) [39–41]. Boucher and co-workers have demonstrated that PD is considerably lower in the small airways than in the trachea, suggesting that water removal from the airway lumen increases going from the small airways to the trachea [42].
Mucins can be generally separated into two families: cell-associated mucins that range between 100–500 nm in length and contain a transmembrane domain, and secreted mucins that are up to several microns long [43–45]. In the gastrointestinal and cervicovaginal tracts, the two distinct mucin types facilitate the formation of two planes of mucus (a cell-adherent layer and a non-adherent, luminal “sloppy” layer), which is critical to the excellent lubrication properties of mucus. When mucus is rapidly sheared, for example during copulation, swallowing, or peristalsis, a slippage plane of low viscosity forms between the cell-associated, unstirred layer and the non-adherent, stirred layer of mucus [6]. As long as mucus is sheared, the viscosity within the slippage plane remains low, and the non-adherent, stirred layer of mucus is rapidly transported by the aforementioned processes.
It is important to note that the mucus mesh is composed primarily of entangled mucins and other mucus constituents with reversible linkages, rather than non-reversible, covalently cross-linked polymers. A drop of mucus in water or saline will initially swell but eventually reach complete dissolution, whereas crosslinked gels do not dissolve [46]. This distinction is further highlighted by experimental observations that, unlike other cross-linked gels that tear irreversibly upon shear, the viscoelasticity of mucus recovers rapidly and reversibly, typically restoring much of its viscous and elastic properties within seconds [6]. This rapid recovery is critical to mucociliary transport and prevents mucus sheared by coughing from flowing downward to the alveoli by gravity. In addition to physical entanglement, low-affinity non-covalent bonds [20] and stronger disulfide bonds [47] between mucin fibers as well as other mucus constituents may further contribute to the viscoelasticity, and these bonds may re-associate after shearing, reaching full recovery more slowly (over minutes to hours).
3.3 DNA
In most mucus samples obtained from healthy subjects, DNA represents approximately 0.02% of the mucus by mass, with the vast majority originating from debris of shed epithelial cells [48, 49]. In certain disease conditions, DNA accumulation can directly increase the viscosity of mucus [49, 50]. When DNA was added to cystic fibrosis sputa, an increase of 30% in both elasticity and viscosity was observed [51]. Secondary infections in cystic fibrosis, which cause neutrophil lysis and further increase the DNA content to up to 0.5–1.5% of the mucus by weight, are suggested to be directly responsible for the 10–100 fold further increase in the mucus viscoelasticity associated with the disease [52]. Treatment of CF sputum with DNAse markedly reduces the bulk rheology [14].
3.4 Lipids
Lipids, with a mass ratio up to 1–2%, represent a high percentage of the molecules present in mucus [48, 49]. Most of the lipid content is associated with hydrophobic domains of mucin glycoproteins [53, 54]. The lipids were found to contribute greatly to the rheological properties of mucus [8, 55]. In particular, extracting the lipids from dog gastric mucus reduced the steady shear viscosity by 80–85% [56]. Much of the decrease in mucus viscoelasticity can be recovered upon addition of lipids. Higher total lipid content was also correlated to increased viscoelasticity in purulent cystic fibrosis secretions [12]. Phosphatidylethanolamine, sphingomyelins and lysophosphatidylcholine were all found to increase the viscosity of cystic fibrosis sputa, whereas phosphatidylglycerol reduced the viscosity [12, 57].
3.5 Salts
Changes in ionic strength can directly lead to shrinkage or swelling of mucus and, thus, significantly alter mucus viscoelasticity. In general, various mineral salts account for up to 1% of the mucus mass [48, 49]. Studies on how mono-, di- and trivalent ions affect the rheological properties of purified mucins suggest that, in general, increases in ion concentration correlate with a decrease in the viscosity of mucus [46]. The elasticity of mucus also increases with greater ion valency [58, 59]. High concentrations of multivalent cations, such as calcium and magnesium, can collapse the mucus gel entirely and facilitate reversible cross-links between mucin monomers [60]. High acidity, which reduces the negative charges of the carboxyl groups on sialic acids along the glycosylated regions of mucin fibers, can also increase the viscoelasticity of mucus, a phenomenon commonly observed with gastric mucus [61].
3.6 Proteins
Girod and coworkers have demonstrated that the addition of pure proteins, such as immunoglobulins A and M (IgA and IgM) or lysozyme, can considerably increase the viscoelasticity of reconstituted lyophilized sputum [8]. This finding is in good agreement with other observations, where increasing IgM concentration correlated with increased viscosity of cystic fibrosis sputa [8, 62]. It was suggested that these proteins increase the viscous and elastic moduli of mucus by serving as restructuring molecules [8, 63]. This model is partially supported by the reduced transport rate observed for IgM and small aggregates of IgA, which were slowed 3- to 5-fold in mucus compared to their diffusion in water [64, 65]. Since these immunoglubulins are significantly smaller than viruses that move in mucus at the same rate they move through pure water, they must be slowed by low-affinity bonds with mucins. Interactions between immunoglubulins and mucins is in good agreement with the ability of anti-sperm antibodies to trap vigorously motile sperm in mucus [64, 66, 67]. It was further suggested that the Fc region of antibodies is primarily responsible for interactions with mucins [64]; in combination with the finding that a number of other proteins are not significantly slowed in mucus [64, 65], this suggests the effect on the rheological properties of mucus is highly dependent on the biochemistry of individual proteins.
3.7 Cells & cellular debris
The exact contribution of cells and cellular debris to the viscoelasticity of mucus remains unclear. Adhesive interactions between cells and other mucus constituents may significantly affect the viscoelasticity of mucus. However, identifying the rheological contribution of cells remains difficult, since it is impossible to isolate cells from physiological mucus without altering the physical properties of mucus. The extent to which cellular debris contribute to the total content of DNA, actin, proteins and lipids in mucus, hence mucus viscoelasticity, also is not known.
4. Microrheology of mucus
4.1 Microrheology of human mucus
Mucus is not a homogenous fluid, but rather comprises nanoscopically heterogeneous environments (Figure 3A). Therefore, when the length scale approaches the dimensions of the mucin fiber mesh, particulate permeability in mucus is expected to be reduced due to increased steric obstruction and, thus, a higher apparent viscosity [68, 69]. At the length scales of macromolecules such as proteins (<10 nm), resistance to their Brownian diffusion largely reflects viscous drag by water, assuming these molecules have negligible affinity to mucus constituents [64, 65]. When length scales are further increased to the dimensions of small capsid viruses, their diffusion rates in mucus were not reduced compared to water. Specifically, Norwalk virus (38 nm) and human papilloma virus (HPV) (55 nm) were both observed to diffuse in human cervical mucus as rapidly as they do in water [64]. This directly implies that at length scales up to 55 nm, the microviscosity of mucus remains similar to the viscosity of water.
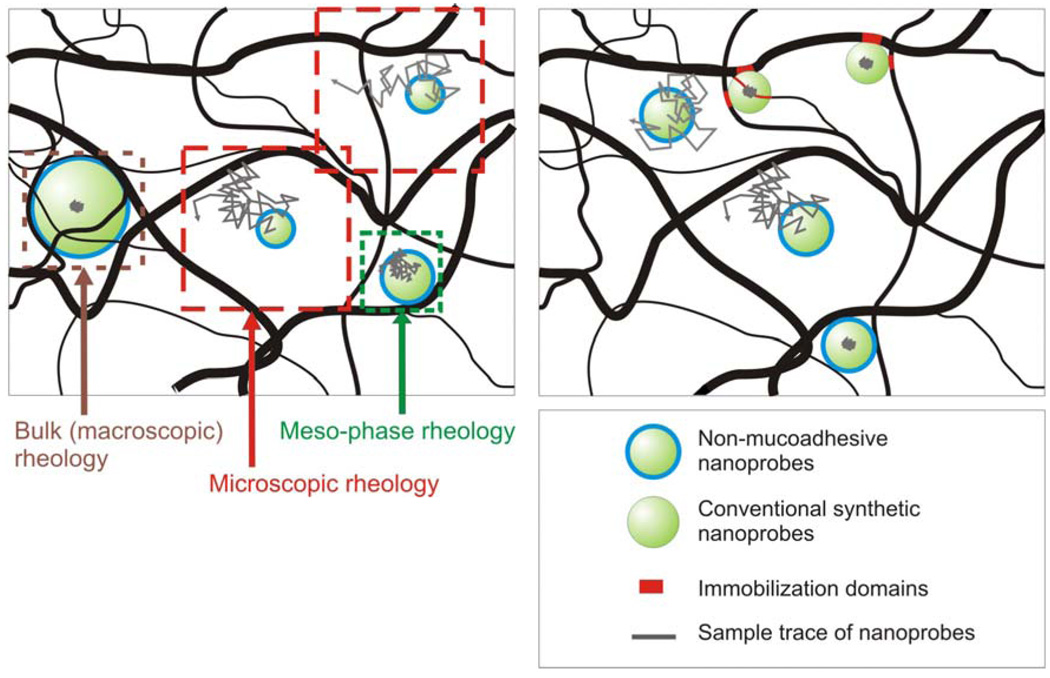
Figure 3.
(A) Schematic representation of the length scale dependence of viscosity in a nanoscopically heterogeneous fluid. Non-adhesive particles that are significantly smaller than the mesh spacing undergo Brownian diffusion and probe the microscopic rheology. As the particle size approaches the dimensions of the mesh spacing, particle movement becomes hindered by the mesh microstructure at short time scales, leading to a mesophase rheology regime. Particles that are significantly larger than the mesh spacing probe the bulk or macroscopic rheology of the gel. (B) Schematic comparison of non-mucoadhesive rheological nanoprobes and conventional polymeric particles. Conventional nanoprobes are immobilized to mucin fibers via adhesive interactions. Their strongly hindered motion, as reflected by the small dimensions of the traces, suggest a markedly higher viscoelastic environment than the true local viscoelasticity of mucus. In contrast, the motion of non-mucoadhesive nanoprobes correctly reflects the local viscous and elastic contributions from the mucus mesh architecture.
The finding that larger, 180 nm herpes simplex virus are slowed, even in thinner regions of mucus, at least 100–1000 fold compared to water is in good agreement with an interfiber spacing estimate of roughly 100 nm for mucus [64]. In turn, this implies that the effective viscosity for length scales 200 nm and larger is expected to greatly increase. Recently, this assumption was challenged by the engineering of 200 and 500 nm polymeric nanoparticles capable of traversing human mucus with effective diffusivities only 6-fold and 4-fold reduced compared to water, respectively [70]. The rapid diffusion of these particles, significantly larger than the previously estimated average mucus fiber spacing, strongly suggests that the mucus mesh architecture is radically different from the majority of previous estimates derived from EM and virus diffusion studies. This work confirms the hypothesis that mucus microviscosity is similar to that of water [64] but greatly shifts the length scale of the low viscosity regime from ∼55 nm to the range of ∼500 nm. The microviscosity at length scales significantly longer than 500 nm is expected to deviate from a low viscosity fluid, since particles significantly larger than the average interfiber spacing will encounter extensive steric hindrance that contributes to markedly higher viscoelastic moduli. In preliminary studies, the increase in microviscosity becomes evident at length scales near 1 µm, as 1 µm particles coated with a similar muco-inert coating as 200 and 500 nm non-mucoadhesive particles exhibit greatly reduced transport rates relative to water.
Besides cervicovaginal mucus, the microrheology of cystic fibrosis sputum has also been studied. The microviscosities of cystic fibrosis sputum, probed by MPT of 100 and 200 nm polystyrene particles, were 15- and 7-fold lower, respectively, than the bulk viscosity measured by a cone and plate rheometer. However, as these studies were performed with particles that may be mucoadhesive, their transport rates in mucus may largely reflect contributions by adhesive interactions and not the local viscoelastic moduli (Figure 3B). The apparent viscosity encountered by small dextran molecules in cystic fibrosis sputum, studied by fluorescence recovery after photobleaching (FRAP), is approximately 2.9-fold higher than water [71]. The higher microviscosity of cystic fibrosis sputum compared to that of cervicovaginal mucus, which remained roughly the same as water, may be a consequence of greater DNA, mucin, and actin contents in the fluid between mesh structures. Our latest studies with non-mucoadhesive nanoprobes suggest that the microviscosities of fresh undiluted cystic fibrosis sputum at length scales of 200 nm may be only 13–66 fold higher than water (Suk, Lai and Hanes, unpublished results), in sharp contrast to the 20,000-fold greater bulk viscosity.
Despite vast differences in the relevant length scales, microrheological properties of mucus may be reflected in part by their macrorheological characteristics. For example, Sanders and co-workers correlated variations in the elastic modulus (G′) of cystic fibrosis sputum samples to particle diffusion and found that the percentage of nanoparticles (124–270 nm) transported through mucus increased with increasing elasticity (G′ > 100 Pa-s) [72]. This unexpected finding was explained by a possible increase in the heterogeneity of the mucus mesh, leading to larger pores for particle diffusion as elasticity increases.
