Abstract
Protection of transplanted cells from the host immune system using immunoisolation technology will be important in realizing the full potential of cell-based therapeutics. Microencapsulation of cells and cell aggregates has been the most widely explored immunoisolation strategy, but widespread clinical application of this technology has been limited, in part, by inadequate transport of nutrients, deleterious innate inflammatory responses, and immune recognition of encapsulated cells via indirect antigen presentation pathways. To reduce mass transport limitations and decrease void volume, recent efforts have focused on developing conformal coatings of micron and submicron scale on individual cells or cell aggregates. Additionally, anti-inflammatory and immunomodulatory capabilities are being integrated into immunoisolation devices to generate bioactive barriers that locally modulate host responses to encapsulated cells. Continued exploration of emerging paradigms governed by the inherent challenges associated with immunoisolation will be critical to actualizing the clinical potential of cell-based therapeutics.
Keywords: Immunoisolation, Cell encapsulation, Cell transplantation, Conformal coating, Cell surface modification, Pancreatic islet, Immunomodulation, Anti-inflammatory
1. Introduction
Transplantation of primary or genetically engineered cells of allo- or xenogenic origin has emerged as a promising approach for the localized and regulated ‘de novo’ delivery of a nearly unlimited number of therapeutic agents with potential capacity to cure or treat virtually any disease or disorder, including, but not limited to, those related to the endocrine system (diabetes, hypoparathyroidism, adrenal insufficiency), central nervous system (Parkinson’s, Alzheimer’s, ALS, Huntington’s), as well as cancer, kidney failure, and heart disease [1–8]. However, widespread clinical application of cell transplantation remains limited, in part, by the deleterious side effects of current immunosuppressive regimens necessary to prevent host rejection of transplanted cells. It has long been postulated [9] that systemic immunosuppression could be eliminated if transplanted cells and tissues were physically isolated from the host immune system using a semipermeable membrane, or immunoisolation device. Since the pioneering work by Chick et al. in the development of a bioartificial pancreas [10] and Lim and Sun’s introduction of alginate-encapsulated islets [11], decades of extensive research has focused on the design and application of immunoisolation devices capable of protecting transplanted allo- and xenogenic cells from the host, while facilitating adequate transport of oxygen, nutrients, and secreted therapeutic molecules.
To date, a variety of polymeric and inorganic matrices and membranes have been utilized to produce immunoisolation devices of diverse physiochemical properties and geometries [12], which include vascular perfusion devices, avascular diffusion chambers, macrocapsules, and microcapsules (Fig. 1) [13,14]. The need for vascular surgery, and associated risks of surface-induced thrombosis, has largely limited the development of vascular perfusion devices [14]. Though notable exceptions exist [15,16], clinical application of macrocapsules and avascular diffusion chambers has been hampered by insufficient oxygen and nutrient transport to cells in the center of such devices [14]. Consequently, most immunoisolation strategies have employed microcapsules consisting of cells or cell clusters entrapped within a spherical semipermeable membrane, an inherently favorable geometry for diffusive nutrient transport that can be implanted with minor surgery [13,14]. Though this review will mostly address limitations in current microencapsulation strategies, the challenges and considerations discussed herein are generally applicable to a variety of immunoisolation devices.
Fig. 1.
Devices for cell transplantation have traditionally included vascular perfusion devices, avascular macrocapsules, and microcapsules. In an effort to reduce the size and void volume of immunoisolation devices, conformal coatings, cell-surface PEGylation, and nanoencapsulation have recently been explored.
Transplantation of encapsulated pancreatic islets for the treatment of diabetes mellitus has been the most common application of cell transplant and immunoisolation technology, and, consequently, will be an area of focus within this review. However, widespread clinical application of cell-based therapy requires an inexhaustible source of cells or tissues capable of delivering the therapeutic agent in an appropriate form, often in response to physiological cues. Genetic engineering has emerged as a promising tool for addressing this challenge [17–19], but genetically engineered cells often arise from an allogenic or xenogenic source and will also require protection from the host immune system. Thus, the lessons afforded by efforts directed towards islet encapsulation will prove to be highly valuable as genetically engineered cells gain in clinical relevance.
While far from a comprehensive history of islet encapsulation, Table 1 summarizes the ability of some notable microcapsules to restore normoglycemia in several animal models of diabetes. The large number of reports and variable success rates attest to the promise and inherent challenges of immunoisolation. As non-encapsulated islet allografts are generally destroyed within two weeks and xenografts within one week, immunoisolation significantly improves the survival of transplanted tissue. However, insulin independence, particularly in larger animals or more rigorous small animal models is of limited duration. Consequently, despite remarkable advances in immunoisolation technology, only a few clinical trials have been performed with immunoisolated islets [20–22] or other cell types [1,15,23–25]. While many of these studies continue to fall short of long-term clinical objectives, early evidence of graft function and improved patient health have demonstrated the potential significance of cell-based therapeutics, providing motivation for further elucidation of mechanisms that limit graft survival with a goal of further optimization of immunoisolation strategies.
Table 1.
