Abstract
A remarkable feature of the adaptive immune system is the speed at which small numbers of antigen-specific lymphocytes can mediate a successful immune response. Rapid expansion of T and B lymphocyte clones that have receptors specific for a particular antigen is one of the primary means by which a swift response is generated. Although much of this clonal expansion is caused by the division of antigen-specific cells, here we demonstrate an additional mechanism by which the pool of effector T cells against a viral infection can quickly enlarge. Our data show that virus-specific CD8+ cytotoxic T lymphocytes (CTL) can transfer their T cell receptors (TCR) to recipient CTL of an unrelated specificity that, as a consequence, gain the antigen specificity of the donor T cell. This process occurs within minutes via membrane exchange and results in the recipient CTL acquiring the ability to recognize and eliminate cells targeted by the donor TCR, while still retaining the antigen specificity of its own TCR. Such receptor sharing allows rapid, proliferation-independent expansion of virus-specific T cell clones of low frequency and plays a highly significant antiviral role that can protect the host from an otherwise lethal infection.
Keywords: antigen receptor transfer, clonal selection theory, poxvirus, trogocytosis, clonal expansion
A fundamental feature of the adaptive immune system is that each T and B lymphocyte expresses an antigen receptor with unique antigen specificity, a concept originally proposed in Burnet's clonal selection theory (1). Indeed, a large body of evidence indicates that lymphocytes express a remarkably diverse repertoire of antigen receptors, a mechanism that ensures that the immune system can respond to a large variety of potential infections, although only a small number of precursor lymphocytes can recognize any given pathogen. In the case of viruses, protection of the host critically depends on CD8+ cytotoxic T lymphocyte (CTL) function. During an infection, rare T cell clones that can recognize, via their T cell receptor (TCR), virus-derived peptides in association with major histocompatibility complex (MHC) class I molecules, rapidly expand many thousand-fold. This dramatic expansion in the number of virus-specific CD8+ T cells is generally thought to be via proliferation of antigen-specific T cells (2, 3).
Recently we reported that activated B lymphocytes rapidly transfer their B cell receptor for antigen (BCR) to bystander B cells, with the transferred BCR allowing recipient B cells to capture and present specific antigen to antigen-specific CD4+ T cells (4). Based on these data we hypothesized that, to rapidly contain a viral infection, viral antigen-specific CD8+ T cells may use a similar mechanism to recruit neighboring CD8+ T cells of irrelevant specificity by sharing their TCR and thereby dramatically, but transiently, expanding the pool of virus-specific effector cells in a division-independent manner. To test this idea we used ectromelia virus (ECTV), a virulent natural mouse pathogen that causes mousepox, a disease similar to smallpox (5, 6). Viral control and recovery from ECTV infection unconditionally relies on CD8+ T cells (7), and the antiviral effector function of these cells depends on perforin (Prf)-mediated cytotoxicity (8–10). This model therefore provides an ideal system to test whether TCR sharing occurs between CD8+ CTL and determine its potential biological significance in terms of effector function and antiviral protection.
Results and Discussion
Transfer of Antigen Specificity Between CD8+ CTL Can Control a Lethal ECTV Infection.
It has been known for many years that adoptive transfer of WT ECTV-immune CD8+ T cells provides protection in recipient mice infected with a lethal dose of ECTV (11) that would otherwise kill mice ≈6 days after infection. Furthermore, transfer of WT, but not Prf-deficient (Prf−/−), ECTV-immune CD8+ T cells results in a significant reduction in viral load (P < 0.05; Fig. 1 A and ref. 9), consistent with our previous study showing that cytolytic activity, rather than cytokine production, is responsible for the control of an ECTV infection by CD8+ T cells (9). Indeed, the inability of the transferred Prf−/− cells to control virus correlates with their failure to lyse ECTV-infected target cells in vitro (Fig. 1B), which occurs despite normal clonal expansion of virus-specific CD8+ T cells, based on MHC-tetramer enumeration of CD8+ T cells recognizing the immunodominant ECTV determinant (12) (Fig. 1C). To test whether Prf−/− ECTV-immune cells could recruit activated WT CD8+ T cells of irrelevant specificity and control ECTV load in an antigen-specific manner, the following experiment was performed (Fig. S1). WT or Prf−/− mice were infected with avirulent, thymidine kinase-deficient ECTV (ECTV-TK−) or WT mice with influenza A (A/PR/8/34) virus (IAV). Five days later, CD8+ T cells purified from the spleens of these mice were transferred into recipient mice infected 24 h earlier with a lethal dose of virulent ECTV, with virus clearance and splenic CTL activity being measured 3 days later.