4.2 Importance of mucus microrheology
The efficient delivery of drugs and genes to mucosal tissues, including the airways, gastrointestinal tract, cervicovaginal tract, nasal tract, and eyes, is often confounded by the mucus barrier. By administering therapeutics topically to mucosal surfaces, increased local drug concentration and reduced systemic side effects can be achieved, leading to improved efficacy. Furthermore, nanoparticle systems may facilitate the delivery of encapsulated therapeutic molecules that otherwise suffer from low stability or low absorption in mucosal fluids, as well as provide controlled and sustained delivery to underlying epithelia. However, nanoparticles must efficiently overcome the mucus barrier to avoid rapid clearance [73, 74]. To engineer nanoparticles capable of crossing this highly viscoelastic and adhesive barrier, it is imperative to understand the biophysical properties of mucus, namely their viscosity and elasticity, at length scales relevant to nanoparticulate delivery systems.
Previously, the development of polymeric and liposomal delivery systems for mucosal drug or gene delivery was strongly discouraged by the slow transport of herpes simplex virus (HSV; d∼180 nm) in cervicovaginal mucus. The few HSV particles that were not immobilized experienced an effective viscosity similar to the bulk viscosity, nearly 100–1000 times greater than water [64]. We recently engineered muco-inert nanoparticles as large as 500 nm to rapidly cross fresh undiluted human cervicovaginal mucus [70], disproving the notion that particles larger than 100 nm are limited by viscous and elastic forces in their penetration of human mucus. This finding has important implications to the design of drug and gene delivery systems. Research efforts on engineering transmucosal delivery systems should now focus on reducing nanoparticle adhesion to mucus. Likewise, the low diffusion rates observed for some macromolecules are clearly a reflection of affinity to mucus constituents rather than viscous drag from high microviscosity.
Improved characterization of microrheology of mucus may also provide further insight into the various biological processes within the mucus “ecosystem”. For example, the low transport rates observed for herpes simplex virus in cervicovaginal secretions indicate they are highly immobilized by mucus, presumably due to hydrophobic interactions between naked protein domains found on mucins and the viral envelope [6, 64]. This in turn suggests that mucus may have evolved to efficiently remove particular infectious pathogens via adhesive interactions. Microrheological characterizations of other human mucus secretions beyond cervicovaginal mucus, as well as mucus associated with specific disease conditions, are expected to contribute further important insights into disease pathology, mucosal immunity, and mucus physiology.
5. Macrorheology of mucus
5.1 Macrorheology of human mucus
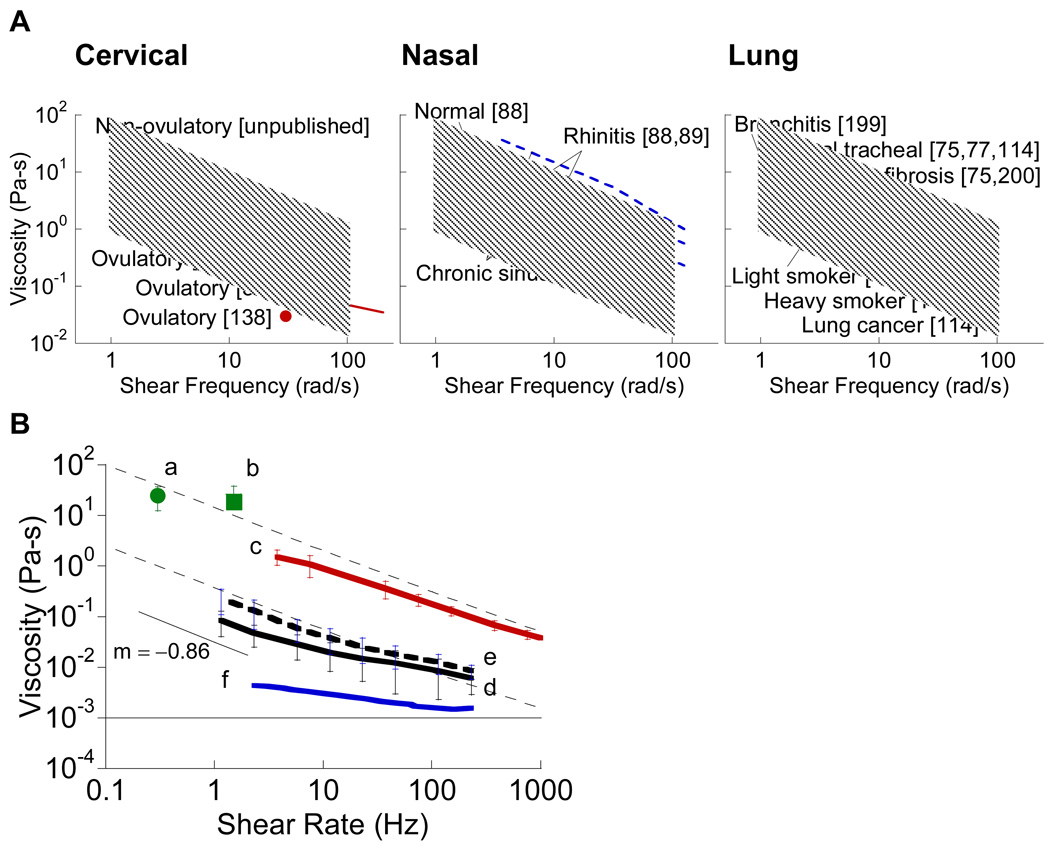
Despite the diversity in mucin glycoproteins, the similar total mucin content among mucus originating from various mucosal organs leads to similar rheological behavior. The viscoelasticity of mucus is characterized by a log-linear shear thinning of viscosity (Figure 4). The average steady-shear viscosity is found to vary for frequencies between 10−4 and 102 Hz, spanning viscosity values as high as 103 Pa-s and as low as 10−2 Pa-s. In general, at low shear rates, the viscosity of human mucus is as high as 104−106 times that of water [6]. However, at shear rates near the physiological maximum of 103−104 per second (achieved during blinking, coughing, or copulation), the viscous drag of mucus is greatly reduced, and mucus becomes a low viscosity fluid. The slope of the viscosity versus shear rate for mucus is commonly within the range of −1 to −0.5, with an average of −0.85 [46]. Less viscoelastic mucus secretions, such as tears, saliva, and ovulatory mucus, have significantly lower viscosity, typically no more than 102 to 103 times that of water at low shear rates and approaching that of water at higher shear rates [6].
Figure 4.
Viscosity of various types of human mucus. (A) Dynamic oscillatory viscosity as a function of shear frequency for cervical mucus (red, [37, 138, 201]), nasal mucus (blue, [88–93]), and lung mucus (black, [75, 78, 114, 199, 200]). Solid lines correspond to normal mucus, while dashed lines indicate mucus under disease conditions. The shaded region represents the range of values for all mucus types shown. (B) Steady shear viscosity as a function of shear rate for (a, b) chronic bronchitis mucus (green circle [202] and square [11]), (c) non-ovulatory cervical mucus (red, [70]), (d) normal gastric mucus (black line, [105]), (e) duodenal ulcer mucus (black dashed line, [105]), and (f) tears (blue, [111]). Thin dashed lines indicate the typical range of viscosity values for human mucus suggested by [6]. The thin solid line represents the viscosity of water (10−3 Pa–s).
The primary challenge in interpreting mucus rheology is the use of different rheological techniques without a standard convention for reporting viscosity, which changes continuously depending on the shear rate (or frequency) or shear stress. Thus, reported viscosity values are apparent viscosities at an arbitrary shear rate or stress. It is also important to distinguish viscosity obtained under steady shear vs. strain-controlled dynamic oscillatory shear. Under dynamic oscillatory shear, mucus is subjected only to small oscillations of fixed amplitude (strain) at different shear frequencies. By minimizing the deformation, these measurements best reflect mucus rheology in the native, unperturbed state. This shear frequency-dependent apparent viscosity is directly derived from the viscous modulus, G″, and typically denoted as η″. In contrast, when mucus is subjected to continuous steady shear across various shear rates, the measured physical properties largely mirror those of mucus during activities such as peristalsis and copulation. The steady shear apparent viscosity, η, is derived from the shear stress necessary to maintain a specific shear rate. Due to differences in rheological characterization and choice of shearing conditions, the viscosity of mucus can not be compared at face value without properly accounting for shearing effects. Summarized in Figure 4 is the viscosity of human mucus obtained from literature, distinguishing between dynamic oscillatory shear and steady shear viscosity.
Interpretation of the literature is further complicated by the fact that the macrorheological properties are subject to natural, spontaneous changes due to the method of handling, such as the application of stress [11]. Careful experimentation by several investigators indicate that deep freezing and thawing alters mucus viscosity, presumably by altering the chemical or physicochemical configuration of the complex molecules of the mucus polymers. Nonetheless, in two recent independent works related to the viscoelasticity of cystic fibrosis mucus, short term storage of the mucus up to 30 days at −20 °C did not reduce the bulk, macroviscoelasticity of mucus compared to fresh samples [69, 72]. Reconstitution of mucus, on the other hand, leads to markedly different viscoelastic properties in human cervical mucus [37].
5.1.1 Human respiratory mucus
Tracheobronchial mucus from healthy human lung airways is not readily available due to difficulty in collection. Limited characterization of the physical properties of airway mucus can be performed with mucus collected by an endotracheal tube [75], screens [76, 77], or a specimen brush [78]. In these studies, viscosity in the range of 12–15 Pa-s, with a relaxation time roughly 40 s and an elastic modulus of 1 Pa, was suggested to represent an optimal rheological profile for mucociliary clearance [79]. The rheological properties of respiratory mucus vary depending on the anatomical site in the lung. In particular, the elasticity of mucus collected from the small airways was significantly less than that from the trachea, a finding attributed to the lower solid content in small airway mucus (12.1±3.5% vs. 16.5±2.7%) [80].
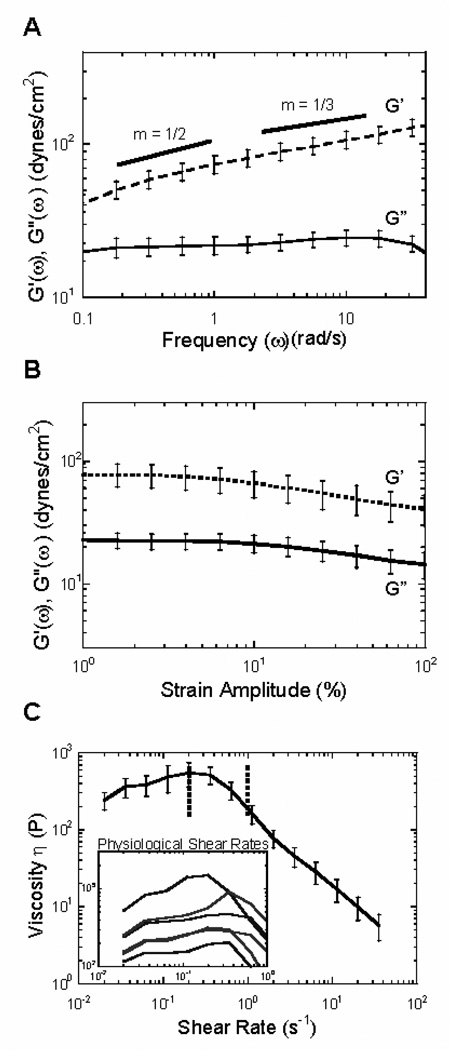
Pulmonary disease conditions, such as cystic fibrosis (CF), chronic obstructive pulmonary disorder (COPD), and asthma, generally result in an increase in the viscoelasticity of mucus, owing in part to reduced water content and an increased fraction of glycoproteins [81, 82]. Careful collection of the mucus secretions (sputum) from these patients is necessary to avoid saliva contamination, and these mucus secretions likely do not mimic healthy mucus. Under oscillatory, controlled strain shear, CF sputum is significantly more elastic than viscous across all frequencies, with a phase angle of 16.2 ± 0.6° at a shear frequency of 1 rad/s (Figure 5A–B). Typical of other mucus fluids, CF sputum undergoes significant shear-thinning [69, 83–85], with representative steady shear viscosities of ∼110 and ∼14 Pa-s at shear rates of 0.1 and 1s−1, respectively (Figure 5C). Viscoelasticity as well as relaxation times are commonly higher in patients with more advanced disease. Despite the biochemical variations between sputum and healthy human secretions, the slope of the viscosity versus shear rate is similar to healthy human mucus and confined within the range of −0.9 to −0.72, with a mean of −0.86. In specific diseases such as bronchorrhea, very low values of viscosity (< 5 Pa-s) may be observed as well [8].
Figure 5.
Macrorheology of human cystic fibrosis sputum. (A) The frequency dependent elastic, G’(ω), and viscous moduli, G”(ω), of CF samples (n=6) were recorded at a constant strain amplitude of 1%. (B) Strain-dependent elastic, G’(ω), and viscous moduli, G”(ω), from 0.1–100% strain amplitude. (C) The steady state viscosity of CF sputum at shear rates between 10− 2−102 rad/s. Physiological rates in the normal lung are represented by the dotted line. Inset: viscosities of individual CF sputum samples at physiological shear rates. Figure obtained from ref [14].