Maintenance of normoglycemia after transplantation of microencapsulated islets
| Donor | Recipient | Capsule Type | Tx site | Islet dose | Normoglycemia (avg. days) | Normoglycemia (max. days) | Normoglycemia (range) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Allogenic | ||||||||
| B6AF1 mice | NOD mice | Alginate/Ba2+ | Peritoneum | 900–1000 | >350a | >385 | 350–>385 | [56] |
| DA rat | Lewis rat | Alginate/Ba2+ | Peritoneum | 5000 IE | 54±6 | 91 | 15–91 | [253] |
| Lewis rat | AO rat | APA | Peritoneum | 3500–4200 | 102.6±9.7 | >200 | 42–>200 | [163] |
| Canine | Canine | Agarose | Omentum | 4300–11,000 IE/kg | 20.5±20.9 | 49 | 28–49 | [276] |
| Xenogenic | ||||||||
| Porcine | Canine | Agarose-based | Peritoneum | 4000–45,000/kg | 49.7±21.7 | 119 | 7–119 | [277] |
| Hamster | Wistar rat | Agarose-based | Peritoneum | 3000 | 124.5±44.6 | 175 | 77–175 | [278] |
| Porcine | NOD mice | APA | Peritoneum | 11,341+/−3293 IE | 13.2±1.2 | NR | NR | [248] |
| Porcine | NOD mice | Alginate/Ba2+ | Peritoneum | 5000 IE | 15±1 | 184 | NR | [239] |
| Porcine | Primate | APA | Peritoneum | 3–7×104b | NR | 804 | 120–804 | [51] |
| Porcine | B6AF1 mice | Alginate/Ba2+ | Peritoneum | 10,000 | NR | 140c | NR | [279] |
| SD rat | Balb/c mice | Alginate/Ba2+ | Peritoneum | 1800 | NR | 270d | 90–270 | [162] |
| Human | Balb/c mice | Alginate/Ba2+ | Peritoneum | 1800 | NR | 268d | 115–268 | [162] |
| SD rat | C57BL/6 mice | Alginate/PMCG | Peritoneum | 1000 | NR | >300 | 20–>300 | [43] |
| SD rat | NOD mice | Alginate/PMCG | Peritoneum | 1000 | NR | 180 | 40–180 | [43] |
| Canine | NOD mice | APA | Peritoneum | 4×103–1.2×10 | 11.5±3 | 17 | 5–17 | [245] |
| Canine | C57BL/6 mice | APA | Peritoneum | 4×103–1.2×10 | 51 ±8 | 68 | 36–68 | [245] |
| Lewis rat | NOD mice | APA | Peritoneum | 1800–2000 | 10±2 | 17 | 6–17 | [245] |
| Bovine | Balb/c mice | alginate/Ca2+ | Peritoneum | 2–4×103 | NR | >70 | 30–>70 | [160] |
| Wistar rat | Balb/c mice | APA | Peritoneum | 900–1000 | 219.8±46.2 | 308 | 172–308 | [280] |
| SD rat | Balb/c mice | Agarose-based | Peritoneum | 500 | 79±32 | 134 | 47–134 | [281] |
NR: not reported. APA: alginate/poly(L-lysine)/alginate. PMCG: poly-methyl-co-guanidine. IE: islet equivalents.
Natural lifespan of mice precluded further investigation.
Multiple transplants used.
Study terminated at 140 days.
Ongoing graft survival at time of report.
The objective of this review is not to provide a comprehensive survey of over thirty years of immunoisolation research, but rather to present a summary of current principal obstacles to the clinical application of immunoisolation technology. These challenges include mass transport limitations and cell hypoxia, deleterious innate inflammatory responses to device materials and/or encapsulated cells, and immune-mediated inflammatory responses elicited via the indirect antigen presentation pathway. Moreover, this review highlights current areas of investigation that hold particular promise for circumventing these limitations, with an emphasis on biomaterial- and tissue engineered-based approaches.
2. Mass transport limitations in microencapsulation
Although the high surface-to-volume ratio provided by microencapsulation considerably improves mass transport relative to macrocapsules or extravascular diffusion chambers [13,26], the relatively large size of conventional microcapsules, typically 400–800 µm in diameter, continues to impose transport limitations that, if not accounted for, may adversely effect cell survival and function. Experimental evidence and mathematical models demonstrate that oxygen concentration decreases radially within cylindrical or spherical devices due to the consumption of oxygen by the encapsulated cells [27–30]. Therefore, if oxygen levels are insufficient at the site of transplantation, cell density must be reduced as device diameter increases to minimize hypoxia of centrally located cells. Even sublethal levels of hypoxia can have deleterious effects on ATP-dependent cell functions, such as insulin secretion [31], and may also induce expression of inflammatory mediators [32]. Consequently, the number of cells that may be transplanted within a given microcapsule is limited both by device size and the related metabolic profile of the donor cells, often leading to an increase in cell transplant volume and an associated increased incidence of device defects [33] and surgical risk [34].
Effective cell-based therapy often relies on the ability of transplanted cells to respond to physiological stimuli in a concentration- and time-dependent manner [8]. The characteristic time for diffusion through a sphere of radius, R, scales as R2/D, where D is the diffusivity of the solute through the encapsulation matrix and/or immunoisolation membrane [35]. Therefore, cells in the center of the device will experience a given solute concentration at a later time than those on the periphery, leading to a lag in response time [30]. Moreover, depending on the pore size and other physiochemical properties of the immunoisolation membrane and cell immobilization matrix, the diffusivity of important solutes such as glucose, insulin, and oxygen may be substantially less than their diffusivity in water [30,36–39], further delaying responses as compared to those observed for non-encapsulated tissue. This is perhaps most clearly illustrated by microencapsulated islets, where the distance between the capsule surface and outer cell layer of the islet may be on the order of 100– 400 µm, creating a void space which glucose and insulin must cross prior to transport in or out of the device. Indeed, delayed in vitro insulin secretion in response to step changes in glucose have been observed for a variety of different capsule formulations [11,40–43]. Decreasing capsule size has been shown to minimize this delay [44–46].
Arguably more detrimental than diffusion limitations inherent to conventional microcapsules are constraints imposed by the transplant sites necessary to accommodate the volume of microencapsulated cells. For example, current clinical islet transplantation protocols require ∼600–700 thousand islets, a volume of roughly 5–10 mL [47]. In contrast, a current clinical trial using islets entrapped in 500 µm microcapsules requires a transplant volume of 50mL [21], representing approximately a 5– 10 fold increase in transplant volume. Consequently, most microencapsulated cells have been transplanted into sites that have a relatively limited vascular supply, such as the omentum [48,49] or peritoneal cavity [20,21,50,51]. The anatomy of the peritoneal cavity does not facilitate instantaneous transport of insulin or glucose to and from the systemic circulation, as insulin must be absorbed by the peritoneum and extracted by the liver [52,53]. As such, insulin production within the peritoneal cavity results in a delayed systemic response relative to intraportal insulin production [54,55], thereby impairing metabolic control. In response to a meal challenge, Tatarkiewicz et al. observed blunted C-peptide concentrations in animals transplanted with non-encapsulated, syngeneic islets in the peritoneal cavity, indicating that transplantation site is critical to proper maintenance of metabolic processes [41]. Though successful reversal of diabetes has been achieved despite impaired insulin and C-peptide responses [41,42,56], it is unclear whether metabolic control will be sufficiently robust to minimize the chronic complications of diabetes [57–59].
Viability and function of microencapsulated cells transplanted into relatively avascular sites may be further exacerbated by partial pressures of oxygen which are 40% of that found in arterial circulation [27]. Microencapsulated islet autografts retrieved from the peritoneum upon graft failure often have necrotic cores [60], a hallmark of hypoxia [32]. Interestingly, core necrosis may be observed even in the absence of encapsulation [60], corroborating previous findings that the peritoneal cavity provides a suboptimal environment for islet transplantation [61–63].
