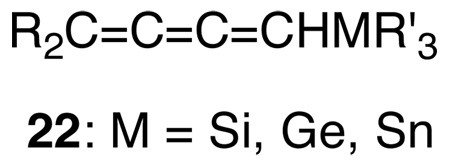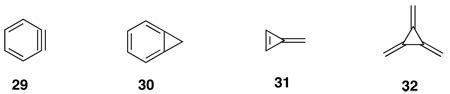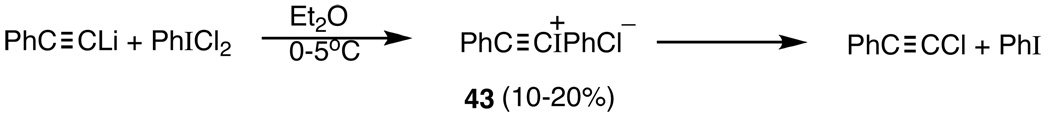Abstract
My sojourn from classical physical-organic chemistry and solvolysis to self-assembly and supramolecular chemistry, over the last forty years, is described. My contributions to unsaturated reactive intermediates, namely vinyl cations and unsaturated carbenes, along with my decade long involvement with polyvalent iodine chemistry, especially alkynyliodonium salts, as well as my more recent research with metal-ligand, coordination driven and directed self-assembly of finite supramolecular ensembles are discussed.
Introduction
During a forty-year career of active research, I have had the pleasure of being involved in and making contributions in three distinct research areas. In the first third of my career I was a classical physical-organic chemist in the area of unsaturated reactive intermediates. In the middle portion I was involved in polyvalent iodine as well as some organometallic chemistry. Since the early 1990's my contributions have primarily been in supramolecular chemistry and self-assembly. In this perspective I shall discuss how and why I got involved in each area, what the critical prior discoveries were and literature results that provided the background and groundwork for our contributions and what our own seminal contributions were to the field. During each period there were also key persons who had an impact and influence on the course of events that I shall mention as well. However, as will be seen, this is a personal narrative based on recollection of events, dependent upon the frailties of human memory, beclouded by the passage of time.
I first became interested in chemistry in high school in Hungary where I grew up. As a freshman in Gymnasium in 1955 I had a chemistry course that was the equivalent of what we now teach in general chemistry to first year students in college in this country. Moreover, in those days in Hungary there were no restrictions on the purchase, even by a teenager, of common chemicals such as inorganic salts, NaOH, common acids, etc., easily available in the corner drug store and elsewhere, so that I was able to set up my own home laboratory and experiments. I clearly recall making “slow burning” black gunpowder from readily available potassium nitrate, sulfur and charcoal powder. Phenolphthalein, used as a primitive laxative by the then pharmacist and as an indicator by the chemist, was available for acid-base titrations and the fascinating color changes that it underwent. When my family moved to the U.S.A. in 1956 I continued with chemistry in high school. I was also fortunate to have a dedicated, inspiring college chemistry teacher Robert C. Miller who invited me to do research with him in organophosphorous chemistry in my sophomore year at DePaul University. He also motivated his students to go to the best graduate schools anywhere in the country.
I was a graduate student at UC-Berkeley from the fall of 1963 until the fall of 1966 in the group of Andrew Streitwieser, Jr. My Ph.D. Dissertation was on the “Kinetics and Mechanism of Boron Fluoride-Alcohol Alkylations.” A classical physical-organic study of Friedel-Crafts alkylations1 including an investigation of the stereochemistry of the alkylation of benzene with chiral 1-propanol-1-d3.2 During this period the Streitwieser and Noyce groups held joint research group meetings. Among the interests of Professor D. Noyce and his group were electrophilic additions to alkynes and vinyl cations.3 Early work on vinyl cations involved essentially only electrophilic additions to alkynes.4 Unlike the rich chemistry on the solvolytic generation of carbonium ions5 little was known about the direct solvolytic generation of vinyl cations. Only systems substituted by an aromatic ring,6 a vinyl group,7 or a cyclopropyl ring8 directly attached to the carbon bearing the leaving group, underwent solvolysis, resulting in “stabilized” vinyl cations. Even the normally highly reactive arenesulfonates, like the 1-cyclohexenyl tosylate and brosylate and the cis-2-buten-2-yl tosylate and brosylate, reacted via an addition-elimination mechanism rather than unimolecular ionization and vinyl cations.9
Sometime during this period, Streitwieser visited the DuPont Company where he was a consultant, and became aware of trifluoromethanesulfonic acid, CF3SO3H, also known as triflic acid.10 Triflic acid, first reported by Haszeldine and Kidd,11 is known to be the strongest Brønsted acid, much stronger than such common acids as HNO3, H2SO4 or even HClO4.10 I remember Streitwieser requesting a sample of Ba(OSO2CF3)2 from the 3M Company in St. Paul, Minnesota, from which the acid was obtained by reaction with fuming H2SO4 and distillation. He and co-workers12 used CF3SO3H to prepare CH3CH2OSO2CF3, as well as the deuterio analogs CD3CH2OTf, and CH3CD2OTf, and via solvolytic studies establish that the reaction occurs via “a transition state with comparatively little positive charge and substantial bonding to a nucleophilic solvent molecule.” In other words, mostly an SN2 process with little, if any SN1 character,12 despite the superior leaving ability of the triflate group.
By December 1966 I had obtained my Ph.D. and left for Princeton for postdoctoral studies with Paul v.R. Schleyer. The 50's and 60's were the golden age of physical-organic chemistry and among the challenges was the so-called nonclassical ion13 problem, typified by the 2-norbornyl system. Hence, I became involved in the study of “remote” methoxy groups as probes for delocalized cations and their substituent effects on 2-norbornyl solvolysis rates.14 However, Schleyer also encouraged his co-workers and, in particular, postdoctoral fellows to develop independent projects.
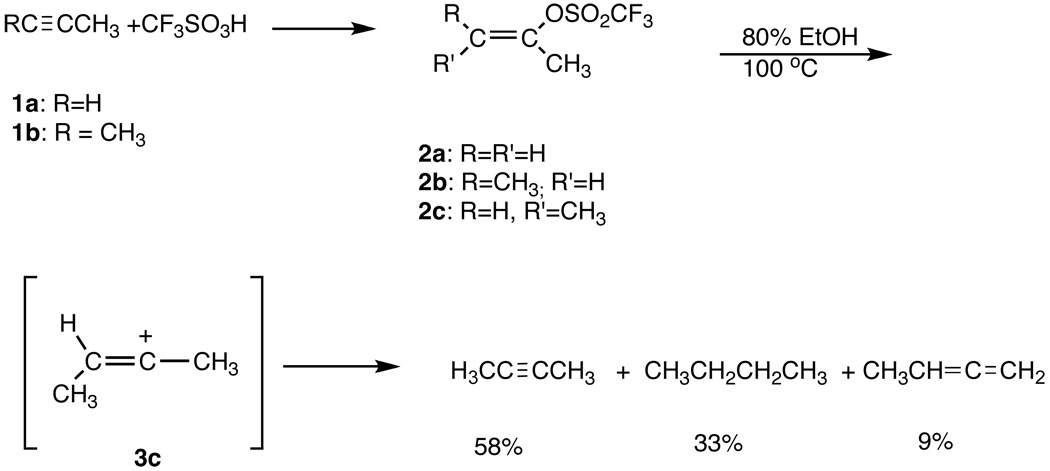
It was at this point that I put two and two together to see if one could generate simple alkylvinyl cations via solvolysis. I told Schleyer about triflic acid and encouraged him to obtain some Ba(OSO2CF3)2 from 3M, which he did. From literature data,15 as well as work in progress in both the Streitwieser12 and Schleyer16 groups, it was known that a triflate was almost 104 times more reactive17 than a comparable tosylate in solvolytic reactions. So the challenge was to prepare a simple alkylvinyl triflate and investigate its solvolytic behavior. Ultimately we succeeded by the direct addition of CF3SO3H to alkynes, in a sealed tube, and established18 that cis-2-buten-2-yl tiflate, 2c, reacted in aqueous ethanol via an SN1 process and the elusive simple alkylvinyl cation, 3c Scheme 1.
Scheme 1.
Solvolytic Generation of a Vinyl Cation18
This was the first example of the preparation of a vinyl trifluoromethanesulfonate (of immense importance in cross coupling reactions, vide infra) and the observation of a simple alkylvinyl cation generated via solvolysis and bond heterolysis.
Throughout my graduate and postdoctoral period I was pretty sure I was interested in an academic career. I liked research, I liked the opportunity of working with young people, I enjoyed teaching (as a TA in Berkeley and as instructor for a year at Princeton) and above all I appreciated the challenge and independence that an academic career afforded. By the late 60's the academic market started to tighten (one of those periodic cycles that seem to occur every 12–15 years). In 1967/68 I interviewed at 6–9 places (I no longer recall the exact number) and received four offers. I was particularly attracted by Utah for a number of reasons: (1) it was a growing department on the move; (2) it had just received an NSF Centers of Excellence Grant; (3) it had just attracted Robert W. Parry from the University of Michigan, a distinguished inorganic chemist, founding editor of Inorganic Chemistry (later ACS President) and was negotiating with Cheves Walling from Columbia University, a distinguished physical-organic chemist and a pre-eminent free-radical expert (already a member of the U.S. National Academy of Sciences, later Editor of JACS); (4) it just completed in 1968 a brand new chemistry building with excellent facilities, (5) my wife to be, Christine, whom I met at Berkeley, although originally from Germany, grew up in San Francisco and was a westerner (as was I after having been in the midwest, B.S. in Chicago, the west at Berkeley and the east at Princeton) and Salt Lake was as far “east” as she was willing to move; (6) I found it to be a very collegial place. So in late 1968 I accepted the offer from the University of Utah, effective July 1 of 1969. I have never regretted this decision and after 40 years and a half dozen outside offers (three as chair of major research institutions) I am still at Utah and will likely remain here for the rest of my career. Many colleagues and friends in the chemical profession thrive by moving, with the challenges and opportunities that a new position and place offer. However, just as many, or even more, thrive by staying at the same place, developing their career parallel with the growth and development of the department at their “home” institution.
Independent Career
First Period
Like all young assistant professors I was now faced with starting my own research. I decided to investigate the generation, nature and chemistry of vinyl cations. It was an area that was different from my Ph.D. work, different from the main thrust of my postdoctoral work but still in the realm of physical-organic chemistry that I was trained in, knew something about, and enjoyed. Moreover, and most important, it was my own idea, developed all on my own from careful observations at Berkeley, reading the literature and with encouragement from my mentors. Furthermore, I had excellent preliminary results as described above,18 that served as a platform for starting an independent career in, at that time, a very active and highly visible area.
The challenge was to generalize the formation of vinyl triflates so that any and all types of alkenyl triflates could be made and investigated, not just the limited number and type accessible by addition of CF3SO3H to alkynes. We were fortunate to discover (independently, but simultaneously with Schleyer and Hanack) that this could be accomplished by reacting enolizable carbonyl compounds, such as aldehydes and ketones with triflic anhydride, (CF3SO2)2O, in the presence of a weak base.17,19 To this date, this is the way all vinyl(enol) triflates are prepared and currently widely used in cross-coupling reactions.20 For us, this opened up the entire spectrum of solvolytic generation of a variety of vinyl cations4a,21 and allowed us to examine cyclic vinyl cations;22 rearrangements of vinyl cations;19b,23 deuterium isotope effects in the generation of vinyl cations24 and the effect different substituents, various solvents and nucleophiles25 have on their generation. We also examined Friedel-Crafts type alkylation of aromatic substrates using enol triflates.26
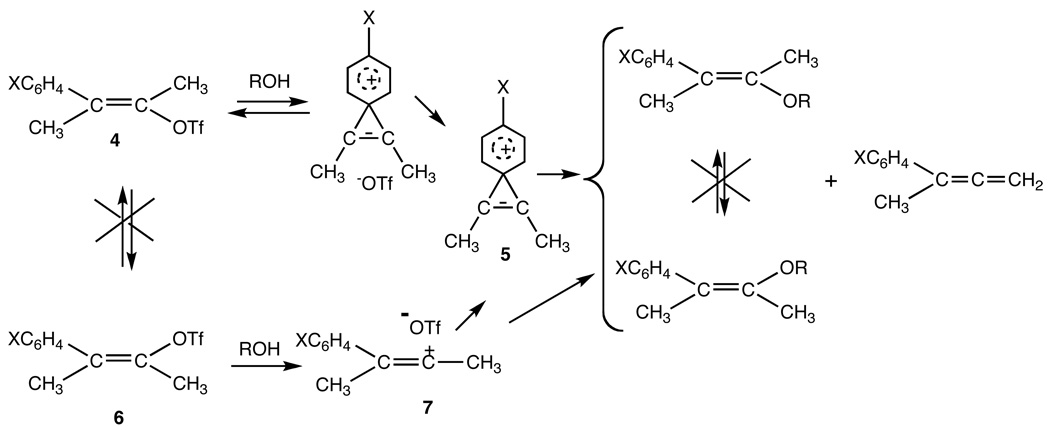
A typical example of our investigations of vinyl cations was the detailed study of the solvolysis of cisand trans-3-phenyl-2-buten-2-yl triflates. Using kinetic deuterium isotope effects, Hammett sigma-rho effects, stereochemistry and product studies, we established that the E-isomer, 4, reacted via a vinylidene phenonium ion, 5, analogous to the well-known σ-bridged phenonium ion,27 first prepared by Cram,28 whereas the Z-isomer, 6, reacted via the open vinyl cation, 7, as shown in Scheme 2.24a,29
Scheme 2.
Mechanism of reaction of E-3, and Z-3-aryl-2-buten-2-yl triflates.29
Of course we were not alone in our study of vinyl cations; as in any active area there were many investigators, key among them, C. Grob (Basel), M. Hanack (Tübingen); G. Modena (Padova); R.G. Bergman (then Caltech); P.v‥ Schleyer (then Princeton), and Z. Rappoport (Jerusalem), as described and summarized in our two monographs on this topic.21b,c Hence, by the late 70's and early 80's vinyl cations were fully accepted members of the family of reactive intermediates, and in fact observed30 by NMR, analogous to the NMR observation of carbocations in the seminal work of Olah.5 Therefore, it was time to move on to a different area. Moreover, one needed to be active and productive in more than one area to be successful in academia.
While investigating vinyl cations, taking advantage of the superior17 leaving group ability (nucleofugality) of triflates, it occurred to us that this superior nucleofugality of triflates could also be applied to the generation of unsaturated carbenes from primary vinyl triflates and cognates.
A large amount of work had been done in carbene chemistry since the pioneering studies of H. Meerwein, J. Hine, W. v. E. Doering, P. Skell and G. Closs.31 Besides simple carbenes, 8, vinylidene carbenes, 9a, and allenidene carbenes, 9b, were already known. Vinylidene carbenes, 9a, were generated via the elimination of HX from primary vinyl halides by bases,32 deamination of vinyl amines,33 photofragmentation,34 and base decomposition of 5,5-disubstituted N-nitrosooxazolidones,35 none of which were ideal, whereas allenidene carbenes, 9b were mostly generated from popargyl halides and/or haloallenes in the presence of bases.36
In the 70's and early 80's we established37 that primary vinyl triflates,38 (R)2C=CHOSO2CF3, and silylvinyl triflates,39 (R)2C=C(SiMe3)OSO2CF3, serve as progenitors par excellence for vinylidene carbenes, 9a. We showed that triflate generated carbenes are singlets,40 electrophilic41 and behave as free carbenes42 rather than carbenoids.31,43 We demonstrated via stereochemical and computational investigations that R2C=C: addition to alkenes is stereoselective and proceeds via a “C2-inward” transition state.44 Via isotopic labeling we determined45 that the rearrangement of β-aryl or β-alkyl vinylidene carbenes to afford alkynes occur via the free carbene rather than by the Fritsch-Buttenberg-Wiechell type of organometallic intermediate46 and a carbenoid.
Addition of R2C=C: to π-systems provided a simple and general means of entry to interesting small and strained ring compounds,47 including alkyledenecyclopropenes,48 10 and m-xylylene,49 11a. The parent m-xylylene, 11b, was just recently spectroscopically fully characterized and investigated via matrix isolation.50 Likewise, reaction with azoarenes afforded 2(H)-Indazoles, 12, via an ylide type process.51 Furthermore, reaction with isonitriles gave heterocumulenes,52 13, and interaction with isocyanates resulted in vinyl carbamates,53 14.
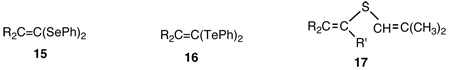
Insertion of R2C=C: into diphenyl diselenide and diphenyl ditelluride yielded little known phenylseleno, 15, and phenyltelluro, 16, ketals, respectively.54
We also established that insertion of unsaturated carbenes into Si-H bonds occurs stereospecifically via a concerted three-centered transition state55 analogous to the insertion of Br2C: and Cl2C: into chiral NpPhMeSiH.56 Insertion of R2C=C: into enethiols gave divinylsulfides, 17.57
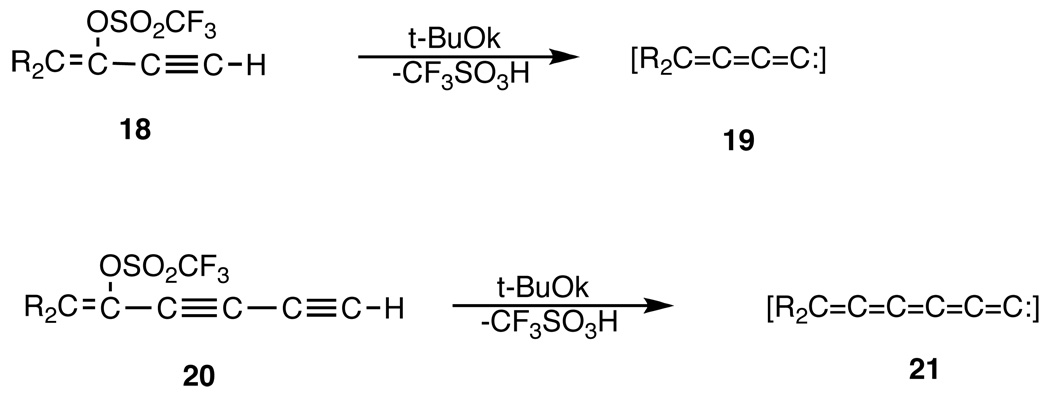
We were also able to extend our unsaturated carbene studies to previously unknown, longer, congeners, alkadienylidenecarbenes, 19, and alkatetraenylidenecarbenes, 21 as shown in Scheme 3.37 Carbene 19 was readily available from 1-(ethynyl)-vinyl triflates,58 18, and 21 from 1-(butadiynyl)-vinyl triflates,59 20. As previously, we established60,61 that both 19 and 21 are singlets, electrophilic and free carbenes. Insertion of 19 into group 4 hydrides resulted in the formation of novel Si, Ge, and Sn functionalized cumulenes, 22.62
Scheme 3.
Generation of extended unsaturated carbenes.
