Abstract
Increasing evidence indicates that an important consequence of protein posttranslational modification (PTM) is the creation of a high affinity binding site for the selective interaction with a PTM-specific binding protein (BP). This PTM-mediated interaction is typically required for downstream signaling propagation and corresponding biological responses. Because the vast majority of mammalian proteins contain PTMs, there is an immediate need to discover and characterize previously undescribed PTMBPs. To this end, we developed and validated an innovative in vivo approach called mammalian tethered catalysis (MTeC). By using methylated histones and methyl-specific histone binding proteins as the proof-of-principle, we determined that the new MTeC approach can compliment existing in vitro binding methods, and can also provide unique in vivo insights into PTM-dependent interactions. For example, we confirmed previous in vitro findings that endogenous HP1 preferentially binds H3K9me3. However, in contrast to recent in vitro observations, MTeC revealed that the tandem tudor domain-containing proteins, JMJD2A and 53BP1, display no preferential H4K20 methyl-selectivity in vivo. Last, by using MTeC in an unbiased manner to identify H3K9 methyl-specific PTMBPs, we determined that endogenous G9a binds methylated H3K9 in vivo. Further use of MTeC to characterize this interaction revealed that G9a selectively binds H3K9me1 in vivo, but not H3K9me2, contrary to recent in vitro findings. Although this study focused solely on methylated histones, we demonstrate how the innovative MTeC approach could be used to identify and characterize any PTMBP that binds any PTM on any protein in vivo.
Keywords: 53BP1, G9a, histone, JMJD2A, methylation
Eukaryotic cells have developed intricate and distinct cellular signaling cascades to translate particular intra or extracellular stimuli into an appropriate biological response. These signaling pathways rely heavily on enzymes that create specific posttranslational modifications (PTMs) on certain proteins of the pathway. These PTMs, themselves, are typically required for signal propagation and the desired biological response, indicating that the PTM of proteins is a central component of most normal cellular programs. Recent advances in proteomics demonstrate that the vast majority of eukaryotic proteins are posttranslationally modified in vivo, presenting investigators with the formidable challenge of identifying the enzymes responsible for each PTM and, importantly, determining the biological significance of each PTM on each protein.
Increasing evidence indicates that one common outcome of protein PTM is the creation of a high affinity binding site for the selective interaction with a specific PTM-specific binding protein (BP). The interaction between the PTMBP and the modified protein is often a critical step for downstream signaling and the biological response. Based on these observations, many have attempted to discover and characterize PTMBPs by using various classic in vitro approaches. Although these methods are typically amenable to high throughput screens, they are also subject to numerous inherent limitations and because of these restrictions, have resulted in the identification of only a relatively small number of bona fide PTMBPs.
To overcome some of the limitations of in vitro approaches, a previously undescribed in vivo method called yeast tethered catalysis was developed (1). Briefly, an expressed fusion protein containing a target peptide sequence was tethered to an enzyme resulting in the constitutive PTM of the peptide and, thereby, served as the bait in yeast two-hybrid screens for putative PTMBPs. Although this technique was used successfully to identify yeast PTMBPs, the ability to detect PTMBPs in higher eukaryotes is constrained by the limitations of the yeast two-hybrid system.
By expanding on the principles of tethered catalysis, we have developed and validated a previously undescribed in vivo approach designed specifically for the discovery and characterization of endogenous PTMBPs in mammalian cells, which we termed mammalian tethered catalysis (MTeC). Methylated histones were chosen as the proof-of-principle for MTeC, because increasing evidence indicates that the major role of histone methylation is to bind distinct PTMBPs that, in turn, function to regulate a specific DNA-templated process such as transcription, replication, or repair (2). By testing various MTeC bait fusion proteins, we demonstrate that this approach can be consistently used to predictably modify MTeC bait fusion proteins in vivo. We also demonstrate that this new technique can compliment existing in vitro binding assays, and importantly, can provide previously undescribed in vivo insights into PTM-dependent interactions. Last, we show that MTeC was used in an unbiased manner to identify an H3K9me1-specific binding protein in vivo. Collectively, our findings indicate that MTeC could be used as a new tool to identify and characterize PTMBPs that bind any PTM on any protein in vivo.
Results
Principles and Implementation of MTeC.