3. Transplantation of microencapsulated cells into a microvascular bed: intraportal islet transplantation
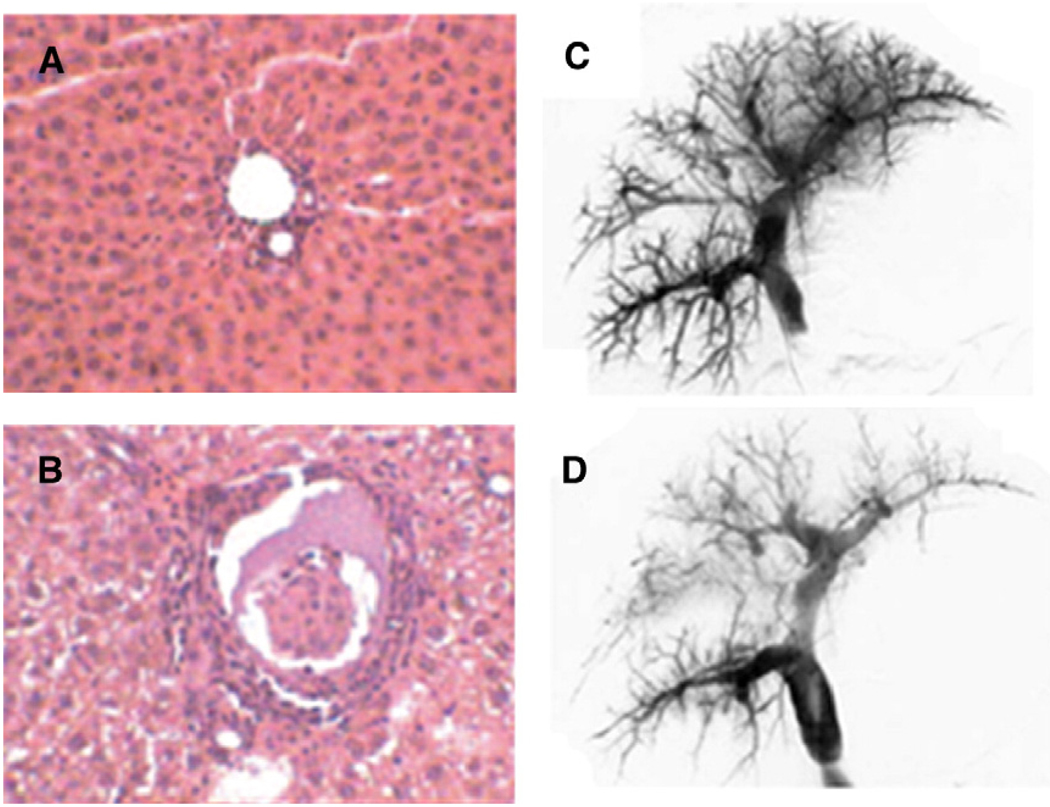
Though several clinical trials and large animal studies have demonstrated the potential efficacy of intraperitoneally transplanted encapsulated islets [20,21,50,51,64], the International Islet Registry reports that, compared to other sites, transplantation of islets into the portal vein is associated with the highest success rate one year after transplantation [65]. Thus, the portal bed remains the clinically preferred site for islet transplantation [47,66,67]. By means of a minimally invasive procedure, isolated and purified islets are infused into the portal vein of the liver where they lodge at distal portal venules [66]. While direct islet-blood contact has been shown to mediate thrombosis [68– 70], the portal vein offers an oxygen and nutrient rich environment and provides physiologically normal drainage of insulin, minimizing delayed insulin secretion in response to glucose. The encouraging early results of the Edmonton Protocol, which combined intraportal islet transplantation with less diabetogenic, steroid free immunosuppression [47], demonstrated the potential of islet transplantation [71]. However, most conventional microcapsules are not suitable for transplantation into microvascular beds due to their large diameter [72– 74]. Intraportal infusion of 420 µm microparticles has been shown to result in dangerous elevations of intraportal pressure and, in some instances, increased mortality in animal models [73]. Bottino et al. have observed impaired hyaluronic acid clearance after intraportal infusion of both islets and an equivalent volume of microparticles, indicating that portal vein endothelial cells are injured in response to particle infusion in a non-specific manner [75]. Schneider et al. have recently demonstrated impaired engraftment of islets encapsulated in 350 µm alginate/Ba2+ microcapsules transplanted into the portal vein compared to non-encapsulated controls, apparently due to occlusion of small and medium sized portal venules and subsequent islet hypoxia (Fig. 2A, B) [74]. Clearly, encapsulation strategies for transplantation of cells into microvascular beds must minimize transplant volume.
Fig. 2.
Transplantation of 350–400 µm microcapsules into the liver microvasculature. Histological evaluation of the liver of a SD rat before (A) and after (B) intraportal transplantation of islets encapsulated within 350 µm alginate/Ba2+ microcapsules. Intraportal transplantation is associated with increased inflammatory cell recruitment and occlusion of portal venules (reprinted from [74] with permission of Wiley-Blackwell Publishing Ltd). Portography before (C) and 15 min after (D) intraportal injection of empty 400 µm microcapsules. Injection of capsules decreases the number of small portal veins; however, this reduction is comparable to that observed during intraportal transplantation of non-encapsulated islets (reprinted from [96] with kind permission from Springer Science and Business Media).
4. Improving the vascularization of immunoisolation devices
Though immunoisolation precludes integration of blood vessels with transplanted cells and tissues, effective diffusion of oxygen and nutrients from capillaries to neighboring cells can occur over a maximum distance of about 200 µm [76]. Therefore, to circumvent mass transport limitations associated with transplantation into relatively avascular sites, recent efforts have sought to induce neovascularization of extravascular diffusion devices through control of surface microarchitecture [77–80]. Vascularization of membranes may be further enhanced through infusion or controlled release of angiogenic factors, such as vascular endothelial growth factor (VEGF) [81–84] or basic fibroblast growth factor (bFGF) [85]. Interestingly, despite the chemoattractive properties of VEGF, Sigrist et al. observed significantly reduced macrophage adhesion to an AN69 membrane containing allogenic islets [82,83]. To achieve sustained angiogenesis and support existing vasculature, continuous delivery of factors may be necessary [82,83,85]. Towards this objective, Miki et al. have recently demonstrated enhanced vascularization of an ethylene vinyl alcohol membrane through co-delivery of bFGF-impregnated gelatin microspheres and encapsulated vascular endothelial TMNK-1 cells, which provide continuous biochemical support for the neovascularized membrane [85].