The impact and legacy of this work in unsaturated reactive intermediates is interesting. Vinyl (enol) triflates, that are the precursors to the solvolytic generation of vinyl cations and that we were the first to prepare and report,18,19 are now widely used in metal catalyzed cross coupling protocols.20 Enol triflates serve as the premier electrophilic coupling partners in a variety of cross coupling reactions that are of importance in natural product synthesis63 as well as materials science.
Unsaturated carbenes have recently been found and are among the most abundant carbon containing species in interstellar space.64,65 Moreover, metal vinylidenes and allenylidenes and metallacumulenes are extensively employed in alkene metathesis66,67 and various catalytic reactions,68 including pericyclic reactions69 and substitution reactions.70
Middle Period
By the mid-1980's, it became obvious to me that we had exhausted the chemistry of unsaturated reactive intermediates. Moreover, in the late 1970's, early 1980's, a senior colleague at Utah wisely counseled me to broaden my horizons and look beyond the generation, nature and chemistry of reactive intermediates as, by this time, the bloom was off classical physical-organic chemistry and, in particular, carbocations and carbenes. At the time, I thought that there may still be interest in the solvolytic generation of the elusive alkynyl cations RC≡C+, and I describe below how our quest for this high-energy species led us to polyvalent iodine chemistry. However, I also took this advice to heart and began looking for new areas. Furthermore, I enjoyed the challenge of venturing into new territory and learning and mastering new chemistry. Initially, we explored two areas that were tangential to and an outgrowth of our previous work. First, our interest in selenium, tellurium, germanium and tin compounds, 15, 16, and 22, led us to organometallic chemistry. Hence, we explored the interaction of various organometallic nucleophiles with our vinyl, 2, ethynylvinyl, 18, and butadiynylvinyl, 20, triflates. We discovered that these precursors readily undergo reactions with cobalt species to give vinyl-cobaloxime complexes,71 23, and σ-butatrienyl
 |
cobaloxime complexes72 24. Likewise, vinyl triflates as well as, 18, readily react with (Ph3P)4Pt to yield four-coordinate cationic σ-vinyl Pt(II) complexes73 and σ-butatrienyl cationic Pt(II) complexes.74 Similarly, we explored the reaction of Vaska’s compound, (Ph3P)2Ir(CO)Cl, with various triflates.75 We examined the mechanism of formation and the chemistry of vinylic acyl Pt(II) complexes.76 We investigated the formation of heterobimetallic Ir-Pt complexes.77 We prepared and studied Rh and Pt π- cumulene complexes,78,79 25, and 26 and 27, 28 below. We also investigated the mechanism, oxidative addition80 and reductive elimination,81 of metal mediated vinylic cross-coupling reactions, using our previously prepared, readily available vinyl(enol) triflates.
This brief excursion into organometallics at this stage of my career, and what I learned about various metals and their complexes, in particular platinum chemistry, served me well later when we employed metal-ligand interactions in abiological self-assembly (vide infra).
Second, a combination of my interest in small and strained ring chemistry via alkylidene carbine additions to π-bonds, briefly described above, and a fortuitous event in 1982, got us involved in alkylidenecycloproparenes. That year Professor Brian Halton, from Victoria University in Wellington, New Zealand, spent a year with me at Utah as a senior Fulbright Scholar. Brian’s interest and expertise was in strained organic molecules and, in particular, cycloproparenes.82 Because of their strain and consequent high energy, such molecules have novel physical, spectral and chemical properties that are both of theoretical as well as synthetic interest and challenge.83 Among the intriguing and more strained hydrocarbons are the ortho-bridged aromatics. The parent member of the family, benzyne, 29 is easily generated and well known as a transient intermediate,84 but observed spectroscopically only in an Armatrix at 8K.85 Cyclopropabenzene, 30, the next homologue, is an isolable compound, despite its nearly 70 kcal/mole strain energy.82 The cross-conjugated fulvenes,86 31, and radialenes, 32, represent another class of alluring strained hydrocarbons.83
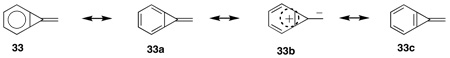
Hence, Brian and I decided to combine our expertise to investigate the preparation, characterization, properties and chemistry of the then unknown alkylidenecycloproparenes, e.g. 33, a unique class of strained hydrocarbons that combine into a single molecular framework the features of the ortho-bridged benzene 30, and the cross-conjugated triafulvene, 31, and 32:
Due to the then unknown nature of 33, and its anticipated high strain of about 80 kcal/mole, and hence possible instability at room temperature, we decided to focus our initial efforts on 38 for two reasons. First, strained derivatives of naphthalene are known84 to be more stable than those of benzene and, furthermore, alkyl substituents stabilize double bonds. Second, we anticipated that 37 would be easily accessible via an alkylidecarbene, 34, addition to tetrahydronaphthalene, 35, a reaction known87 to give adduct, 36, followed by oxidation to the desired 38 as shown in Scheme 4. However, all attempts to oxidize 36 to 38, with numerous reagents under a wide variety of conditions, failed. Only unreacted starting material and/or partially oxidized hydrocarbon, 37, was observed.88
Scheme 4.
Preparation of alkylidenecycloproparene 38
Brian’s year at Utah, from a chemical perspective, was a year of frustration, with no progress and publication. However, after Brian’s return to Wellington, the alternative Peterson olefination89 of 39 worked well88; that started a decade-plus long trans-oceanic collaboration90 with Brian, which resulted not only in nearly 20 joint publications, but also a lifelong friendship. In a collaborative fashion we investigated the electrochemical properties,91 photoelectron spectra,92 polarity,93 13C-NMR94 and ambiphilicity95 of these novel strained hydrocarbons. We explored their reactions with both electrophiles96 and nucleophiles.97 We used them as unique ligands in the formation of interesting Pt, Pd, and Rh-complexes.98 We prepared and characterized related benzocalicenes and benzotriaheptafulvalenes.99
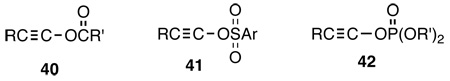
During this period, we were still intrigued by the possible solvolytic generation of alkynyl cations, RC≡C+, the only remaining member of the family of ubiquitous carbocations that were not known in solution. Although they were detected in interstellar space,100 and in the gas phase by mass spectrometry,101 there was no evidence of their formation in the condensed phase. In fact, both experimental data and theoretical calculations indicated that the parent ethynyl cation, HC≡C+ has a heat of formation of about 400 kcal/mole.102 However, given the superior leaving ability of triflate, I reasoned that alkynyl triflates, like ArC≡COSO2CF3 might afford this elusive species in solution. Unfortunately, alkynyl esters of any kind, carboxylate, 40, sulfonate, 41, or phosphate, 42, were unknown compounds prior to the 1980's. At this point two serendipitous events occurred that resulted in a major change and new direction in my research. I had a phone conversation with a former Ph.D. student of mine, Albert G. Anderson (Ph.D. 1977, University of Utah), who was at Central Research at DuPont, and I mentioned the fact that alkynyl esters were unknown. He suggested that I consider using iodonium species as possible progenitors and called my attention to the seminal work of Koser103 on alkynyl tosylates that appeared in 1981. This was the beginning of my interest in polyvalent iodine chemistry that occupied my interest for over a decade, from the mid 1980's onward and ultimately also led to my involvement in abiological self-assembly (vide infra). My interest and involvement in polyvalent iodine species was further facilitated by the arrival of a very talented postdoctoral fellow, Dr. Viktor Zhdankin from N. Zefirov’s group at Moscow State University in Russia, during this time. He was not only very talented, dedicated, hardworking and creative, but was already familiar with polyvalent iodine species as well as the extensive Russian literature in this area.
Iodine was discovered in 1811 and most commonly occurs in the monovalent form with an oxidation state of −1 in organoiodine compounds. However, because it is the largest, most polarizable and least electronegative of the halogens, iodine also readily forms stable, polycoordinate, high-valent compounds, generally as I(III) and I(V) species. Over 120 years ago, the German chemist, C. Willgerodt reported104 the preparaton of PhICl2, followed by the preparation105 of PhI(OAc)2 and numerous106 diaryliodonium salts, Ar2I+X−. Since then, interest and activity in multi-coordinate iodine species waxed and waned until the early 1980's when the chemistry of hypervalent, multi-coordinate iodine experienced a renaissance.107
Our primary interest in this area was focused on alkynyliodonium species,108 RC≡CI+Ph, as potential progenitors of alkynyl sulfonate esters, 41 and, in particular, alkynyl triflates as possible solvolytic precursors of RC≡C+. Beringer and Galton109 were the first to report an alkynyliodonium species, 43, but only in 10–20% yield (Scheme 5). Moreover, 43 decomposed at room temperature in a few hours, as shown in Scheme 5. The first stable, crystalline iodonium salt, 44,
 |
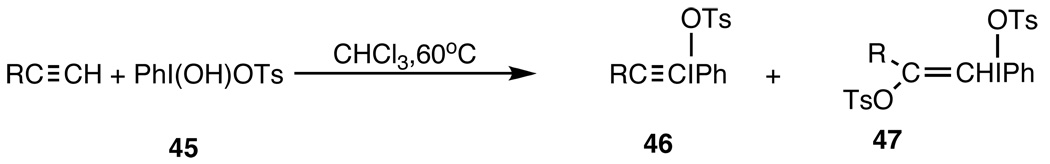
was reported by Merkushev and co-workers,110 but it was characterized only by IR and its hydrolysis product. A major advance was made by Koser and co-workers103 in the formation of alkynyl(phenyl)iodonium tosylates, 46, via addition of what has become known as “Koser’s reagent,” 45,111 to terminal alkynes. Although this early work had serious limitations,108 it galvanized the field. Improvements rapidly followed so that by the mid-1980's a variety of alkynyl(phenyl)iodonium salts were readily available as stable microcrystalline compounds.112,113
Scheme 5.
Formation and decomposition of 43.
Arguably, the most general and widely used methods114 of preparing alkynyliodonium species involves alkynylsilanes and in situ Zefirov’s reagent,115 48, or the use of stannylalkynes,116 49, and iodonium triflate, 50,117 as shown in Scheme 7. This later methodology affords a wide variety of β- functionalized alkynyliodonium salts, 51. This procedure is also applicable to the preparation of the parent system,118 HC≡CI+Ph −OTf, as well as PhI+C≡CI+Ph 2−OTf,119 and other bis-alkynyliodonium compounds.120 Similar procedures have been developed for the ready preparation of alkenyliodonium species,121–123 as well as heteroaryliodonium salts.124
Scheme 7.
Formation of alkynyl(phenyl)iodonium triflates.
All pure alkynyliodonium species are microcrystalline solids sparingly soluble in most organic solvents, the best solvent being acetonitrile. Their stability depends both on the counter-anion and the β-substituent on the alkyne. The more nucleophilic the counter-ion, the less stable the alkynyliodonium compound, the most stable and hence most widely employed being the triflates, tetrafluoroborates and tosylates. They have highly characteristic signals in both the IR and NMR; in particular C-13 NMR.125 X-ray analysis indicates a pseudobipyramidal or T-shaped structure, 52, with the alkyne and counter ion in an axial arrangement, and the phenyl group and two lone pairs on the iodine in the equatorial position,118a,126 in accord with the hypervalent (10-I-3) nature of these salts.127
The reactions of alkynyliodonium salts may be described in three broad classes: (1) interaction with nucleophiles; (2) cycloadditions; (3) cross coupling reactions. In a formal sense alkynyliodonium salts can serve as electrophilic acetylene equivalents or as “alkynylating” agents “RC≡C+”. Indeed they undergo reactions with a wide variety of nucleophiles, including C, N, O, S, P, As, Se, Te, and organometallic nucleophiles as described below. The actual mechanism of reaction of course does not involve the high-energy alkynyl cation but goes through ylids and carbenes as summarized in Scheme 8.
Scheme 8.
The mechanism of alkynyliodonium salts via ylids and carbenes.
The preponderance of evidence107c,d indicates that the mechanism of these reactions involves a conjugate addition of the nucleophile to the electron-deficient β-carbon to form an iodonium ylide, 53, (Scheme 8). Loss of iodobenzene forms the alkylidenecarbene, 54, which rearranges via migration of either R or the nucleophile to provide the final product, 56. Among the evidence for this mechanism is that in the presence of acid, ylide 53 can be trapped to give stable alkenyliodonium salts, 55. Moreover, if either the alkyl group or the Nu on the β-carbon of 54 has a 1,5-C-H bond, the carbene can insert and provide a cyclopentene or five-membered heterocycle 57 (vide infra). Both the ylide, 53, and carbene, 54, have been trapped.128 The addition of the nucleophile to the β-alkynyl carbon (Michael type addition) is facilitated by the strong inductive electron-withdrawing nature of the PhI+ moiety (σI=1.24 for PhI+ vs. σI=0.39 for I-itself).129
Only “soft” carbon nucleophiles, such as β-dicarbonyl compounds, react with alkynyliodonium salts; enolates of simple aldehydes, or ketones analogous to hard nucleophiles, like RO−, result in decomposition products. This reaction represents a convenient way of directly introducing the RC≡C-group into a variety of β-dicarbonyl compounds.130
Reaction with R1R2NLi affords aminoalkynes RC≡CNR1R2.131 Sulfur nucleophiles provide ready access to RC≡CSCN,132 RC≡CS(O)2Ar,133 RC≡CSS(O)2Ar,134 and RC≡CP(S)(OR1)2.135 Phosphorus nucleophiles provide alkynyl phosphonium salts, RC≡CP+Ph3X−,136 and RC≡CP(O)(OR1)2.137 Acetylenic arsonium salts, RC≡CAs+Ph3BF4−,138 selenides, RC≡CSeAr,139 and tellurides, RC≡CTeAr139 are obtained from reactions with Ph3As, NaSeAr and NaTeAr, respectively. Organometallic nucleophiles, such as (Ph3P)2M(CO)(Cl) M=Ir, Rh, and others, provide interesting organometallic complexes.120c,140,141
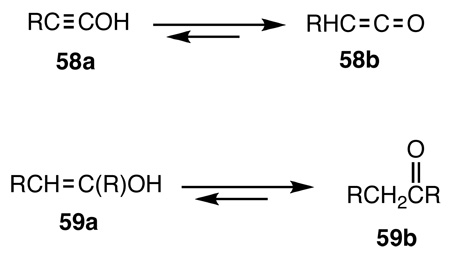
However, the most interesting reaction is the formation of the previously unknown alkynyl esters, 40–42, that combine two of the most common and important functional groups in organic chemistry, namely the carbon-carbon triple bond and esters into a single moiety.142 Simple carboxylate, phosphate and sulfonate esters are important in synthetic, mechanistic and biochemistry and are so ubiquitous that they are often taken for granted by most chemists. As described in all introductory text books of organic chemistry, esters are most readily made by the reaction of acid halides, RC(O)X, (RO)2P(O)X, RS(O)2X, with an alcohol. Likewise, vinyl(enol) esters are made by the reaction of enolates with acid halides. Therein was the problem. The preparation of alkynyl esters by an analogous process requires hydroxyalkynes, 58a, or ynols that are the triple-bond analogs of enols, 59a. Whereas enols are readily accessible,143 in the case of ynols the tautomeric equilibrium is completely on the side of ketenes, 58b, and hence ynols 58a are not available. Theoretical calculations144 indicate that the ketene-ynol, CH2=C=O  HC≡COH, energy difference is 37 kcal/mole in favor of the ketene, while the energy difference between CH3CHO and CH2=CHOH is only 14 kcal/mole.
HC≡COH, energy difference is 37 kcal/mole in favor of the ketene, while the energy difference between CH3CHO and CH2=CHOH is only 14 kcal/mole.
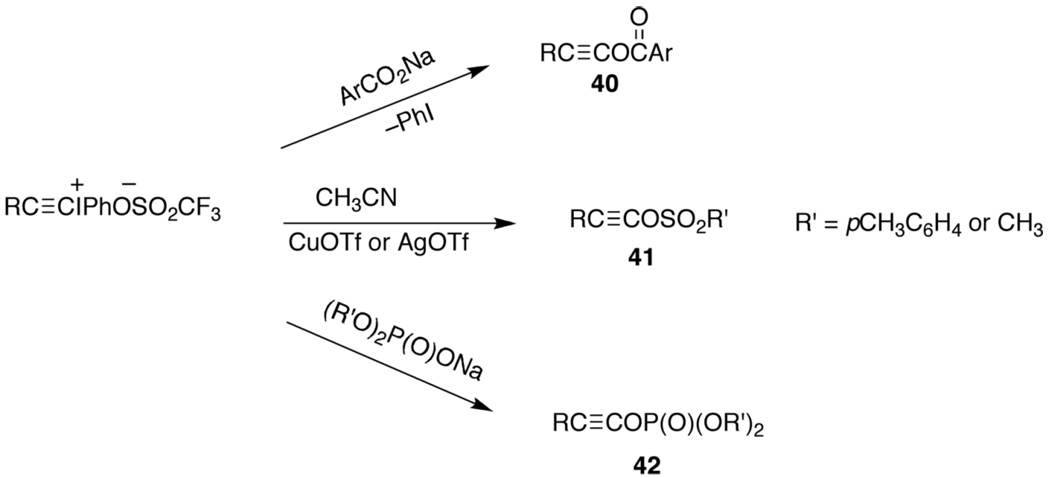
We prepared these unique esters by interaction of alkynyl(phenyl)iodonium triflates with ArCO2Na for the carboxylate esters,145 40, CuOTf or AgOTf for the sulfonate esters,112a,b 41, and (R′O)2P(O)ONa for the phosphate esters,146 42, as shown in Scheme 9. X-ray structure determination of the carboxylate ester,147 40, and the sulfonate ester,148 41, established, as anticipated, that the Csp–O bond is considerably shorter than the Csp2–O bond of ordinary esters with a value of 1.366 Å vs 1.445 Å for carboxylate esters and 1.331 Å vs. 1.465 Å for the sulfonate esters respectively.147,148
Scheme 9.
Preparation of alkynyl esters 40–42.
Acid-catalyzed hydrolysis of these esters proceeds via an AdE2 process involving a rate-limiting proton transfer to the β-carbon and formation of a vinyl cation that rapidly reacts with H2O to give the respective acids as final products.149 Whereas the base-catalyzed reaction involves –OH attack on the acyl moiety, C=O, P=O, SO2, and the usual standard mechanistic steps.149 Since alkynyl esters are electron-rich acetylenes they do not undergo Diels-Alder type cycloadditions but do react with an azete to give interesting Dewar pyridines.150
These alkynyl esters, 40–42, also have interesting biological activity. Both alkynyl carboxylates, 40, and alkynyl phosphates, 42, are potent serine protease inhibitors.151 Moreover, the alkynylphosphate esters, 42, are potent inhibitors of bacterial phosphotriesterase.152 For example, 1-hexynyl diethyl phosphate, n-BuC≡COP(O)(OEt)2 effectively inhibits the phosphotriesterase from Pseudomonas diminuta in less than one minute with <1% residual activity.152b,c As I had no background in biochemistry these studies were carried out in collaboration with Y. Shalitin at the Technion in Isreal and F. Raushel at Texas A. & M.