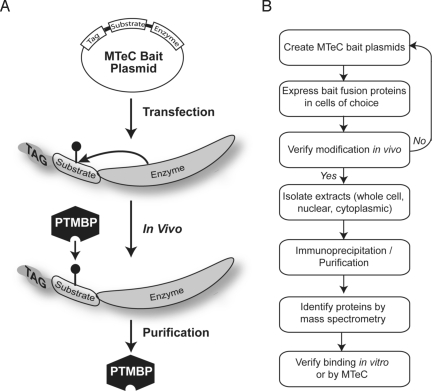
The overall goals of MTeC are to discover previously undescribed proteins, or validate those, that selectively bind to a target peptide sequence in vivo only when the sequence possesses a specific PTM. As shown in Fig. 1A, MTeC begins with the creation of a bait expression plasmid that includes, in tandem, an affinity epitope tag followed by a peptide sequence containing the target amino acid to be modified and, finally, the catalytic domain of an enzyme known to modify the target residue of interest within the peptide sequence. The MTeC bait plasmid is then introduced into the cells of choice where, once in the cell, the catalytic domain of the expressed fusion protein is able to selectively posttranslationally modify the target residue within the peptide sequence. The expressed MTeC fusion protein is then available to interact in vivo with proteins that bind this particular modified peptide sequence. The cellular proteins bound to the modified MTeC fusion protein can then be purified from cells by using standard biochemical techniques, including fractionation of specific subcellular compartments, affinity immunoprecipitation, and/or various other available chromatographic steps (Fig. 1B). Once this material is isolated, the purified proteins are analyzed by MS for identification of the bound proteins. Importantly, the experiments are performed in parallel with two critical controls. The first is an MTeC bait fusion protein lacking the catalytic domain of the enzyme, allowing subtraction of proteins that may bind both the modified and unmodified peptide sequence. The second control is an MTeC bait fusion protein where the modified target residue is eliminated and, therefore, unavailable to be posttranslationally modified, allowing subtraction of interacting proteins that may bind another portion of the peptide sequence and/or the catalytic domain of the tethered enzyme. Once the putative PTMBPs are identified, well-established in vitro binding methods can be used to confirm the modification-specific interaction and/or MTeC could also be used to verify these interactions in vivo.
Fig. 1.
Principles of MTeC. (A) An MTeC bait plasmid is composed of an affinity tag fused in tandem with a peptide sequence possessing an amino acid to be posttranslationally modified in vivo followed by the catalytic domain of an enzyme known to modify the residue. (B) The MTeC bait plasmids are expressed in the cells of choice and, after the MTeC bait fusion protein is confirmed to be properly modified in vivo, the PTMBPs are biochemically purified. After SDS/PAGE of the purified material, visible protein bands can be isolated and identified by MS, or alternatively, complete sample mixtures can be submitted for protein identification. MTeC can also be used to verify and characterize PTM-dependent binding in vivo.
MTeC Bait Fusion Proteins Achieve Specific Degrees of H3K9 Methylation in Vivo.
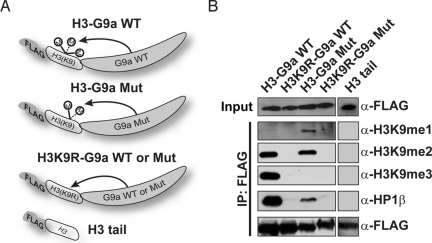
To test the ability of MTeC bait fusion proteins to acquire the appropriate PTM in vivo, we capitalized on recent discoveries and characterizations of enzymes that modify specific histone residues. In particular, we first focused on the well-described methylation of histone H3 lysine 9 (H3K9) by the G9a methyltransferase (3, 4). An MTeC bait plasmid containing a FLAG epitope and the first 44 aa of histone H3 were cloned upstream of the catalytic SET domain of the human G9a. Because we previously demonstrated that G9a is responsible for global dimethylation (H3K9me2) in mammalian cells, and, in vitro, has also been shown to cause trimethylation (H3K9me3), it was unclear which H3K9 methylated form would be observed in an H3-G9a WT MTeC bait fusion protein (Fig. 2A) (5, 6). To determine the methyl status of the H3-G9a WT construct, the bait plasmid was transfected into HEK 293 cells, and after a FLAG immunoprecipitation, Western blot analysis on the FLAG-bound material using a panel of H3K9 methyl-specific antibodies demonstrated that both H3K9me2 and H3K9me3 were clearly achieved; however, monomethylation (H3K9me1) was not detected (Fig. 2B). To create an MTeC fusion protein possessing H3K9me1, we capitalized on a previous report demonstrating that an F->Y mutation in the catalytic portion of the G9a SET domain caused an enzymatic shift in specificity toward H3K9me1 in vitro (7). Consistent with the in vitro results, we found that a G9a F->Y mutant MTeC bait fusion protein achieved H3K9me1 and H3K9me2 in vivo, but lacked detectable H3K9me3 (Fig. 2B). The MTeC bait fusion proteins containing only the H3 sequence or the H3K9R-G9a failed to achieve any detectable degree of methylation in vivo confirming the utility of these plasmids as negative controls in further experiments (Fig. 2B). Collectively, these findings demonstrate that, depending on the inherent catalytic property of the fused enzyme, MTeC can be used to selectively and predictably posttranslationally modify a target amino acid within a given peptide sequence in vivo.