Some studies have suggested that cystic fibrosis and perhaps normal bronchial mucus display reversible thixotropical properties, meaning that the steady state viscosity increases with increasing shear rate initially and then decreases [8, 69, 86] (Figure 5C). The critical shear rate for healthy and cystic fibrosis respiratory mucus is approximately 1 s−1 [8, 69, 87]; the steady state viscosity first increases in response to an applied shear rate up to 1 s−1, and then the mucus undergoes progressive shear-thinning at higher shear rates [8]. By modeling the tracheobronchial regions of the lungs as cylindrical tubes, average critical shear rates were estimated as 0.91 s−1 (large bronchi), 0.78 s−1 (medium bronchi), and 0.25 s−1 (small bronchi) [87]. Thus, mucus acts elastically at physiological shear rates in the upper airways, thereby maintaining the shape of mucus and the structure of the mucus fiber network.
A review of the literature suggests nasal mucus to be more viscoelastic than tracheobronchial mucus (Figure 4A), with nasal mucus from healthy subjects exhibiting viscosities of 46 and 1.3 Pa-s at shear frequencies of 1 and 100 rad/s, respectively [88]. In patients with rhinitis or bronchitis, the viscoelasticity of nasal mucus decreases dramatically, resulting in decreased clearability [88, 89]. Much of the viscoelasticity of CS mucus was thought to be attributed to mucin glycoproteins [90], which was supported by the reduced viscoelasticity of nasal mucus in CS patients treated with mucolytic drugs, such as serratiopeptidase or L-cysteine ethyl ester hydrochloride [91, 92].
Variations in rheological properties from patient to patient and also day to day appear to be a trait of both healthy and diseased respiratory mucus. Large discrepancies in sputum viscoelasticity are found in patients with chronic bronchitis, ranging from 1–80 Pa-s for viscosity at a constant shear rate of 0.4 s [8]. In healthy subjects, it was suggested that large deviations in nasal mucus transport are a direct consequence of the highly variable rheological properties of mucus, and not to differences in ciliary activity. King and coworkers observed a large variation in human tracheobronchial mucus viscoelasticity, both between subjects, with a coefficient of variation (CV) of 130%, and within the same subject on different days, with a CV of 55% [78]. In chronic sinusitis (CS) patients, nasal mucus viscosity also varies considerably, ranging from ∼1.5 to 23 Pa-s at a shear frequency of 6.28 rad/s [90–93]. No significant difference was found in respiratory mucus rheology between men and women.
5.1.2 Human gastrointestinal mucus
Mucus secretions from different regions along the gastrointestinal tract are expected to exhibit similar rheological properties [94]. This is inferred from studies of pig gastrointestinal mucus secretions, which exhibit overall structural similarities, suggesting similar rheological properties [95–99]. Indeed, gastric, duodenal and colonic mucus all exhibit similar rheological profiles [94]. Adherent gastric mucus gel from humans and pigs, obtained by gently scraping the washed mucosal surfaces of the stomach, both showed comparable viscoelasticity, with the elastic modulus greater than the viscous modulus throughout the frequency range studied (10−2 to 102 rad/s) [100–104]. The viscosity of healthy gastric mucus has been reported to be only ∼0.085 Pa-s at a shear rate of 1.15 s−1, but may increase significantly during duodenal ulceration [105].
5.1.3 Human cervicovaginal and other mucus
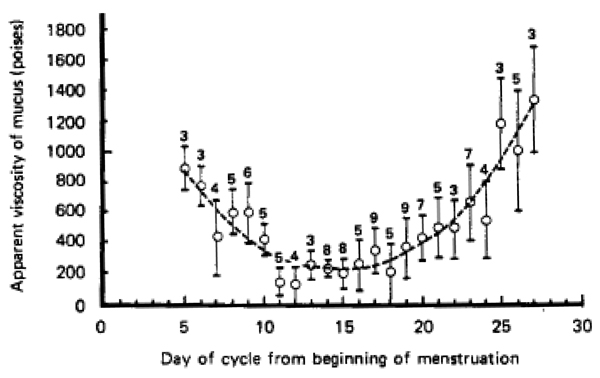
Undiluted human cervicovaginal mucus at physiologically relevant conditions can be obtained by a recently described procedure using a menstrual collection device [106]. These cervicovaginal fluids exhibit viscosity within the range of typical human mucus secretions (Figure 4B). The rheological properties of mucus from thirteen non-ovulating individuals were remarkably similar. Given the reproducible rheology, similar biochemistry to a variety of mucus secretions, large sample volumes, and ease of collection, nonovulatory human cervicovaginal mucus represents an excellent model for a variety of mucus studies. Ovulatory mucus, as previously discussed, possesses significantly lower viscosity due in large part to increased hydration [107]. The cyclic transition between non-ovulatory and ovulatory mucus results in a U-shape curve when plotting the apparent viscosity versus the day of the cycle from the beginning of menstruation [108–110] (Figure 6).
Figure 6.
Variations in the viscosity of cervical mucus from healthy, non-pregnant subjects with day in menstruation cycle (119 samples). The dotted line is drawn to emphasize the main feature of the graph. The number above each point indicates the number of samples averaged. Each vertical line indicates ± one standard deviation of individual readings about the mean. Figure obtained from [108].
Human tears exhibit markedly lower viscoelasticity than other mucus secretions; a viscosity of roughly 0.006 Pa-s was found in freshly-collected samples of normal tears [111]. Although marginally-dry eyes appear to exhibit a 5-fold increase in viscosity, both mucus types show shear-thinning that could be fitted to a power-law equation [111].
5.2 Importance of understanding the macrorheology of mucus
Careful elucidation of the macroscopic viscous and elastic properties of mucus has greatly improved our understanding of lung function, including its barrier properties against inhaled particulates. Rapid mucus clearance in the respiratory tract, from the lung airways and sinuses towards the pharynx and stomach for eventual gastric sterilization and digestion, is an important component of the mucosal defense against foreign pathogens [1]. Airway mucus is primarily cleared by the systematic and rapid unidirectional sweeping motion of cilia, which are thin, tail-like projections extending approximately 5 to 10 µm outwards from the airway epithelia [2]. An intermediate viscoelasticity of the mucus gel, as well as a low viscosity periciliary fluid (sol) layer, are essential for optimal mucociliary transport [9, 32]. When the tips of the cilia sweep through the surface of the mucus gel layer, the shearing motion is fast enough (i.e. high shear rate) that the mucus layer acts similarly to a low viscosity fluid, hence facilitating efficient transport of the mucus layer. If the viscoelasticity of respiratory mucus becomes too low, however, the elasticity is insufficient for mucus to withstand gravitational pulling, causing mucus to run out of the sinuses as well as slide down into the lung and flood the alveoli. In contrast, when lung disease develops that leads to the formation of a highly viscoelastic mucus layer and the depletion of the sol layer, the sweeping force exerted by cilia is incapable of transporting the thick mucus and mucociliary clearance ceases. In such cases, airflow interactions, such as coughing, assume great importance in mucus transport [112]. In healthy individuals, respiratory mucus is likely more viscoelastic than the optimal physical properties for maximum mucociliary transport to allow regulation of mucus clearance in response to environmental stimuli [6]. For example, when irritants, such as cigarette smoke or particular allergens, are encountered, more dilute mucus is secreted, hence decreasing the viscoelasticity for faster clearance [89, 113]. However, with prolonged exposure to irritants or disease, for example with long-term smoking or lung cancer [114], mucociliary clearance can decrease to sub-optimal levels.
Macrorheological characterization of mucus has also greatly improved our understanding of reproductive biology. In the cervicovaginal tract, the difference in viscoelasticity between ovulatory and non-ovulatory mucus is sufficient to make nonovulatory mucus virtually impenetrable to sperm. Therefore, changes in vaginal discharge quality, regardless of the exact timing relative to ovulation, have been widely suggested as a better predictor of conception than classical attempts to time (or avoid) intercourse during the six-day fertile interval ending on the day of ovulation [108, 115]. Similarly, highly viscoelastic cervical mucus has been suggested as a important factor for the low fertility rates of women with cystic fibrosis [116, 117]. Differences in viscoelastic properties also enable the differentiation of mucus with increased levels of estrogen, which increases cervical mucus production, from mucus with high levels of progesterone, which lowers cervical mucus production [118]. Similarly, infertility occurs in males with cystic fibrosis apparently because epididymal mucus becomes too viscoelastic to be transported by peristalsis, leading to blockage and atrophy of the epididymus and vas deferens [119, 120].
Insights into the bulk rheology of mucus are also highly relevant to our understanding of disease transmission and pathology. Many mucus secretions are sufficiently thick to block the motility of various types of bacteria. For example, the viscosity of rat epididymal mucus is thick enough to block motile Escherichia coli as well as sperm [121]. A systematic investigation relating bacterial migration and mucus-simulant viscoelasticity suggests that a viscosity higher than 8 Pa-s serves as an efficient bacterial barrier and filter, whereas low viscosity allows for rapid progression and growth of bacteria [8]. For this reason, most intestinal bacteria populate the outer luminal surface layer of the mucus blanket. Mucus may also alter its rheological properties in response to pathogens; for example, the viscoelasticity of gastrointestinal mucus increases in the presence of Helicobacter pylori [57]. Nonetheless, if mucus becomes sufficiently thick to disable mucociliary transport in the lung, the blocked flow actually leads to bacterial overgrowth and infections, a primary complication found in cystic fibrosis patients [122, 123]. In particular, persistent lung infection may be attributed to inefficient neutrophil-mediated killing of bacteria, since the high viscoelasticity impairs neutrophil migration [124].
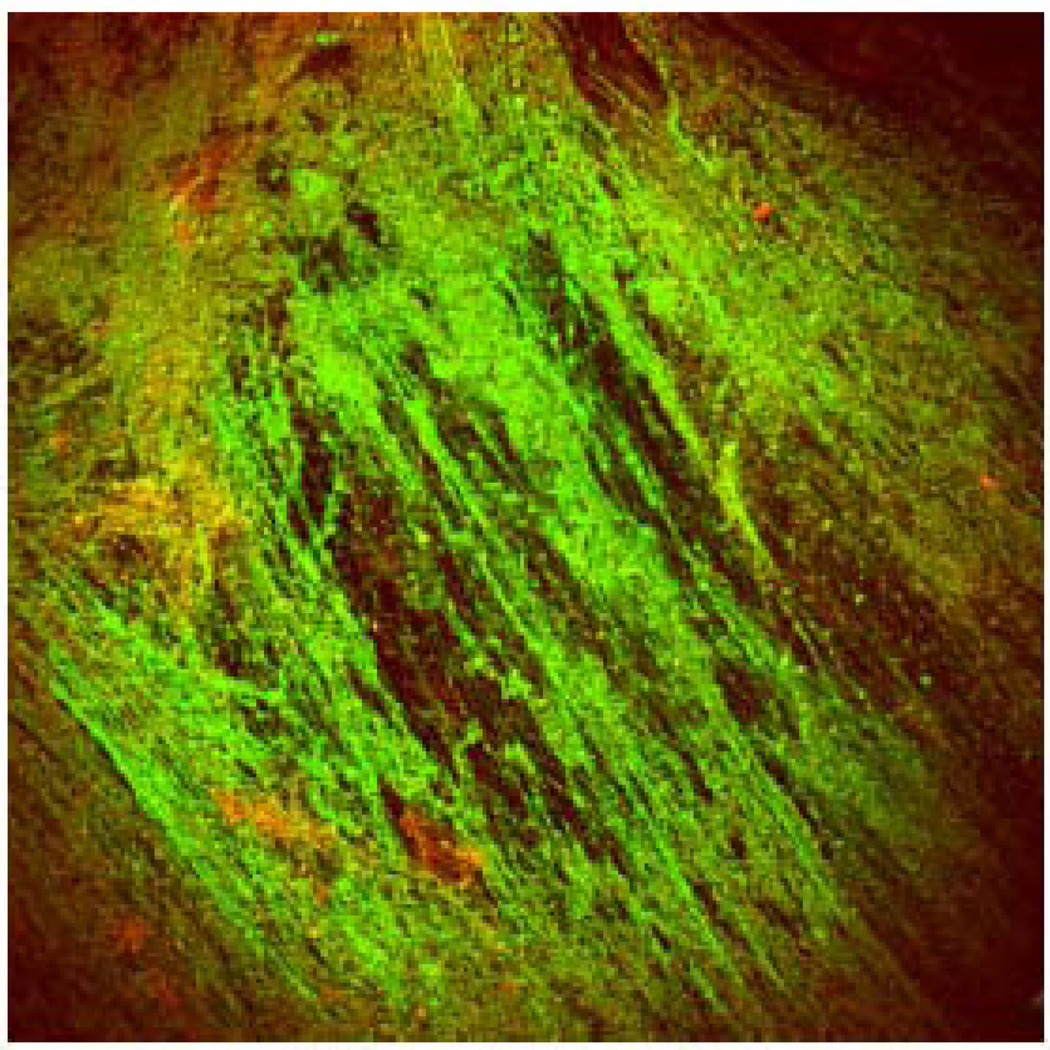
Last but not least, understanding the biochemical basis of the macrorheology of mucus has important consequences to the development and selection of potential therapeutic strategies. For instance, cystic fibrosis patients often need to inhale specific mucolytics for enzymatic cleavage of mucus constituents to facilitate mucus clearance from lungs by coughing. Commonly used mucolytics include N-acetylcysteine (NAC) [125, 126] and recombinant human DNase [127, 128]. NAC decreases viscoelasticity by substituting the disulfide bonds in the mucin networks with free sulfhydryl groups, hence disrupting the structure of the mucus gel. Despite in vitro mucolytic activity and a long history of use, there are no data demonstrating that aerosolized NAC is an effective therapy for cystic fibrosis [125, 129]. This may be attributed to the depolymerization of essential mucin polymer structures that decreased mucus viscoelasticity beyond the optimal rheological properties for clearance. Alternatively, stable cystic fibrosis mucus may express reduced mucin content and far greater amounts of DNA and actin [81, 130] (Figure 7). Indeed, DNase, which decreases the size of neutrophil-derived DNA fragments and hence the viscoelasticity, has demonstrated far greater clinical efficacy than NAC [125]. Likewise, actin released from necrosing cells can further copolymerize with DNA and entangle with mucin networks to increase the viscoelasticity of mucus [131]. Therefore, Gelsolin [132] and Thymosin β4 [133], two agents that efficiently depolymerize extracellular actins, are among the various mucolytics currently under development for cystic fibrosis therapy. Readers are referred to excellent reviews on mucolytics for further details [125, 134–136].