However, as shown in Scheme 8, the reaction of a nucleophile with alkynyl iodonium salts, besides resulting in a wide variety of functionalized acetylenes, 56, as just described, can take an alternative route. If there is a 1,5-C-H bond available the intermediate carbene, 54, instead of rearranging to 56, will undergo intramolecular C-H insertion, resulting in a cyclopentene, 57. This tandem Michael-addition/carbene-insertion process provides an efficient means to some interesting cyclopentene derivatives.153 We have taken advantage of this process to prepare154 a variety of interesting cyclopentenones with yields of 44–75% at room temperature in CH2Cl2. This provides a nice alternative to the Nazarov155 and Pauson-Khand156,157 reactions for the synthesis of cyclopentenones. Feldman and co-workers have also used this tandem Michael-addition/carbene-insertion procedure to prepare highly substituted dihydropyrrole derivatives158 as well as polycyclic alkaloids.159 This method has also been used to prepare thiazoles160 a well as benzofurans,161 furopyridines162 and 2-mercaptothiazoles.163
The second type of reaction that alkynyl(phenyl)iodonium species undergo is cycloadditions, β- Functionalized alkynyliodonium salts, 51, undergo Diels-Alder cycloadditions with symmetrical dienes114c under mild conditions, to give cycloadducts, 60, and 61, in good yields as shown in Scheme 10, whereas unsymmetrical dienes164 result in mixtures of products with little, if any, regioselectivity. Similarly bisiodonium triflate 62 reacts with furan or cyclopentadiene, at room temperature to give 63 (Scheme 10).119b,165
Scheme 10.
Diels-Alder reactions involving alkynyliodonium salts.
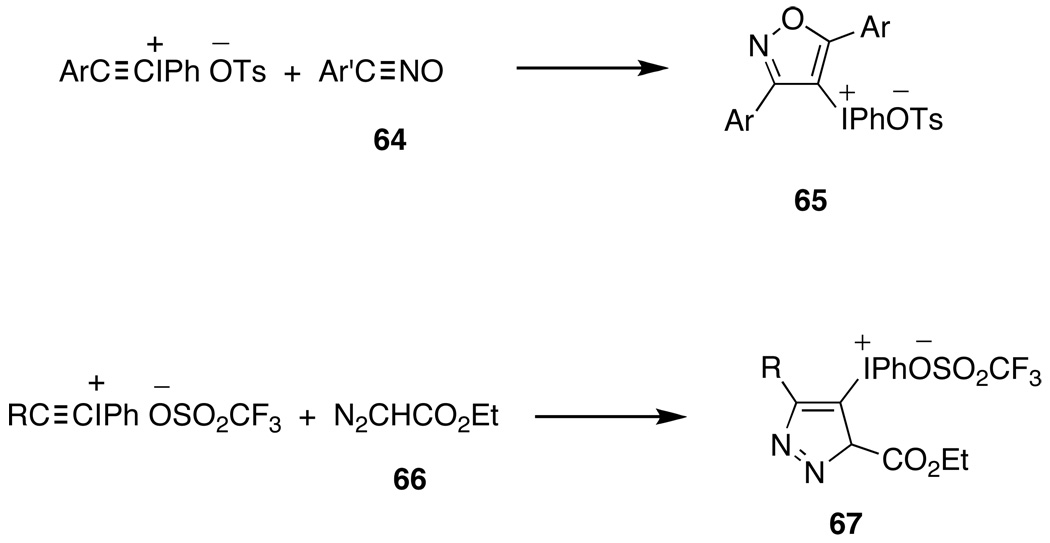
Since alkynyliodonium salts are also excellent 1,3-dipolarophiles, various [3+2]-cycloadditions have been studied. Nitrile oxides, 64, give cycloadducts,166 65, as sole products, whereas ethyl diazoacetate, 66, yields regioisomeric pyrazoles, 67, in only moderate yields as shown in Scheme 11. Organic azides, PhN3 and CH3N3, give triazolyliodonium salts, and diazocarbonyl compounds afford pyrazolyliodonium salts, but only in low yields.168
Scheme 11.
[3+2]-cyclo-additions involving alkynyliodonium salts as 1,3-dipolarophiles.
Because of the superior leaving ability of the PhI+ moiety, various iodonium species are excellent electrophilic partners in a range of metal-catalyzed cross-coupling reactions.169,170 Perhaps the most interesting of these is the preferential and selective coupling of alkenyliodonium salt, 68, with alkynes, in the presence of a triflate in the same molecule, to give conjugated enynes, 69, stereospecifically,171 as shown in Scheme 12.
Scheme 12.
Coupling of alkenyliodonium salt 68 with alkynes.
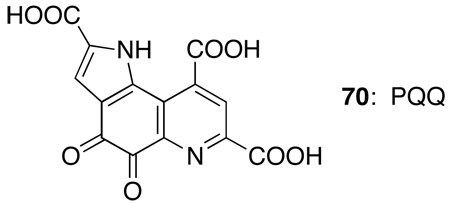
Finally, alkynyliodonium and other iodonium salts have significant biological activity;107b,c specifically alkynyl and aryl mono- and diiodonium salts are effective inhibitors of PQQ, 70, an organic co-factor in certain biological redox processes,172 and have very potent in vitro activity against oral and dental anaerobes.173
Although we did not succeed in our original goal of the generation of alkynyl cations, RC≡C+, that has only been accomplished174 via nuclear decay processes involving tritium and 3He, we have uncovered some very interesting chemistry. The legacy and significance of this work is the increasing use and involvement of polyvalent iodine species in contemporary organic chemistry.175 Examples include Feldman and Cutarrellis116 elegant synthesis of pareitropone,176 a potent anti-cancer alkaloid, the synthesis of discorhabdin,177 and morphinandienone and neospirinedienone178 and many others. Most recently, the unique reactivity of polyvalent iodine reagents in conjunction with Pd(O) and Pd(II) complexes has been exploited for a wide range of synthetically useful organic transformations.179
Third Period
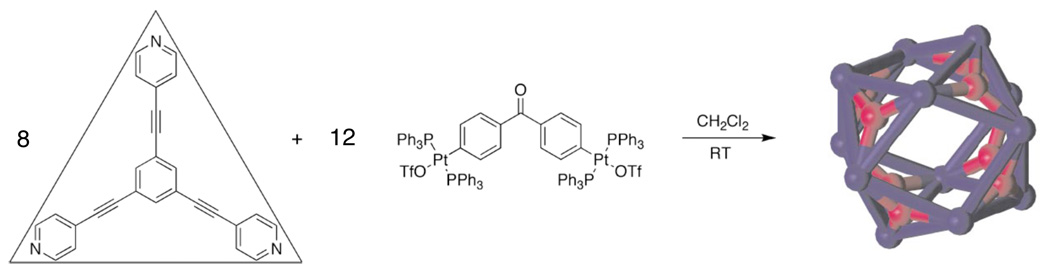
At this juncture, another serendipitous and fortunate event occurred that started and influenced my current interest and involvement in abiological self-assembly. In 1991 I visited the University of Nevada at Reno and gave a seminar on the above described polyvalent iodine chemistry. During the question and answer period, Professor Lawrence Scott (then at Reno, now at Boston College) asked whether, given the pseudotrigonal pyramidal nature and concomitant 90° angle of alkynyliodonium species, 52, I had thought of making macrocyclic systems. I had not, but immediately grasped the significance of his suggestion and, upon returning to Utah, discussed it with my theoretical colleague Jack Simons and with V. Zhdankin, my postdoctoral fellow already mentioned. In collaboration with Jack, we examined the structures, stabilities and vibrational frequencies of square planar tetraalkynyl and tetraaryl tetraiodonium salts,180 71–73, and with Dr. Zhdankin started the synthesis of the triflate salt of 73 as shown in Scheme 13. Although we succeeded in the preparation of 78 from commercially available 74 in just three steps, the overall yield was only 7–10%.181 Moreover, getting satisfactory structure proof (mass spec, NMR, etc) proved to be a challenge and frustration due to the insolubility of 78 and hence this even delayed publication.181 Although more recently we succeeded182 in improving the overall yield for the formation of 78 to 70%; at the time this made me think of how one could make such macrocycles in a simpler, better way in much higher yields. My aforementioned experience in organometallic chemistry, and in particular Pt(II) chemistry and the square planar nature and concomitant 90° angle of Pt(II) complexes, provided the insight that transition metal chemistry, metal-ligand dative interactions183 and likely self-assembly were an answer.
Scheme 13.
Synthesis of macrocyclic tetraaryltetraiodonium compound 78.
Self-assembly is a process whereby appropriate complementary molecular subunits spontaneously assemble according to the specific information encoded within their structures. Nature is the supreme and consummate master of self-assembly and supramolecular chemistry. It embraces the principles of, and adroitly exploits, noncovalent interactions of all types in a multitude of ways to enable and facilitate countless biological processes. All living organisms, from the simplest to humans, depend upon some form of molecular self-assembly. Nature performs the most amazing feats of self-assembly with an artistry and facility we can only admire and all too often take for granted. Protein folding,184 nucleic acid assembly and tertiary structures,185 phospholipid membranes,186 ribosomes, microtubules, etc. are but some representative examples of self-assembly in nature that are of critical importance in living organisms.187 The protein coat of all viruses around their nucleic acid consists of a self-assembled spherical capsid, in the shape of either a dodecahedron or an icosahedron.188
The power and beauty of spontaneous self-assembly derives from its ability to rapidly, and deceptively simply, generate large, complex and sophisticated “supramolecules” from easily available building blocks with maximum efficiency, generally under mild conditions (at or near room temperature, at 1-atm.) in water or other common solvents. Self-assembly depends upon appropriate direction and control being exerted at all stages of the process via preprogramming of the subunits or building blocks such that the requisite recognition elements for self-assembly are contained in the subunits. Nature’s repertoire of information to guide self-assembly includes hydrogen bonding, π-π stacking, hydrophobic-hydrophilic interactions, electrostatic and van der Waals interactions, conformations, etc., all commonly referred to as “weak interactions.” Among the difficulties encountered in our attempts to mimic nature’s elegant self-assembly processes, particularly in the formation of ensembles with well defined shapes and sizes such as polyhedra like dodecahedra, is our inability to use the directionality of the “weaker interactions” nature employs. In contrast, because of d-orbital involvement, dative, metal-ligand bonds are highly directional. Moreover, 3rd row metal ligand bonds have bond energies of ca. 15–25 kcal/mol, much less than covalent bonds (ca. 60–120 kcal/mol) but stronger than the weak interactions of biology (ca. 0.5–10 kcal/mol). Hence, coordination kinetics can be modulated to engage in self-repair to achieve thermodynamic control of superstructure. Furthermore, by being stronger than weak interactions one dative metal-ligand bond can replace several hydrogen bonds in the self-assembly process.
An important and critical feature of coordination driven, and indeed all self-assembly processes, is that they must occur under thermodynamic control with kinetically rapid reversible equilibria between starting materials, intermediates and products. As the equilibrium is reversible, the process is self-correcting: an “incorrectly” formed bond can dissociate and reassociate “correctly.” However, to be practically useful, any thermodynamic self-assembly process must generate one product, which is substantially more stable than any starting material, intermediate or competitors, thus ensuring a near-quantitative yield. This is generally, albeit not always, the case in coordination driven self-assembly. In such a thermodynamically controlled, kinetically rapid, self-assembly process, all components are continuously being formed and are in thermodynamic equilibrium.189,190
Elegant, pioneering work by J.-M. Lehn and J.-P. Sauvage (Strasbourg) demonstrated191 the feasibility and usefulness of coordination driven self-assembly in the formation of infinite helicates, grids, ladders and racks.192 Inspired by this work, my interest in molecular squares such as 71–73, and my experience with square planar Pt(II), the logical place to start was the formation of supramolecular squares via coordination driven self-assembly. Indeed, reaction of dppp-chelated Pt and Pd bis-triflates 79 with dipyridine afforded193 the desired squares 80, in essentially quantitative yields as determined by NMR and high isolated yields, as shown in Scheme 14. Furthermore, X-ray data unambiguously established the structure and exact geometry (Figure 1a) as well as the novel solid-state packing of the cationic portion of molecular square 80a (Figure 1b).
Scheme 14.
Self-Assembly of dppp-chelated Pt and Pd macrocyclic molecular squares 80.
Figure 1.
(a) Geometry of Pt-square 80a based upon X-ray data; (b) solid-state stacking diagram of the cationic part of 80a.
This initial success was rapidly followed by the formation of a wide variety of squares of different sizes and composition194 such as mixed,195 neutral-charged Pt-Pt and Pt-Pd, as well as hybrid196 Pt-iodonium, early-late197 transition metal, large nanoscale198 and prophyrin199 based squares, all in essentially quantitative yields.
These achievements in turn made me think of the broader implications and advantages of metal directed and driven abiological self-assembly and in particular of different types of finite, closed, rigid 2D and 3D architectures with pre-designed shapes and sizes. As a consequence, together with Bogdan Olenyuk (then a graduate student at Utah, now an Assistant Professor of Chemistry at the University of Arizona) we devloped200 the so called “directional bonding” approach for the ready, modular assembly of a wide range of 2D polygons and 3D cages as illustrated in Figure 2. This methodology combines rigid electron-poor metal centers and complementary rigid electron-rich organic donors. The two most significant factors that largely determine the supramolecular structure so obtained are the shape and size of the individual component-building units (tectons). The shape of the donor/acceptor units is dominated by the turning angle, defined as the angle formed between the open valencies of a ditopic or tritopic donor or acceptor. For example, as seen in Figure 2, four 90° square planar Pt-units in combination with four linear donor units, such as bipyridine, afford a A24L24 molecular square or eight mutually perpendicular, tritopic angular, units in combination with 12 ditopic, linear units, can form a A38L212 molecular cube. Furthermore, the size of the individual building units determines the overall size of the self-assembled supramolecular species. As previously described,200b symmetry considerations also play an important role in this abiological self-assembly protocol.
Figure 2.
Directional bonding approach to the formation of 2D polygons and 3D cages via the combination of various angular (A) and linear (L) tectons.
Our “directional bonding” approach, which has been widely used and adopted,201 allowed us to prepare other polygons such as triangles,202 various rhomboids,203 diverse rectangles204 and hexagons,205 all in near quantitative yields as expected for a self-correcting, self-healing process.
Of course, as in all active, cutting-edge research such as coordination driven self-assembly, we were and are not alone in the field. M. Fujita (University of Tokyo) and co-workers were pioneers in this area and reported the self-assembly of the first molecular square206 as well as many other interesting metallacycles and metallacages.207 K. Raymond (UC-Berkeley) and co-workers208 have developed and applied a protocol based upon incommensurate n-fold symmetry axes, and C. Mirkin (Northwestern) and co-workers pioneered a methodology based upon the weak-link approach209 and, more recently, a new protocol using a halide induced ligand rearrangement reaction.210 The late Professor Albert Cotton and co-workers employed di-metal building blocks in their approach.211
We proceeded to investigate both 2D212 and 3D213 chiral self-assembled supramolecular species. Chiral supramolecular systems214 are particularly interesting because of their applications in asymmetric catalysis, chiral-host-guest events, chiral sensing, and their potential to mimic complex biological processes.
Likewise, functionalized supramolecular metal-organic assemblies are of considerable interest because of their potential uses in a variety of electronic, magnetic, catalytic, photonic, mechanic and sensor applications.215 There are at least three ways of incorporating functionalities into metal-organic supramolecular architectures: (1) edge or corner functionalization; (2) “inside” or endo functionalization via covalent attachment; (3) “outside” or exo functionalization via covalent linking as shown in Figure 3.
Figure 3.
Different ways of incorporating functionality into self-assembled supramolecular metallacycles.
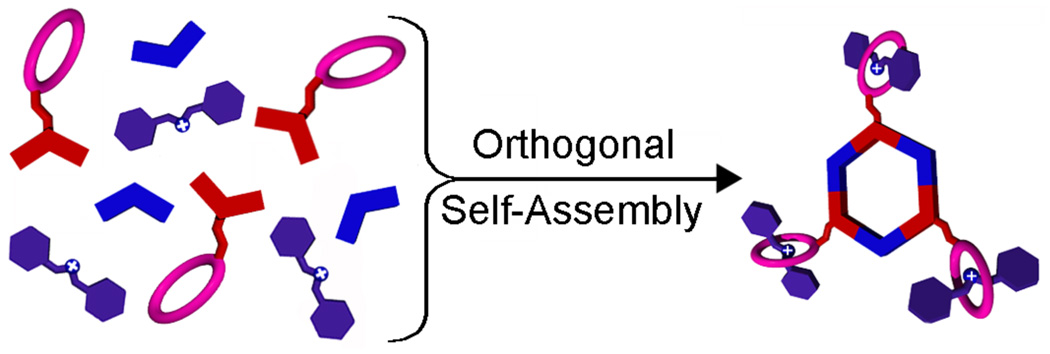
Initially we prepared corner functionalized ferrocene216 and crown ether and calixrene217 systems; more recently we self-assembled various edge functionalized metal-organic architectures.218 Most recently we self-assembled a series of dendrimer,219 ferrocene,220 and crown-ether221 exo-functionalized architectures. A novel aspect of this work is the direct “one pot” self-assembly, via orthogonal none-covalent interactions of hexagonal cavity-cored poly[2]pseudorotaxanes221 as shown in Figure 4.
Figure 4.
Orthogonal, one-pot self-assembly of poly[2]pseudorotaxanes.
Similarly we have developed a functionalized rectangle as a chromogenic supramolecular optical sensor for Ni(II), Cd(II) and Cr(III) as illustrated in Scheme 15.
Scheme 15.
Supramolecular chromogenic sensor for Ni, Cd, and Cr.
The vast majority of self-assembled supramolecular metallacycles are charged.223 However, recently we224 and others225 have also prepared a range of neutral supramolecular ensembles via self-assembly. Similarly, ambidentate ligands, usually a pyridyl carboxylate, have also been employed226,227 in the formation of self-assembled metallacycles.
In some cases, instead of a simple product, there is an equilibrium between two species such as a triangle and square202b or a rhomboid and hexagon.228 In some instances there is also restricted rotation around the Pt-N bond199b,229 resulting in AB multiplets due to the inequivalent α- and β- protons of the pyridyl moiety. This has been investigated using variable temperature NMR by us230 and others,231 and a free energy of activation, ΔG‡, for the rotation of the bipyridine unit was determined to be about 74 kJ/mol.