Fig. 2.
H3-G9a MTeC bait fusion proteins attain specific degrees of methylation in vivo and selectively bind endogenous HP1β in a methyl-dependent manner. (A) MTeC bait fusion proteins containing a FLAG affinity tag fused to the first 44 aa of the H3 N-terminal tail (where K9 is WT or mutated to arginine) followed by the catalytic SET domain of WT G9a (WT) or a F->Y G9a mutant (Mut). (B) Western blot analysis of FLAG immunoprecipitated MTeC bait fusion proteins described above from HEK 293 nuclear lysates by using FLAG, HP1β, or H3K9 methyl-specific antibodies.
Endogenous Heterochromatin Protein (HP)1β Binds H3-G9a MTeC Bait Fusion Proteins in a Methylation-Dependent Manner.
Once we showed that the H3-G9a MTeC bait fusion proteins could achieve specific degrees of H3K9 methylation in vivo, it was next necessary to determine whether these fusion proteins could selectively interact with an endogenous protein known to bind H3K9 in a methylation-dependent manner. Previous in vitro binding studies showed that the chromodomain of HP1 binds H3K9me3 > H3K9me2 > H3K9me1 (8–11). Based on these findings, we predicted that endogenous HP1β would preferentially bind the H3-G9a WT MTeC bait fusion protein compared with the H3-G9a mutant when expressed in cells. To test this hypothesis, Western blot analysis was performed on the FLAG-bound immunoprecipitated material by using an HP1β antibody (Fig. 2B). As predicted, endogenous HP1β was clearly bound to the H3-G9a WT MTeC bait fusion protein possessing H3K9me3 and H3K9me2, but was absent in the H3K9R-G9a WT and H3 tail-only MTeC control fusion proteins. These findings confirm that HP1β selectively binds methylated H3K9 in vivo. Importantly, we determined that HP1β binding was detectable, but significantly reduced, in the H3-G9a mutant MTeC bait plasmid possessing H3K9me2 and H3K9me1, confirming recent studies that HP1β preferentially binds H3K9me3 in vivo (12, 13). These findings indicate that properly modified MTeC fusion proteins expressed in cells can be successfully used as bait for the binding, purification, and identification of endogenous PTMBPs.
Histone H4K20 MTeC Bait Fusion Proteins Are Differentially Methylated by Distinct Enzymes.
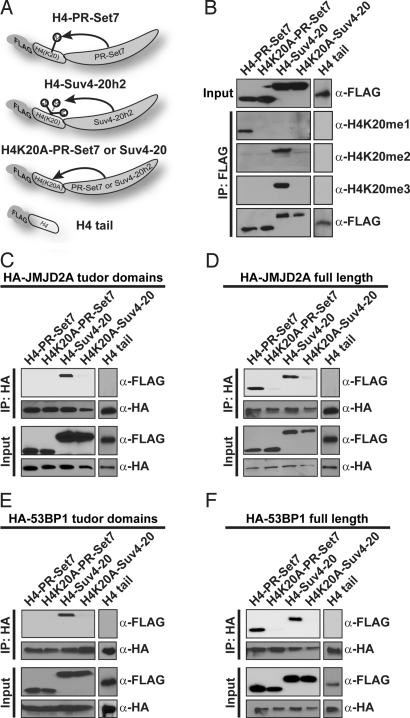
To validate that MTeC could be applied successfully for modified peptide substrates other than H3K9, we shifted our focus to the N-terminal tail of histone H4. It was previously reported that lysine 20 of histone H4 (H4K20) is selectively monomethylated (H4K20me1) by the PR-Set7/KMT5a enzyme, whereas the Suv4-20/KMT5b/KMT5c enzymes are responsible for di and trimethylation (H4K20me2 and H4K20me3) in mammals (14–16). Based on these findings, MTeC bait plasmids were created by using the FLAG affinity tag in tandem with the first 44 aa of H4 followed by the catalytic SET domain of either human PR-Set7 or Suv4-20h2 (Fig. 3A). The control plasmids for nonspecific binding included the H4 tail alone or where K20 was replaced by alanine. As described above, HEK 293 cells were transiently transfected with these MTeC plasmids, and a FLAG affinity immunoprecipitation was performed on their nuclear lysates. As predicted, Western blot analysis of the FLAG-bound material confirmed that H420me1 was selectively enriched in the H4-PR-Set7 MTeC fusion protein, but H4K20me2 and H4K20me3 were not detected (Fig. 3B). In contrast, H4K20me2 and H4K20me3 were selectively enriched in the H4-Suv4-20h2 MTeC fusion protein, but H4K20me1 was not detected. Importantly, H4K20 methylation was not detected in the H4 tail only or H4K20A mutant MTeC fusion proteins. Collectively, our findings demonstrate that selective degrees of methylation of a target residue within an MTeC fusion protein can be consistently achieved in vivo by employing the specific catalytic domains of different methyltransferases.