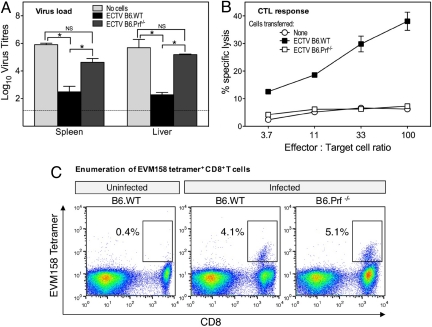
Fig. 1.
Failure of Prf-deficient CD8+ CTL to control an ECTV infection. B6.WT and B6.Prf−/− mice were infected i.v. with 5 × 105 PFU of avirulent ECTV-TK−. Five days later mice were killed, and splenic CD8+ T cells were enriched by using Dynabeads. Enriched CD8+ T cells (>80% purity) were injected i.v. into recipient B6.WT mice 24 h after infection with 5 × 104 PFU of virulent ECTV. Groups of mice received 107 cells of either population or no cells. (A) Viral titers in spleen and liver 4 days after infection and 3 days after cell transfer, as determined by viral plaque assays. *, P < 0.05; NS, not significant. (B) CTL activity of unfractionated splenocytes from the different recipients, 3 days after cell transfer, as measured by 51chromium release assay using uninfected and ECTV-infected MC57G fibrosarcoma target cells. (C) Clonal expansion of ECTV-specific CD8+ T cells in B6.WT and B6.Prf−/− mice 5 days after ECTV infection. CD8+ T cells recognizing the immunodominant ECTV determinant (TSYKFESV) (12), encoded by ORF EVM158, were enumerated by MHC class I-tetramer staining.
WT ECTV-immune CD8+ T cells reduced ECTV titers in recipient mice by ≈1,000-fold (P < 0.05), whereas the Prf−/− CD8+ T cells were ineffective and the IAV-immune CD8+ T cells tended to enhance virus titers (Fig. 2A). In contrast, mice receiving a mixture of IAV-immune and Prf−/− ECTV-immune CD8+ T cells were able to reduce virus titers to similar levels as WT ECTV-immune CD8+ T cells. The cotransfer of the IAV-immune and Prf−/− ECTV-immune CD8+ T cells also resulted in the appearance of ECTV-specific CTL activity in the spleen of recipient mice that approached the cytolytic activity of splenocytes from mice receiving WT ECTV-immune CD8+ T cells (Fig. 2B), whereas splenocytes from mice receiving either of these two T cell populations alone had minimal CTL activity. Flow cytometric separation, using CD45 allotype markers, of IAV and ECTV-immune CD8+ T cells from mice that received the cotransferred cells revealed that the IAV-immune CD8+ T cells had gained the ability to specifically kill ECTV-infected target cells (Fig. 2C). In contrast, the Prf−/− ECTV-immune T cells had low killing activity that was not enhanced when they were cotransferred with IAV-immune T cells. These results indicate that the noncytolytic Prf−/− CTL were able to transfer their antigen specificity to cytolytic CD8+ T cells of an unrelated specificity.
Fig. 2.
Virus-specific CD8+ T cells donate their antigen specificity to CD8+ T cells of unrelated specificity in vivo, a process that can control an ECTV infection. Enriched splenic CD8+ T cells (Fig. S1) from ECTV-immune B6.SJL or B6.Prf−/− mice and IAV-immune B6.SJL mice were adoptively transferred into recipient B6.WT mice 24 h after infection with 5 × 104 PFU of virulent ECTV. Groups of mice received 107 cells of any one T cell population or a mixture of 0.5 × 107 B6.Prf−/− ECTV-immune and 0.5 × 107 B6.SJL IAV-immune cells. (A) Splenic viral titers 4 days after infection and 3 days after cell transfer. *, P < 0.05; NS, not significant. (B) CTL activity of unsorted splenocytes against ECTV-infected MC57G fibrosarcoma target cells. (C) CTL activity, against the same ECTV-infected targets, of CD8+ T cells FACS separated based on their CD45 allotypes.