The mutual recognition of complementary components within a complex mixture, that is self-sorting, is a critical phenomenon in biological systems. Self-sorting allows for the formation of multiple higher-order supramolecular structures from complex multicomponent mixtures when specific recognition information is encoded within the structure of molecular subunits.232 Therefore, we,233 and many other groups,234 investigated self-sorting in metal-ligand coordination driven abiological self-assembly. As shown in Scheme 16, only head-to-tail ensembles, 83 and 85 were observed,233c with no trace of head-to-head analogs in the reaction of the ambidentate pyridylcarboxylate unit 81 with acceptors 82 and 84, respectively. Likewise, over 90% self-recognition, with but minor amounts of oligomers, and formation of the respective rectangle 87, square 88, and triangle 89, were observed,233d after equilibration for about 120 hours, in the reaction of mixtures of tectons, 82, 84, and 86 with 4,4′-bipyridine, as shown in Scheme 17. Hence, these coordination driven abiological self-assembly processes undergo self-selection via self-recognition analogous to biological systems and provide further evidence for the thermodynamic control, via fairly rapid kinetic pathways, in the self-assembly of these nanoscale supramolecular ensembles.
Scheme 16.
Self-selection in self-assembly.
Scheme 17.
Self-recognition in self-assembly.
Molecular-based devices,235 e.g. molecules that possess the ability to act as sensors, switches, motors, machines, memory components, etc. - represent perhaps the ultimate in minaturization of functional machinery. Such devices, when properly designed and controlled, constitute the backbone of the nascent nanotechnology that has the potential to revolutionize manufacturing and consumer goods in the 21st century. Nanotechnology, however, will only develop to its full potential if preceded by nanoscience: a solid foundation and understanding of the fundamental properties of molecules at the nanoscale and of “bottom up” nanofabrication.236
As already described above, the synthesis and characterization of self-assembled 2D and 3D coordination based supramolecular architectures is well established, as are their solution and solid-state properties.229 Very little is known, however, about their behavior on surfaces. While solution phase studies are essential for the establishment and understanding of many of the configurational, physical, optical, and dynamic properties of molecules, and solid-state studies provide unequivocal structural information, neither phase is ideal for the design and fabrication of molecular devices.237 Where the solution state lacks coherence and orientational control, and the solid state often imparts too much rigidity and confinement, the deposition of molecules and supramolecules on surfaces provides238 a means of controlling and integrating them with other known materials in typical device settings, provided that they can be aligned, distributed, and addressed appropriately. For these reasons, investigating the behavior and properties of these species on surfaces will provide the necessary nanoscience foundation that is critical for the design, development, and fabrication of nanomolecular devices.
The advantages of incorporating functional organic and organometallic molecules into device settings by integrating them onto/into surfaces have been known for some time. Surface confined molecular films that contain functional electronic materials include photovoltaic devices, optical and non-linear optical materials, light emitting diodes, and field-effect transistors.239 The reduced symmetry of surface confined, molecular film-based components and devices offers the opportunity to systematically manipulate and tune their collective optical, electronic and other properties through molecular design in a manner that is often more effective than may be achieved by the same components in the less coherent solution phase. Even with the great many successes239 of molecular film-based devices, the use of self-assembled supramolecular metallacycles provide additional advantages owing to the ease with which large, symmetric, structurally complex polygons and polyhedra can be prepared. It is in this regard that the incorporation of metallacycles onto surfaces can be considered multi-tiered self-assembly: the supramolecules are first themselves self-assembled and then they are collectively self-assembled onto the desired surface. Taking advantage of thermodynamically-driven self-assembly processes at each step avoids many of the time consuming and costly elements of device fabrication, such as multistep covalent synthetic procedures, high-vacuum sublimation-deposition of monolayers, harsh surface treatments necessary for etching, and the like.
The primary method of characterizing and understanding surface confined nanoscale molecules is scanning probe microscopy237 and, in particular, the primary instruments are scanning tunneling microscopes (STM) and electrochemical STM (ECSTM).240 STM has unparalleled spacial resolution and is thereby able to provide real-time, real-space, 3D surface structural information up to atomic resolution in the exploration of single molecules and their aggregates on surfaces.240
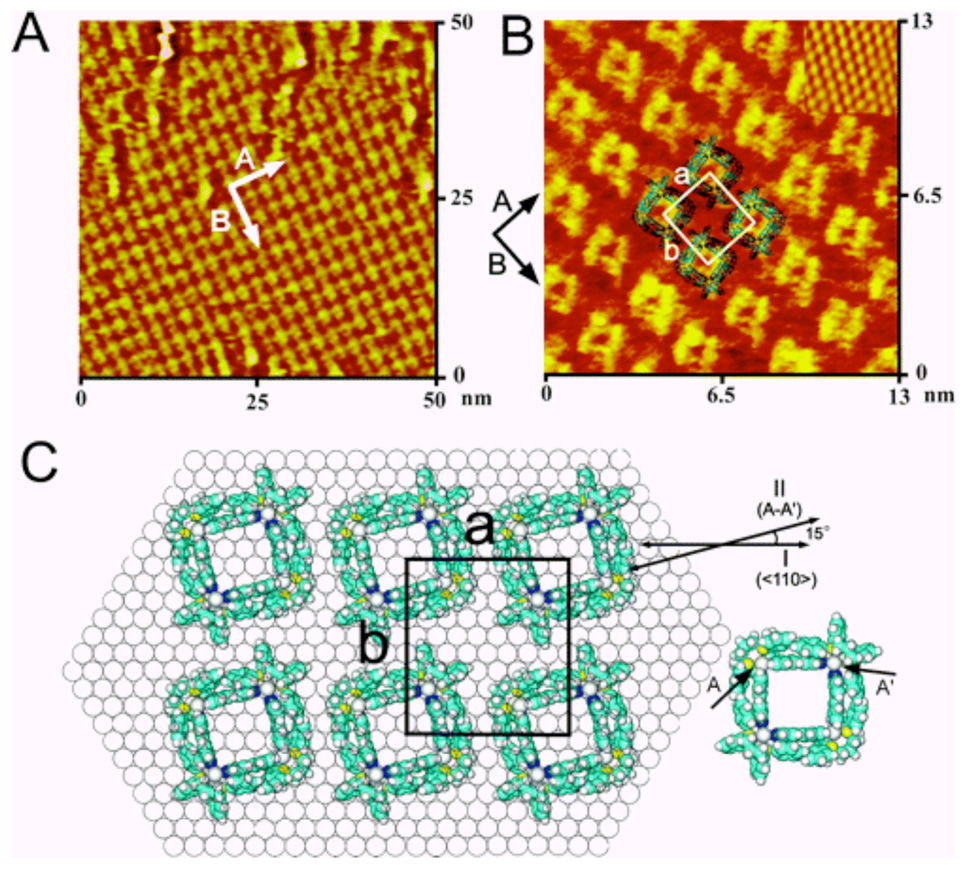
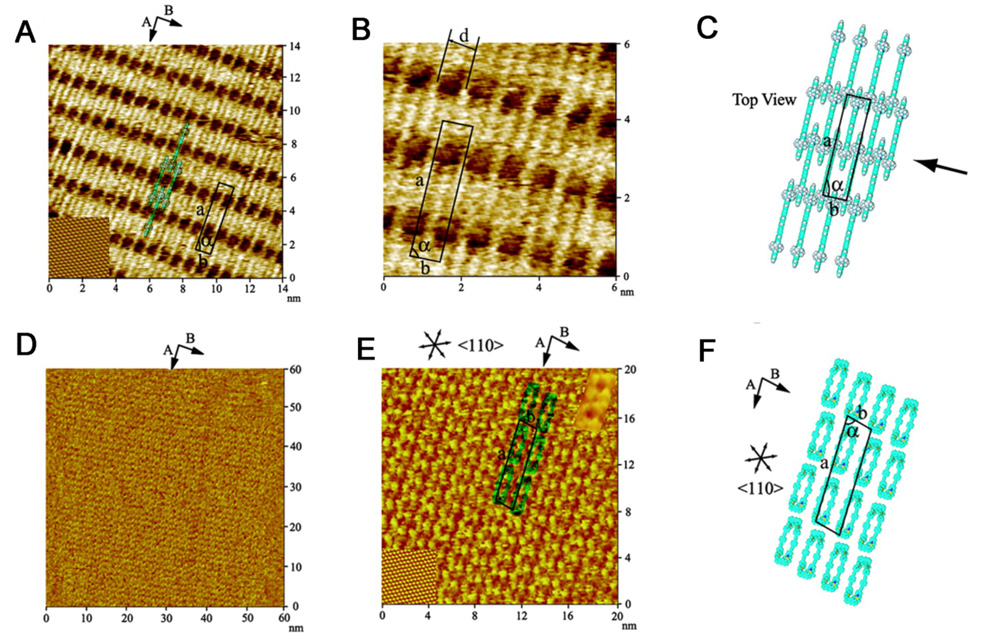
To date, a large number of surface-confined complex organic, inorganic, and biological molecules have been studied.241 There have been, however, very few investigations of the structure and properties of nanosized 2D metallacycles or 3D metallasupramolecular cages on surfaces.242 Therefore we,243 in collaboration with Professor L. J. Wan at the Institute of Chemistry in Beijing, China, and others244 investigated surface confined metallacycles and metallacages. Figure 5 demonstrates that molecular square, 80a, physisorbs on a Au(111) surface and, as seen in Figure 6, supramolecular rectangles physisorb on both HOPG and on Au(111) surfaces and self-organize to form highly ordered adlayers. Interestingly, as seen in Figure 6, the rectangles uniformly adopt an edge on orientation on HOPG but a flat orientation on Au(111). The reason for this difference of orientation on the two different surfaces, graphite and gold, is not fully understood, but likely has to do with the stronger π-Au interactions on gold vs. the weaker substrate-surface interaction on graphite. These results nicely demonstrate that nanoscale supramolecular metallacycles do indeed form well-ordered adlayers on surfaces and hence it will be possible to integrate these molecules into devices such as molecular sensors, switches, etc.
Figure 5.
(A) Large-scale STM image of self-assembled squares adsorbed on a Au(111) surface. (B) High-resolution STM image of the adlayer also showing the underlying Au(111)-(1x1) lattice in the upper right corner. (C) Proposed structural model for the adlayer.
Figure 6.
(A) Low-resolution image of a supramolecular rectangle on HOPG, showing the underlying HOPG surface in the lower left corner. (B) High-resolution STM image of the supramolecular rectangle on HOPG. (C) A proposed structural model for the adlayer of the rectangle on HOPG. (D) A large-scale (60 × 60 nm) image of the same supramolecular rectangle on Au(111). (E) High-resolution image of the rectangle adlayer, showing the underlying Au(111) lattice in the lower left corner. (F) A proposed structural model for the adlayer of the rectangle on the Au(111) surface.
A fascinating and important aspect of abiological coordination driven self-assembly is the formation of three dimensional supramolecular polyhedra and cages.223,245 Such systems may be self-assembled via either a face-directed or edge/corner directed approach. We have used the face directed approach to self-assemble a truncated tetrahedron246 and cuboctahedra247 (Scheme 18) as well as various molecular prisms.231c,248
Scheme 18.
Face directed self-assembly of a cuboctahedron from triangle-tectons and 12 connectors.
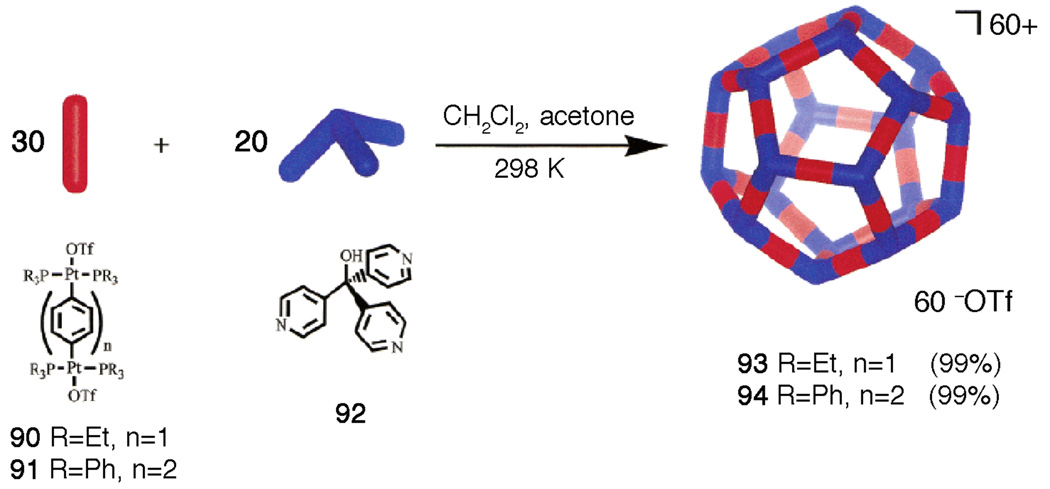
The edge/corner directed approach was used in the self-assembly of a variety of 3D cages249 as well as dodecahedra250 (Scheme 19).
Scheme 19.
Self-assembly of a nanoscale dodecahedra.
This last reaction is truly remarkable as fifty individual building units have to combine in just the right way to form the desired dodecahedron in quantitative yield as observed.250 Although we do not have an X-ray structure of the dodecahedra, various spectral techniques, including spin-echo NMR and TEM (Figure 7) establish their structure with a high level of confidence.
Figure 7.
TEM micrograph of individual dodecahedra deposited on a carbon film at low concentrations and dried at −130 °C.250a
Similarly, Fujita251 and Raymond252 have self-assembled a remarkable array of interesting metallacages and investigated their host-guest properties and used them as nano reaction vessels.
These rationally designed self-assembled coordination based metallacages are also related to metal-organic-frameworks (MOF) that are currently very topical because of their potential use in gas (H2, CO2, etc.) uptake and storage.253
Our own contributions to this burgeoning field of abiological self-assembly via coordination has been the introduction of the “directional bonding” approach for the rational design of metallacycles and metallacages; the self-assembly of the first chiral architectures and the demonstration that they form highly ordered adlayers on HOPG and gold surfaces. The significance of this work lies in providing insights, by analogy, into biological self-assembly processes and, more importantly, in the nascent nanotechnology area by providing a means of rationally designing new materials, methods for “bottom-up” fabrication and new platforms for devices such as biomedical sensors, molecular machines and switches etc.
Summary and Conclusions
I have been fortunate to make contributions in three distinct areas in three different phases of my career. Early on I was involved in physical-organic chemistry and unsaturated reactive intermediates, in particular vinyl cations and unsaturated carbenes that has enriched the family of reactive carbon intermediates. Perhaps the most important legacy of this work is the discovery and first preparation of vinyl(enol) trifluoromethanesulfonates (triflates) that have become important and widely used in contemporary metal catalyzed cross coupling reactions. In the middle of my career I was involved in polyvalent iodine chemistry and some organometallic chemistry as well as strained, unusual aromatic hydrocarbons. The significance of this work is our discovery of several new iodonium reagents, in particular PhICNOTf, and a general method for the ready formation of alkynyliodonium species that are increasingly being employed in synthetic organic chemistry. For the last 15–18 years I have been involved in supramolecular chemistry and self-assembly where we have developed an entire new paradigm for the rational design of complex, nanoscale, finite metallacycles and metallacages that has impact upon nanoscience and nanotechnology and inspired numerous young scientists to enter the field.254 A common thread in all three areas has been my “mechanistic” thinking and approach acquired during my training as a physical-organic chemist. I continue to see a bright future for physical-organic chemistry and the rigorous thinking and approach that it demands and affords. My own involvement for the remainder of my active career will undoubtedly be in the area of supramolecular chemistry and self-assembly where there is a wealth of exciting and new things to be discovered and exploited.
Scheme 6.
Formation of alkynyl(phenyl)iodonium tosylates.
ACKNOWLEDGMENT
I am most grateful to the NIH and NSF that have continuously supported my research through all its phases, as described above, during my nearly 40-year career. Likewise, all the credit for all the work described belongs to a very able and dedicated international group of coworkers, some mentioned in the text and many others as cited in the references. I am also greatly indebted to Professors Robert C. Miller, Andrew Streitwieser, Jr., and Paul v. R. Schleyer my undergraduate, graduate and postdoctoral advisors, respectively, who were mentors par excellence during my professional education. I am also greatful to my collegues at Utah for a collegial and friendly environment that makes it a joy to do chemistry.
Footnotes
Presented at the 236th ACS National Meeting August 17–21, 2008, Philidelphia, PA.; Division of Organic Chemistry Centenial Symposium.
References
- 1.Olah GA, editor. Friedel-Crafts and Related Reactions. Vol.1–4. New York-London: Wiley-Interscience; 1963. [Google Scholar]
- 2.Streitwieser A, Jr., Stang PJ. J. Am. Chem. Soc. 1965;87:4953. [Google Scholar]
- 3.Inter allia: Noyce DS, Matesich MA, Peterson PE. J. Am. Chem. Soc. 1967;89:6225. Noyce DS, Schiavelli MD. J. Am. Chem. Soc. 1968;90:1020. Noyce DS, DeBruin KE. J. Am. Chem. Soc. 1968;90:372.
- 4.Reviews: Stang PJ. Prog. Phys. Org. Chem. 1973;10:205. Modena G, Tonellato U. Adv. Phys. Org. Chem. 1971;9:185.
- 5.(a) Olah GA, Schleyer PvR, editors. Carbonium Ions. Vol.1–5. New York-London: Wiley-Interscience; 1968. [Google Scholar]; (b) Olah GA, Prakash GKS, editors. Carbocation Chemistry. Hoboken, N. J.: Wiley; 2004. [Google Scholar]
- 6.(a) Grob CA, Cseh G. Helv. Chim. Acta. 1964;47:194. [Google Scholar]; (b) Capozzi G, Melloni G, Modena G, Piscitelli M. Tetrahedron Lett. 1968:4039. [Google Scholar]; (c) Miller LL, Kaufman DA. J. Am. Chem. Soc. 1968;90:7282. [Google Scholar]; (d) Jones WM, Maness DD. J. Am. Chem. Soc. 1969;91:4314. [Google Scholar]
- 7.Grob CA, Spaar R. Tetrahedron Lett. 1969:1439. [Google Scholar]
- 8.(a) Sherrod SA, Bergman RG. J. Am. Chem. Soc. 1969;91:2115. [Google Scholar]; (b) Hanack M, Bässler T. J. Am. Chem. Soc. 1969;91:2117. [Google Scholar]
- 9.Peterson PE, Indelicato JM. J. Am. Chem. Soc. 1968;90:6515. [Google Scholar]
- 10.Review: Howells RD, McCown JD. Chem. Rev. 1977;77:69. and references therein.
- 11.Haszeldine RN, Kidd JM. J. Chem. Soc. 1954:4228. [Google Scholar]
- 12.Streitwieser A, Jr., Wilkins CL, Kiehlmann E. J. Am. Chem. Soc. 1968;90:1598. [Google Scholar]
- 13.Bartlett PD, editor. Nonclassical Ions. New York: Benjamin, Inc; 1965. [Google Scholar]
- 14.Schleyer PvR, Stang PJ, Raber DJ. J. Am. Chem. Soc. 1970;92:4725. [Google Scholar]
- 15.Hansen RL. J. Org. Chem. 1965;30:4322. [Google Scholar]
- 16.Su TM, Sliwinski WF, Schleyer PvR. J. Am. Chem. Soc. 1969;91:5386. [Google Scholar]
- 17.Review: Stang PJ, Hanack M, Subramanian LR. Synthesis. 1982:85.