Fig. 3.
Distinct enzymes used in MTeC to vary the degree of H4K20 methylation demonstrate that full-length tandem tudor domain-containing proteins bind all three H4K20 methylated forms in vivo. (A) Schematic representation of MTeC bait fusion proteins. FLAG affinity tag followed by the first 44 aa of the WT H4 N-terminal tail (where K20 is WT or mutated to alanine) followed by the catalytic SET domain of PR-Set7 or Suv4-20h2. (B) Western blot analysis of the FLAG-immunoprecipitated MTeC bait fusion proteins from HEK 293 nuclear lysates by using FLAG or H4K20 methyl-specific antibodies. An HA-tagged JMJD2A tandem tudor domain only (C) or full-length (D) plasmid were cotransfected in HEK 293 cells with the histone H4 MTeC bait plasmids. Western blot analysis of the HA-immunoprecipitates from nuclear lysates were preformed by using FLAG or HA antibodies. Similar experiments were performed by using an HA-tagged 53BP1 tandem tudor domain only (E) of full-length plasmid (F).
Tandem Tudor Domain-Containing Proteins Do Not Exhibit Differential H4K20 Methyl-Selective Binding Properties in Vivo.
Increasing evidence indicates that the tandem tudor domain located within conserved chromatin-associated proteins has the ability to selectively bind methylated histone residues in vitro (17, 18). For example, the tandem tudor domain of the JMJD2A histone demethylase was recently found to bind H4K20me3 and H4K20me2 in vitro, but not H4K20me1 (17, 19). To confirm this interaction in vivo, a plasmid expressing only the tandem tudor domain of JMJD2A was cotransfected in HEK 293 cells with the various histone H4 MTeC plasmids. HA-immunoprecipitations of these nuclear lysates verified that the JMJD2A tandem tudor domain preferentially binds the H4-Suv4-20h2 MTeC bait fusion protein containing only H4K20me2 and H4K20me3 in vivo (Fig. 3C). Surprisingly, when a full-length JMJD2A expression plasmid was used in these experiments, JMJD2A also bound the H4-PR-Set7 MTeC bait fusion protein containing only H4K20me1 (Fig. 3D). These findings demonstrate that WT JMJD2A does not display selective binding properties for higher degrees of H4K20 methylation, rather, has the capacity to interact with all three methylated forms in vivo.
To determine whether these results were consistent for another tandem tudor domain-containing protein, similar studies were performed by using the tandem tudor domain of the 53BP1 DNA repair protein, which was previously reported to bind H4K20me2 > H4K20me1 in vitro, but did not bind H4K20me3 (17, 20). Consistent with the in vitro results, the 53BP1 tandem tudor domain alone preferentially bound the H4-Suv4-20h2 MTeC bait fusion protein possessing H4K20me2 (Fig. 3E). Similar to what was observed for full-length JMJD2A, full-length 53BP1 also bound the H4-PR-Set7 MTeC bait fusion protein containing only H4K20me1, indicating that WT 53BP1 has the capacity to interact with all three H4K20 methylated forms in vivo (Fig. 3F). In contrast to in vitro binding studies, these findings indicate that full-length JMJD2A and 53BP1 can bind any methylated form of H4K20 in vivo, and suggests that similar binding properties will be observed for other tandem tudor domain-containing proteins. Collectively, these findings highlight the use of MTeC to accurately characterize PTM-specific binding properties in an in vivo context.
Full-Length G9a Selectively Binds H3K9me1 in Vivo.