To further investigate this transfer of antigen specificity we used ovalbumin (OVA)-specific TCR-transgenic CD8+ T cells (OT-I) in conjunction with recombinant ECTV expressing OVA (ECTV-OVA), where OVA is seen by the immune system as a viral antigen. The cotransfer of in vitro-activated Prf−/− OT-I CD8+ T cells with a population of polyclonal IAV-immune CD8+ T cells resulted in a significant reduction of viral load (P < 0.05; Fig. 3A) and the appearance of potent OVA-specific CTL activity in the spleen of recipient mice (Fig. 3B). An interesting feature of this experiment was that it was performed in lymphocyte-deficient RAG-1−/− recipients, indicating that endogenous lymphocytes are not required for transfer of antigen specificity. To eliminate the possibility that transfer of antigen specificity was a consequence of Prf deficiency, WT in vitro-activated OT-I CD8+ T cells were cotransferred with IAV-immune CD8+ T cells into ECTV-OVA-infected WT mice and the two populations were separated 3 days later based on CD45 allotypes. The IAV-immune population, when injected alone, exhibited negligible OVA-specific CTL activity but when cotransferred with OVA-specific OT-I CD8+ T cells displayed remarkable CTL activity against OVA-expressing targets (Fig. 3C). It should be noted that in this experiment the separated OT-I population contained a large number of recipient CD8+ T cells that carried the same CD45 allotype (CD45.2), hence much higher effector/target cell ratios were needed for cytotoxic activity to be observed.
Fig. 3.
Antigen-specific TCR transgenic CD8+ T cells facilitate viral clearance through transfer of their antigen specificity to CD8+ T cells of unrelated specificity in vivo. Enriched splenic CD8+ T cells from IAV-immune B6.SJL mice (Fig. S1) and/or FACS purified Prf−/− or WT OT-I cells that had been activated in vitro with SIINFEKL-pulsed splenic dendritic cells for 5 days (Fig. S2) were transferred into either B6.RAG-1−/− or WT recipient mice 24 h after infection with 5 × 104 PFU ECTV-OVA. (A and B) Splenic viral load (A) (*, P < 0.05; NS, not significant) and CTL activity (B) were measured 4 days after infection and 3 days after transfer of Prf−/− OT-I and/or IAV-immune cells into RAG-1−/− mice. (C) The cytotoxic activity of FACS sorted splenic CD8+ T lymphocytes, based on CD45 allotypes, was tested on SIINFEKL-pulsed targets 4 days after infection and 3 days after transfer of WT OT-I and/or IAV-immune cells to WT recipient mice.
Transfer of Antigen Specificity Between CD8+ CTL Involves Acquisition of Foreign TCR via Membrane Exchange.
To formally demonstrate that the extraordinary transfer of antigen specificity depicted in Figs. 2 and 3 was caused by TCR transfer, we established an in vitro transfer model that was also used to probe aspects of the transfer process itself (Fig. S2). Prf−/− OT-I CD8+ T cells and P14 TCR transgenic CD8+ T cells specific for the lymphocytic choriomeningitis virus (LCMV) peptide gp33 (13) were first activated separately in vitro with dendritic cells pulsed with their cognate peptides. They were then mixed and cocultured for various times, and transfer of antigen specificity and TCR were assessed by CTL activity and flow cytometry, respectively. When cultured separately for 5 days the Prf−/− OT-I and P14 T cells showed undetectable cytotoxic activity against OVA-expressing target cells (Fig. 4A). However, when these two T cell populations were mixed on day 3 and cocultured for 2 days potent OVA-specific CTL activity was observed that was comparable to WT OT-I CTL. Separation of the two T cell populations by flow cytometry after coculture revealed that the P14 T cells gained the ability to lyse OVA-expressing target cells (Fig. 4B). The Prf−/− OT-I T cells also showed slightly enhanced cytolytic activity for the OVA-peptide pulsed targets by an unknown mechanism; however, this was observed in in vitro cocultures but not in vivo (Fig. 2C). The P14 T cells retained their ability to lyse gp33 peptide-pulsed targets after coculture with the