- 18.Stang PJ, Summerville R. J. Am. Chem. Soc. 1969;91:4600. [Google Scholar]
- 19.(a) Dueber TE, Stang PJ, Pfeifer WD, Summerville RH, Imhoff MA, Schleyer PvR, Hummel K, Bocher S, Harding CE, Hanack M. Angew. Chem. Int. Ed. Engl. 1970;9:521. [Google Scholar]; (b) Imhoff MA, Summerville RH, Schleyer PvR, Martinez AG, Hanack M, Dueber TE, Stang PJ. J. Am. Chem. Soc. 1970;92:3802. [Google Scholar]; (c) Stang PJ, Dueber TE. Organic Syntheses. 1974;54:79. [Google Scholar]; (d) Stang PJ, Treptow W. Synthesis. 1980:283. [Google Scholar]; (e) Stang PJ, Dueber TE. Org. Syn. Coll. 1988;6:757. [Google Scholar]
- 20.Reviews: Diederich F, Stang PJ, editors. Metal-catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 1998. de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd Ed. Weinheim: Wiley VCH; 2004.
- 21.Reviews: Stang PJ. Acc. Chem. Res. 1978;11:107. Stang PJ, Rappoport Z, Hanack M, Subramanian LR. Vinyl Cations. New York: Academic Press; 1979. Rappoport Z, Stang PJ, editors. Dicoordinated Carbocations. Chichester: J. Wiley and Sons; 1997.
- 22.(a) Pfeifer WD, Bahn CA, Schleyer PvR, Bocher S, Harding CE, Hummel K, Hanack M, Stang PJ. J. Am. Chem. Soc. 1971;93:1513. [Google Scholar]; (b) Hargrove RJ, Stang PJ. Tetrahedron. 1976;32:37. [Google Scholar]; (c) Ladika M, Stang PJ, Schiavelli MD, Kowalski MH. J. Am. Chem. Soc. 1984;106:6372. [Google Scholar]
- 23.(a) Martinez AG, Hanack M, Summerville RH, Schleyer PvR, Stang PJ. Angew. Chem. Int. Ed. Engl. 1970;9:302. [Google Scholar]; (b) Stang PJ, Dueber TE. Tetrahedron Lett. 1977;18:563. [Google Scholar]
- 24.(a) Hargrove RJ, Dueber TE, Stang PJ. Chem. Comm. 1970:1614. [Google Scholar]; (b) Stang PJ, Dueber TE. J. Am. Chem. Soc. 1973;95:2686. [Google Scholar]; (c) Stang PJ, Hargrove RJ, Dueber TE. J. Chem. Soc. Perkins II. 1974:843. [Google Scholar]; (d) Stang PJ, Hargrove RJ, Dueber TE. J. Chem. Soc. Perkin. 1977;2:1486. [Google Scholar]; (e) Ladika M, Schiavelli MD, Kowalski MH, Stang PJ. J. Org. Chem. 1985;50:4397. [Google Scholar]
- 25.(a) Summerville RH, Senkler CA, Schleyer PvR, Dueber TE, Stang PJ. J. Am. Chem. Soc. 1974;96:1100. [Google Scholar]; (b) Ladika M, Stang PJ, Schiavelli MD, Hughey MR. J. Org. Chem. 1982;47:4563. [Google Scholar]; (c) Schiavelli MD, Jung DM, Vaden AK, Stang PJ, Fisk TE, Morrison DS. J. Org. Chem. 1981;46:92. [Google Scholar]
- 26.(a) Stang PJ, Anderson AG. Tetrahedron Lett. 1977;17:1485. [Google Scholar]; (b) Stang PJ, Anderson AG. J. Am. Chem. Soc. 1978;100:1520. [Google Scholar]
- 27.Reviews: Lancelot CJ, Cram DJ, Schleyer PvR. In: Carbonium Ions. Olah GA, Schleyer PvR, editors. Vol. III. New York: Wiley-Insterscience; 1972. Ref. 13 and references therein. Capon CQ. Rev. Chem. Soc. 1964;18:45.
- 28.(a) Cram DJ. J. Am. Chem. Soc. 1948;70:4244. doi: 10.1021/ja01192a078. [DOI] [PubMed] [Google Scholar]; (b) Cram DJ. J. Am. Chem. Soc. 1949;71:3863. [Google Scholar]; (c) Cram DJ. J. Am. Chem. Soc. 1949;71:3875. [Google Scholar]; (d) Cram DJ. J. Am. Chem. Soc. 1952;74 2129, 2137, 2149. [Google Scholar]
- 29.(a) Stang PJ, Dueber TE. J. Am. Chem. Soc. 1973;95:2683. [Google Scholar]; (b) Stang PJ, Dueber TE. J. Am. Chem. Soc. 1977;99:2602. [Google Scholar]
- 30.Review: (a) Inter alia: Siehl H-U. In: Stable Carbocation Chemistry. Prakash GKS, Schleyer PvR, editors. New York: Wiley; 1997. pp. 165–196. Müller T, Meyer R, Lennartz D, Siehl H-U. Angew. Chem. Int. Ed. 2000;39:3074. Kaufmann F-P, Siehl H-U. J. Am. Chem. Soc. 1992;114:4937. Siehl H-U, Mayr H. J. Am. Chem. Soc. 1982;104:909.
- 31.Reviews: Hine J. Divalent Carbon. New York: Ronald Press; 1964. Gilcrist TL, Rees CW. Carbenes, Nitrenes and Arynes. New York: Appleton-Century-Crofts; 1969. Kirmse W. Carbene Chemistry. 2nd Ed. New York: Academic Press; 1971. Jones M, Jr., Moss RA. Carbenes. Vol. I. New York: Wiley; 1973.
- 32.Review: Köbrich G. Angew. Chem. Int. Ed. 1967;6:41.
- 33.Curtin DY, Kampeier JA, O’Connor BR. J. Am. Chem. Soc. 1965;87:863. [Google Scholar]
- 34.Gilbert JC, Butler JR. J. Am. Chem. Soc. 1970;92:7493. [Google Scholar]
- 35.(a) Newman MS, Okorodudu OM. J. Am. Chem. Soc. 1968;90:4189. [Google Scholar]; (b) Newman MS, Patrick TB. J. Am. Chem. Soc. 1969;97:5677. [Google Scholar]
- 36.(a) Hartzler HD. J. Am. Chem. Soc. 1971;93:4527. and references therein. [Google Scholar]; (b) LeNoble WJ, Tatsukani Y, Morris HF. J. Am. Chem. Soc. 1970;92:5681. [Google Scholar]
- 37.Reviews: Stang PJ. Chem. Rev. 1978;78:383. Stang PJ. Acc. Chem. Res. 1982;15:348. Stang PJ. Pure and Appl. Chem. 1983;55:369.
- 38.Stang PJ, Mangum MG, Fox DP, Haak P. J. Am. Chem. Soc. 1974;96:4562. [Google Scholar]
- 39.(a) Stang PJ, Fox DP. J. Org. Chem. 1977;42:1667. [Google Scholar]; (b) Fox DP, Bjork JA, Stang PJ. J. Org. Chem. 1983;48:3994. [Google Scholar]
- 40.Stang PJ, Mangum MG. J. Am. Chem. Soc. 1975;97:1459. [Google Scholar]
- 41.(a) Stang PJ, Madsen JR, Mangum MG, Fox DP. J. Org. Chem. 1977;42:1802. [Google Scholar]; (b) Apeloig Y, Karni M, Christensen SB, Stang PJ. Tetrahedron Lett. 1986;27:6115. [Google Scholar]
- 42.Stang PJ, Mangum MG. J. Am. Chem. Soc. 1975;97:6478. [Google Scholar]
- 43.Closs GL, Moss RA. J. Am. Chem. Soc. 1964;86:4042. [Google Scholar]
- 44.(a) Apeloig Y, Karni M, Stang PJ, Fox DP. J. Am. Chem. Soc. 1983;105:4781. [Google Scholar]; (b) Fox DP, Stang PJ, Apeloig Y, Karni M. J. Am. Chem. Soc. 1986;108:750. [Google Scholar]; (c) Apeloig Y, Schrieber R, Stang PJ. Tetrahedron Lett. 1980;21:411. [Google Scholar]
- 45.(a) Stang PJ, Davis J, Fox DP. J. Chem. Soc. Chem. Commun. 1975:17. [Google Scholar]; (b) Stang PJ, Fox DP, Collins CJ, Waston CR., Jr. J. Org. Chem. 1978;43:364. [Google Scholar]
- 46.(a) Bothner-By AA. J. Am. Chem. Soc. 1955;77:3293. [Google Scholar]; (b) Curtin DY, Flynn EW, Nystrom RF. J. Am. Chem. Soc. 1958;80:4599. [Google Scholar]
- 47.(a) Stang PJ. Israel J. Chem. 1981;21:119. [Google Scholar]; (b) Kaftory MI, Agmon Ladika M, Stang PJ. J. Am. Chem. Soc. 1987;109:782. [Google Scholar]; (c) Learned AE, Arif AM, Stang PJ. J. Org. Chem. 1988;53:3122. [Google Scholar]
- 48.Stang PJ, Mangum MG. J. Am. Chem. Soc. 1975;97:3854. [Google Scholar]
- 49.Gajewski JJ, Chang MJ, Stang PJ, Fisk TE. J. Am. Chem. Soc. 1980;102:2096. [Google Scholar]
- 50.Neuhaus P, Grote D, Sander W. J. Am. Chem. Soc. 2008;130:0000. doi: 10.1021/ja073453d. [DOI] [PubMed] [Google Scholar]
- 51.(a) Stang PJ, Mangum MG. J. Am. Chem. Soc. 1977;99:2597. [Google Scholar]; (b) Krageloh K, Anderson GH, Stang PJ. J. Am. Chem. Soc. 1984;106:6015. [Google Scholar]
- 52.Stang PJ, Bjork JA. J. Chem. Soc. Chem. Commun. 1978:1057. [Google Scholar]
- 53.Stang PJ, Anderson GH. J. Org. Chem. 1981;46:4585. [Google Scholar]
- 54.Stang PJ, Roberts KA, Lynch LE. J. Org. Chem. 1984;49:1653. [Google Scholar]
- 55.Stang PJ, Learned AE. J. Am. Chem. Soc. 1987;109:5019. [Google Scholar]
- 56.Sommer LH, Ulland LA, Parker GA. J. Am. Chem. Soc. 1972;94:3469. [Google Scholar]
- 57.Stang PJ, Christensen SB. J. Org. Chem. 1981;46:823. [Google Scholar]
- 58.Stang PJ, Fisk TE. Synthesis. 1979:438. [Google Scholar]
- 59.Stang PJ, Ladika M. Synthesis. 1981:29. [Google Scholar]
- 60.(a) Stang PJ, Fisk TE. J. Am. Chem. Soc. 1979;101:4772. [Google Scholar]; (b) Stang PJ, Fisk TE. J. Am. Chem. Soc. 1980;102:6813. [Google Scholar]
- 61.(a) Stang PJ, Learned AE. J. Chem. Soc. Chem. Commun. 1988:301. [Google Scholar]; (b) Stang PJ, Ladika M. J. Am. Chem. Soc. 1980;102:5406. [Google Scholar]; (c) Stang PJ, Ladika M. J. Am. Chem. Soc. 1981;103:6437. [Google Scholar]
- 62.Stang PJ, White MR. J. Am. Chem. Soc. 1981;103:5429. [Google Scholar]
- 63.Reviews: Nicolauo KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. Dounay DB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h.
- 64.McCarthy MC, Thaddeus P. Chem. Soc. Rev. 2001;30:177. [Google Scholar]
- 65.Thaddeus P, Gottlieb CA, Mollaaghababa R, Vrtilek JM. J. Chem. Soc. Faraday Trans. 1993;89:2125. [Google Scholar]
- 66.Reviews: Grubbs RH. Angew. Chem. Int. Ed. 2006;45:3760. doi: 10.1002/anie.200600680. Bruneau C, Dixneuf PH. Angew. Chem. Int. Ed. 2006;45:2176. doi: 10.1002/anie.200501391. Touchard D, Dixneuf PH. Coord. Chem. Rev. 1998;178:409.
- 67.Trnka TM, Morgan JP, Sandford MS. J. Am. Chem. Soc. 2003;125:2546. doi: 10.1021/ja021146w. [DOI] [PubMed] [Google Scholar]
- 68.Reviews: Seleque JP. Coord. Chem. Rev. 2004;248:1543. Cadierna V, Gamasa MP, Gimeno J. Coord. Chem. Rev. 2004;248:1627. Ilg J, Werner H. Chem. Eur. J. 2002;8:2812. doi: 10.1002/1521-3765(20020617)8:12<2812::AID-CHEM2812>3.0.CO;2-C.
- 69.Varela JA, Saá C. C. Chem. Eur. J. 2006;12:6450. doi: 10.1002/chem.200600388. [DOI] [PubMed] [Google Scholar]
- 70.Yamauchi Y, Yuki M, Tanabe T, Miyake Y, Inada Y, Uemura S, Nishibayashi Y. J. Am. Chem. Soc. 2008;130:2908. doi: 10.1021/ja710013y. [DOI] [PubMed] [Google Scholar]
- 71.Stang PJ, Datta AK. J. Am. Chem. Soc. 1989;111:1358. [Google Scholar]
- 72.(a) Stang PJ, Wistrand LG. J. Organomet. Chem. 1981;204:405. [Google Scholar]; (b) Stang PJ, Datta AK, Dixit V, Wistrand LG. Organometallics. 1989;8:1020. [Google Scholar]; (c) Stang PJ, Dixit V. Tetrahedron Lett. 1985;26:2301. [Google Scholar]
- 73.(a) Kowalski MH, Stang PJ. Organometallics. 1986;5:2392. [Google Scholar]; (b) Stang PJ, Kowalski MH, Zhong Z. Organometallics. 1990;9:833. [Google Scholar]; (c) Kowalski MH, Arif AM, Stang PJ. Organometallics. 1988;7:1227. [Google Scholar]; (d) Zhong Z, Stang PJ, Arif AM. Organometallics. 1990;9:1703. [Google Scholar]; (e) Zhong Z, Hinkle RJ, Arif AM, Stang PJ. J. Am. Chem. Soc. 1991;113:6196. [Google Scholar]
- 74.(a) Stang PJ, Cao DH, Poulter GT, Arif AM. Organometallics. 1995;14:1110. [Google Scholar]; (b) Stang PJ, Datta AK. Organometallics. 1989;8:1024. [Google Scholar]
- 75.(a) Stang PJ, Schiavelli MD, Chenault HK, Breidegam JL. Organometallics. 1984;3:1133. [Google Scholar]; (b) Stang PJ, Dixit V, Schiavelli MD, Drees P. J. Am. Chem. Soc. 1987;109:1150. [Google Scholar]
- 76.(a) Stang PJ, Zhong Z, Arif AM. Organometallics. 1992;11:1017. [Google Scholar]; (b) Stang PJ, Zhong Z. Organometallics. 1992;11:1026. [Google Scholar]
- 77.(a) Stang PJ, Song L, Halton B. J. Organomet. Chem. 1990;388:215. [Google Scholar]; (b) Huang Y-H, Stang PJ, Arif AM. J. Am. Chem. Soc. 1990;112:5648. [Google Scholar]; (c) Stang PJ, Huang YH, Arif AM. Organometallics. 1992;11:231. [Google Scholar]
- 78.(a) Stang PJ, White MR, Maas G. Organometallics. 1983;2:720. [Google Scholar]; (b) White MR, Stang PJ. Organometallics. 1983;2:1382. [Google Scholar]
- 79.Song L, Arif AM, Stang PJ. J. Organomet. Chem. 1990;395:219. [Google Scholar]
- 80.Stang PJ, Kowalski MH, Schiavelli MD, Longford D. J. Am. Chem. Soc. 1989;111:3347. [Google Scholar]
- 81.Stang PJ, Kowalski MH. J. Am. Chem. Soc. 1989;111:3356. [Google Scholar]
- 82.Review: Halton B. Chem. Rev. 1973;73:113. Billups WE. Acc. Chem. Res. 1978;11:245. Halton B. Chem. Rev. 2003;103:1327. doi: 10.1021/cr010009z.
- 83.Greenberg A, Liebman JF. Strained Organic Molecules. New York: Academic Press; 1978. [Google Scholar]
- 84.Review: Hoffman RW. Dehydrobenzene and Cycloalkynes. New York: Academic Press; 1967.
- 85.Chapman OL, Mattes K, McIntosh CL, Pacansky J, Calder GV, Orr GJ. J. Am. Chem. Soc. 1973;95:6134. [Google Scholar]
- 86.Review: Halton B. Eur. J. Org. Chem. 2005:3391.
- 87.Newman MS, Patrick TB. J. Am. Chem. Soc. 1969;91:6461. [Google Scholar]
- 88.(a) Halton B, Randall CJ, Stang PJ. J. Am. Chem. Soc. 1984;106:6108. doi: 10.1021/ja00279a046. [DOI] [PubMed] [Google Scholar]; (b) Halton B, Randall CJ, Gainsford GJ, Stang PJ. J. Am. Chem. Soc. 1986;108:5949. doi: 10.1021/ja00279a046. [DOI] [PubMed] [Google Scholar]; (c) Halton B, Lu Q, Stang PJ. Aust. J. Chem. 1990;43:1277. [Google Scholar]
- 89.Weber WP. Silica-Reagents for Organic Synthesis. New York: Springer-Verlag; 1983. [Google Scholar]
- 90.Reviews: Halton B, Stang PJ. Acc. Chem. Res. 1987;20:443. Halton B, Stang PJ. Synlett. 1997:145.