As an alternative to inducing vascularization of the immunoisolation device per se, others have sought to generate highly vascularized subcutaneous, intramuscular, or intraperitoneal sites for cell transplantation using stainless steel [86], polyethylene terephthalate [87], or polytetrafluoroethylene (PTFE) [88] mesh, respectively. Generation of such sites may allow microencapsulated cells to be removed and replaced, and may potentially circumvent complications associated with transplantation of encapsulated cells into native tissue [73,74]. Delivery of angiogenic factors, such as bFGF [87], acidic fibroblast growth factor [88], and VEGF [89] has been used as an adjunct to improve vascularization of these artificial cell-recipient beds. Islet transplantation into prevascularized sites dramatically improves graft survival and function relative to transplantation into non-modified tissue [86–88]. Moreover, syngeneic islets transplanted into prevascularized sites perform comparably to portal vein transplants, though some impairment in glucose clearance was still observed [86,88]. de Vos et al. have sought to minimize this effect by generating vascularized beds in the immediate vicinity of the liver to promote integration with the liver vasculature and facilitate portal drainage of insulin [89]. It remains unclear whether such sites can be rendered large enough to accommodate the large graft volumes associated with conventional microen-capsulation devices.
5. Strategies to reduce microcapsule size
5.1. Microfabrication of capsules
An obvious but non-trivial approach to improving transport properties of microcapsules is to produce smaller capsules by optimizing the process parameters used in traditional microcapsule fabrications. Traditional microcapsules have been mostly commonly produced using a coaxial air flow system [14]. Recently, Sugiura et al. have developed an analogous microfluidics device that utilizes an array of microfabricated silicon nozzles and airflow channels. Using 60 µm nozzles and appropriate airflow rates, genetically modified CHO cells could be encapsulated in nearly monodisperse 150 µm alginate– calcium microcapsules, and subsequently coated with a poly(L-lysine) (PLL) /alginate permselective membrane [90]. While microfabricated nozzles smaller than 50–100 µm are clearly not amenable to islet encapsulation, this approach holds considerable promise for reducing the transplant volume of microencapsulated genetically engineered cells. Ross and Chang have reported increased beta-glucuronidase production by genetically engineered fibroblasts using 100–200 µm alginate microcapsules, owing to tighter packing of smaller capsules and increased viable cell density [91]. In particular, smaller capsules may have significant advantages in the treatment of CNS diseases, where injection of microcapsules into the subarachnoid space or the brain parenchyma [3] excludes large transplant volumes. Additionally, smaller microcapsules might be used to facilitate intraportal transplantation of insulin producing cell lines, such as MIN6 [92] and βTC3 cells [93].
Several groups have addressed the feasibility of intraportal transplantation of microcapsules slightly smaller than conventional microcapsules. Hallé et al. have generated 300–350 µm microcapsules using a high voltage electrostatic pulse system [73,94,95]. Injection of 10,000 315 µm diameter microcapsules into the portal vein of rats resulted in only modest and transient increases in portal pressure [73]. Similarly, Toso et al. intraportally injected 10,000 400 µm alginate/poly(methylene-co-guanidine) hydrochloride microcapsules per kilogram body weight (Fig. 2C, D). While portal pressures were elevated immediately post-implant, the increase was comparable to that observed during clinical islet transplantation and returned to normal after three months [96]. However, these [96] and other intraportally infused microcapsules [97] elicited a significant foreign body reaction. The dose of microcapsules used in both of these studies is comparable to the islet dose used in clinical islet transplantation [47], demonstrating the potential to infuse 300–400 µm capsules in vascularized sites. However, decreasing the size of alginate microcapsules from 800 to 500 µm is associated with a ∼4 fold increase in the percentage of incompletely encapsulated islets [98]. Host response to even 2–10% of encapsulated islets has been shown to result in destruction of 40% of the graft [60,99]. Therefore, complete encapsulation of all transplanted cells remains an important prerequisite for successful immunoisolation.
5.2. Conformal coating via fluidic entrainment and interfacial precipitation
To circumvent limitations associated with random entrapment of cell aggregates within microparticles, several investigators have deposited coatings of defined thickness that conform to the surface of the cell or tissue. Transplant volume is, therefore, defined only by the size of the object being coated and the thickness of the coating, significantly reducing void volume while retaining the presence of a polymer barrier to provide immunoisolation.
Fluidic entrainment of polymer solution around cell aggregates followed by interfacial precipitation of the polymer has been most commonly utilized to generate conformal coatings. In light of the promise and widespread use of alginate-based microcapsules, Zekorn et al. [100], and subsequently Park et al. [101], have fabricated conformal alginate hydrogel coatings on the surface of individual pancreatic islets. To accomplish this, they utilized a discontinuous density centrifugation gradient composed of a top layer of islets suspended in sodium alginate, followed by denser spacer layers composed of dextran or Ficoll, one of which contained a divalent cation (CaCl2 or BaCl2). During centrifugation, islets approach and deform the alginate–dextran (Ficoll) interface, entraining a film of alginate around the islet provided drainage between the islet and interface is sufficiently slow. When the islet and entrained alginate cross into the layer containing the divalent cation the alginate is crosslinked, resulting in a 5– 10 µm film of alginate that largely conforms to the shape of the islet. Islets coated in this manner demonstrated normal biphasic insulin secretion in a dynamic perfusion assay, indicating that islet function and viability are preserved and that the thin coating prevents the lag in insulin secretion often observed with larger alginate microcapsules [100]. In a similar manner, Sefton et al. have used density centrifugation to coat islets [102] and HegG2 cell aggregates (Fig. 3C) [103] with water insoluble poly(hydroxyethyl methacrylate-co-methyl methacrylate) (HEMA-MMA) copolymers by interfacial precipitation, generating polymer films as thin as 1.5 µm. The non-aqueous nature of HEMA-MMA is anticipated to improve stability relative to hydrogels, which are susceptible to hydrolytic degradation. However, conformal coating of cells with HEMA-MMA results in a significant degree of cell death, owing to the need to expose cells to an organic solvent (polyethylene glycol, MW 200 Da) [104]. Recently, improved cell viability has been achieved by decreasing coating times and pre-encapsulating cells in agarose beads prior to HEMA-MMA conformal coating to minimize exposure to non-aqueous solvents [104].