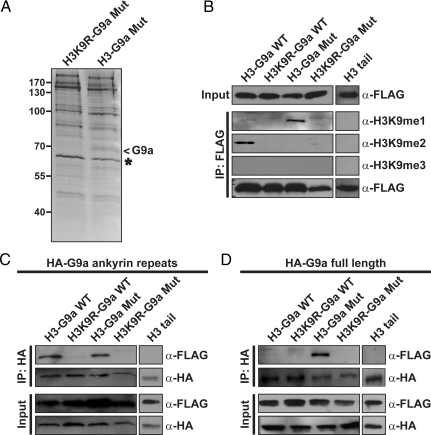
An important utility of MTeC is to identify previously undescribed endogenous proteins that selectively bind a specific posttranslationally modified peptide sequence in vivo. To test MTeC in this application, we returned to the H3-G9a mutant MTeC bait plasmid in an attempt to discover H3K9me1/me2 binding proteins (Fig. 2). This plasmid or the H3K9R-G9a mutant control MTeC bait plasmid were transiently transfected into HEK 293 cells, and the bound MTeC-associated proteins from nuclear lysates were purified by FLAG-immunoprecipitation. After extensive washing, the MTeC-associated proteins were eluted with a FLAG peptide and resolved by SDS/PAGE (Fig. 4A). Comparison of the banding patterns between the two samples revealed a single band of ≈70 kDa found exclusively in the H3-G9a mutant MTeC sample (arrow). This band and its size equivalent from the control sample were excised from the gel for protein identification by MS, which resulted in the detection of 5 peptide fragments from only the H3-G9a mutant MTeC sample that uniquely aligned to the C-terminal of G9a (Fig. S1). These findings were unexpected, because full-length G9a is ≈130 kDa, suggesting that our results were artifacts of contamination by the abundant amounts of the MTeC bait fusion protein and/or potential homodimerization of the G9a SET domains, as was previously reported (21). However, G9a peptide fragments were not detected by MS in the H3K9R-G9a mutant MTeC negative control gel slice, indicating that the observed binding was most likely due to differences in H3K9 methylation and not due to contamination or to dimerization of G9a SET domains. The putative G9a-H3K9 methyl-dependent interaction was confirmed in coimmunoprecipitation experiments by using full-length G9a and the various H3 MTeC bait plasmids in HEK 293 cells (Fig. S2).
Fig. 4.
MTeC reveals that full-length G9a selectively binds H3K9me1 in vivo. (A) Silver stain of FLAG-purified H3-G9a mutant and H3K9R-G9a mutant control MTeC bait fusion proteins (asterisk) from HEK 293 nuclear lysates. MS identified ≈70-kDa band as G9a (arrow). (B) Western blot analysis of FLAG-purified H3-G9a MTeC bait fusion proteins from COS7 nuclear lysates indicates a distinct H3K9 methylation pattern compared with HEK 293 cells (Fig. 2B). (C) Western blot analysis of HA-purified COS7 nuclear lysates cotransfected with the indicated FLAG-tagged H3-G9a MTeC bait plasmids and either an HA-tagged G9a ankyrin repeats only or full-length HA-G9a plasmid (D).
Although the origin of the observed truncated G9a remains unclear, we calculated that it contains the SET domain and all seven ankyrin repeats (Fig. S1). Consistent with our findings, it was recently shown that the ankyrin repeats of G9a bind H3K9me1 and H3K9me2 with relatively high affinity in vitro (22). To confirm this interaction in vivo, MTeC was again used by cotransfecting an HA-tagged plasmid expressing only the G9a ankyrin repeats with the different H3-G9a MTeC bait plasmids in COS7 cells. By switching the experimental cell line, we serendipitously discovered that the expression of MTeC bait fusion proteins in different cell lines can have dramatic effects on the modification characteristics of the target substrate. In contrast to the observed H3K9 methylation patterns in HEK 293 cells (Fig. 2B), the H3-G9a WT MTeC fusion protein displayed only H3K9me2, and the H3-G9a mutant MTeC fusion protein displayed only H3K9me1 in COS7 cells (Fig. 4B). Western blot analysis performed on the HA-immunoprecipitated material from COS7 nuclear lysates confirmed the in vitro results that the G9a ankyrin repeats bind both H3K9me1 and H3K9me2 in vivo (Fig. 4C).
Given the findings above with the tandem tudor domain-containing proteins, the in vivo binding properties of full-length G9a were further investigated by using MTeC. Western blot analysis performed on the HA-immunoprecipitated material from COS7 nuclear lysates cotransfected with an HA-tagged full-length G9a and the various H3-G9a bait plasmids revealed that full-length G9a selectively binds the H3-G9a mutant MTeC bait fusion protein containing only H3K9me1 in vivo (Fig. 4C). It is interesting to note that although full-length tandem tudor domain-containing proteins are less selective for histone methyl-binding compared with the tudor domains alone, full-length G9a displayed a pronounced degree of methyl-selectivity compared with the G9a ankyrin repeats alone as it preferentially bound H3K9me1 in vivo. Collectively, these findings indicate that MTeC can be used in an unbiased manner to identify endogenous modification-specific binding proteins in vivo and can also be subsequently used to accurately characterize the modification-dependent interaction in vivo.