- 91.(a) Ashley K, Foley JK, Mei Q, Ghoroghchian J, Sarfarazi F, Cassidy J, Halton B, Stang PJ, Pons S. J. Org. Chem. 1986;51:2089. [Google Scholar]; (b) Ashley K, Sarfarazi F, Buckland SJ, Foley JK, Mei Q, Halton B, Stang PJ, Pons S. Can. J. Chem. 1987;65:2062. [Google Scholar]; (c) McNichols AT, Stang PJ, Halton B, Kay AJ. Tetrahedron Lett. 1993;34:3131. [Google Scholar]
- 92.Koenig V, Curtiss T, Winter R, Ashley K, Mei Q, Stang PJ, Pons S, Buckland SJ, Halton B, Rolison D. J. Org. Chem. 1988;53:3735. [Google Scholar]
- 93.Halton B, Buckland SJ, Lu Q, Mei Q, Stang PJ. J. Org. Chem. 1988;53:2418. [Google Scholar]
- 94.Halton B, Lu Q, Stang PJ. J. Org. Chem. 1990;55:3056. [Google Scholar]
- 95.Halton B, Lu Q, Stang PJ. J. Chem. Soc. Chem. Commun. 1988:879. [Google Scholar]
- 96.Buckland SJ, Halton B, Mei Q, Stang PJ. Aust. J. Chem. 1987;40:1375. [Google Scholar]
- 97.Buckland SJ, Halton B, Stang PJ. Aust. J. Chem. 1988;41:845. [Google Scholar]
- 98.(a) Stang PJ, Song L, Halton B. J. Organomet. Chem. 1990;388:215. [Google Scholar]; (b) Stang PJ, Song L, Lu Q, Halton B. Organometallics. 1990;9:2149. [Google Scholar]; (c) Song Li, Arif AM, Stang PJ. Organometallics. 1990;9:2792. [Google Scholar]
- 99.(a) Halton B, Buckland SJ, Mei Q, Stang PJ. Tetrahedron Lett. 1986;27:5159. [Google Scholar]; (b) McNichols AT, Stang PJ, Addington DM, Halton B. Tetrahedron Lett. 1994;35:437. [Google Scholar]
- 100.(a) Mul PM, McGowan JW. Astrophys. 1980;237:749. [Google Scholar]; (b) Herbst E. Astrophys J. Suppl. Ser. 1983;53:41. [Google Scholar]; (c) Krolik JH, Kallman TR. Astrophys. J. 1983;267:610. [Google Scholar]
- 101.Burgers PC, Holmes JL, Mommers AA, Szulejko JE. J. Am. Chem. Soc. 1984;106:521. [Google Scholar]
- 102.Apeloig Y. Chapt.2. In: Rappoport Z, Stang PJ, editors. Theory and Calculations in Dicoordinated Carbocations. Chichester: Wiley; 1997. pp. 9–104. [Google Scholar]
- 103.(a) Koser GF, Rebrovic L, Wettach RH. J. Org. Chem. 1981;46:4324. [Google Scholar]; (b) Rebrovic L, Koser GF. J. Org. Chem. 1984;49:4700. [Google Scholar]
- 104.Willgerodt C. J. Prakt. Chem. 1886;33:155. [Google Scholar]
- 105.Willgerodt C. Chem. Ber. 1892;25:3494. [Google Scholar]
- 106.Willgerodt C. Die Organischen Verbindugen mit Mehrwertigen Iod. Stuttgart: Enke; 1914. [Google Scholar]
- 107. Varvoglis A. Hypervalent Iodine in Organic Synthesis. London: Academic Press; 1997. Varvoglis A. The Organic Chemistry of Polycoordinate Iodine. New York: VCH Publishers; 1992. Stang PJ, Zhdankin VV. Chem. Rev. 1996;96:1123. doi: 10.1021/cr940424+. Zhdankin VV, Stang PJ. Chem. Rev. 2002;102:2523. doi: 10.1021/cr010003+. Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis, Wirth T, editor. Top. Curr. Chem. 2003. p. 224.
- 108.Review: Stang PJ. Angew. Chem. Int. Ed. Engl. 1992;31:274.
- 109.Beringer FM, Galton SA. J. Org. Chem. 1965;30:1930. doi: 10.1021/jo00826a001. [DOI] [PubMed] [Google Scholar]
- 110.Merkushev EB, Karpitskaya LG, Novosel’tseva GI. Dokl. Akad. Nauk SSSR. 1979;245:607. [Google Scholar]
- (a).Neiland O, Karele B. J. Org. Chem. USSR (Eng. Transl.) 1970;6:889. [Google Scholar]; (b) Moriarty RM, Vaid RK, Koser GF. Synlett. 1990:365. [Google Scholar]
- 112.(a) Stang PJ, Surber BW. J. Am. Chem. Soc. 1985;107:1452. [Google Scholar]; (b) Stang PJ, Surber BW, Chen ZC, Roberts KA, Anderson AG. J. Am. Chem. Soc. 1987;109:228. [Google Scholar]; (c) Stang PJ, Kitamura T. Org. Syn. 1991;70:215. [Google Scholar]; (d) Kitamura T, Stang PJ. J. Org. Chem. 1988;53:4105. [Google Scholar]; (e) Stang PJ, Roberts KA. J. Am. Chem. Soc. 1986;108:7832. doi: 10.1021/ja00284a057. [DOI] [PubMed] [Google Scholar]
- 113.Ochiai M, Kunishima M, Sumi K, Nagao K, Fujita E, Ariomoto M, Yamaguchi H. Tetrahedron Lett. 1985;26:4501. [Google Scholar]
- 114.(a) Bachi MD, Bar-Ner N, Crittell CM, Stang PJ, Williamson BL. J. Org. Chem. 1991;56:3912. [Google Scholar]; (b) Stang PJ, Zhdankin VV, Williamson BL. J. Am. Chem. Soc. 1991;113:5870. [Google Scholar]; (c) Williamson BL, Stang PJ, Arif AM. J. Am. Chem. Soc. 1993;115:2590. [Google Scholar]
- 115.Zefirov NS, Zhdankin VV, Dan’kov YY, Kozmin AS. Zh. Org. Khim. 1984;20:446. [Google Scholar]
- 116.Williamson BL, Stang PJ. Synlett. 1992:199. [Google Scholar]
- 117.(a) Zhdankin VV, Crittell CM, Stang PJ, Zefirov NS. Tetrahedron Lett. 1990;31:4821. [Google Scholar]; (b) Zhdankin VV, Scheuller MC, Stang PJ. Tetrahedron Lett. 1993:6853. [Google Scholar]; (c) Stang PJ, Zhdankin VV, Tykwinski R, Zefirov NS. Tetrahedron Lett. 1992;33:1419. [Google Scholar]
- 118.(a) Stang PJ, Arif AM, Crittell CM. Angew. Chemie Int. Ed. English. 1990;29:287. [Google Scholar]; (b) Ochiai M, Ho T, Takaoka Y, Masaki Y, Kunishima M, Tani S, Nagao Y. J. Chem. Soc. Chem. Commun. 1990:118. [Google Scholar]
- 119.Stang PJ, Zhdankin VV. J. Am. Chem. Soc. 1990;112:6437. [Google Scholar]; (b) Stang PJ, Zhdankin VV. J. Am. Chem. Soc. 1991;113:4571. [Google Scholar]
- 120.(a) Stang PJ, Zhdankin VV, Arif AM. J. Am. Chem. Soc. 1991;113:8997. [Google Scholar]; (b) Stang PJ, Ullmann J. Synthesis. 1991:1073. [Google Scholar]; (c) Stang PJ, Tykwinski R, Zhdankin VV. J. Org. Chem. 1992;57:1861. [Google Scholar]; (d) Tykwinski R, Stang PJ. Organometallics. 1994;13:3203. [Google Scholar]; (d) Kitamura T, Furuki R, Taniguchi H, Stang PJ. Mendeleev Commun. 1991:148. [Google Scholar]; (e) Stang PJ, Zhdankin VV, Arif AM. Mendeleev Commun. 1992:158. [Google Scholar]
- 121.(a) Stang PJ, Ullmann J. Angew. Chem. Int. Ed. Engl. 1991;30:1469. [Google Scholar]; (b) Hinkle RJ, Stang PJ. Synthesis. 1994:313. [Google Scholar]; (c) Kasumov TM, Pirguliyev NS, Brel VK, Grishin YK, Zefirov NS, Stang PJ. Tetrahedron. 1997;53:13139. [Google Scholar]
- 122.Ochiai M, Suni K, Takuoka Y, Kunishima Y, Nagao Y, Shiro M, Fujita E. Tetrahedron. 1998;54:4095. [Google Scholar]
- 123.(a) Kitamura T, Furuki R, Taniguchi H, Stang PJ. Tetrahedron Lett. 1990;31:703. [Google Scholar]; (b) Kitamura T, Furuki R, Nagata K, Taniguchi H, Stang PJ. J. Org. Chem. 1992;57:6810. [Google Scholar]; (c) Kitamura T, Furuki R, Taniguchi H, Stang PJ. Tetrahedron. 1992;48:7149. [Google Scholar]
- 124.(a) Stang PJ, Tykwinski R, Zhdankin VV. J. Heterocyc. Chem. 1992;29:815. [Google Scholar]; (b) Stang PJ, Olenyuk B, Chen K. Synthesis. 1995:937. [Google Scholar]
- 125.Stang PJ. In: Modern Acetylene Chemistry. Stang PJ, Diederich F, editors. Weinheim: VCH Publishers; 1995. [Google Scholar]
- 126.Stang PJ, Wingert H, Arif AM. J. Am. Chem. Soc. 1987;109:7235. [Google Scholar]
- 127.Martin JC. Science. 1983;221:509. doi: 10.1126/science.221.4610.509. [DOI] [PubMed] [Google Scholar]
- 128.(a) Kitamura T, Stang PJ. Tetrahedron Lett. 1988;29:1887. [Google Scholar]; (b) Ochiai M, Kunishima M, Fuj K, Nagao Y. J. Org. Chem. 1988;53:6144. [Google Scholar]
- 129.Mironova AA, Maletina II, Iksanova SV, Yagapulski LM. Zh. Org. Khim. 1989;25:306. [Google Scholar]
- 130.(a) Ochiai M, Ito T, Takaoka Y, Masuki Y, Kunishima M, Tani S, Nagao Y. J. Chem. Soc. Chem. Commun. 1990:118. [Google Scholar]; (b) Susuki T, Uozumi T, Shibasaki M. J. Chem. Soc. Chem. Commun. 1991:1593. [Google Scholar]; (c) Bachi MD, Bar-Ner N, Stang PJ, Williamson BL. J. Org. Chem. 1993;58:7923. [Google Scholar]; (d) Kitamura T, Nagata K, Taniguchi H. Tetrahedron Lett. 1995:1081. [Google Scholar]; (e) Kitamura T, Fukuoka T, Zheng L, Fujimoto T, Taniguchi H, Fujiwara Y. Bull. Soc. Chem. Jpn. 1996;69:2649. [Google Scholar]
- 131.(a) Witulski B, Stengel T. Angew. Chem. Int. Ed. 1998;37:489. doi: 10.1002/(SICI)1521-3773(19980302)37:4<489::AID-ANIE489>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]; (b) Witulski B, Stengel T. Angew. Chem. Int. Ed. 1999;38:2426. [PubMed] [Google Scholar]; (c) Witulski T, Gossman M. J. Chem. Soc. Chem. Commun. 1999:1897. [Google Scholar]; (d) Murch P, Williamson BL, Stang PJ. Synthesis. 1994:1255. [Google Scholar]
- 132.Fischer DR, Williamson BL, Stang PJ. Synlett. 1992:535. [Google Scholar]
- 133.Tykwinski RR, Williamson BL, Fischer DR, Stang PJ, Arif AM. J. Org. Chem. 1993;58:5235. [Google Scholar]
- 134.Williamson BL, Murch P, Fischer DR, Stang PJ. Synlett. 1993:858. [Google Scholar]
- 135.Liu ZD, Chen ZC. J. Org. Chem. 1993;58:1924. [Google Scholar]
- 136.(a) Stang PJ, Crittell CM. J. Org. Chem. 1992;57:4305. [Google Scholar]; (b) Schmidpeter A, Mayer P, Stocker J, Roberts KA, Stang PJ. Heteroatom Chem. 1991;2:569. [Google Scholar]; (c) Laali KK, Regitz M, Birkel M, Stang PJ, Crittell CM. J. Org. Chem. 1993;58:4105. [Google Scholar]
- 137.Lodoya JS, Koser GF. J. Org. Chem. 1993;58:1924. [Google Scholar]
- 138.Nagaoka T, Sueda T, Ochiai M. Tetrahedron Lett. 1995:261. [Google Scholar]
- 139.Stang PJ, Murch P. Synthesis. 1997:1378. [Google Scholar]
- 140.(a) Stang PJ, Crittell CM. Organometallics. 1990;9:3191. [Google Scholar]; (b) Stang PJ, Tykwinski R. J. Am. Chem. Soc. 1992;114:4411. [Google Scholar]; (c) Stang PJ, Crittell CM, Arif AM. Organometallics. 1993;12:4799. [Google Scholar]
- 141.(a) Kunz R, Fehlhanmer WP. Angew. Chem. Int. Ed. 1994;33:330. [Google Scholar]; (b) Dembinski R, Lis T, Szafert S, Mayne CL, Bartik T, Gladysz JA. J. Organomet. Chem. 1999;578:229. [Google Scholar]
- 142.Review: Stang PJ. Acc. Chem. Res. 1991;24:304.
- 143.Review: Rappoport Z, editor. The Chemistry of Enols. London: Wiley-Interscience; 1990.
- 144.(a) Smith BJ, Radom L, Kresge AJ. J. Am. Chem. Soc. 1989;111:8297. [Google Scholar]; (b) Bouma WJ, Nobes RH, Radom L, Woodward CE. J. Org. Chem. 1982;47:1869. [Google Scholar]; (c) Holmes JL, Lossing FP. J. Am. Chem. Soc. 1982;104:2648. [Google Scholar]
- 145.(a) Stang PJ, Boehshar M, Lin J. J. Am. Chem. Soc. 1986;108:7832. doi: 10.1021/ja00284a057. [DOI] [PubMed] [Google Scholar]; (b) Stang PJ, Boehshar M, Wingert H, Kitamura T. J. Am. Chem. Soc. 1988;110:3272. [Google Scholar]
- 146.(a) Stang PJ, Kitamura T, Boehshar M, Wingert H. J. Am. Chem. Soc. 1989;111:2225. [Google Scholar]; (b) Zhdankin VV, Stang PJ. Tetrahedron Lett. 1993;34:1461. [Google Scholar]; (c) Tykwinski RR, Stang PJ. Tetrahedron. 1993;49:3043. [Google Scholar]
- 147.Stang PJ, Kitamura T, Arif AM, Karni M, Apeloig Y. J. Am. Chem. Soc. 1990;112:374. [Google Scholar]
- 148.Stang PJ, Crittell CM, Arif AM, Karni M, Apeloig Y. J. Am. Chem. Soc. 1991;113:7461. [Google Scholar]
- 149.Allen AD, Roberts KA, Kitamura T, Stang PJ, Tidwell TT. J. Am. Chem. Soc. 1988;110:622. [Google Scholar]; (b) Allen AD, Kitamura T, McClelland RA, Stang PJ, Tidwell TT. J. Am. Chem. Soc. 1990;112:8873. [Google Scholar]
- 150.(a) Maas G, Regitz M, Rahm R, Schneider J, Stang PJ, Crittell CM. J. Chem. Soc. Chem. Commun. 1990:1456. [Google Scholar]; (b) Maas G, Rahm R, Krebs F, Regitz M, Stang PJ, Crittell CM. Chem. Ber. 1991;124:1661. [Google Scholar]
- 151.(a) Segal D, Shalitin Y, Wingert H, Kitamura T, Stang PJ. FEBS. Lett. 1989;247:217. doi: 10.1016/0014-5793(89)81338-9. [DOI] [PubMed] [Google Scholar]; (b) Segal D, Shalitin C, Shalitin Y, Fischer DR, Stang PJ. FEBS Lett. 1996;392:117. doi: 10.1016/0014-5793(96)00795-8. [DOI] [PubMed] [Google Scholar]
- 152.(a) Blenkenship JN, Abu-Soud H, Francisco WA, Raushel FM, Fischer DR, Stang PJ. J. Am. Chem. Soc. 1991;113:8560. [Google Scholar]; (b) Banzon JA, Kuo JM, Miles B, Fischer DR, Stang PJ, Raushel FM. Biochemistry. 1995;34:743. doi: 10.1021/bi00003a006. [DOI] [PubMed] [Google Scholar]; (c) Banzon JA, Kuo J-M, Fischer DR, Stang PJ, Raushel FM. Biochemistry. 1995;34:750. doi: 10.1021/bi00003a007. [DOI] [PubMed] [Google Scholar]
- 153.(a) Ochiai M, Kunishima M, Tani S, Nagao Y. J. Am. Chem. Soc. 1991;113:3135. [Google Scholar]; (b) Ochiai M, Kunishima M, Nagao Y, Fuji M, Shiro M, Fujita E. J. Am. Chem. Soc. 1986;108:8221. [Google Scholar]; (c) Tykwinski RR, Stang PJ, Persky NE. Tetrahedron Lett. 1994;35:23. [Google Scholar]
- 154.Williamson BL, Tykwinski RR, Stang PJ. J. Am. Chem. Soc. 1994;116:93. [Google Scholar]
- 155.Reviews: Pellissier H. Tetrahedron. 2005;61:6479. Denmark SE. In: Comprehensive. Trost BM, Fleming I, editors. Vol. 5. New York: Pergamon; 1991. pp. 751–784.
- 156.Reviews: Gibson SE, Stevenazzi A. Angew. Chem. Int. Ed. 2003;42:1800. doi: 10.1002/anie.200200547. Brummond KM, Kent JL. Tetrahedron. 2000;56:3263.