OT-I T cells, indicating that acquisition of the OVA specificity did not compromise the original LCMV specificity of the P14 T cells (Fig. 4C). Interestingly, the cocultured Prf−/− OT-I T cells also gained the ability to lyse gp33 peptide-pulsed targets (Fig. 4C), implying that TCR transfer occurred in both directions. Blocking studies with peptide-MHC class I tetramers confirmed that the CTL activity being observed for each T cell population was antigen-specific (P < 0.05; Fig. 4D) and that each CTL population had acquired dual antigen specificity (Fig. 4 B–D). Furthermore, flow cytometric analysis revealed substantial TCR transfer between the two activated TCR transgenic T cell populations after 2-day coculture (Fig. 5A), consistent with acquisition of antigen specificity of the apposing CTL, although recipient T cells only needed to gain ≈4% of the TCR expression of the donor CTL to become effectors (based on mean fluorescence intensity of OT-I TCR). In contrast, polyclonally activated CD8+ T cells were unable to transfer their TCR; only T cells activated by their cognate antigen presented by dendritic cells were effective TCR donors (Fig. S3A). With appropriately activated CTL, TCR transfer occurred within minutes of coculture in the absence of additional antigen (Fig. 5 B and C), concurrently with other membrane components such as CD45 molecules and the membrane intercalating dye PKH-26 (Fig. 5B), and required cell–cell contact (Fig. 5C), transfer not being observed with the culture supernatant of activated CD8+ T cells (Fig. S3 B and C) or when the donor and recipient CTL were separated by a semipermeable membrane (Fig. S3C), data consistent with TCR transfer occurring via membrane transfer. Although the results shown demonstrate membrane exchange between activated CD8+ T cells, our in vitro studies also suggested that CTL can transfer membrane molecules to bystander naive CD8+ T cells (Fig. S4). However, because these naive CD8+ T cells are not cytolytic, membrane transfer may not have functional consequences. Whether such membrane transfer occurs between activated and naive CD8+ T cells in vivo is not known and remains to be determined. Such intercellular transfer of membrane components between immune cells (14) has been extensively reported before through processes involving trogocytosis and nanotubes (reviewed in refs. 15–18). Indeed, treatment with an inhibitor of actin polymerization, previously shown to inhibit CD8+ T cell trogocytosis (19), completely blocked the transfer of TCR between the two T cell populations (Fig. 5D). Precisely where TCR sharing occurs in vivo between donor and recipient CTL remains to be elucidated. However, because TCR sharing proceeds rapidly, it is conceivable that in our adoptive transfer experiments some TCR transfer occurred during the short period donor and recipient CTL were mixed immediately before injection. We believe that this is unlikely to be important as the recipient CTL were able to lyse ECTV-infected targets 3 days after injection, a result that would require the acquired TCR to persist for many days on recipient T cells. It is more likely that TCR transfer occurs in vivo in peripheral lymphoid organs or at sites of infection.
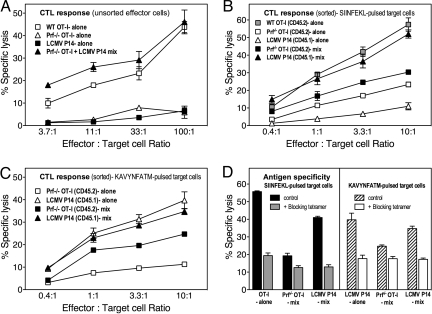
Fig. 4.
Activated CD8+ T cells can exchange antigen specificity in vitro by TCR transfer. FACS-purified WT OT-I, Prf−/− OT-I and LCMV P14 CD8+ TCR transgenic T lymphocytes were cultured with cognate peptide-pulsed primary dendritic cells for 5 or 3 days and then mixed (Prf−/− OT-I and LCMV P14) and cultured for another 2 days (Fig. S2). (A) CTL activity of unsorted splenocytes against SIINFEKL-pulsed targets. (B) CTL activity of FACS-sorted cells against SIINFEKL-pulsed targets. (C) CTL activity of FACS-sorted cells against KAVYNFATM (gp33 peptide)-pulsed targets. (D) The dual specificity of sorted CD8+ T cells was established by using Kb-SIINFEKL or Kb-KAVYNFATM tetramers to block killing in a 51chromium release assay at a 100:1 effector/target cell ratio.
Fig. 5.