Fig. 3.
Conformal coating of islets and cell aggregates. (A) Alginate/Ba2+ coating formed on a HEK 293 cell spheroid using an aqueous two phase emulsion (reprinted from [106] with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc). (B) PEG hydrogel coating fabricated on porcine islets via interfacial polymerization (reprinted from [109] with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc). (C) Electron micrograph of HepG2 cell aggregates coated with HEMA-MMA via density centrifugation and interfacial precipitation (Reprinted from [103] with permission from Elsevier). (D) Islets coated with PEG hydrogel via selective withdrawal (reprinted from [108] with permission of Wiley-VCH).
Emulsification has also been used to generate a conformal coating on islets and cell aggregates [105,106]. Calafiore et al. placed islets into an alginate/polyethylene glycol (PEG)/Ficoll emulsion, whereby alginate-containing Ficoll droplets suspended in a continuous PEG phase coalesced on the islet surface, engulfing individual islets in a layer of alginate, which was subsequently crosslinked with calcium chloride and coated with a poly(L-ornithine)/alginate bilayer [105]. This conformal barrier prevented direct cell-islet and antibody-islet contact, and did not impair insulin secretion in response to glucose stimulation [72]. Leung et al. have recently optimized this emulsion process to minimize the incidence of incomplete and non-uniform coating, and have coated cell clusters with 20–25 µm thin films of alginate (Fig. 3A) [106]. Importantly, conformally coated canine islets transplanted intraportally into a porcine model were viable and free of inflammatory reaction 15 days post transplant; however, a dense inflammatory infiltrate was observed 15 days later [107]. Nonetheless, these studies demonstrate the feasibility of using conformal barriers in intravascular cell transplantation.
Selective withdrawal of one fluid through a second immiscible fluid has recently been used to encapsulate pancreatic islets (Fig. 3D, Fig. 4A) [108]. Islets suspended in PEG-diacrylate were layered onto a more dense, immiscible oil phase, and fluid was withdrawn through a tube placed immediately below the interface. At an appropriate flow rate, the PEG-diacrylate solution, containing islets, was entrained in a thin spout within the oil phase. When the diameter of an islet is greater than that of the spout, surface tension causes the spout to break at both ends, leaving a thin layer of polymer solution around the islet. PEG-diacrylate was subsequently photocros-slinked, resulting in ∼10 µm thick coatings independent of the size of the encapsulated islet. The investigators found it necessary to repeat this process in order to prevent coating defects, ultimately generating ∼20 µm thick coatings. In principle, sequential coating may allow for the generation of composite coatings with each layer having independent properties designed to elicit a preferred biological response. For example, the authors cite examples in which the outer layer may contain anti-inflammatory or pro-angiogenic molecules, while the inner layer may contain molecules to improve islet function. The authors found that two-layer conformal coatings inhibited the transport of a 140 kDa macromolecule and enabled normal dynamic insulin secretion in response to glucose stimulation (Fig. 4B). Despite these promising results, ∼75% of islets were lost during the coating process, a problem that must be remedied in light of donor shortage for human islet transplantation. Moreover, scalability of the technique to allow for encapsulation of a clinically relevant number of islets in a timely manner must be addressed.
Fig. 4.
Selective withdrawal of one fluid through a second immiscible fluid can be used to fabricate conformal coatings on pancreatic islets. (A) Schematic representation of encapsulation apparatus. Islets in the upper fluid are enveloped with a thin film of precursor solution (PEG-diacrylate and photoinitiator) as they are drawn across the interface into a withdrawal tube. The thin film is crosslinked upon exposure to an argon-ion laser. (B) Islets coated with a conformal PEG coating demonstrate normal dynamic insulin secretion behavior in response to glucose and membrane depolarization (reprinted from [108] with permission of Wiley-VCH).
5.3. Conformal coating via interfacial polymerization
Conformal coating strategies have also utilized the islet surface as a template upon which coatings may be grown or chemically deposited. Hubbell et al. have generated ∼35–50 µm thick PEG-diacrylate coatings (Fig. 3B) on both porcine [109–111] and human [112] islets through a process of interfacial polymerization. In this polymerization scheme, eosin Y, a photoinitiator, is non-specifically adsorbed to the islet surface, and islets are placed in a solution containing PEG-diacrylate and triethanolamine. Upon illumination with light, eosin Y is excited and donates an electron to triethanolamine, which initiates the free-radical polymerization of PEG-diacrylate at the islet-macromer interface [110]. Through parametric optimization of key process variables, greater than 90% islet viability and encapsulation efficiency was reported [110], and conformally coated islets were shown to behave comparably to non-coated islets in a dynamic glucose perfusion experiment and intraperitoneal glucose tolerance test [109]. Preclinical trials in diabetic cynomolgus monkeys and baboons demonstrated function of subcutaneously transplanted conformally coated islets for up to 20 months, despite discontinuation of low dose immunosupression (cyclosporine) one month post-transplant [112]. This technology is currently the basis for phase I/II clinical trials by Novocell for encapsulated human islet allografts, which began in 2005 [113]. Although patients are currently receiving transplants in a subcutaneous site, the low void volume of the graft and the high blood compatibility of PEG may also facilitate transplantation into the portal bed.
5.4. Molecular camouflage and nanoencapsulation
While conformal coatings on cell aggregates offer a significant decrease in void volume relative to conventional microcapsules, immunoisolation may, in principle, be accomplished using coatings or membranes of submicron, or nanoscale, thickness. A common approach to generating such barriers has been through immobilization of PEG chains to the cell or tissue surface, creating a molecular barrier of PEG to prevent molecular recognition between cell surface receptors and soluble ligands [114–120]. This has generally been accomplished through covalent coupling of PEG to amines of cell surface proteins or carbohydrates, or by direct insertion of PEG-lipid conjugates into the cell membrane [119]. PEG is a hydrated, flexible polymer chain due to repeating, highly mobile ether units, which allows the polymer chain to act as a steric barrier on the cell surface [121]. Through proper control of polymer chain length and surface grafting density, cell surface PEGylation has been shown to camouflage antigenic sites, alter surface charge, and attenuate cell-cell and receptor– ligand interactions [122]. In an effort to create a universal red blood cell (RBC) donor, PEGylation of RBCs has been shown to mask major and minor blood group antigens from host antibodies [116,123,124]. Evidence has recently emerged that conjugation of PEG to human peripheral blood mononuclear cells [115] and isolated murine splenocytes [114] can interrupt a number of receptor–ligand interactions important in allorecognition, including weakening CD28-B7 costimulation, resulting in T cell apoptosis. Furthermore, transplantation of PEGylated C57BL/6 splenocytes into lethally irradiated Balb/c mice significantly abrogated donor T cell proliferation and improved survival rates in this model of graft versus host disease relative to non-PEGylated controls [114].