Discussion
Here, we illustrate an innovative in vivo method, MTeC, developed specifically for the discovery and characterization of previously undescribed PTMBPs. The MTeC approach can be used as an unbiased method to detect and identify endogenous PTMBPs in vivo. For example, by engineering and employing the H3-G9a bait fusion proteins in MTeC, we discovered that G9a binds H3K9 in a methylation-dependent manner. We also established that MTeC can be subsequently used to validate and characterize the in vivo PTM-dependent binding properties of PTMBPs. For example, we confirmed the H3K9 methyl-dependent binding of both G9a and HP1 using MTeC and elucidated their H3K9 mono- and trimethylation-selective binding properties in vivo, respectively. Futhermore, we described how MTeC can be applied to compliment existing in vitro binding assays and can provide important perspectives into in vivo PTM-specific interactions. For example, we demonstrated that the ankyrin repeats of G9a bind both H3K9me1 and H3K9me2 in vivo, consistent with previous in vitro results (22). We also confirmed in vitro findings that the tandem tudor domains of JMJD2A and 53BP1 selectively bind H4K20me2 and H4K20me3 in vivo (19, 20). However, MTeC also provided previously undescribed insights into the in vivo binding properties of these proteins when expressed as full-length proteins rather than when constrained to small domains. For example, we demonstrated that full-length JMJD2A and 53BP1 display relaxed methyl-binding characteristics by binding all three methylated forms of H4K20 compared with their more methyl-selective tandem tudor domains. In contrast, we found that full-length G9a displayed enhanced binding selectivity for H3K9me1 in vivo compared with the less methyl-selective ankyrin repeats.
These findings emphasize that significantly different and, perhaps, contradictory PTM-dependent binding properties of full-length proteins may be observed using the in vivo MTeC method compared with conventional in vitro binding assays. The differences could be due to several physiologically relevant factors typically missing from in vitro conditions that are known to dramatically alter protein binding properties, such as the association of the PTMBP with a multiprotein complex and/or the PTM of the PTMBP, itself. For example, it was recently reported that the N-terminal portion of G9a, which does not include the ankyrin repeats, can be methylated in vivo (23). The methylation of G9a results in the recruitment of HP1 and, most likely, other associated factors that may explain the observed H3K9me1 selectivity of full-length G9a in vivo. This evidence highlights another useful application of MTeC in which to determine the minimal region of the PTMBP required for in vivo PTM-dependent binding selectivity by characterizing truncation mutants of the PTMBP.
Although this study focused solely on histone methylation and histone methyl-specific binding proteins, MTeC can theoretically be used to discover and characterize PTMBPs that bind any PTM on any target peptide sequence in vivo. The first step in MTeC is engineering the bait plasmid containing the target peptide sequence in tandem with an appropriate enzyme followed by the confirmation that the bait fusion protein is sufficiently modified in vivo. Although we did not vary the size of the H3 or H4 target peptides, it seems likely that shorter substrates will reduce potential nonspecific binding, and also, it may be best to avoid peptide substrates with complex secondary sequences that may structurally inhibit catalysis by the tethered enzyme in vivo. Similar strategies should be applied when tethering the enzyme into the MTeC bait plasmid. However, we found that the length of the enzyme required for maximal substrate modification in vivo usually had to be determined empirically. Unlike methyltransferases that are restrictive in terms of substrate peptide sequence and degree of methylation, more promiscuous PTM-creating enzymes with robust activity, such as kinases or acetyltransferases, could be used in MTeC to effectively modify a target peptide sequence even if the enzyme responsible for the endogenous modification is unknown. Last, the ability of the bait fusion protein to be sufficiently modified when expressed in the experimental cell system of choice must be assessed. Although we were able to use commercially available histone methyl-specific antibodies for this purpose, such antibodies may not be available for other modified peptides. In these cases, it should be possible to employ established MS approaches on the purified MTeC bait fusion protein to estimate the ratio of unmodified:modified substrate. Once a satisfactory degree of peptide PTM has been attained for the experimental bait fusion protein, the negative control bait plasmids can then be engineered.