Figure 7.
Confocal micrograph of CF sputum showing DNA polymers in green (Yoyo-1 stain) and minimal mucin staining in red (UAE-Texas red). Figure obtained from [81].
5.3 Macrorheology of animal mucus
Animal mucus models are invaluable in instances where human mucus is not readily available in easily obtainable and/or sufficient quantities, or when a dynamic in vivo measurement, such as mucociliary clearance rate, is desired. For example, animal models are frequently used in studies investigating disease pathogenesis and the effects of pharmacological agents, where the bulk rheological properties of mucus are usually considered an important indicator of disease state or drug action. Similar to human mucus, animal mucus is a viscoelastic gel with reduced viscoelasticity under increasing shear rates. The most commonly studied animal models for mucus are dogs, pigs, and rats, although mucus from a wide range of animals has been characterized as well, including horses [137], cows [138], cats [139], rabbits [140] and ferrets [140]. Typical values for the rheological parameters of various types of animal mucus are given in Table 1 as a function of shear frequency.
Table 1.
Typical rheological values for various types of animal mucus.
| Animal | Type of Mucus | ω (rad/s) | η″ (Pa-s) | G* (Pa) | G′ (Pa) | G″ (Pa) | δ | Refs. | |
|---|---|---|---|---|---|---|---|---|---|
| Dog | Respiratory | Subglottis | 1 | 1.0–20 | 4.8–66 | 4.7–63 | 1.0–20 | 12–18 | [144, 194] |
| Tracheal | 0.05–400 | 0.038–32 | 2.1–85 | 2.0–80 | 0.50–40 | 9–54 | [141, 146, 162, 194] | ||
| Bronchial | 1 | 14–120 | [144] | ||||||
| Pig | Gastrointestinal | Gastric | 0.01–100 | 0.13–63 | 3.3–34 | 3.2–32 | 0.63–13 | 11–22 | [103, 195] |
| Small Intestinal | 0.01–100 | 0.063–5.0 | 0.19–12 | 0.18–10 | 0.050–6.3 | 16–32 | [103, 148] | ||
| Colonic | 0.01–100 | 0.16–1000 | 64–160 | 63–160 | 10–16 | 6–9 | [148] | ||
| Rat | Respiratory | Nasal | 1–100 | 0.0012–4.4 | 0.45–11 | 0.44–8.2 | 0.062–8.4 | 8–60 | [151–154] |
| Tracheal | 1–100 | 0.0050–2.8 | 1.6–9.1 | 1.5–8.2 | 0.17–5.8 | 6–43 | [153] | ||
| Horse | Respiratory | Tracheal | 10 | 0.61–1.2 | 19–36 | 18–34 | 6.1–12 | 19–20 | [137] |
| Rabbit | Respiratory | Tracheal | 1 | 35–130 | [140] | ||||
| Ferret | Respiratory | Tracheal | 1 | 12–110 | [140] | ||||
η″ = apparent viscosity; G* = complex shear modulus; G′ = elastic modulus; G″ = viscous modulus; δ = phase angle. Where possible, variables were calculated from reported values according to the following equations: ;; G' = G* cos δ; G" = G* cos δ = η" ω; and . Ranges are provided based on reported mean ± SD values and figures in the referenced studies.
Rheological studies of canine mucus have largely been focused on respiratory mucus, since the dog lung is similar to the human lung in anatomy and overall size. Indeed, based on a review by Tomkiewicz et al. [140], dog tracheal mucus showed the most similar rigidity (log G*) to human mucus out of four animal models (dog, ferret, rabbit, and rat). Rheological characterization of canine lung mucus typically focuses on the effect of various stimulants, such as mucolytics [141–144], cholinergic agents [145], and anesthesia [146], which are correlated with mucociliary or cough clearance. Measured values may vary depending on the region of the respiratory tract sampled, but it is difficult to identify statistically significant differences due to the wide variability of reported values.
Pig mucus is a popular model for characterizing gastrointestinal tract mucus. Two rheologically distinct types of mucus have been identified in pig gastric mucus, a firmly adherent mucus that is resistant to shear and a loose, sloppy mucus that is shear-compliant [147]. The rheological properties of mucus vary throughout the entire gastrointestinal tract. Both pig gastric and colonic mucus are highly viscoelastic, while pig small intestinal mucus is a comparably weaker gel structure, with G′ and G″ profiles closer together in value. This reduced viscoelasticity was attributed to the presence of a large number of mucosal cells [148]. A limited number of studies have characterized the rheology of pig tracheal pouch mucus, which has a viscosity of 102–103 Pa-s at near zero shear rates [149, 150].
Mucus derived from the nares, lung, and gastrointestinal tract of rats are frequently used in a wide range of studies. Rat nasal mucus has been used to study the relationship between mucus rheology and mucociliary transport [151, 152], disease [153], and sex [154], but appears at least 10-fold less viscoelastic compared to human nasal mucus (Figure 4A and Table 1). At a shear stress of 50 Pa, the average apparent viscosity of rat gastric mucus was found to be 7800 Pa-s compared to 39 Pa-s for duodenal mucus [155]. For both types of mucus, the slope of log viscosity vs. log shear rate was in the range of −0.82 and −0.93, in agreement with the average slope of −0.85 for human mucus [46].
As is the case with human mucus, significant variations in measured values are often observed intersubject, intrasubject, and even within the same animal mucus sample [156] (see also Table 1). Due to the large variability in reported values, it is difficult to determine an appropriate animal model strictly based on macrorheological properties. Nevertheless, it is generally assumed that similarity in organ size is a good predictor of mucus physiology [140, 142]. Collection of larger volumes of mucus is also easier with larger animals.
6. Techniques to characterize rheology of mucus
The rheological characterization of mucus was described as early as 1924, when Szegvari measured the recoil of mucus extruded along an open capillary [108]. Since that time, a variety of rheological techniques have been developed and adapted to characterize mucus secretions from humans and animals.
6.1 Classical macro-rheological techniques
The cone & plate rheometer is the most commonly used instrument for sensitive characterization of biological liquids. The apparatus consists of suspending the mucus volume between a flat plate and a shallow, inverted cone. Under oscillation, the plate is rotated with known amplitude and shear rate, and the resulting torque and input strain can be used to calculate shear stress, phase angle, apparent viscosity, and viscous and elastic moduli. The cone & plate rheometer is particularly convenient for the analysis of non-Newtonian liquids such as mucus since the frequency and rate of shear can be varied over a wide range of values [157, 158]. The primary disadvantage of cone & plate rheometry is the relatively large volumes required, typically on the order of hundreds of microliters.
In addition to the cone & plate rheometer, a number of other biophysical techniques are also used to characterize the macrorheology of mucus. The first quantitative characterization of the physical properties of mucus was performed using a capillary viscometer [108], which can be used to determine steady state viscosity of mucus from simple measurements of fluid velocity at known applied pressures [157, 158]. The simplicity of the device, in addition to the small volume (∼20 µL) required, has led to its popular use in characterizing a variety of animal and human mucus [157, 159, 160]. Nevertheless, the technique does not afford rapid characterization over a range of shear rates, since typical capillary viscometers are limited to measuring viscosity for one shear rate at a time.
The magnetic microrheometer, pioneered by King and coworkers [161, 162], is an elegant technique for measuring rheological properties of small volumes of mucus. Consequently, magnetic microrheometry has primarily been used for the rheological analysis of respiratory mucus, in part because of the difficulty of obtaining large quantities of airway mucus. The apparatus involves the placement of a 50–150 µm diameter steel microsphere in mucus in a clear-bottomed container on a microscope stage. The displacements of the sphere, subjected to oscillation by the application of magnetic fields of varying strength, are monitored via high resolution video microscopy. The amplitude and phase lag of the displacement can be extracted from a plot of displacement vs. applied force to calculate the viscous and elastic moduli.
The primary advantage to the magnetic microrheometer is the small sample volume required, as little as 2–5 uL, since large volumes of mucus are difficult to obtain from mucosal tissues such as those in healthy lung airways and the eye. Nevertheless, there are a number of drawbacks associated with magnetic microrheometry, including the risk of rapid dehydration due to the very small sample size [8], which can greatly influence the rheological properties of mucus, as well as difficulty in applying the technique to pathologically heterogeneous samples [157]. Furthermore, the mucus sample must be clear or moderately opaque, and the technique may not be accurate in probing very high viscoelasticities [161]. King et al. suggested that the precision of the magnetic microrheometer is likely lower than that of other rheological devices, making it perhaps more appropriate for studying the effects of pharmacological intervention or disease on mucus rheology than for quantitative characterization of mucus [162]. Lastly, it is difficult to exert sufficient force on the steel microsphere to observe shear-thinning at the moderate to high shear rates that occur physiologically.
Another technique that has been widely used is the filancemeter, a device that measures spinnability (also known as Spinnbarkeit) of mucus. Spinnability, which quantifies the extent to which mucus can be drawn into threads under traction, is correlated with the fluid’s elasticity. Typically, a small mucus volume (10–30 µL) is stretched at a rate of 1 cm/s until the mucus thread is broken, and spinnability is defined as the thread length at the moment of rupture [155, 163]. Although spinnability has been primarily used to evaluate properties of cervical mucus throughout the menstrual cycle, it has also been used to characterize other mucus fluids such as respiratory and nasal mucus [32, 155, 163]. Spinnability may be a sensitive measure to changes in mucus structure [164] and is an excellent complement to the capillary viscometer for viscous and elastic characterization of mucus.
Particle tracking microrheology (PTM) can also be used to characterize the mechanical properties of complex fluids with the accuracy of traditional bulk-fluid rheological techniques, but with low volume requirements [165]. PTM is discussed in detail in the next section.
6.2 Characterization of microrheology via Particle Tracking Microheology (PTM)
The dynamic motions of different sized non-mucoadhesive particles in mucus, obtained by Multiple Particle Tracking (MPT), can be analyzed to extract the local viscous and elastic forces as a function of length scale. This method, called Particle tracking microrheology (PTM), was first described in the viscoelastic characterization of concentrated solutions of high molecular weight polyethylene oxide [166], DNA [167]and actin [168]. It has since been applied to study the biophysical properties of various complex biological environments, including the nucleoplasm [169] and cytoplasm of living cells [170–174], including in disease [175] and in vivo [176], and healthy and diseased synovial fluid [177]. PTM has also been used to characterize the micromechanical properties of solutions of biologically relevant polymers, such as reconstituted actin filaments [165, 174, 178, 179], DNA [180–182]and cardiac thin filaments [183].
The basis of PTM rests on the assumption that particle (or bead) transport in complex environments is controlled by the local properties of the material. Beads that are smaller than the effective mesh spacing of the network and, importantly, non-adherent experience the low viscosity of the interstitial suspending liquid. For example, globular proteins are small enough to diffuse rapidly and in a largely unrestricted manner in biological environments, such as mucus or cell cytoplasm. The motion of beads larger than the effective mesh spacing can rapidly become restricted. These beads are subjected to small random forces induced by the small spontaneous movements of surrounding macromolecules as well as solvent (e.g. water) molecules. In a purely viscous liquid (no elasticity), such as water or glycerol, the mean squared displacement of beads in suspension increases linearly with time. After a finite displacement, the probing beads lose all memory of their previous location and undergo classical random walks. In this case, the shear viscosity of the suspending liquid can be computed directly from the (constant) slope of the mean squared displacement using the Stokes-Einstein relation. , which directly relates the diffusion coefficient, D, of a bead with radius a to the viscosity of the liquid, η, for a given thermal energy, kBT. In contrast, each displacement of a bead in a highly elastic material is accompanied by a restoring opposite force that pushes the bead instantaneously back to its original position. Here the bead has a perfect memory of its previous location. The mean squared displacement of the bead is finite and constant. That constant, r02, is simply inversely proportional to the elasticity, G0, of the material: [184]. Finally, for complex materials, such as mucus or cell cytoplasm, the mean squared displacement of beads comparable to or larger than the mesh spacing may display a time-dependent profile (see more below) since these materials are both viscous and elastic.