Based on such promising findings, several groups have demonstrated that PEG can be grafted to islets without compromising viability or function [117,118,125] and have began to explore whether or not PEG grafting provides a mechanism of preventing, or at least attenuating, host response to both allo-[126–131] and xenografts [120,125]. Lee et al. have recently reported some efficacy of this strategy in a rat allograft model of islet transplantation into the kidney capsule [128–131]. In their most recent experience, islets were serially PEGylated three times in an effort to increase PEG surface density and improve uniformity [128]. In contrast to non-PEGylated controls, which all mice rejected within one week, 3 of 7 recipients transplanted with PEGylated islets provided maintenance of normoglycemia for more than 100 days without any immunosuppression. Histological evaluation demonstrated that PEGylation prevented graft infiltration by host immune cells, but did not prevent host cell recruitment to the graft site. Similarly, Contreras et al. have used islet surface PEGylation in a xenogenic model of intraportal islet transplantation [120]. While the authors did not monitor engraftment beyond two weeks, animals that received islets treated with PEG presented significantly better control of blood glucose levels than animals receiving non-modified islets. PEG with a molecular weight of 5 kDa performed slightly better than 2 kDa PEG, and capping of surface grafted PEG with albumin proved most efficacious, which was attributed to the enhanced capacity of thicker films and/or larger steric barriers to shield islets from complement and xenoreactive antibodies [120,125].
Despite these encouraging results, it is unclear whether or not surface grafted PEG will remain stable enough to provide protection for the anticipated lifetime of the graft. Covalently modified cell surfaces are likely to be turned over and remodeled with time due to endocytosis and/or shedding of PEG-conjugated cell surface macromolecules. Moreover, PEG on the surface of cells with a finite lifecycle is likely to be lost and replaced with fresh tissue, thereby restoring immunogenicity [121]. Lee et al. have demonstrated via avidin staining of biotinylated PEG that PEG is present for at least one month [131], but further time points were not explored.
The efficacy of PEGylation may also be limited, in part, by the lack of a defined pore structure and dependence on a steric exclusion effect to provide an immunoprotective barrier. Therefore, several groups have recently began to explore the possibility of constructing nano-thin films of controlled permeability and surface chemistry directly on the surface of cells and tissues via layer-by-layer polymer self-assembly [132], effectively creating conformal, permselective membranes of nanoscale thickness. Elbert et al. assembled an alginate/PLL polyelectrolyte multilayer (PEM) film on gelatin to limit cell adhesion to the proteinaceous surface [133]. Likewise, Thierry et al. have coated damaged endothelium with PEM films consisting of chitosan and hyaluronic acid to inhibit platelet deposition [134]. PEM films have also been assembled on the surface of gluteraldehyde-fixed red blood cells [135], yeast [136,137], and bacteria [138], but their assembly on viable mammalian cells and tissues has proven more challenging due to the well documented toxicity of most polycations [139–141]. Germain et al. have explored the possibility of encapsulating MELN and HeLa cell lines with various polycations and anionic poly(styrene sulfonate) (PSS) [142]. Most polycations explored were extremely cytotoxic, though nine bilayers of poly (diallyldimethylammonium chloride)/PSS could be assembled with only a ∼25% decrease in cell viability. Coated cells demonstrated impaired receptor mediated endocytosis of transferrin (80 kDa) and a lectin (64 kDa), suggesting that multilayer films may provide a barrier for transport of these molecules. Similarly, Veerabadran et al. have recently reported the assembly of PLL/hyaluronic acid multilayer films on mouse mesenchymal stem cells [143]. Krol et al. have attempted to extend this concept to coat human pancreatic islets, demonstrating a minimal loss of islet function and viability when coated with a poly(allylamine hydrochloride) (PAH)/PSS/PAH multilayer film [283]. This finding is surprising given the reported toxicity of PAH to pancreatic islets [128]. A closer analysis of their data indicate that the PAH is localized both intra- and extracellularly, consistent with polycation-induced pore formation, diffusion of polymers into the cytoplasm, and subsequent cell death [140]. This apparent discrepancy deserves further consideration and exploration. Miura et al. have attempted to assemble an alginate/PLL/alginate multilayer on islets by first inserting a lipid–PEG–NH2 conjugate into islet cell membranes, thereby generating a positively charged islet surface to facilitate electrostatic binding of negatively charged alginate [144]. Islets coated with this membrane appear to function normally in a static glucose stimulation assay, but no attempts to characterize the barrier capacity of this coating were made. Nonetheless, deposition of thin, multilayer films directly on the surface of cells and tissues provides a promising approach to minimizing void volume while generating a true permselective membrane.
Though PEGylation and nanoencapsulation remain immature technologies, the nanoscale thickness of such coatings offer a number of potential advantages relative to traditional immunoisolation or conformal coating approaches. Stuhlmeir and Yin demonstrated that PEGylation of endothelial cells inhibited binding of immunoglobulins and TNF-α, and reasoned that perfusion of hearts with reactive PEG might attenuate hyperacute xenograft rejection [145]. Though in vivo results were discouraging, this study exemplifies the potential utility of nanoassembled coatings to immunoprotect whole organs.
6. Early inflammatory events in transplantation of immunoisolated cells and tissue
Non-specific inflammatory responses to immunoisolated cells and tissue may also result in limited graft survival and function (Fig. 5). The inflammatory response to immunoisolated cells may be mediated by a foreign body response to the device itself, the surgical procedure necessary for implantation of the device, or the release of inflammatory mediators from the encapsulated cells. Cellular overgrowth of devices generates additional physical and metabolic barriers to the effective transport of metabolites, further exacerbating the aforementioned mass transport limitations. Moreover, localized acute or chronic inflammation can generate a sea of cytotoxic molecules capable of diffusion across immunoisolation devices, potentially damaging the encapsulated graft.
Fig. 5.