The ability to successfully purify PTMBPs by MTeC relies mainly on the abundance of the endogenous PTMBP and the strength of the interaction between the modified substrate and the PTMBP in vivo. Although we were able to easily observe endogenous HP1 binding the H3-G9a MTeC bait fusion proteins by Western blot analysis, we were unable to use this method to detect the binding of other endogenous PTMBPs. Although this phenomenon could be due to the quality of the antibodies, it is likely that these PTMBPs are in lower abundance compared with HP1 and/or the interaction may be more transient in nature. We were able to consistently overcome this obstacle by performing coimmunoprecipitations with HA-tagged versions of the putative PTMBPs to verify and characterize their PTM-dependent interactions. However, this approach can only be used when the putative PTMBP is already known. One possible way to counter this difficulty may be to use a tandem affinity tag that will likely result in significantly less background after the immunoprecipitation step and reveal a larger number of putative PTMBPs. Also, stable cell lines containing the experimental and control MTeC bait plasmids could be created; thereby, providing a virtually unlimited pool of input material to identify PTMBPs that are in low abundance. Also, the use of cross-linking reagents could also be used before the purification process to capture PTMBPs that transiently bind the modified MTeC bait fusion protein (24). A more modern and sophisticated way to avoid both of these potential problems would be to use the stable isotope labeling with amino acids method (SILAC) followed by MS to identify proteins specifically associated with the experimental MTeC sample, but absent in control samples (25). In this case, the experimental MTeC cell lines would be cultured in medium containing “heavy” 13C6-lysine, whereas the control MTeC cell lines would be cultured in regular media containing the normal “light” lysine. After the purification steps, the heavy bound proteins from the experimental MTeC sample and the light bound proteins from the control sample would be mixed 1:1 and processed for in solution digest for MS analysis. Peptides fragments containing light lysine would be considered nonspecific, because they were derived from the control sample. However, peptide fragments that contain only heavy lysine would be considered putative PTMBPs for further analysis.
Compared with current in vitro methods, the MTeC approach offers the advantages of identifying endogenous PTMBPs in an unbiased manner, and importantly, all experiments are performed in a cellular context allowing for the more physiologically relevant in vivo characterization of PTM-dependent interactions. Although we demonstrated that MTeC can be used to vary the degree of methylation of MTeC bait fusion proteins, it is conceivable that even more complex PTMs, such as SUMOylation or ubiquitination, could also be achieved in vivo by tethering the target peptide to an appropriate ligase. Also, it should be possible to create and investigate a dual modified peptide in vivo by flanking the MTeC target peptide sequence with different enzymes. Although the experiments in this study were performed in mammalian cell lines, the MTeC bait plasmids could easily be converted for use in transgenic systems for whole animal studies or investigating tissue-specific PTM-dependent interactions. Collectively, this study demonstrates that the MTeC approach provides investigators with an innovative biological tool to gain important insights into in vivo PTM-dependent interactions in various different eukaryotic cell types.
Materials and Methods
Plasmids.
MTeC bait plasmids were created by first cloning a FLAG affinity tag into the NotI and AgeI sites of the pQCXIP vector (Clontech). The first 44 aa of histone H3 or H4 WT or mutants were then cloned in frame (AgeI and EcoRI). Then, the SET domains of G9a (amino acids 984-1210), PR-Set7 (amino acids 146–317), or Suv4-20h2 (amino acids 1–280) were subcloned into the appropriate MTeC plasmid (EcoRI and BamHI).
Transfections, Immunoprecipitations, and Western Blot Analysis.
Plasmids were transfected into HEK 293 or COS7 cells by using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Nuclei were collected 48 h posttransfection as previously described (26), and 2 × 107 nuclei/sample were resuspended in IP buffer (50 mM Tris·HCl, pH 7.0/150 mM NaCl/0.5 mM DTT/1% Nonidet P-40/1 mM PMSF/1 μg/mL pepstatin/1 μg/mL aprotinin/leupeptin) followed by an overnight 4 °C incubation with 20 μL preequilibrated ANTI-FLAG M2 or ANTI-HA affinity beads (Sigma). Beads were washed extensively with IP buffer before eluting bound material by boiling in SDS loading dye and fractionation by SDS/PAGE. Western blot analysis was performed as previously described (26) by using FLAG, HP1β (Sigma), HA (Santa Cruz), H3K9 methyl-specific (Millipore), or H4K20 methyl-specific (Active Motif) antibodies.
Immunoaffinity Purification.