- 157.Pauson PL. Tetrahedron. 1985;41:5855. [Google Scholar]
- 158.(a) Feldman KS, Mareska DA. J. Org. Chem. 1999;64:5650. doi: 10.1021/jo9907538. [DOI] [PubMed] [Google Scholar]; (b) Feldman KS, Mareska DA. Tetrahedron Lett. 1998:4781. [Google Scholar]; (c) Feldman KS, Mareska DA. J. Am. Chem. Soc. 1998;120:4027. [Google Scholar]
- 159.(a) Schildknegt K, Bohnstedt AC, Feldman KS, Sambandam A. J. Am. Chem. Soc. 1995;117:7544. [Google Scholar]; (b) Feldman KS, Bruendl MM, Schildknegt K. J. Org. Chem. 1995;60:7722. [Google Scholar]
- 160.Wipf P, Venkatraman S. J. Org. Chem. 1996;61:8004. doi: 10.1021/jo961681c. [DOI] [PubMed] [Google Scholar]
- 161.(a) Kitamura T, Zheng L, Fukuoka T, Fujiwara Y, Taniguchi H, Sakurai M, Tanaka R. J. Chem. Soc. Perkin Trans. 1997;2:1511. [Google Scholar]; (b) Nikas S, Rodios N, Varvoglis A. Molecules. 2000;5:1182. [Google Scholar]
- 162.Kitamura T, Tsuda K, Fujiwara Y. Tetrahedron. Lett. 1998:5375. [Google Scholar]
- 163.Zhang PF, Chen ZC. Synthesis. 2001:238. [Google Scholar]
- 164.Murch P, Arif AM, Stang PJ. J. Org. Chem. 1997;62:5959. [Google Scholar]
- 165.(a) Stang PJ, Schwarz A, Blume T, Zhdankin VV. Tetrahedron Lett. 1992;33:6759. [Google Scholar]; (b) Stang PJ, Blume T, Zhdankin VV. Synthesis. 1993;1:35. [Google Scholar]
- 166.Kotali E, Varvoglis A, Bozopoulos A. J. Chem. Soc., Perkin Trans. 1989;1:827. [Google Scholar]
- 167.Stang PJ, Murch P. Tetrahedron Lett. 1997:8793. [Google Scholar]
- 168.Maas G, Regitz M, Moll U, Rahm R, Krebs F, Hector R, Stang PJ, Crittell CM, Williamson BL. Tetrahedron. 1992;48:3527. [Google Scholar]
- 169.(a) Moriarty RM, Epa WR, Awasthi AK. J. Am. Chem. Soc. 1991;113:6315. [Google Scholar]; (b) Kang SK, Lee HW, Jang SB, Kim TJ, Pyun SJ. J. Org. Chem. 1996;61:260. doi: 10.1021/jo951923t. [DOI] [PubMed] [Google Scholar]; (c) Ho PS. J. Org. Chem. 1996;61:9082. doi: 10.1021/jo960195m. [DOI] [PubMed] [Google Scholar]; (d) Kang SK, Ryu HC, Lee SW. J. Chem. Soc. Perkin Trans. 1999;1:2661. [Google Scholar]; (e) Huang X, Xu X. J. Chem. Soc., Perkin Trans. 1998;1:3321. [Google Scholar]; (f) Roh KR, Kim JY, Kim YH. Tetrahedron Lett. 1999:1903. [Google Scholar]; (g) Hinkle RJ, Levi A, David GA, Erwin WM. Org. Lett. 2002;4:1521. doi: 10.1021/ol005682+. [DOI] [PubMed] [Google Scholar]
- 170.(a) Hinkle RJ, Poulter GT, Stang PJ. J. A. Chem. Soc. 1993;115:11626. [Google Scholar]; (b) Ryan JH, Stang PJ. J. Org. Chem. 1996;61:6162. doi: 10.1021/jo960750k. [DOI] [PubMed] [Google Scholar]; (c) Ryan JH, Stang PJ. Tetrahedron Lett. 1997;38:5061. [Google Scholar]; (d) Radhakrishnan U, Stang PJ. Org. Lett. 2001;3:859. doi: 10.1021/ol015555t. [DOI] [PubMed] [Google Scholar]; (e) Kitamura T, Tanaka T, Taniguchi H, Stang PJ. J. Chem. Soc. Perkin, Trans. I. 1991:2892. [Google Scholar]; (f) Stang PJ, Kitamura T. J. Am. Chem. Soc. 1987;109:7561. [Google Scholar]; (g) Kitamura T, Mihara I, Tamisuchi H, Stang PJ. J. Chem. Soc. Chem. Commun. 1990:614. [Google Scholar]
- 171.Pirguliyev NSh, Brel VK, Zefirov NS, Stang PJ. Tetrahedron. 1999;55:12377. [Google Scholar]
- 172.Gallop PM, Paz MA, Fückiger R, Stang PJ, Zhdankin VV, Tykwinski RR. J. Am. Chem. Soc. 1993;115:11702. [Google Scholar]
- 173.Goldstein EJC, Citron DM, Warren Y, Merriam CV, Tyrrell K, Fernandez H, Radhakrishnan U, Stang PJ, Conrads G. Antimicrobial Agents and Chemotherapy. 2004;48:2766. doi: 10.1128/AAC.48.7.2766-2770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Angelini G, Hanack M, Vermehren J, Speranza M. J. Am. Chem. Soc. 1988;110:1298. see also Zhdankin VV, Stang PJ, Zefirov NS. Chem. Comm. 1992:578.
- 175.Reviews: Moriarty RM. J. Org. Chem. 2005;70:2893. doi: 10.1021/jo050117b. Stang PJ. J. Org. Chem. 2003;68:2997. doi: 10.1021/jo030022e. Zhdankin VV, Stang PJ. Tetrahedron. 1998;54:10927. Wirth T. Angew. Chem. Int. Ed. 2005;44:3656. doi: 10.1002/anie.200500115. Chaudhari SS. Synlett. 2000:278.
- 176.Feldman KS, Cutarelli TD. J. Am. Chem. Soc. 2002;124:11600. doi: 10.1021/ja0277430. [DOI] [PubMed] [Google Scholar]
- 177.Tohma H, Harayama Y, Hashizume M, Iwata M, Kiyono Y, Egi M, Kita Y. J. Am. Chem. Soc. 2003;125:11235. doi: 10.1021/ja0365330. [DOI] [PubMed] [Google Scholar]
- 178.Hamamoto H, Shiozaki Y, Nambu H, Hata K, Tohma H, Kita Y. Chem. Eur. J. 2004;10:4977. doi: 10.1002/chem.200400358. [DOI] [PubMed] [Google Scholar]
- 179.Reviews: Deprez NR, Sanford MS. Inorg. Chem. 2007;46:1924. doi: 10.1021/ic0620337. Zhdankin VV, Stang PJ. Chem. Rev. 2009;109:000. In Press.
- 180.Boldyrev AI, Zhdankin VV, Simons J, Stang PJ. J. Am. Chem. Soc. 1992;114:10569. [Google Scholar]
- 181.Stang PJ, Zhdankin VV. J. Am. Chem. Soc. 1993;115:9808. [Google Scholar]
- 182.Radhakrishnan U, Stang PJ. J. Org. Chem. 2003;68:9209. doi: 10.1021/jo030246x. [DOI] [PubMed] [Google Scholar]
- 183.(a) Collman JP, Hegedus LS, Norton JR, Finke RG. Principles and Applications of Organotransition Metal Chemistry. Mill Valley, CA: University Science Books; 1987. [Google Scholar]; (b) Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 5th Ed. New York: Wiley-Interscience; 1988. [Google Scholar]
- 184.Reviews: Gellman SH. Guest Ed. Chem. Rev. 1997;97:1231. doi: 10.1021/cr970328j.
- 185.Neidle S. Oxford Handbook of Nucleic Acid Structure. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 186.Jones MN, Chapman D. Micelles, Monolayers, and Biomembranes. New York: Wiley-Liss; 1995. [Google Scholar]
- 187.Kauffman S. At Home in the Universe: the Search for Laws of Self-Organization and Complexity. New York: OUP; 1995. [Google Scholar]
- 188.Cann AJ. Principles of Molecular Virology. New York: Academic Press; 1997. [Google Scholar]
- 189.Ercolani G. J. Am. Chem. Soc. 2003;123:16097. doi: 10.1021/ja038396c. [DOI] [PubMed] [Google Scholar]
- 190.Lehn JM. Proc. Natl. Acad. Sci. 2002;99:4763. doi: 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lehn JM. Chem. Eur. J. 6:2097. 200. [Google Scholar]; (c) Lehn J-M. Proc. Natl. Acad. Sci. USA. 1997;94:2106. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reviews: Chambron JC, Dietrich-Buchecker C, Sauvage JP. In: Comprehensive Supramolecular Chemistry. Chapt 2. Lehn J-M, Atwood JL, Davis JED, MacNicol DD, Vögtle, editors. Vol. 9. Oxford: Pergamon Press; 1996. p. 43. Baxter PNW. Chapt. 5. Ibid. 1996;9:165. Constable EC. Ibid. 1996;9(Chapt 6):213.
- 192.Reviews: Steel PJ. Acc. Chem. Res. 2005;38:243. doi: 10.1021/ar040166v. Swieger GF, Malefetse TJ. Chem. Rev. 2000;1000:3483. doi: 10.1021/cr990110s.
- 193.(a) Stang PJ, Cao DH. J. Am. Chem. Soc. 1994;116:4981. [Google Scholar]; (b) Stang PJ, Cao DH, Saito S, Arif AM. J. Am. Chem. Soc. 1995;117:6273. [Google Scholar]
- 194.Cao DH, Chen K, Fan J, Manna J, Olenyuk B, Whiteford JA, Stang PJ. Pure Appl. Chem. 1997;69:1979. [Google Scholar]
- 195.Stang PJ, Whiteford JA. Organometallics. 1994;13:3776. [Google Scholar]
- 196.(a) Stang PJ, Chen K. J. Am. Chem. Soc. 1995;117:1667. [Google Scholar]; (b) Stang PJ, Chen K, Arif AM. J. Am. Chem. Soc. 1995;117:8793. [Google Scholar]; (c) Whiteford JA, Lu CV, Stang PJ. J. Am. Chem. Soc. 1997;119:2524. [Google Scholar]; (d) Whiteford JA, Stang PJ, Huang SD. Inorg. Chem. 1998;37:5595. doi: 10.1021/ic980727c. [DOI] [PubMed] [Google Scholar]
- 197.Stang PJ, Persky NE. J. Chem. Soc. Chem. Commun. 1997:77. [Google Scholar]
- 198.(a) Manna J, Whiteford JA, Stang PJ, Muddiman DC, Smith RD. J. Am. Chem. Soc. 1996;189:8731. [Google Scholar]; (b) Manna J, Kuehl CJ, Whiteford JA, Stang PJ, Muddiman DC, Hofstadler SA, Smith RD. J. Am. Chem. Soc. 1997;119:11611. [Google Scholar]
- 199.(a) Stang PJ, Fan J, Olenyuk B. J. Chem. Soc. Chem. Commun. 1997:1453. [Google Scholar]; (b) Fan J, Whiteford JA, Olenyuk B, Levin MD, Stang PJ, Fleischer EB. J. Am. Chem. Soc. 1999;121:2741. [Google Scholar]
- 200.(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Stang PJ. Chem. Eur. J. 1998;4:19. [Google Scholar]; (c) Olenyuk B, Fechtenkötter A, Stang PJ. Dalton Transactions. 1998:1707. [Google Scholar]
- 201.Reviews: Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. Sweigers GF, Malefetse TJ. Chem. Rev. 2000;100:3483. doi: 10.1021/cr000023w.
- 202.(a) Schweiger M, Seidel SR, Arif AM. Angew. Chem. Int. Ed. Engl. 2001;40:3467. doi: 10.1002/1521-3773(20010917)40:18<3467::aid-anie3467>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; (b) Schweiger M, Seidel SR, Arif AM, Stang PJ. Inorg. Chem. 2002;41:2556. doi: 10.1021/ic0112692. [DOI] [PubMed] [Google Scholar]; (c) Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 203.(a) Schmitz M, Leininger S, Fan J, Arif AM, Stang PJ. Organometallics. 1999;18:4817. [Google Scholar]; (b) Tabellion FM, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2001;123:7740. doi: 10.1021/ja015784a. [DOI] [PubMed] [Google Scholar]; (c) Campbell K, Kuehl CJ, Ferguson MJ, Stang PJ, Tykwinski RR. J. Am. Chem. Soc. 2002;124:7266. doi: 10.1021/ja025773x. [DOI] [PubMed] [Google Scholar]; (d) Chatterjee B, Noveron JC, Resendiz MJE, Lui J, Yamamoto T, Parker D, Cinke M, Nguyen CV, Arif AM, Stang PJ. J. Am. Chem. Soc. 2004;126:10645. doi: 10.1021/ja0388919. [DOI] [PubMed] [Google Scholar]
- 204.(a) Kuehl CJ, Mayne CL, Arif AM, Stang PJ. Org. Lett. 2000;2:3727. doi: 10.1021/ol006638x. [DOI] [PubMed] [Google Scholar]; (b) Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]; (c) Addicott C, Oesterling I, Yamamoto T, Mullen K, Stang PJ. J. Org. Chem. 2005;70:797. doi: 10.1021/jo048239b. [DOI] [PubMed] [Google Scholar]; (d) Kaim W, Schwederski B, Dogan A, Fiedler J, Kuehl CJ, Stang PJ. Inorg. Chem. 2002;41:4025. doi: 10.1021/ic020122n. [DOI] [PubMed] [Google Scholar]
- 205.(a) Stang PJ, Persky NE, Manna J. J. Am. Chem. Soc. 1997;119:4777. [Google Scholar]; (b) Leininger S, Schmitz M, Stang PJ. Org. Lett. 1999;1:1921. doi: 10.1021/ol9911425. [DOI] [PubMed] [Google Scholar]; (c) Chi KW, Addicott A, Arif AM, Das N, Stang PJ. J. Org. Chem. 2003;68:9798. doi: 10.1021/jo0353785. [DOI] [PubMed] [Google Scholar]
- 206. Fujita M, Yazaki J, Ogura K. J. Am. Chem. Soc. 1990;112:5645. Fujita M, Kwon YJ, Washizu S, Ogura K. J. Am. Chem. Soc. 1994;116:1151. See however Stricklen PM, Vocko EJ, Verkade JG. J. Am. Chem. Soc. 1983;105:2494. for an early example of a neutral, tetranuclear, organometallic macrocycle.
- 207.Reviews: Kawano M, Fujita M. Coord. Chem. Rev. 2007;251:2592. Maurizot V, Yoshizawa M, Kawano M, Fujita M. Dalton. Trans. 2006:2750. doi: 10.1039/b516548m. Fujita M, Tominaga M, Hori A, Therien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509. Fujita M. Chem. Soc. Rev. 1998;6:417.
- 208.Reviews: Caulder DL, Raymond KN. Acc. Chem. Res. 1999;32:975. Caulder DL, Raymond KN. J. Chem. Soc., Dalton Trans. 1999:1185. Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. doi: 10.1021/ar040152p.
- 209.Reviews: Holliday B, Mirkin CA. Angew. Chem. Int. Ed. 2001;40:2022. Gianneschi NC, Maser MS, Mirkin CA. Acc. Chem. Res. 2005;38:825. doi: 10.1021/ar980101q.
- 210.Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. doi: 10.1021/ar800025w. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reviews: Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759. doi: 10.1021/ar010062+. Cotton FA, Lin C, Murillo CA. Proc. Natl. Acad. Sci. USA. 2002;99:4810. doi: 10.1073/pnas.012567599.
- 212.(a) Stang PJ, Olenyuk B, Arif AM. Organometallics. 1995;14:5281. [Google Scholar]; (b) Stang PJ, Olenyuk B. Angew. Chem. Int. Ed. Engl. 1996;35:732. [Google Scholar]; (c) Stang PJ, Whiteford JA, Olenyuk B. J. Am. Chem. Soc. 1996;118:8221. [Google Scholar]; (d) Müller C, Whiteford JA, Stang PJ. J. Am. Chem. Soc. 1998;120:9287. [Google Scholar]; (e) Das N, Ghosh A, Singh OM, Stang PJ. Org. Lett. 2006;8:1701. doi: 10.1021/ol060365+. [DOI] [PubMed] [Google Scholar]
- 213.(a) Stang PJ, Olenyuk B, Muddiman DC, Smith RD. Organometallics. 1997;16:3094. [Google Scholar]; (b) Schweiger M, Seidel S, Schmitz M, Stang PJ. Org. Lett. 2000;2:1255. doi: 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]; (c) Caskey DC, Yamamoto T, Addicott C, Shoemaker RK, Vacek J, Hawkridge AM, Muddiman DC, Kottas GS, Michl J, Stang PJ. J. Am. Chem. Soc. 2008;130:7620. doi: 10.1021/ja710715e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Reviews: Lee SJ, Lin W. Acc. Chem. Res. 2008;41:521. doi: 10.1021/ar700216n. Seeber G, Tiedmann BEF, Raymond KN. Top. Curr. Chem. 2006;265:147.
- 215.Reviews: Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008 doi: 10.1039/b811712h. 000 In Press. Lee SJ, Hupp JT. Coord. Chem. Rev. 2006;250:1710. Thanasekaran P, Liao RT, Liu Y-H, Rajendran T, Rajagopal S, Lu KL. Coord. Chem. Rev. 2005;249:1085. Würthner F, You CC, Saha-Möller CR. Chem. Soc. Rev. 2004;33:133. doi: 10.1039/b300512g. Dunbar KR. Foundations of Nanoscience Proc. 2004:171. Keefe MH, Benkstein KD, Hupp JT. Coord. Chem. Rev. 2000;205:201.
- 216.Stang PJ, Olenyuk B, Fan J, Arif AM. Organometallics. 1996;15:904. [Google Scholar]
- 217.Stang PJ, Cao DH, Chen K, Gray GM, Muddiman DC, Smith RD. J. Am. Chem. Soc. 1997;119:5163. [Google Scholar]
- 218.(a) Chi KW, Addicott C, Stang PJ. J. Org. Chem. 2004;69:2910. doi: 10.1021/jo049883t. [DOI] [PubMed] [Google Scholar]; (b) Jude H, Disteldörf H, Fischer S, Wedge T, Hawkridge AH, Arif AM, Hawthorne MF, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2005;127:12131. doi: 10.1021/ja053050i. [DOI] [PubMed] [Google Scholar]; (c) Yang H, Das N, Huang F, Hawkridge AM, Diaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J. Org. Chem. 2006;71:6644. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]; (d) Jude H, Sinclair DJ, Das N, Sherburn MS, Stang PJ. J. Org. Chem. 2006;71:4155. doi: 10.1021/jo060133o. [DOI] [PubMed] [Google Scholar]; (e) Northrop BH, Glöckner A, Stang PJ. J. Org. Chem. 2008;73:1787. doi: 10.1021/jo702380b. [DOI] [PubMed] [Google Scholar]
- 219.(a) Yang H-B, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ.J. Am. Chem. Soc 20061281001416881621 [Google Scholar]; (b) Yang HB, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]
- 220.(a) Yang H-B, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ. J. Am. Chem. Soc. 2008;130:839. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh K, Zhao Y, Yang H-B, Northrop BH, White HS, Stang PJ. J. Org. Chem. 2008;73:8553. doi: 10.1021/jo801692v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.(a) Yang H-B, Ghosh K, Northrop BH, Zheng YR, Lyndon MM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:14187. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh K, Yang H-B, Northrop BH, Lyndon MM, Zheng YR, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2008;130:5320. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]
- 222.Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org. Lett. 2004;6:651. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]
- 223.Review: Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324.
- 224.(a) Das N, Mukherjee PS, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:13950. doi: 10.1021/ja037788g. [DOI] [PubMed] [Google Scholar]; (b) Mukherjee PS, Das N, Kryschenko Y, Arif AM, Stang PJ. J. Am. Chem. Soc. 2004;126:2464. doi: 10.1021/ja039235b. [DOI] [PubMed] [Google Scholar]; (c) Mukherjee PS, Min KS, Arif AM, Stang PJ. Inorg. Chem. 2004;43:6345. doi: 10.1021/ic0493088. [DOI] [PubMed] [Google Scholar]; (d) Das N, Arif AM, Stang PJ, Sieger M, Sarkar B, Kaim W, Fiedler J. Inorg. Chem. 2005;44:5798. doi: 10.1021/ic0481834. [DOI] [PubMed] [Google Scholar]; (e) Das N, Ghosh A, Arif AM, Stang PJ. Inorg. Chem. 2005;44:7130. doi: 10.1021/ic050919p. [DOI] [PubMed] [Google Scholar]; (f) Das N, Stang PJ, Arif AM, Compana CF. J. Org. Chem. 2005;70:10440. doi: 10.1021/jo0517085. [DOI] [PubMed] [Google Scholar]; (g) Huang F, Yang H, Maran U, Arif AM, Gibson HW, Stang PJ. J. Org. Chem. 2006;71:6623. doi: 10.1021/jo061007n. [DOI] [PubMed] [Google Scholar]
- 225.Inter alia Cotton FA, Donahue JP, Murillo CA. J. Am. Chem. Soc. 2003;125:5436. doi: 10.1021/ja029101i. Cotton FA, Lin C, Murillo CA. Inorg. Chem. 2001;40:478. doi: 10.1021/ic001016t. Cotton FA, Lin C, Murillo CA. Inorg. Chem. 2001;40:6413. doi: 10.1021/ic0104959. Cheng H, Chung-Ying D, Chen-jie F, Yong-jiang L, Qingjin M. J. Chem. Soc. Dalton. Trans. 2000:1207. Cotton FA, Daniels LM, Lin C, Murillo CA. J. Am. Chem. Soc. 1999;121:4538. Cotton FA, Lin C, Daniels ML, Murillo CA, Yu S-Y. J. Chem. Soc. Dalton Trans. 2001:502. Bala M, Thanasekaran P, Rajendran T, Liao RT, Liu YH, Lee GH, Peng SM, Rajagopal S, Lu KL. Inorg. Chem. 2003;42:4795. doi: 10.1021/ic034172j. Manimaran B, Rajendran T, Lu YL, Lee GH, Peng SM, Lu KL. Eur. J. Inorg. Chem. 2001;3:633. Slone RV, Hupp JT, Albrecht-Schmitt TE. Inorg. Chem. 1996;35:4096. doi: 10.1021/ic960464r. Saalfrank RW, Reimann U, Goritz M, Hampel E, Scheurer A, Heinemann FW, Busches M, Daub J, Schunemann V, Trautwein AX. Chem. Eur. J. 2002;8:3614. doi: 10.1002/1521-3765(20020816)8:16<3614::AID-CHEM3614>3.0.CO;2-3. Saalfrank RW, Trummer S, Reimann U, Chowdhry MM, Hampel F, Waldmann O. Angew. Chem. Int. Ed. 2000;39:3492. doi: 10.1002/1521-3773(20001002)39:19<3492::aid-anie3492>3.0.co;2-g.