The viscous (G″) and elastic (G′) moduli of complex fluids can be inferred from the extent by which the motion of embedded probe beads is restricted [182, 185]. The Laplace transform of the time-dependent mean squared displacement, 〈Δr2(s)〉, can be directly related to the viscoelastic spectrum of the fluid, G(s), by the equation , where s is the Laplace frequency. The elastic and viscous moduli can then be calculated as the real and imaginary parts, respectively, of the complex modulus G*(ω), which is the projection of G(s) in Fourier space. In the case of a viscous liquid, the elastic modulus is zero and the viscous modulus is proportional to the shear viscosity of the liquid, η, and the rate of deformation applied to the liquid, ω: G″ = ηω and G′ = 0. In the case of an elastic solid, such as rubber, the viscous modulus is zero and the elastic modulus is the elasticity of the solid, G0: G″ = 0 and G′ = G0. In the case of mucus or cytoplasm, G′ and G″ are both non-zero functions of ω: G″ = G″(ω) and G′ = G′(ω). If G′>G″, the material is a visco-elastic solid; if G″>G′, the material is a visco-elastic liquid.
An important feature of microviscosity is that the measured values are highly dependent on both the size of the probe particles as well as the time scale (Figure 3A). In nanoscopically heterogeneous environments such as mucus, a fraction of particles may move with Brownian or near-Brownian trajectories, indicating that they are localized to fluid regions (pores) of the sample. Assuming the particles are non-adhesive and sufficiently small to move freely in the interstitial fluid, their trajectories would represent the same Brownian motion expected from diffusion of particles in a homogenous fluid. In other words, the Stokes-Einstein relation, based on local viscosity, governs particle dynamics in this regime.
When the diameter of probe particles approaches the dimensions of the mesh spacings, particle transport is strongly affected by the mesh microstructure. In this regime of “mesoscopic” transport, the particle motion at early time scales may appear hindered or caged. However, at longer time scales, changes in the microstructure due to mucin polymer relaxation and untangling can lead to the appearance of diffusive motion, as a probe particle moves from one cage to the next. Particles undergoing such anomalous diffusion often display a biphasic diffusivity, which initially decreases with time scale and then approaches a constant value at long time scales, reflecting a mesoscopic diffusion coefficient.
By increasing the diameter of tracked particles to dimensions significantly larger than the average fluid pore size, it becomes possible to probe the bulk biophysical property of the fluid, which appears as a homogeneous solution to probe particles. In this macroscopic domain, PTM can be used to determine the bulk-fluid rheological properties with the accuracy of traditional techniques, such as strain-controlled cone and plate rheometry [182]. Since the average interfiber spacing in mucus can be more than 300 nm, macrorheological characterization via PTM is restricted to large probe particles. Readers are referred to articles on MPT and PTM for more detailed technical instructions on rheological characterization at the micro-, meso- and macro-scopic level [174, 178, 179, 186].
For PTM-based characterization of mucus, the development of muco-inert nanoprobes has been a primary challenge due to the highly adhesive nature of mucus. Indeed, adhesive interactions between conventional polymeric beads and mucus often lead to overestimates of the true mucus viscoelasticity at the relevant length scales (Figure 3B). This problem was resolved recently with the discovery that a low molecular weight polyethylene glycol coating can greatly reduce mucoadhesion [70]. Nevertheless, here are aspects to PTM that can be further improved for mucus characterization. First, PTM relies on measuring deviations from thermally-driven Brownian diffusion of non-interacting probes. Due to low thermal energy, these particles cannot probe macro- or microrheological properties under the application of high stress. The low stress condition may reduce the accuracy of elastic measurements of highly viscoelastic gels [187], a problem likely common to all microscopy-based rheological techniques. Second, there are also restrictions on the maximum viscosity that can be probed. Larger probe particles have a smaller sensitivity range and detection limit than smaller probe particles, since large beads undergo inherently smaller displacements. For example, the detection limit for viscosity achievable with 500 nm probe particles and a tracking resolution of 5 nm is roughly 10 Pa-s at a shear rate of 1 s−1, which may be inadequate for characterizing cystic fibrosis sputum with viscosities up to 70 Pa-s [69]. In addition, it may not be possible to probe very high or very low shear rates with 2D PTM due to limits on the temporal resolution or duration of tracking, respectively. For microrheological characterization of low viscosity fluid channels, the rapid transport by probe particles out of the focal plane limits the rheological characterization at low shear rates. These problems are expected to be resolvable with improvements in microscopy equipment that can afford greater temporal resolution and/or prolonged 3D tracking.
6.3 Characterization of microrheology by other microscopy techniques
In addition to PTM, the microrheological properties of mucus has been probed using alternative microscopy techniques, including fluorescence recovery after photobleaching (FRAP) and fluorescence imaging of concentration profiles (FIP). Both methods rely on measuring changes in the concentration profiles of fluorescently labeled solutes or particles, followed by fitting to specific models that estimate diffusivity and the immobilized fraction. Among the first to do such work in the field, Saltzman and coworkers applied both FRAP and FIP to characterize the diffusion of various macromolecules in low viscosity channels within human cervical mucus [65]. Cone and coworkers similarly used FRAP to demonstrate the low viscosity nature of mucus fluids at length scales up to 55 nm [64]. FRAP has also been used for characterizing the diffusion of particles in cystic fibrosis sputum [71]. The ubiquitous use of FRAP to study mobility characteristics of molecules and particles in biological fluids have led to an increasing number of different FRAP models [71, 188–191].
An important distinction between FRAP and PTM is that FRAP provides only ensemble-averaged diffusion rates. In contrast, PTM captures information at the individual particle level that provides insight into the true local viscous and elastic contributions affecting their diffusion. In particular, PTM yields detailed characterization of variations of local microrheological properties, and may be less subject to errors from fluctuations in local particle concentrations. PTM also characterizes the fractions of anomalous diffusive particles that are hindered at early time scales but regain diffusive motion at longer time scales, measurements that are related to the relaxation times of the mucus mesh matrix. Nevertheless, FRAP may be more useful for investigating mucus transport in cases where prolonged tracking in a 2D focal plane is impossible, either due to rapid photobleaching or rapid particle movement in and out of the focal plane. Further improvements in microscopy for 3D MPT may alleviate some of the current shortcomings.
5. Current limitations on the rheological characterization of mucus
Beyond the limitations inherent to specific rheological measurement techniques, current rheological characterization of mucus is largely hampered by difficulty in obtaining high quality native physiological mucus in sufficient volumes. With the exception of cervicovaginal mucus, there is no simple method of collecting normal mucus, e.g., from the lungs or the gastrointestinal tract, in sufficient quantities to make repeated or even single measurements. This is particularly problematic in the case of respiratory mucus, where any expectorate may be contaminated with saliva or represent a pathological condition and possibly overproduction of mucus. Thus, materials obtained by cough are expected to be quite different in structure and properties from healthy mucus secretions. Normal mucus in the lungs of animals or man are typically collected either via a bronchoscopy brush or endotracheal tube techniques [78, 114, 162, 192, 193]. These methods are not only limited by the small volume collected, but also may exert mechanical stimulation that can induce water secretion and hence considerably alter the rheological properties of bronchial secretions. Finally, it is also widely recognized that in vitro studies of lung mucus may give a true picture of in vivo conditions [79] as only small mucus samples can be obtained from normal healthy mucosa and the proportion derived from the ‘gel’ layer or from the periciliary layer cannot be determined.
6. Conclusion
The dynamic and selective barrier and lubricant properties of mucus are intimately related to its viscoelasticity, which changes as a function of stress amplitude, shear rate, environmental stimuli, and length scale. The macrorheological characterization of human and animal mucus, often using well-established rheological methods, has contributed greatly to our understanding physiological processes such as mucociliary clearance and sexual reproduction, as well as the pathology of diseases affecting mucosal surfaces. Recent advances in light microscopy microrheological techniques and the development of non-mucoadhesive probes have made possible the rheological characterization of mucus at the micro-and nano- length scales. Together, the rheological characterization of mucus across vastly different length scales is expected to contribute critical insight into mucus function, pathogen transmission, and the development of nanoparticle therapeutics as well as prophylactic strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chilvers MA, O'Callaghan C. Local mucociliary defence mechanisms. Paediatr Respir Rev. 2000;1:27–34. doi: 10.1053/prrv.2000.0009. [DOI] [PubMed] [Google Scholar]
- 3.McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strombeck DR, Harrold D. Binding of cholera toxin to mucins and inhibition by gastric mucin. Infect Immun. 1974;10:1266–1272. doi: 10.1128/iai.10.6.1266-1272.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreyling WG, Semmler M, Moller W. Dosimetry and toxicology of ultrafine particles. J Aerosol Med. 2004;17:140–152. doi: 10.1089/0894268041457147. [DOI] [PubMed] [Google Scholar]
- 6.Cone R. Mucus. In: Michael WS, Lamm E, McGhee Jerry R, Mayer Lloyd, Mestecky Jiri, Bienenstock John, editors. Mucosal Immunlogy. San Diego: Academic Press; 1999. pp. 43–64. [Google Scholar]
- 7.Florey H. Mucin and the protection of the body. Proc R Soc Lond B Biol Sci. 1955;143:147–158. doi: 10.1098/rspb.1955.0001. [DOI] [PubMed] [Google Scholar]
- 8.Girod S, Zahm JM, Plotkowski C, Beck G, Puchelle E. Role of the physiochemical properties of mucus in the protection of the respiratory epithelium. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 1992;5:477–487. [PubMed] [Google Scholar]
- 9.Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35:20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slomiany BL, Slomiany A. Role of mucus in gastric mucosal protection. J Physiol Pharmacol. 1991;42:147–161. [PubMed] [Google Scholar]
- 11.Dulfano MJ, Adler K, Philippoff W. Sputum viscoelasticity in chronic bronchitis. The American Review of Respiratory Disease. 1971;104:88–98. doi: 10.1164/arrd.1971.104.1.88. [DOI] [PubMed] [Google Scholar]
- 12.Galabert C, Jacquot J, Zahm JM, Puchelle E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clinica Chimica Acta; International Journal of Clinical Chemistry. 1987;164:139–149. doi: 10.1016/0009-8981(87)90065-9. [DOI] [PubMed] [Google Scholar]
- 13.Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic Fibrosis Sputum: A Barrier to the Transport of Nanospheres. American Journal of Respiratory and Critical Care Medicine. 2000;162:1905–1911. doi: 10.1164/ajrccm.162.5.9909009. [DOI] [PubMed] [Google Scholar]
- 14.Dawson M, Wirtz D, Hanes J. Enhanced Viscoelasticity of Human Cystic Fibrotic Sputum Correlates with Increasing Microheterogeneity in Particle Transport. Journal of Biological Chemistry. 2003;278:50393–50401. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- 15.Olmsted SS, Meyn LA, Rohan LC, Hillier SL. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sexually Transmitted Diseases. 2003;30:257–261. doi: 10.1097/00007435-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Tuteja A, Mackay ME, Narayanan S, Asokan S, Wong MS. Breakdown of the continuum stokes-einstein relation for nanoparticle diffusion. Nano Lett. 2007;7:1276–1281. doi: 10.1021/nl070192x. [DOI] [PubMed] [Google Scholar]