Cytotoxic inflammatory responses may be initiated and/or propagated via surgery associated with device implantation, the foreign body response to the implanted material, and/or the release of inflammatory mediators by immunoisolated cells.
6.1. Inflammation induced by devices and surgery
The inflammatory, or foreign body, response to implanted biomaterials, reviewed thoroughly elsewhere [146–149], is generally described as a dynamic biochemical process, initiated by non-specific adsorption of proteins to the material surface, followed by recruitment of neutrophils and macrophages to the implant site, and the subsequent attachment and overgrowth of the device by macrophages, foreign body giant cells, and fibroblasts [150]. While the severity of foreign body responses to immunoisolation devices is dependent on transplantation site and material properties, such as surface charge, porosity, roughness, surface chemistry and free energy, and implant size [146], this generalized response has been observed on a variety of immunoisolation devices [147], including the commonly employed alginate/PLL/alginate (APA) microcapsule [151,152] and the TheraCyte device produced by Baxter Healthcare [153]. For many years, the inherent biocompatibility of immunoisolation devices was implicated as the principle cause for capsular overgrowth and subsequent graft failure [154]; accordingly, many groups focused their efforts on improving the biocompatibility of immunoisolation membranes and encapsulation devices. Surface PEGylation has been used to improve the biocompatibility of synthetic immunoisolation membranes [155,156], as well as APA microcapsules [157]. Use of highly purified alginate of appropriate composition has improved the biocompatibility of alginate-based microencapsulation devices [76]. The use of polycations, in particular PLL, in membrane forming processes has been shown to mediate adhesion of fibroblasts and macrophages [158] and induce cytokine production [159], and many groups have recently abandoned use of polycations in microcapsule formulations [56,160–162]. However, several polycation-containing microcapsules have been optimized such that fibrotic overgrowth of empty capsules is minimized [43,163–165]. Most notably, poly (L-ornithine) (PLO) and PLL containing alginate capsules are currently being utilized in clinical trials of encapsulated islets [21,22].
The surgical procedure associated with device implantation, though often minimally invasive, generates an inflammatory response necessary to facilitate normal wound healing [146]. Indeed, Robitalle et al. have recently observed significant neutrophil infiltration and inflammatory cytokine expression (IL-1β, IL-6) in the 17 h immediately after intraperitoneal injection of saline [152]. Similarly, Roth et al. have reported early and transient NF-κB expression during sham saline injections into fat pads [166]. While it is unclear if this ‘intrinsic’ inflammatory response is capable of adversely effecting graft function, it likely initiates or propagates a foreign body response to the device [146,152], as well as inflammatory responses to the immunoisolated cells [154], as discussed below.
6.2. Inflammation mediated by immunoisolated cells
Encapsulated cells may produce low molecular weight inflammatory mediators capable of diffusion across immunoisolation membranes, potentially triggering inflammatory cell recruitment and activation [40,56,167–170]. Isolated pancreatic islets, for example, have been shown to produce a number of proinflammatory molecules (Table 2) [32,171–184]. Consequently, transplantation of non-encapsulated islets is associated with elevated inflammatory responses in the immediate post-transplant period, characterized by intense macrophage infiltration [185,186], increased expression of endothelial cell and leukocyte adhesion molecules (E-selectin, ICAM-1) [186], and elevation of proinflammatory mediators (TNF-α, IL-1β, and nitric oxide) [75,186,187]. Significantly, recent data suggests that levels of inflammatory molecules produced by islet preparations pre-transplant may correlate with the outcome of clinical islet transplantation [188].
Table 2.
Soluble inflammatory mediators expressed by pancreatic islets
| Inflammatory mediator | Molecular weight (Da) | Ref. |
|---|---|---|
| TNF-α | 51,000a | [176–179] |
| IL-6 | 21,500–28,000 | [176,180] |
| IL-1β | 17,000 | [175–177,179] |
| Macrophage migration inhibitory Factor (MIF) | 12,000 | [175] |
| CXCL9 (MIG) | 11,700 | [181] |
| CXCL10 | 10,000 | [181] |
| IL-8 | 8000 | [175,180] |
| CCL5 (RANTES) | 8000 | [181] |
| MIP-1α | 7800 | [178] |
| MCP-1 | 6000–7000 | [175,179,180,182,183] |
| CXCL2 (MIP-2α) | 6000 | [181] |
| Nitric oxide | 30 | [177,184] |
Soluble trimeric form.
Not surprisingly, similar mechanisms appear to be operative when islets are encapsulated. Co-culture of isolated peritoneal macrophages and syngeneic islets encapsulated in APA microcapsules mediates islet-specific macrophage activation accompanied by TNF-α and IL-1β release, suggesting that factors other than alloantigens are capable of diffusing across the capsular membrane to activate macrophages [189]. Similarly, co-culture of conformally coated islets and macrophages results in the production of several inflammatory mediators, including MIP-2, IL-1, TNF-α, and IL-6 [190].
6.3. Effects of the inflammatory environment on immunoisolated cells
Generation of such an inflammatory milieu may be highly detrimental to graft performance and longevity [172,187,191]. This is exemplified in an experimental model of syngeneic islet transplantation into non-autoimmune diabetic mice, whereby ∼60% of transplanted islet tissue was lost 3 days post transplantation [192], demonstrating that early islet destruction is not allo- or autoantigen-specific, but rather mediated, in large part, by generation of cytotoxic inflammatory molecules [172,187]. Elegant studies by de Vos et al. suggest that comparable mechanisms may be active in the failure of encapsulated islet grafts [60,99,189]. Examination of encapsulated islet allografts retrieved from the peritoneum revealed that only ∼10% of capsules were overgrown with fibrotic tissue [60,99,163,193]; however, this was accompanied by a 40% loss of viable cells within the first 4 weeks of transplantation [60]. Notably, encapsulated islet autografts performed comparably, suggesting that graft failure was mediated by non-specific mechanisms [163,193]. Histological evaluation indicated that activated macrophages were the predominant cell type attached to overgrown capsules, and co-culture of macrophages and encapsulated islets resulted in macrophage activation, cytokine production, and impaired islet function [60,189]. Similarly, de Groot et al. co-cultured encapsulated islets overgrown with host cells retrieved from the peritoneum with freshly encapsulated islets at a 1:9 ratio (i.e. 10% overgrowth) [194]. Impaired insulin secretion in response to glucose, decreased beta cell replication, and increased cell necrosis occurred after 48 h of co-culture. IL-1β, TNF-α, and nitrite, a marker for nitric oxide (NO), were elevated in the culture media, and analysis of mRNA expression profiles of encapsulated islets suggested that NO mediated islet damage [194]. While some immunoisolation membranes have been reported to protect cells from IL-1β and/or TNF-α [40,169], blockade of free radical diffusion is not likely. Indeed, Wiegand et al. and Chae et al. have demonstrated that, despite its short half-life, NO can destroy encapsulated islets [195,196]. This has recently been supported by a mathematical model of free radical diffusion through a spherical matrix containing pancreatic islets [197]. Not surprisingly, depletion of macrophages improves engraftment of both encapsulated and non-encapsulated cells [75,198]. Hence, significant evidence has emerged that limited survival and function of immunoisolated cells is at least partially mediated by non-immune, inflammatory responses.