Transfer of TCR between CD8+ CTL populations is rapid and involves membrane exchange. Purified OT-I and B6.SJL P14 CD8+ T cells, stimulated separately as in Fig. 4 for 3 days, were cocultured and assessed for TCR and membrane transfer. (A) TCR exchange (dot plot and histogram), as detected using antibodies specific for OT-I (Vβ5.2) or P14 (Vβ8.1) TCR and populations discriminated by CD45 allotype. Histograms show percentage of CD8+ cells in coculture expressing exogenous TCR. (B) PKH-26 (membrane stain), CD45.2, and Vβ5.2 are rapidly and simultaneously transferred between cocultured OT-I cells and P14 cells. Values are percentage of P14 CD8+ cells in coculture acquiring the OT-I-specific markers. (C) Confocal images of interaction between stimulated OT-I and P14 cells, stained with PKH-26 and BODIPY, respectively. Arrows show merged membranes (yellow areas). (Scale bar: 10 μM.) (D) TCR transfer from stimulated OT-I cells to stimulated P14 cells (1-h coculture) in the presence of latrunculin B (Lat-B) or DMSO as control.
To combat a primary viral infection, rapid and dramatic expansion in the number of virus-specific CD8+ T cells is required. For instance, in a naïve animal the precursor frequency of LCMV-specific CD8+ T cells is only ≈1–2% (20, 21). However, at the peak of the response to an infection the proportion of CD8+ T cells exhibiting an effector phenotype and specificity for LCMV dramatically rises to 85–95% (3). Our data suggest that in addition to proliferation mediated clonal expansion (2, 3) antigen-specific CD8+ T cells can recruit adjacent CD8+ T cells by TCR sharing, thereby further increasing the number of antigen-specific effector T cells available to promptly control an infection.
Our finding that the transfer of a relatively small number of TCR results in bystander CTL gaining the antigen specificity of the donor CTL may explain why this phenomenon has not been detected previously when MHC class I tetramers or TCR-specific antibodies have been used to detect antigen-specific CD8+ T cells. It is also possible that only a fraction of the TCR transferred to bystander CTL are able to deliver an activation signal as, in the case of trogocytosis, transferred receptors often do not gain access to the cytoplasm of recipient cells (22). In the case of TCR this would not pose a problem as it has been estimated that <10 TCR/effector T cells need to engage MHC-cognate peptide complexes to deliver an activation signal (23).
The process of trogocytosis is now well recognized as a feature of T, B, and NK cell biology, although the functional consequences and physiological relevance of this phenomenon have been poorly understood. Although there is ample evidence that cell-surface proteins can be transferred between cells, both in vitro and in vivo, our study demonstrates its importance and functional relevance during the course of an immune response in vivo. We have shown that TCR sharing between CD8+ T cells has significant biological consequences and can play a pivotal role in protecting the host from an otherwise lethal viral infection. Indeed our data provides an insight into the versatility of the host immune response to virus infection.
Materials and Methods
Mice.
Female C57BL/6J WT (B6.WT; CD45.2), B6.129S7-Rag1tm1Mom/J (B6.RAG-1−/−) (24), B6.SJL-Ptprca Pep3b/BoyJ (B6.SJL; CD45.1), Prf-deficient B6 (B6.Prf−/−; CD45.2) (25), B6-Tg (TcraTcrb)1100Mjb (WT OT-I; CD45.2) (26), Prf-deficient OT-I (Prf−/− OT-I), B6 Tg (TCR LCMV P14)-Tcratm1Mom (B6.P14), and B6.SJL P14 transgenic mice (13) were used at 6–10 weeks of age. All animal experimental protocols were approved by the Australian National University Animal Experimentation Ethics Committee and were carried out according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Viruses.
ECTV-WT (virulent), ECTV-TK− (avirulent), and recombinant ECTV-OVA (virulent) (27) were prepared in African green monkey kidney epithelial BS-C-1 cells (28), and influenza A/PR/8/34 was grown in 10-day-old embryonated eggs (29) by using published protocols.
CD8+ T Cell Purification and Activation.
Splenic CD8+ T cells were first enriched by Dynabead (Dynal) negative selection (30) and further purified from recipient mice by FACS using CD45 allotypic markers, with cell purity being >98%. TCR transgenic CD8+ cells, purified by negative FACS (30), were activated with Nycodenz-purified (31) and peptide (1 μM)-pulsed splenic dendritic cells in the presence of 5 units/mL recombinant IL-2 (Peprotech). Fluorochrome-conjugated MHC class I-peptide tetramers were generated at the Australian Cancer Research Foundation Biomolecular Resource Facility, John Curtin School of Medical Research.