- 17.Carlstedt I, Sheehan JK. Structure and macromolecular properties of cervical mucus glycoproteins. Symp Soc Exp Biol. 1989;43:289–316. [PubMed] [Google Scholar]
- 18.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- 19.Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28:5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- 20.Carlstedt I, Sheehan JK. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Foundation Symposium. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- 21.Lamblin G, Lhermitte M, Klein A, Houdret N, Scharfman A, Ramphal R, Roussel P. The carbohydrate diversity of human respiratory mucins: a protection of the underlying mucosa? The American Review of Respiratory Disease. 1991;144:S19–S24. doi: 10.1164/ajrccm/144.3_pt_2.S19. [DOI] [PubMed] [Google Scholar]
- 22.Masson PL, Heremans LF. Sputum proteins. In: Dulfano MF, editor. Fundamentals and Clinical Pathology. Springfield: Charles C. Thomas; 1973. pp. 412–474. [Google Scholar]
- 23.Puchelle E, Zahm JM, Havez R. Biochemical and rheological data in sputum. 3. Relationship between the biochemical constituents and the rheological properties of sputum. Bulletin de physio-pathologie respiratoire. 1973;9:237–257. [PubMed] [Google Scholar]
- 24.Markesich DC, Anand BS, Lew GM, Graham DY. Helicobacter pylori infection does not reduce the viscosity of human gastric mucus gel. Gut. 1995;36:327–329. doi: 10.1136/gut.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollerstrom N, Lord PW, Whimster WF. A difference in the composition of bronchial mucus between smokers and non-smokers. Thorax. 1977;32:155–159. doi: 10.1136/thx.32.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Vidriero MT, Reid L. Bronchial mucus in health and disease. Br Med Bull. 1978;34:63–74. doi: 10.1093/oxfordjournals.bmb.a071461. [DOI] [PubMed] [Google Scholar]
- 27.Roberton AM, Wiggins R, Horner PJ, Greenwood R, Crowley T, Fernandes A, Berry M, Corfield AP. A novel bacterial mucinase, glycosulfatase, is associated with bacterial vaginosis. J Clin Microbiol. 2005;43:5504–5508. doi: 10.1128/JCM.43.11.5504-5508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 1992;30:663–666. doi: 10.1128/jcm.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol. Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 30.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. The Biochemical Journal. 1983;211:13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao C-CW, Butala SM, Herp A. Studies on the isolation and composition of human ocular mucin. Experimental Eye Research. 1988;47:185–196. doi: 10.1016/0014-4835(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 32.Quraishi MS, Jones NS, Mason J. The rheology of nasal mucus: a review. Clinical otolaryngology and allied sciences. 1998;23:403–413. doi: 10.1046/j.1365-2273.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- 33.Samet JM, Cheng PW. The role of airway mucus in pulmonary toxicology. Environmental Health Perspectives. 1994;102:89–103. doi: 10.1289/ehp.9410289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao C-CW, Vergnes J-P, Brown SI. Fractionation and partial characterization of macromolecular components from human ocular mucus. Experimental Eye Research. 1983;36:139–150. doi: 10.1016/0014-4835(83)90097-0. [DOI] [PubMed] [Google Scholar]
- 35.Engel E, Guth PH, Nishizaki Y, Kaunitz JD. Barrier function of the gastric mucus gel. Am J Physiol Gastrointest Liver Physiol. 1995;269:G994–G999. doi: 10.1152/ajpgi.1995.269.6.G994. [DOI] [PubMed] [Google Scholar]
- 36.Gipson IK. Mucins of the human endocervix. Frontiers in bioscience: a journal and virtual library. 2001;6:1245–1255. doi: 10.2741/gipson. [DOI] [PubMed] [Google Scholar]
- 37.Wolf DP, Blasco L, Khan MA, Litt M. Human cervical mucus. I. Rheologic characteristics. Fertility and Sterility. 1977;28:41–46. [PubMed] [Google Scholar]
- 38.Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl-and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boucher RC, Jr, Bromberg PA, Gatzy JT. Airway transepithelial electric potential in vivo: species and regional differences. J Appl Physiol. 1980;48:169–176. doi: 10.1152/jappl.1980.48.1.169. [DOI] [PubMed] [Google Scholar]
- 40.Boucher RC, Stutts MJ, Bromberg PA, Gatzy JT. Regional differences in airway surface liquid composition. J Appl Physiol. 1981;50:613–620. doi: 10.1152/jappl.1981.50.3.613. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson I, Nadel JA. Movement of electrolytes and fluid across airways. Lung. 1984;162:125–137. doi: 10.1007/BF02715640. [DOI] [PubMed] [Google Scholar]
- 42.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 43.Dekker JWA, Rossen J, Buller AWC, Einerhand HA. The MUC family: an obituary. Trends in Biochemical Sciences. 2002;27:126–131. doi: 10.1016/s0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- 44.Thornton DJ, Carlstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J. 1996;316(Pt 3):967–975. doi: 10.1042/bj3160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Klinken BJ, Dekker J, Buller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 46.Yeates DB, Besseris GJ, Wong LB. Physicochemical Properties of Mucus and Its Propulsion. In: Crystal RG, West JB, et al., editors. THE LUNG: Scientific Foundations. Philadelphia: Lippincott - Raven Publishers; 1997. pp. [Google Scholar]
- 47.Meyer FA, Silberberg A. The rheology and molecular organization of epithelial mucus. Biorheology. 1980;17:163–168. doi: 10.3233/bir-1980-171-217. [DOI] [PubMed] [Google Scholar]
- 48.Matthews LW, Spector S, Lemm J, Potter JL. Studies on Pulmonary Secretions. I. the over-All Chemical Composition of Pulmonary Secretions from Patients with Cystic Fibrosis, Bronchiectasis, and Laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 49.Potter JL, Matthews LW, Spector S, Lemm J. Studies on pulmonary secretions. II. Osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. The American Review of Respiratory Disease. 1967;96:83–87. doi: 10.1164/arrd.1967.96.1.83. [DOI] [PubMed] [Google Scholar]
- 50.Boat TF, Cheng PW, Leigh MW. Biochemistry of mucus. In: Takishima T SS, editor. Airway Secretion: Lung Biology in Health and Disease. New York: Dekker; 1994. pp. 217–282. [Google Scholar]
- 51.Lethem MI, James SL, Marriott C. The role of mucous glycoproteins in the rheologic properties of cystic fibrosis sputum. The American Review of Respiratory Disease. 1990;142:1053–1058. doi: 10.1164/ajrccm/142.5.1053. [DOI] [PubMed] [Google Scholar]
- 52.Mrsny RJ, Daugherty AL, Short SM, Widmer R, Siegel MW, Keller GA. Distribution of DNA and alginate in purulent cystic fibrosis sputum: implications to pulmonary targeting strategies. J Drug Target. 1996;4:233–243. doi: 10.3109/10611869608995625. [DOI] [PubMed] [Google Scholar]
- 53.Nadziejko CE, Slomiany BL, Slomiany A. Most of the lipid in purulent sputum is bound to mucus glycoprotein. Exp Lung Res. 1993;19:671–684. doi: 10.3109/01902149309064364. [DOI] [PubMed] [Google Scholar]
- 54.Slomiany BL, Tsukada H, Slomiany A. Cotranslational attachment of fatty acids to nascent peptides in gastric mucus glycoprotein. Biochem Biophys Res Commun. 1986;141:387–393. doi: 10.1016/s0006-291x(86)80381-3. [DOI] [PubMed] [Google Scholar]
- 55.Widdicombe JG. Role of lipids in airway function. European Journal of Respiratory Diseases. 1987;Supplement. 153:197–204. [PubMed] [Google Scholar]
- 56.Murty VL, Sarosiek J, Slomiany A, Slomiany BL. Effect of lipids and proteins on the viscosity of gastric mucus glycoprotein. Biochem Biophys Res Commun. 1984;121:521–529. doi: 10.1016/0006-291x(84)90213-4. [DOI] [PubMed] [Google Scholar]
- 57.Girod S, Galabert C, Lecuire A, Zahm JM, Puchelle E. Phospholipid composition and surface-active properties of tracheobronchial secretions from patients with cystic fibrosis and chronic obstructive pulmonary diseases. Pediatr Pulmonol. 1992;13:22–27. doi: 10.1002/ppul.1950130107. [DOI] [PubMed] [Google Scholar]
- 58.Crowther RS, Marriott C, James SL. Cation induced changes in the rheological properties of purified mucus glycoprotein gels. Biorheology. 1984;21:253–263. doi: 10.3233/bir-1984-211-227. [DOI] [PubMed] [Google Scholar]
- 59.Steiner CA, Litt M, Nossal R. Effect of Ca++ on the structure and rheology of canine tracheal mucin. Biorheology. 1984;21:235–252. doi: 10.3233/bir-1984-211-226. [DOI] [PubMed] [Google Scholar]
- 60.Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. Journal of Biological Chemistry. 2003;278:28703–28710. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- 61.Lamont JT. Mucus: the front line of intestinal mucosal defense. Ann N Y Acad Sci. 1992;664:190–201. doi: 10.1111/j.1749-6632.1992.tb39760.x. [DOI] [PubMed] [Google Scholar]
- 62.Puchelle E, Jacquot J, Beck G, Zahm JM, Galabert C. Rheological and transport properties of airway secretions in cystic fibrosis–relationships with the degree of infection and severity of the disease. European Journal of Clinical Investigation. 1985;15:389–394. doi: 10.1111/j.1365-2362.1985.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 63.Harbitz O, Jenssen AO, Smidsrød O. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. European Journal of Respiratory Diseases. 1984;65:512–520. [PubMed] [Google Scholar]
- 64.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical Journal. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody Diffusion in Human Cervical-Mucus. Biophysical Journal. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jager S, Kremer J, Kuiken J, Mulder I. The significance of the Fc part of antispermatozoal antibodies for the shaking phenomenon in the sperm-cervical mucus contact test. Fertility and Sterility. 1981;36:792–797. doi: 10.1016/s0015-0282(16)45927-3. [DOI] [PubMed] [Google Scholar]
- 67.Kremer J, Jager S. The sperm-cervical mucus contact test: a preliminary report. Fertility and Sterility. 1976;27:335–340. doi: 10.1016/s0015-0282(16)41726-7. [DOI] [PubMed] [Google Scholar]
- 68.Saxton MJ. Anomalous diffusion due to obstacles: a Monte Carlo study. Biophys J. 1994;66:394–401. doi: 10.1016/s0006-3495(94)80789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson M, Wirtz D, Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. Journal of Biological Chemistry. 2003;278:50393–50401. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- 70.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braeckmans K, Peeters L, Sanders NN, De Smedt SC, Demeester J. Three-Dimensional Fluorescence Recovery after Photobleaching with the Confocal Scanning Laser Microscope. Biophysical Journal. 2003;85:2240–2252. doi: 10.1016/s0006-3495(03)74649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162:1905–1911. doi: 10.1164/ajrccm.162.5.9909009. [DOI] [PubMed] [Google Scholar]
- 73.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev Accepted. 2008 doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev Accepted. 2008 doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubin BK, Ramirez O, Zayas JG, Finegan B, King M. Collection and analysis of respiratory mucus from subjects without lung disease. The American Review of Respiratory Disease. 1990;141:1040–1043. doi: 10.1164/ajrccm/141.4_Pt_1.1040. [DOI] [PubMed] [Google Scholar]
- 76.Man SF, Adams GKr, Proctor DF. Effects of temperature, relative humidity, and mode of breathing on canine airway secretions. Journal of applied physiology: respiratory, environmental and exercise physiology. 1979;46:205–210. doi: 10.1152/jappl.1979.46.2.205. [DOI] [PubMed] [Google Scholar]
- 77.Reasor MJ, Adams GKr, Proctor DF, Rubin RJ. Tracheobronchial secretions collected from intact dogs. I. Protein and mucous glycoprotein composition. Journal of applied physiology: respiratory, environmental and exercise physiology. 1978;45:182–189. doi: 10.1152/jappl.1978.45.2.182. [DOI] [PubMed] [Google Scholar]
- 78.Jeanneret-Grosjean A, King M, Michoud MC, Liote H, Amyot R. Sampling technique and rheology of human tracheobronchial mucus. The American Review of Respiratory Disease. 1988;137:707–710. doi: 10.1164/ajrccm/137.3.707. [DOI] [PubMed] [Google Scholar]
- 79.Puchelle E, Zahm JM, Quemada D. Rheological properties controlling mucociliary frequency and respiratory mucus transport. Biorheology. 1987;24:557–563. doi: 10.3233/bir-1987-24606. [DOI] [PubMed] [Google Scholar]
- 80.App EM, Zayas JG, King M. Rheology of mucus and transepithelial potential difference: small airways versus trachea. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 1993;6:67–75. [PubMed] [Google Scholar]