- 226.Ghosh S, Turner DR, Batten SR, Mukherjee PS. Dalton Trans. 2007:1869. doi: 10.1039/b702353g. and references therein. [DOI] [PubMed] [Google Scholar]
- 227.Chi KW, Addicott C, Stang PJ. J. Am. Chem. Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]
- 228.Yamamoto T, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:12309. doi: 10.1021/ja0302984. [DOI] [PubMed] [Google Scholar]
- 229.Megyes T, Jude H, Grosz T, Bako I, Radnai T, Tarkanyi G, Palinkas G, Stang PJ. J. Am. Chem. Soc. 2005;127:10731. doi: 10.1021/ja0523690. [DOI] [PubMed] [Google Scholar]
- 230.230(a) Vacek J, Caskey DC, Horinek D, Shoemaker RK, Stang PJ, Michl J. J. Am. Chem. Soc. 2008;130:7629. doi: 10.1021/ja801341m. [DOI] [PubMed] [Google Scholar]; (b) Tarkanyi G, Jude H, Palinkas G, Stang PJ. Org. Lett. 2005;7:4971. doi: 10.1021/ol051910u. [DOI] [PubMed] [Google Scholar]; (c) Fuss M, Siehl H-U, Olenyuk B, Stang PJ. Organometallics. 1999;18:758. [Google Scholar]
- 231.(a) Park S-J, Shin DM, Sakamoto S, Yamaguchi K, Chung YK, Lah MS, Hong J-I. Chem. Eur. J. 2005;11:235. doi: 10.1002/chem.200400801. [DOI] [PubMed] [Google Scholar]; (b) Tiede DM, Zhang R, Chen LX, Yu L, Lindsey JS. J. Am. Chem. Soc. 2004;126:14054. doi: 10.1021/ja048209q. [DOI] [PubMed] [Google Scholar]
- 232.(a) Lehn J-M. Rep. Prog. Phys. 2004;67:249. [Google Scholar]; (b) Wu A, Isaacs L. J. Am. Chem. Soc. 2003;125:4831. doi: 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]; (c) Lehn J-M. Science. 2002;295:2400. doi: 10.1126/science.1071063. [DOI] [PubMed] [Google Scholar]; (d) Hollingsworth MD. Science. 2002;295:2410. doi: 10.1126/science.1070967. [DOI] [PubMed] [Google Scholar]
- 233.(a) Zheng YR, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (b) Yang H-B, Ghosh K, Northrop BH, Stang PJ. Org. Lett. 2007;9:1561. doi: 10.1021/ol070371l. [DOI] [PubMed] [Google Scholar]; (c) Chi KW, Addicott C, Stang PJ. J. Am. Chem. Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]; (d) Addicott C, Das N, Stang PJ. Inorg. Chem. 2004;43:5335. doi: 10.1021/ic049326p. [DOI] [PubMed] [Google Scholar]; (e) Chi KW, Addicott C, Moon ME, Lee HJ, Yoon SC, Stang PJ. J. Org. Chem. 2006;71:6662. doi: 10.1021/jo060971i. [DOI] [PubMed] [Google Scholar]; (f) Zhao L, Northrop BH, Zheng YR, Yang H-B, Lee HJ, Lee YM, Park JY, Chi KW, Stang PJ. J. Org. Chem. 2008;73:6580. doi: 10.1021/jo800957r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhao L, Northrop BH, Stang PJ. J. Am. Chem. Soc. 2008;130:11886. doi: 10.1021/ja805770w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Inter alia: Langner A, Tait SL, Lin N, Rajadurai C, Ruben M, Kern K. Proc. Natl. Acad. Sci. USA. 2007;104:17927. doi: 10.1073/pnas.0704882104. Saur I, Scopelliti R, Severin K. Chem. Eur. J. 2006;12:1058. doi: 10.1002/chem.200500621. Kamada T, Aratani N, Ikeda T, Shibata N, Higuchi Y, Wakamiya A, Yamaguchi S, Kim KS, Yoon ZS, Kim D, Osuka A. J. Am. Chem. Soc. 2006;128:7670. doi: 10.1021/ja0611137. Pinalli R, Cristini V, Sottili V, Geremia S, Campagnolo M, Caneschi A, Dalcanale E. J. Am. Chem. Soc. 2004;126:6516. doi: 10.1021/ja038694+. Kondo T, Oyama K-I, Yoshida K. Angew. Chem. Int. 2001;40:894. doi: 10.1002/1521-3773(20010302)40:5<894::AID-ANIE894>3.0.CO;2-8. Albrecht M, Schneider M, Rötelle H. Angew. Chem. Int. Ed. 1999;38:557. doi: 10.1002/(SICI)1521-3773(19990215)38:4<557::AID-ANIE557>3.0.CO;2-S. Taylor PN, Anderson HL. J. Am. Chem. Soc. 1999;121:11538. Stiller R, Lehn J-M. Eur. J. Inorg. Chem. 1998:977. Enemark EJ, Stack TDP. Angew. Chem. Int. Ed. 1998;37:932. doi: 10.1002/(SICI)1521-3773(19980420)37:7<932::AID-ANIE932>3.0.CO;2-C. Masood MA, Enemark EJ, Stack TDP. Angew. Chem. Int. Ed. 1998;37:928. doi: 10.1002/(SICI)1521-3773(19980420)37:7<928::AID-ANIE928>3.0.CO;2-T. Caulder DL, Raymond KN. Angew. Chem. Int. Ed. 1997;36:1440. Krämer R, Lehn J-M, Marquis-Rigault A. Proc. Natl. Acad. Sci. USA. 1993;90:5394. doi: 10.1073/pnas.90.12.5394.
- 235.Kay ER, Leigh DA, Zerbetto F. Angew. Chem. Int. Ed. 2007;46:72. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]
- 236.(a) Snyder MA, Tsapatsis M. Angew. Chem. Int. Ed. 2007;46:7650. doi: 10.1002/anie.200604910. [DOI] [PubMed] [Google Scholar]; (b) Balzani G, Credi A, Venturi M. Chem. Eur. J. 2002;8:5525. [Google Scholar]; (c) Seeman NC, Belcher AM. Proc. Natl. Acad. Sci. USA. 2002;99:6451. doi: 10.1073/pnas.221458298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gates BG, Xu Q, Stewart M, Ryan D, Wilson CG, Whitesides GM. Chem. Rev. 2005;105:1171. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- 237.Barth JV. Annu. Rev. Phys. Chem. 2007;58:375. doi: 10.1146/annurev.physchem.56.092503.141259. [DOI] [PubMed] [Google Scholar]
- 238.Barth JV, Constantini G, Kern K. Nature. 2005;437:671. doi: 10.1038/nature04166. [DOI] [PubMed] [Google Scholar]
- 239.Hooks DE, Fritz T, Ward MD. Adv. Mater. 2001;13:227. [Google Scholar]
- 240.Wang D, Wan LJ. J. Phys. Chem. C. 2007;111:16109. [Google Scholar]
- 241.Reviews: Barlow SM, Raval R. Surface Science Reports. 2003;50:201. Wan LJ. Acc. Chem. Res. 2006;39:334. doi: 10.1021/ar0501929.
- 242.Li SS, Northrop BH, Yuan QH, Wan LJ, Stang PJ. Acc. Chem. Res. 2009;42 doi: 10.1021/ar800117j. 000 In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.(a) Gong JR, Wan LJ, Yuan QH, Bai CL, Jude H, Stang PJ. Proc. Nat. Acad. Sci. USA. 2005;102:971. doi: 10.1073/pnas.0409145102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yuan QH, Wan LJ, Jude H, Stang PJ. J. Am. Chem. Soc. 2005;127:16279. doi: 10.1021/ja0549300. [DOI] [PubMed] [Google Scholar]; (c) Li SS, Yan HJ, Wan LJ, Yang H-B, Northrop BH, Stang PJ. J. Am. Chem. Soc. 2007;129:9268. doi: 10.1021/ja0733282. [DOI] [PubMed] [Google Scholar]; (d) Yuan QH, Yan CJ, Wan JL, Northrop BH, Jude H, Stang PJ. J. Am. Chem. Soc. 2008;130:8878. doi: 10.1021/ja801934w. [DOI] [PubMed] [Google Scholar]
- 244.(a) Jeohn KS, Shin U-S, Kogej M, Hai NTM, Brockmann P, Jeong N, Krichner B, Reiher M, Schalley C. J. Am. Chem. Soc. 2005;127:17672. doi: 10.1021/ja053781i. [DOI] [PubMed] [Google Scholar]; (b) Menzonni E, Pinalli R, Speets EA, Ravoo BJ, Dalcanale E, Reinhoudt DN. Chem. Eur. J. 2004;10:2199. doi: 10.1002/chem.200305570. [DOI] [PubMed] [Google Scholar]; (c) Safurousky C, Merz L, Rang A, Brockman P, Hermann BA, Schalley CA. Angew. Chem. Int. Ed. 2004;43:1291. doi: 10.1002/anie.200352968. [DOI] [PubMed] [Google Scholar]; (d) Semenau A, Spatz JP, Möller M, Lehn J-M, Sell B, Schubert D, Weidl CH, Schubert US. Angew. Chem. Int. Ed. 1999;38:2547. doi: 10.1002/(sici)1521-3773(19990903)38:17<2547::aid-anie2547>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 245.Review: Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d.
- 246.(a) Schweiger M, Yamamoto T, Stang PJ, Bläser D, Boese R. J. Org. Chem. 2005;70:4861. doi: 10.1021/jo050469i. [DOI] [PubMed] [Google Scholar]; (b) Leininger S, Fan J, Schmitz M, Stang PJ. Proc. Natl. Acad. Sci. USA. 2000;97:1380. doi: 10.1073/pnas.030264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Olenyuk B, Whiteford JA, Fechtenkötter A, Stang PJ. Nature. 1999;398:796. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- 248.Kuehl CJ, Yamamoto T, Seidel SR, Stang PJ. Org. Lett. 2002;4:913. doi: 10.1021/ol017296d. [DOI] [PubMed] [Google Scholar]
- 249.(a) Radhakrishnan U, Schweiger M, Stang PJ. Org. Lett. 2001;3:3141. doi: 10.1021/ol0164684. [DOI] [PubMed] [Google Scholar]; (b) Kuehl CJ, Kryschenko YK, Radhakrishnan U, Seidel SR, Huang SD, Stang PJ. Proc. Natl. Acad. Sci. USA. 2002;99:4932. doi: 10.1073/pnas.012540799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kryschenko YK, Seidel SR, Muddiman DC, Nepomureno AI, Stang PJ. J. Am. Chem. Soc. 2003;125:9647. doi: 10.1021/ja030209n. [DOI] [PubMed] [Google Scholar]; (d) Chi KW, Addicott C, Kryschenko YK, Stang PJ. J. Org. Chem. 2004;69:964. doi: 10.1021/jo035607n. [DOI] [PubMed] [Google Scholar]; (e) Mukherjee PS, Das N, Stang PJ. J. Org. Chem. 2004;69:3526. doi: 10.1021/jo049772u. [DOI] [PubMed] [Google Scholar]; (f) Yang H-B, Ghosh K, Das N, Stang PJ. Org. Lett. 2006;8:3991. doi: 10.1021/ol0614626. [DOI] [PubMed] [Google Scholar]; (g) Yang H-B, Ghosh K, Arif AM, Stang PJ. J. Org. Chem. 2006;71:9464. doi: 10.1021/jo061779j. [DOI] [PubMed] [Google Scholar]
- 250.(a) Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. J. Am. Chem. Soc. 1999;121:10434. [Google Scholar]; (b) Levin MD, Stang PJ. J. Am. Chem. Soc. 2000;122:7428. [Google Scholar]
- 251.(a) Yamauchi Y, Yoshizawa M, Fujita M. J. Am. Chem. Soc. 2008;130:5832. doi: 10.1021/ja077783+. [DOI] [PubMed] [Google Scholar]; (b) Yamaguchi T, Fujita M. Angew. Chem. Int. Ed. 2008;47:2067. doi: 10.1002/anie.200705139. [DOI] [PubMed] [Google Scholar]; (c) Nakabayashi K, Ozak Y, Kawano M, Fujita M. Angew. Chem. Int. Ed. 2008;47:2046. doi: 10.1002/anie.200704924. [DOI] [PubMed] [Google Scholar]; (d) Kikuchi T, Murase T, Sato S, Fujita M. Supramol. Chem. 2008;20:81. [Google Scholar]; (e) Yamashita K, Kawano M, Fujita M. Chem. Comm. 2007:4102. doi: 10.1039/b712529a. [DOI] [PubMed] [Google Scholar]; (f) Suzuki K, Kawano M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:10652. doi: 10.1021/ja073629b. [DOI] [PubMed] [Google Scholar]; (g) Nishioka Y, Yamaguchi T, Yoshizawa M, Fujita M. J. Am. Chem. Soc. 2007;129:7000. doi: 10.1021/ja071591x. [DOI] [PubMed] [Google Scholar]; (h) Suzuki K, Kawano M, Fujita M. Angew. Chem. Int. Ed. 2007;46:2819. doi: 10.1002/anie.200605084. [DOI] [PubMed] [Google Scholar]; (i) Kasai K, Fujita M. Chem. Eur. J. 2007;13:3089. doi: 10.1002/chem.200501067. [DOI] [PubMed] [Google Scholar]; (j) Kamija N, Tominaga M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:3816. doi: 10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]; (k) Ono K, Yoshizawa M, Kato T, Watanabe K, Fujita M. Angew. Chem. Int. Ed. 2007;46:1803. doi: 10.1002/anie.200604790. [DOI] [PubMed] [Google Scholar]; (l) Kobayashi Y, Kawano M, Fujita M. Chem Comm. 2006:4377. doi: 10.1039/b612562j. [DOI] [PubMed] [Google Scholar]; (m) Tashiro S, Kobayashi M, Fujita M. J. Am. Chem. Soc. 2006;128:9280. doi: 10.1021/ja061722e. [DOI] [PubMed] [Google Scholar]; (n) Kawano M, Kobayashi Y, Ozeki T, Fujita M. J. Am. Chem. Soc. 2006;128:6558. doi: 10.1021/ja0609250. [DOI] [PubMed] [Google Scholar]; (o) Tashiro S, Tominaga M, Yamaguchi Y, Kato K, Fujita M. Chem. Eur. J. 2006;12:3211. doi: 10.1002/chem.200501424. [DOI] [PubMed] [Google Scholar]; (p) Yoshizawa M, Kumazawa K, Fujita M. J. Am. Chem. Soc. 2005;127:13456. doi: 10.1021/ja053508g. [DOI] [PubMed] [Google Scholar]; (q) Tominaga M, Suzuki K, Murase T, Fujita M. J. Am. Chem. Soc. 2005;127:11950. doi: 10.1021/ja054069o. [DOI] [PubMed] [Google Scholar]; (r) Nakabayashi K, Kawano M, Fujita M. Angew. Chem. Int. Ed. 2005;44:5322. doi: 10.1002/anie.200501568. [DOI] [PubMed] [Google Scholar]; (s) Yoshizawa M, Fujita M. Pure & Appl. Chem. 2005;77:1107. [Google Scholar]
- 252.(a) Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2008;130:6362. doi: 10.1021/ja076691h. [DOI] [PubMed] [Google Scholar]; (b) Leung DH, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2008;130:2798. doi: 10.1021/ja075975z. [DOI] [PubMed] [Google Scholar]; (c) Davis AV, Fiedler D, Ziegler M, Terpin A, Raymond KN. J. Am. Chem. Soc. 2007;129:15354. doi: 10.1021/ja0764815. [DOI] [PubMed] [Google Scholar]; (d) Biros SM, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2007;129:12094. doi: 10.1021/ja075236i. [DOI] [PubMed] [Google Scholar]; (e) Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2007;129:11459. doi: 10.1021/ja072654e. [DOI] [PubMed] [Google Scholar]; (f) Pluth MD, Bergman RG, Raymond KN. Science. 2007;316:85. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]; (g) Leung DH, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2006;128:9781. doi: 10.1021/ja061412w. [DOI] [PubMed] [Google Scholar]; (h) Fiedler D, Bergman RG, Raymond KN. Angew. Chem. Int. Ed. 2006;45:745. doi: 10.1002/anie.200501938. [DOI] [PubMed] [Google Scholar]; (i) Tiedemann BEF, Raymond KN. Angew. Chem. Int. Ed. 2006;45:83. [Google Scholar]; (j) Davis AV, Raymond KN. J. Am. Chem. Soc. 2005;127:7912. doi: 10.1021/ja051037s. [DOI] [PubMed] [Google Scholar]; (k) Brumaghim JL, Michels M, Pagliero D, Raymond KN. Eur. J. Org. Chem. 2004:5115. [Google Scholar]; (l) Fiedler D, Leung DH, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2004;126:3674. doi: 10.1021/ja039225a. [DOI] [PubMed] [Google Scholar]
- 253.Reviews: Moulton B, Zaworotko MJ. Chem. Rev. 2001;101:1629. doi: 10.1021/cr9900432. Eddaouchi M, Moler DB, Li H, Chen B, Reineke TM, O’Keeffe M, Yaghi OM. Acc. Chem. Res. 2001;34:318. doi: 10.1021/ar000034b. Yaghi OM, Li H, Davies C, Richardson D, Groy TL. Acc. Chem. Res. 1998;31:474.
- 254.An indication of this in the over 1600 citations to date of our chemical review article,223 published in 2000, and the over 600 citations to date of our Accounts article,200a published in 1997, where we first described the directional bonding approach as summarized in Figure 2.