In Vitro TCR Transfer Assay.
TCR transfer was assessed by flow cytometry after coculturing 3-day activated CD8+ T cell populations from B6.SJL P14 and OT-I mice. Cells were stained with CD8-, Vβ5.2-, Vβ8.1-, CD45.1-, and CD45.2-specific antibodies (BD Biosciences) and the viability dye Hoechst 33258 (1 μg/mL; Calbiochem). Activated CD8+ T cells were stained with PKH-26 (Sigma) before coculture. Percentage of cells expressing transferred markers was assessed by Overton subtraction of histogram profiles (32).
Live Cell Confocal Microscopy.
Activated CD8+ T cells were stained with PKH-26 or boron-dipyrromethene (β-BODIPY) 500/510 C12 (5 μg/mL, 12 min, 37 °C; Molecular Probes) and allophycocyanin-Alexa Fluor 750-conjugated anti-CD8 antibodies (eBioscience) to confirm their CD8+ phenotype and analyzed with a Leica TCS SP5 confocal microscope.
CTL Assays.
51Chromium release assays were performed as described (28).
Statistics.
Data were analyzed by using the unpaired, two-tailed, Mann–Whitney test.
Supplementary Material
Acknowledgments.
We thank Ros de Chazal and Megan Glidden for technical assistance, Ian Parish for helpful discussions, and Chris Goodnow for critical reading of the manuscript. This work was supported by National Health and Medical Research Council of Australia project grants (to G.K., G.C., B.J.Q. and C.R.P.), National Institutes of Health Grant R01AI067401 (to G.K.), an International Research Scholars Special Initiative grant on infectious diseases from the Howard Hughes Medical Institute (to G.K.), a National Health and Medical Research Council of Australia program grant (to C.R.P.), a National Health and Medical Research Council of Australia senior research fellowship (to G.K.), a Peter Doherty biomedical fellowship from the National Health and Medical Research Council of Australia (to B.J.Q.), and a C. J. Martin Biomedical Fellowship from the National Health and Medical Research Council of Australia (to Y.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906554106/DCSupplemental.
References
- 1.Burnet FM. A modification of Jerne's theory of antibody production using the concept of clonal selection. Aust J Sci. 1957;20:67–69. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 2.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Murali-Krishna M, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T cell response: It is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quah BJ, et al. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci USA. 2008;105:4259–4264. doi: 10.1073/pnas.0800259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenner F. Mouse pox, infectious ectromelia of mice: A review. J Immunol. 1949;63:341–373. [PubMed] [Google Scholar]
- 6.Esteban DJ, Buller RM. Ectromelia virus: The causative agent of mouse pox. J Gen Virol. 2005;86:2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- 7.Karupiah G, Buller RM, Van Rooijen N, Duarte CJ, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T cell function. J Virol. 2006;80:6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramshaw IA, et al. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 10.Mullbacher A, Hla RT, Museteanu C, Simon MM. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J Virol. 1999;73:1665–1667. doi: 10.1128/jvi.73.2.1665-1667.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanden RV. Mechanisms of recovery from a generalized viral infection: Mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971;133:1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tscharke DC, et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 14.Cho KS, Hill AB. T cell acquisition of APC membrane can impact interpretation of adoptive transfer experiments using CD45 congenic mouse strains. J Immunol Methods. 2008;330:137–145. doi: 10.1016/j.jim.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 16.LeMaoult J, Caumartin J, Carosella ED. Exchanges of membrane patches (trogocytosis) split theoretical and actual functions of immune cells. Hum Immunol. 2007;68:240–243. doi: 10.1016/j.humimm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261–269. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111:5621–5628. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 21.Razvi ES, Welsh RM, McFarland HI. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 22.Williams GS, et al. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic. 2007;8:1190–1204. doi: 10.1111/j.1600-0854.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 23.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 24.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 25.Kagi D, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 26.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Chaudhri G, Jackson RJ, Karupiah G. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J Immunol. 2009 Aug 5; doi: 10.4049/jimmunol.0803985. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhri G, et al. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc Natl Acad Sci USA. 2004;101:9057–9062. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon γ-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belz GT, et al. Cutting edge: Conventional CD8 α+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 31.Vremec D, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: Investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9:619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.