- 81.Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatr Respir Rev. 2007;8:4–7. doi: 10.1016/j.prrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- 83.Davis SS, Dippy JE. The rheological properties of sputum. Biorheology. 1969;6:11–21. doi: 10.3233/bir-1969-6102. [DOI] [PubMed] [Google Scholar]
- 84.Lieberman J. Measurement of sputum viscosity in a cone-plate viscometer. I. Characteristics of sputum viscosity. The American Review of Respiratory Disease. 1968;97:654–661. doi: 10.1164/arrd.1968.97.4.654. [DOI] [PubMed] [Google Scholar]
- 85.Sturgess JM, Palfrey AJ, Reid L. The viscosity of bronchial secretion. Clinical Science. 1970;38:145–156. doi: 10.1042/cs0380145. [DOI] [PubMed] [Google Scholar]
- 86.Puchelle E, Zahm JM, Duvivier C, Didelon J, Jacquot J, Quemada D. Elasto-thixotropic properties of bronchial mucus and polymer analogs. I. Experimental results. Biorheology. 1985;22:415–423. doi: 10.3233/bir-1985-22505. [DOI] [PubMed] [Google Scholar]
- 87.Banerjee R, Bellare J, Puniyani R. Effect of Phospholipid Mixtures and Surfactant Formulations on Rheology of Polymeric Gels, Simulating Mucus, at Shear Rates Experienced in the Tracheobronchial Tree. Biochem Eng J. 2001;7:195–200. [Google Scholar]
- 88.Rubin BK, Druce H, Ramirez OE, Palmer R. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am J Respir Crit Care Med. 1997;155:2018–2023. doi: 10.1164/ajrccm.155.6.9196110. [DOI] [PubMed] [Google Scholar]
- 89.Hattori M, Majima Y, Ukai K, Sakakura Y. Effects of nasal allergen challenge on dynamic viscoelasticity of nasal mucus. Ann Otol Rhinol Laryngol. 1993;102:314–317. doi: 10.1177/000348949310200412. [DOI] [PubMed] [Google Scholar]
- 90.Majima Y, Harada T, Shimizu T, Takeuchi K, Sakakura Y, Yasuoka S, Yoshinaga S. Effect of biochemical components on rheologic properties of nasal mucus in chronic sinusitis. Am J Respir Crit Care Med. 1999;160:421–426. doi: 10.1164/ajrccm.160.2.9805117. [DOI] [PubMed] [Google Scholar]
- 91.Majima Y, Hirata K, Takeuchi K, Hattori M, Sakakura Y. Effects of orally administered drugs on dynamic viscoelasticity of human nasal mucus. Am Rev Respir Dis. 1990;141:79–83. doi: 10.1164/ajrccm/141.1.79. [DOI] [PubMed] [Google Scholar]
- 92.Majima Y, Inagaki M, Hirata K, Takeuchi K, Morishita A, Sakakura Y. The effect of an orally administered proteolytic enzyme on the elasticity and viscosity of nasal mucus. Arch Otorhinolaryngol. 1988;244:355–359. doi: 10.1007/BF00497464. [DOI] [PubMed] [Google Scholar]
- 93.Atsuta S, Majima Y. Nasal mucociliary clearance of chronic sinusitis in relation to rheological properties of nasal mucus. Ann Otol Rhinol Laryngol. 1998;107:47–51. doi: 10.1177/000348949810700109. [DOI] [PubMed] [Google Scholar]
- 94.Allen A, Cunliffe WJ, Pearson JP, Sellers LA, Ward R. Studies on gastrointestinal mucus. Scandinavian journal of gastroenterology. 1984;Supplement. 93:101–113. [PubMed] [Google Scholar]
- 95.Fahim RE, Forstner GG, Forstner JF. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochemical Journal. 1983;209:117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gold DV, Miller F. Characterization of human colonic mucoprotein antigen. Immunochemistry. 1974;11:369–375. doi: 10.1016/0019-2791(74)90190-6. [DOI] [PubMed] [Google Scholar]
- 97.Pearson J, Allen A, Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980;78:709–715. [PubMed] [Google Scholar]
- 98.Schrager J, Oates MD. The isolation and partial characterization of a glycoprotein isolated from human gastric aspirates and from extracts of gastric mucosae. Biochimica et Biophysica Acta. 1974;372:183–195. doi: 10.1016/0304-4165(74)90086-5. [DOI] [PubMed] [Google Scholar]
- 99.Waldron-Edward D, Skoryna SC. Studies on human gastric gel mucin. Isolation and characterization of a major glycoprotein component. Gastroenterology. 1970;59:671–682. [PubMed] [Google Scholar]
- 100.Bell A, Allen A, Morris E, Ross-Murphy S. Functional interactions of gastric mucus glycoprotein. International Journal of Biological Macromolecules. 1984;6:309–315. [Google Scholar]
- 101.Bell AE, Sellers LA, Allen A, Cunliffe WJ, Morris ER, Ross-Murphy SB. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985;88:269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- 102.Sellers LA, Allen A. Gastrointestinal mucus gel rheology. Symposia of the Society for Experimental Biology. 1989;43:65–71. [PubMed] [Google Scholar]
- 103.Sellers LA, Allen A, Morris ER, Ross-Murphy SB. Mechanical characterization and properties of gastrointestinal mucus gel. Biorheology. 1987;24:615–623. doi: 10.3233/bir-1987-24614. [DOI] [PubMed] [Google Scholar]
- 104.Sellers LA, Allen A, Morris ER, Ross-Murphy SB. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydrate Research. 1988;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- 105.Curt JR, Pringle R. Viscosity of gastric mucus in duodenal ulceration. Gut. 1969;10:931–934. doi: 10.1136/gut.10.11.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boskey ER, Moench TR, Hees PS, Cone RA. A Self-Sampling Method to Obtain Large Volumes of Undiluted Cervicovaginal Secretions. Sexually Transmitted Diseases. 2003;30:107–109. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 107.Wolf DP, Sokoloski J, Khan MA, Litt M. Human cervical mucus. III. Isolation and characterization of rheologically active mucin. Fertility and Sterility. 1977;28:53–58. doi: 10.1016/s0015-0282(16)42317-4. [DOI] [PubMed] [Google Scholar]
- 108.Clift AF. Early studies on the rheology of cervical mucus. American Journal of Obstetrics and Gynecology. 1979;134:829–832. doi: 10.1016/0002-9378(79)90956-6. [DOI] [PubMed] [Google Scholar]
- 109.Elstein M. Functions and physical properties of mucus in the female genital tract. British Medical Bulletin. 1978;34:83–88. doi: 10.1093/oxfordjournals.bmb.a071464. [DOI] [PubMed] [Google Scholar]
- 110.Wolf DP, Blasco L, Khan MA, Litt M. Human cervical mucus. II. Changes in viscoelasticity during the ovulatory menstrual cycle. Fertility and Sterility. 1977;28:47–52. doi: 10.1016/s0015-0282(16)42316-2. [DOI] [PubMed] [Google Scholar]
- 111.Tiffany JM. The viscosity of human tears. International Ophthalmology. 1991;15:371–376. doi: 10.1007/BF00137947. [DOI] [PubMed] [Google Scholar]
- 112.King M. Physiology of mucus clearance. Paediatr Respir Rev. 2006;7 Suppl 1:S212–S214. doi: 10.1016/j.prrv.2006.04.199. [DOI] [PubMed] [Google Scholar]
- 113.Rubin BK, Ramirez O, Zayas JG, Finegan B, King M. Respiratory mucus from asymptomatic smokers is better hydrated and more easily cleared by mucociliary action. Am Rev Respir Dis. 1992;145:545–547. doi: 10.1164/ajrccm/145.3.545. [DOI] [PubMed] [Google Scholar]
- 114.Zayas JG, Man GC, King M. Tracheal mucus rheology in patients undergoing diagnostic bronchoscopy. Interrelations with smoking and cancer. The American Review of Respiratory Disease. 1990;141:1107–1113. doi: 10.1164/ajrccm/141.5_Pt_1.1107. [DOI] [PubMed] [Google Scholar]
- 115.Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod. 2004;19:889–892. doi: 10.1093/humrep/deh173. [DOI] [PubMed] [Google Scholar]
- 116.Gervais R, Dumur V, Letombe B, Larde A, Rigot JM, Roussel P, Lafitte JJ. Hypofertility with thick cervical mucus: another mild form of cystic fibrosis? Jama. 1996;276:1638. [PubMed] [Google Scholar]
- 117.Oppenheimer EA, Case AL, Esterly JR, Rothberg RM. Cervical mucus in cystic fibrosis: a possible cause of infertility. Am J Obstet Gynecol. 1970;108:673–674. doi: 10.1016/0002-9378(70)90254-1. [DOI] [PubMed] [Google Scholar]
- 118.Borenstein R, Apelman Z, Lancet M, Ben-Hur H, Hegesh E, Chen M. The clinical significance of rheometric measurement of cervical mucus properties. Gynecologic and Obstetric Investigation. 1982;14:32–38. doi: 10.1159/000299440. [DOI] [PubMed] [Google Scholar]
- 119.Dean M, Santis G. Heterogeneity in the severity of cystic fibrosis and the role of CFTR gene mutations. Hum Genet. 1994;93:364–368. doi: 10.1007/BF00201659. [DOI] [PubMed] [Google Scholar]
- 120.Dumur V, Gervais R, Rigot JM, Delomel-Vinner E, Decaestecker B, Lafitte JJ, Roussel P. Congenital bilateral absence of the vas deferens (CBAVD) and cystic fibrosis transmembrane regulator (CFTR): correlation between genotype and phenotype. Hum Genet. 1996;97:7–10. doi: 10.1007/BF00218824. [DOI] [PubMed] [Google Scholar]
- 121.Usselman MC, Cone RA. Rat sperm are mechanically immobilized in the caudal epididymis by “immobilin,” a high molecular weight glycoprotein. Biol Reprod. 1983;29:1241–1253. doi: 10.1095/biolreprod29.5.1241. [DOI] [PubMed] [Google Scholar]
- 122.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 123.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 124.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol. 2005;175:1090–1099. doi: 10.4049/jimmunol.175.2.1090. [DOI] [PubMed] [Google Scholar]
- 125.Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatr Respir Rev. 2007;8:24–29. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 126.Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in Vitro Reduction in Viscosity of Human Tracheobronchial Secretions by Acetylcysteine. Am Rev Respir Dis. 1964;90:721–729. doi: 10.1164/arrd.1964.90.5.721. [DOI] [PubMed] [Google Scholar]
- 127.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ulmer JS, Herzka A, Toy KJ, Baker DL, Dodge AH, Sinicropi D, Shak S, Lazarus RA. Engineering actin-resistant human DNase I for treatment of cystic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8225–8229. doi: 10.1073/pnas.93.16.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, De Backer W, Lankhorst I, Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 130.Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B Mucins Are Decreased in Cystic Fibrosis Airway Secretions. Am J Respir Cell Mol Biol. 2004;31:86–91. doi: 10.1165/rcmb.2003-0345OC. [DOI] [PubMed] [Google Scholar]
- 131.Sheils CA, Kas J, Travassos W, Allen PG, Janmey PA, Wohl ME, Stossel TP. Actin filaments mediate DNA fiber formation in chronic inflammatory airway disease. Am J Pathol. 1996;148:919–927. [PMC free article] [PubMed] [Google Scholar]
- 132.Vasconcellos CA, Allen PG, Wohl ME, Drazen JM, Janmey PA, Stossel TP. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994;263:969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- 133.Rubin BK, Kater AP, Goldstein AL. Thymosin beta4 sequesters actin in cystic fibrosis sputum and decreases sputum cohesivity in vitro. Chest. 2006;130:1433–1440. doi: 10.1378/chest.130.5.1433. [DOI] [PubMed] [Google Scholar]
- 134.Majima Y. Mucoactive medications and airway disease. Paediatr Respir Rev. 2002;3:104–109. doi: 10.1016/s1526-0550(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 135.Rubin BK. The pharmacologic approach to airway clearance: mucoactive agents. Paediatr Respir Rev. 2006;7 Suppl 1:S215–S219. doi: 10.1016/j.prrv.2006.04.198. [DOI] [PubMed] [Google Scholar]
- 136.Shak S. Aerosolized recombinant human DNase I for the treatment of cystic fibrosis. Chest. 1995;107:65S–70S. doi: 10.1378/chest.107.2_supplement.65s. [DOI] [PubMed] [Google Scholar]
- 137.Gerber V, King M, Schneider DA, Robinson NE. Tracheobronchial mucus viscoelasticity during environmental challenge in horses with recurrent airway obstruction. Equine Vet J. 2000;32:411–417. doi: 10.2746/042516400777591183. [DOI] [PubMed] [Google Scholar]
- 138.Tam PY, Katz DF, Berger SA. Non-linear viscoelastic properties of cervical mucus. Biorheology. 1980;17:465–478. [PubMed] [Google Scholar]
- 139.Leikauf GD, Ueki IF, Nadel JA. Autonomic regulation of viscoelasticity of cat tracheal gland secretions. J Appl Physiol. 1984;56:426–430. doi: 10.1152/jappl.1984.56.2.426. [DOI] [PubMed] [Google Scholar]
- 140.Tomkiewicz RP, Albers GM, De Sanctis GT, Ramirez OE, King M, Rubin BK. Species differences in the physical and transport properties of airway secretions. Can J Physiol Pharmacol. 1995;73:165–171. doi: 10.1139/y95-025. [DOI] [PubMed] [Google Scholar]
- 141.Khan MA, Wolf DP, Litt M. Effect of mucolytic agents on the rheological properties of tracheal mucus. Biochim Biophys Acta. 1976;444:369–373. doi: 10.1016/0304-4165(76)90380-9. [DOI] [PubMed] [Google Scholar]
- 142.Sudo E, Boyd WA, King M. Effects of dextran sulfate on tracheal mucociliary velocity in dogs. J Aerosol Med. 2000;13:87–96. doi: 10.1089/089426800418613. [DOI] [PubMed] [Google Scholar]
- 143.Tomkiewicz RP, App EM, Coffiner M, Fossion J, Maes P, King M. Mucolytic treatment with N-acetylcysteine L-lysinate metered dose inhaler in dogs: airway epithelial function changes. Eur Respir J. 1994;7:81–87. doi: 10.1183/09031936.94.07010081. [DOI] [PubMed] [Google Scholar]