Each MTeC bait plasmid was transfected into 2 × 108 HEK 293 cells as described above. Nuclear pellets were resuspended in 10 mL IP buffer and incubated with 150 μL of preequilibrated ANTI-FLAG M2 affinity beads overnight at 4 °C. Beads were collected by centrifugation, washed extensively, and incubated with 100 μg FLAG Peptide (Sigma) in 150 μL TBS at 4 °C for 12 h. This elution step was performed twice resulting in 300 μL of FLAG-bound material. A portion of this material was resolved by SDS/PAGE before silver staining, and unique bands were excised and destained by using the SilverQuest Silver Staining Kit (Invitrogen) before proteomic analysis. Alternatively, the entire bound material can be precipitated by adding 3 mL ice-cold 10% TCA in acetone, incubated overnight at −20 °C, and collected by centrifugation before proteomic analysis.
Supplementary Material
Acknowledgments.
We thank Jennifer Sims and Julian Desmond for early technical contributions, and the Taplin Mass Spectrometry Facility, Boston, particularly Donald Kirkpatrick, for proteomic analysis and discussion. Reagents were graciously provided by Michael Stallcup, University of Southern California (G9a), Bernard Futscher, University of Arizona, Tucson, AZ (V5-G9a), Xiaodong Cheng, Emory University, Atlanta (G9a mutant), Johnathan Whetstine, Massachusetts General Hospital Cancer Center, Boston (JMJD2A), and Phillip Carpenter, University of Texas Health Science Center, San Antonio, TX (53BP1). This work was supported by the Pew Charitable Trusts and the James H. Zumberge Faculty Research Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907799106/DCSupplemental.
References
- 1.Guo D, et al. A tethered catalysis, two-hybrid system to identify protein-protein interactions requiring post-translational modifications. Nat Biotechnol. 2004;22:888–892. doi: 10.1038/nbt985. [DOI] [PubMed] [Google Scholar]
- 2.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 3.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters AH, et al. Partitioning and plasticity of repressive histone methylation States in Mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 6.Rice JC, et al. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 7.Collins RE, et al. In vitro and in vivo analyses of a Phe/Tyr switch controlling product specificity of histone lysine methyltransferases. J Biol Chem. 2005;280:5563–5570. doi: 10.1074/jbc.M410483200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister AJ, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs SA, Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080–2083. doi: 10.1126/science.1069473. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SA, et al. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 2001;20:5232–5241. doi: 10.1093/emboj/20.18.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 12.Peters AH, et al. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 13.Peters AH, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 14.Couture JF, Collazo E, Brunzelle JS, Trievel RC. Structural and functional analysis of SET8, a histone H4 Lys-20 methyltransferase. Genes Dev. 2005;19:1455–1465. doi: 10.1101/gad.1318405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schotta G, et al. A silencing pathway to induce H3–K9 and H4–K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao B, et al. Specificity and mechanism of the histone methyltransferase Pr-Set7. Genes Dev. 2005;19:1444–1454. doi: 10.1101/gad.1315905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, et al. Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep. 2006;7:397–403. doi: 10.1038/sj.embor.7400625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer-Stroh S, et al. The Tudor domain ‘Royal Family’: Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem Sci. 2003;28:69–74. doi: 10.1016/S0968-0004(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Thompson JR, Botuyan MV, Mer G. Distinct binding modes specify the recognition of methylated histones H3K4 and H4K20 by JMJD2A-tudor. Nat Struct Mol Biol. 2008;15:109–111. doi: 10.1038/nsmb1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botuyan MV, et al. Structural basis for the methylation state-specific recognition of histone H4–K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana M, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3–K9. Genes Dev. 2005;19:815–826. doi: 10.1101/gad.1284005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins RE, et al. The ankyrin repeats of G9a and GLP histone methyltransferases are mono- and dimethyllysine binding modules. Nat Struct Mol Biol. 2008;15:245–250. doi: 10.1038/nsmb.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampath SC, et al. Methylation of a histone mimic within the histone methyltransferase G9a regulates protein complex assembly. Mol Cell. 2007;27:596–608. doi: 10.1016/j.molcel.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland BW, Toews J, Kast J. Utility of formaldehyde cross-linking and mass spectrometry in the study of protein-protein interactions. J Mass Spectrom. 2008;43:699–715. doi: 10.1002/jms.1415. [DOI] [PubMed] [Google Scholar]
- 25.Trinkle-Mulcahy L, et al. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J Cell Biol. 2008;183:223–239. doi: 10.1083/jcb.200805092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sims JK, Houston SI, Magazinnik T, Rice JC. A trans-tail histone code defined by monomethylated H4 Lys-20 and H3 Lys-9 demarcates distinct regions of silent chromatin. J Biol Chem. 2006;281:12760–12766. doi: 10.1074/jbc.M513462200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.