- 144.Tomkiewicz RP, App EM, De Sanctis GT, Coffiner M, Maes P, Rubin BK, King M. A comparison of a new mucolytic N-acetylcysteine L-lysinate with N-acetylcysteine: airway epithelial function and mucus changes in dog. Pulm Pharmacol. 1995;8:259–265. doi: 10.1006/pulp.1995.1035. [DOI] [PubMed] [Google Scholar]
- 145.King M, Viires N. Effect of methacholine chloride on rheology and transport of canine tracheal mucus. J Appl Physiol. 1979;47:26–31. doi: 10.1152/jappl.1979.47.1.26. [DOI] [PubMed] [Google Scholar]
- 146.King M, Engel LA, Macklem PT. Effect of pentobarbital anesthesia on rheology and transport of canine tracheal mucus. J Appl Physiol. 1979;46:504–509. doi: 10.1152/jappl.1979.46.3.504. [DOI] [PubMed] [Google Scholar]
- 147.Taylor C, Allen A, Dettmar PW, Pearson JP. Two rheologically different gastric mucus secretions with different putative functions. Biochim Biophys Acta. 2004;1674:131–138. doi: 10.1016/j.bbagen.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 148.Sellers LA, Allen A, Morris ER, Ross-Murphy SB. The rheology of pig small intestinal and colonic mucus: weakening of gel structure by non-mucin components. Biochim Biophys Acta. 1991;1115:174–179. doi: 10.1016/0304-4165(91)90027-e. [DOI] [PubMed] [Google Scholar]
- 149.Davis SS, Cox A, Marriott C, Readman AS, Barrett-Bee K. A new mucotropic agent–in vitro and in vivo evaluation of 2-alpha-thenoylthiopropionylglycine (bronchoplus) Eur J Respir Dis. 1985;67:94–102. [PubMed] [Google Scholar]
- 150.Martin GP, Loveday BE, Marriott C. Bromhexine plus oxytetracycline: the effect of combined administration upon the rheological properties of mucus from the mini-pig. J Pharm Pharmacol. 1993;45:126–130. doi: 10.1111/j.2042-7158.1993.tb03696.x. [DOI] [PubMed] [Google Scholar]
- 151.Lorenzi G, Bohm GM, Guimaraes ET, Vaz MA, King M, Saldiva PH. Correlation between rheologic properties and in vitro ciliary transport of rat nasal mucus. Biorheology. 1992;29:433–440. doi: 10.3233/bir-1992-29406. [DOI] [PubMed] [Google Scholar]
- 152.Macchione M, King M, Lorenzi-Filho G, Guimaraes ET, Zin WA, Bohm GM, Saldiva PH. Rheological determinants of mucociliary transport in the nose of the rat. Respir Physiol. 1995;99:165–172. doi: 10.1016/0034-5687(94)00080-j. [DOI] [PubMed] [Google Scholar]
- 153.Saldiva PH, King M, Delmonte VL, Macchione M, Parada MA, Daliberto ML, Sakae RS, Criado PM, Silveira PL, Zin WA, et al. Respiratory alterations due to urban air pollution: an experimental study in rats. Environ Res. 1992;57:19–33. doi: 10.1016/s0013-9351(05)80016-7. [DOI] [PubMed] [Google Scholar]
- 154.Saldiva PH, Parada MA, Macchione M, Paiva PS, Guimaraes ET, Lorenzi G, Martins MA, Montes GS, Balbani AP, King M. Nasal mucus clearance in rats: differences with sex and phase of the oestrous cycle. J Appl Toxicol. 1995;15:289–295. doi: 10.1002/jat.2550150410. [DOI] [PubMed] [Google Scholar]
- 155.Zahm JM, Pierrot D, Fuchey C, Levrier J, Duval D, Lloyd KG, Puchelle E. Comparative rheological profile of rat gastric and duodenal gel mucus. Biorheology. 1989;26:813–822. doi: 10.3233/bir-1989-26412. [DOI] [PubMed] [Google Scholar]
- 156.Tippe A, Korbel R, Ziesenis A, Heyder J. Viscoelastic properties of canine tracheal mucus: effects of structural inhomogeneities and storage. Scand J Clin Lab Invest. 1998;58:259–264. doi: 10.1080/00365519850186652. [DOI] [PubMed] [Google Scholar]
- 157.Kim CS, Berkley BB, Abraham WM, Wanner A. A micro double capillary method for rheologic measurements of lower airway secretions. Bulletin Européen de Physiopathologie Respiratoire. 1982;18:915–927. [PubMed] [Google Scholar]
- 158.Srivastava N, Burns MA. Analysis of Non-Newtonian Liquids Using a Microfluidic Capillary Viscometer. Anal. Chem. 2006;78:1690–1696. doi: 10.1021/ac0518046. [DOI] [PubMed] [Google Scholar]
- 159.Passàli D, Bellussi L, Lauriello M. The rheological characteristics of nasal mucus in patients with rhinitis. European Archives of Oto-Rhino-Laryngology. 1995;252:348–352. doi: 10.1007/BF00178275. [DOI] [PubMed] [Google Scholar]
- 160.Patrick G, Stirling C. The Retention of Particles in Large Airways of the Respiratory Tract. Proceedings of the Royal Society of London. 1977;198:455–462. doi: 10.1098/rspb.1977.0109. [DOI] [PubMed] [Google Scholar]
- 161.King M. Magnetic microrheometer. In: Braga PC, Allegra L, editors. Methods in Bronchial Mucology. Raven Press, Ltd.; 1988. pp. 78–83. [Google Scholar]
- 162.King M, Macklem PT. Rheological properties of microliter quantities of normal mucus. Journal of applied physiology: respiratory, environmental and exercise physiology. 1977;42:797–802. doi: 10.1152/jappl.1977.42.6.797. [DOI] [PubMed] [Google Scholar]
- 163.King M. Experimental models for studying mucociliary clearance. Eur Respir J. 1998;11:222–228. doi: 10.1183/09031936.98.11010222. [DOI] [PubMed] [Google Scholar]
- 164.King M, Dasgupta B, Tomkiewicz Robert P, Brown Neil E. Rheology of Cystic Fibrosis Sputum after in vitro Treatment with Hypertonic Saline Alone and in Combination with Recombinant Human Deoxyribonuclease I. Am. J. Respir. Crit. Care Med. 1997;156:173–177. doi: 10.1164/ajrccm.156.1.9512074. [DOI] [PubMed] [Google Scholar]
- 165.Apgar J, Tseng Y, Fedorov E, Herwig MB, Almo SC, Wirtz D. Multiple-particle tracking measurements of heterogeneities in solutions of actin filaments and actin bundles. Biophysical Journal. 2000;79:1095–1106. doi: 10.1016/S0006-3495(00)76363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett. 1995;74:1250–1253. doi: 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- 167.Mason TG, Ganesan K, van Zanten JH, Wirtz D, Kuo SC. Particle Tracking Microrheology of Complex Fluids. Physical Review Letters. 1997;79:3282–3285. [Google Scholar]
- 168.Gittes F, Schnurr B, Olmsted PD, MacKintosh FC, Schmidt CF. Microscopic Viscoelasticity: Shear Moduli of Soft Materials Determined from Thermal Fluctuations. Physical Review Letters. 1997;79:3286–3289. [Google Scholar]
- 169.Tseng Y, Lee JS, Kole TP, Jiang I, Wirtz D. Micro-organization and visco-elasticity of the interphase nucleus revealed by particle nanotracking. J Cell Sci. 2004;117:2159–2167. doi: 10.1242/jcs.01073. [DOI] [PubMed] [Google Scholar]
- 170.Dangaria JH, Butler PJ. Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress. Am J Physiol Cell Physiol. 2007;293:C1568–C1575. doi: 10.1152/ajpcell.00193.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol Biol Cell. 2005;16:328–338. doi: 10.1091/mbc.E04-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rogers SS, Waigh TA, Lu JR. Intracellular microrheology of motile Amoeba proteus. Biophys J. 2008 doi: 10.1529/biophysj.107.123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci U S A. 2008;105:889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tseng Y, Kole TP, Wirtz D. Micromechanical Mapping of Live Cells by Multiple-Particle-Tracking Microrheology. Biophysical Journal. 2002;83:3162. doi: 10.1016/S0006-3495(02)75319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Daniels BR, Masi BC, Wirtz D. Probing single-cell micromechanics in vivo: the microrheology of C. elegans developing embryos. Biophys J. 2006;90:4712–4719. doi: 10.1529/biophysj.105.080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci U S A. 2007;104:6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc Natl Acad Sci U S A. 2004;101:9636–9641. doi: 10.1073/pnas.0308733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tseng Y, Wirtz D. Mechanics and Multiple-Particle Tracking Microheterogeneity of -Actinin-Cross-Linked. Actin Filament Networks Biophysical Journal. 2001;81:1643–1656. doi: 10.1016/S0006-3495(01)75818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goodman A, Tseng Y, Wirtz D. Effect of length, topology, and concentration on the microviscosity and microheterogeneity of DNA solutions. J Mol Biol. 2002;323:199–215. doi: 10.1016/s0022-2836(02)00893-8. [DOI] [PubMed] [Google Scholar]
- 181.Hasnain IA, Donald AM. Microrheological characterization of anisotropic materials. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73:031901. doi: 10.1103/PhysRevE.73.031901. [DOI] [PubMed] [Google Scholar]
- 182.Mason TG, Ganesan K, van Zanten JH, Wirtz D, Kuo SC. Particle Tracking Microrheology of Complex Fluids. Physical Review Letters. 1997;79:3282. [Google Scholar]
- 183.Tassieri M, Evans RM, Barbu-Tudoran L, Trinick J, Waigh TA. The self-assembly, elasticity, and dynamics of cardiac thin filaments. Biophys J. 2008;94:2170–2178. doi: 10.1529/biophysj.107.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rufener K, Palmer A, Xu J, Wirtz D. High-frequency dynamics and microrheology of macromolecular solutions probed by diffusing wave spectroscopy: the case of concentrated solutions of F-actin. Journal of Non-Newtonian Fluid Mechanics. 1999;82:303–314. [Google Scholar]
- 185.Mason TG. Estimating the viscoelastic moduli of complex fluids using the generalized Stokes-Einstein equation. Rheologica Acta. 2000;39:371–378. [Google Scholar]
- 186.Panorchan P, Lee JS, Kole TP, Tseng Y, Wirtz D. Microrheology and ROCK signaling of human endothelial cells embedded in a 3D matrix. Biophys J. 2006;91:3499–3507. doi: 10.1529/biophysj.106.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dawson M. Department of Chemical and Biomolecular Engineering. Baltimore, MD: Johns Hopkins University; 2005. Understanding and Overcoming Mucosal Barriers to Non-Viral Gene Delivery in the Cystic Fibrotic Lung; p. 242. [Google Scholar]
- 188.Gordon GW, Chazotte B, Wang XF, Herman B. Analysis of simulated and experimental fluorescence recovery after photobleaching. Data for two diffusing components. Biophysical Journal. 1995;68:766–778. doi: 10.1016/S0006-3495(95)80251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kubitscheck U, Wedekind P, Peters R. Three-dimensional diffusion measurements by scanning microphotolysis. Journal of Microscopy. 1998;192:126–138. [Google Scholar]
- 190.Lopez A, Dupou L, Altibelli A, Trotard J, Tocanne JF. Fluorescence recovery after photobleaching (FRAP) experiments under conditions of uniform disk illumination. Critical comparison of analytical solutions, and a new mathematical method for calculation of diffusion coefficient D. Biophysical Journal. 1988;53 doi: 10.1016/S0006-3495(88)83177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsay TT, Jacobson KA. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophysical Journal. 1991;60:360–368. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bossi R. Methods for collecting and measuring mucus in humans. In: Braga PC, Allegra L, editors. Methods in Bronchial Mucology. Raven Press; 1988. pp. 13–20. [Google Scholar]
- 193.Proctor DF, Aharonson EF, Reasor MJ, Bucklen KR. A method for collecting normal respiratory mucus. In: Puchelle E, editor. Rheology of bronchial secretions and respiratory functions. Paris: Masson; 1973. pp. 351–356. [PubMed] [Google Scholar]
- 194.App EM, King M. Tracheal mucus rheology and epithelial potential difference in two day old puppies. Biorheology. 1990;27:515–526. doi: 10.3233/bir-1990-273-430. [DOI] [PubMed] [Google Scholar]
- 195.Bell AE, Allen A, Morris E, Rees DA. Rheological studies on native pig gastric mucus gel. Adv Exp Med Biol. 1982;144:97–99. doi: 10.1007/978-1-4615-9254-9_11. [DOI] [PubMed] [Google Scholar]
- 196.Perez-Vilar J, Hill RL. The carboxyl-terminal 90 residues of porcine submaxillary mucin are sufficient for forming disulfide-bonded dimers. J Biol Chem. 1998;273:6982–6988. doi: 10.1074/jbc.273.12.6982. [DOI] [PubMed] [Google Scholar]
- 197.Perez-Vilar J, Eckhardt AE, DeLuca A, Hill RL. Porcine submaxillary mucin forms disulfide-linked multimers through its amino-terminal D-domains. J Biol Chem. 1998;273:14442–14449. doi: 10.1074/jbc.273.23.14442. [DOI] [PubMed] [Google Scholar]
- 198.Sheehan JK, Oates K, Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986;239:147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rubin BK, Ramirez O, Ohar JA. Iodinated glycerol has no effect on pulmonary function, symptom score, or sputum properties in patients with stable chronic bronchitis. Chest. 1996;109:348–352. doi: 10.1378/chest.109.2.348. [DOI] [PubMed] [Google Scholar]
- 200.Shah PL, Scott SF, Knight RA, Marriott C, Ranasinha C, Hodson ME. In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax. 1996;51:119–125. doi: 10.1136/thx.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Litt M, Khan MA, Wolf DP. Mucus rheology: relation to structure and function. Biorheology. 1976;13:37–48. doi: 10.3233/bir-1976-13106. [DOI] [PubMed] [Google Scholar]
- 202.Puchelle E, Zahm JM, Duvivier C. Spinability of bronchial mucus. Relationship with viscoelasticity and mucous transport properties. Biorheology. 1983;20:239–249. doi: 10.3233/bir-1983-20214. [DOI] [PubMed] [Google Scholar]