7. Strategies to abrogate inflammatory responses
7.1. Systemic delivery of anti-inflammatory agents
The inflammatory response to encapsulated islets has been reported to be complete within 1–4 weeks [99,151,158], and, therefore, temporary anti-inflammatory therapy may be sufficient to dramatically reduce early inflammatory events associated with transplantation of immunoisolated cells. Single-dose or short term administration of drugs that inhibit cytokine actions or macrophage function have been found to dramatically improve islet engraftment in experimental models of islet transplantation (Table 3) [75,178,186,199–204]. Despite parallels between primary islet non-function and limited survival of immunoisolated islet grafts, surprisingly little consideration has been given to adjunctive anti-inflammatory therapy for the transplantation of immunoisolated cells and tissues. Omer et al. delivered clodronate liposomes to deplete peritoneal macrophages, reducing capsular overgrowth and improving islet function [198]. While macrophage depletion is not a clinically viable approach, delivery of agents that inhibit or modulate macrophage activation, such as 15-deoxyspergualin [205], in the immediate post-transplant period could prove valuable in abrogating early inflammatory responses and subsequent destruction of immunoisolated tissue.
Table 3.
Anti-inflammatory therapies for improving islet engraftment
| Therapeutic molecule | Treatment regimen | Ref. |
|---|---|---|
| 15-deoxyspergualin | IP injection (day-1 to day 9) | [203] |
| ICAM-1 antisense oligodeoxynucleotide | IP injection (7 days) | [202] |
| anti-ICAM-1 mAb | IP injection (7 days) | [202] |
| anti-IFN-γ mAb | IP injection (day 0,2,4) | [204] |
| anti-IL-1β mAb | IP injection (day 0,2,4) | [204] |
| Acetylsalicyclic acid | Orally (daily for entire experiment) | [199] |
| IL-1ra | IP injection (day-1 and 0, and 4 h) | [199] |
| Pravastatin | Orally (daily for first 14 days) | [200] |
| Anti-MCP-1 mAb | IP injection (3×/week for 2 weeks) | [201] |
| Activated protein C | IV injection (1 h pre-Tx) | [186] |
| Dichloromethylene diphosphonate | IV injection (2 days pre-Tx) | [75] |
| α1-antitrypsin | IP injection (day-1, every 3 days post Tx) | [178] |
IP: intraperitoneal, IV: intravenous.
7.2. Bioactive immunoisolation barriers that locally attenuate inflammatory responses
To avoid potential adverse consequences associated with systemic delivery of anti-inflammatory molecules, recent efforts have focused on integrating anti-inflammatory capabilities into immunoisolation devices. Loading of microcapsules with anti-inflammatory molecules, for example dexamethasone [206], offers a simple approach, but may be limited by undesirable release kinetics and/or cytotoxic intracapsular concentrations. Co-encapsulation of cells and drug delivery vehicles offers a rational alternative for controlled delivery of anti-inflammatory agents. Luca et al. co-encapsulated cellulose acetate microspheres (30–70 µm) containing the antioxidant vitamin D3 with rat islets in alginate/PLO microcapsules [207]. Similarly, Ricci et al. found that microcapsules charged with 5 µm polyester microspheres releasing the non-steroidal anti-inflammatory drug ketoprofen reduced the foreign body response to polycation coated microcapsules [208].
Encapsulated cells may be further protected through immobilization of cells or molecules that scavenge, inhibit, or metabolize cytotoxic molecules that diffuse across the immunoisolation barrier. Wiegand et al. have demonstrated that coencapsulation of islets with autologous erthyrocytes within alginate capsules provided nearly complete protection from macrophage-mediated cell lysis due to the capacity of erthyrocytes to scavenge NO and/or convert it to nitrate [196]. Recently, Chae et al. have extended this concept, replacing erthyrocytes with hemoglobin crosslinked with PEG. APA capsules containing crosslinked hemoglobin protected rat islets and RINm5F insulinoma cells from NO-mediated cellular damage [195]. Significantly, after transplantation of a suboptimal mass of encapsulated islet xenografts these microcapsules were found to prolong normoglyemia and improve glucose clearance relative to capsules formulated without crosslinked hemoglobin [209]. While this effect may have also been mediated by improved oxygen tension in the capsule [209,210], this study exemplifies the potential efficacy of actively antiinflammatory immunoisolation devices.
Our lab has postulated that the native endothelium provides a structural and biochemical model for the design of immunoisolation membranes through its ability to act as a template for the assembly of complex macromolecular structures that regulate inflammatory processes. In this regard, we developed a substrate-supported, polymerizable, cell membrane-mimetic thin film, which can be readily assembled on the surface of conventional APA microcapsules (Fig. 6) [165,211,212]. We have recently shown that microcapsules coated with a membrane-mimetic film are stable and largely free of pericapsular overgrowth after four weeks in the peritoneal cavity [165]. Moreover, membrane-mimetic films have been assembled on microcapsules containing CHO cells [212] and pancreatic islets [211] with minimal loss of cell viability or function. Importantly, we have previously demonstrated the ability of membrane-mimetic films to serve as a template for the assembly of membrane-bound macromolecules that may abrogate inflammatory responses to encapsulated cells, specifically heparin and the transmembrane enzyme thrombomodulin [213–217]. Though perhaps better known for its anticoagulant properties, immobilized heparin can inhibit the formation of NO through its capacity to bind superoxide dismutase [218], and has been shown to limit complement activity by inhibiting the formation of C3 convertase and the assembly of C5b-9 [219–221].
Fig. 6.
Construction of a polymerized, self-assembled, membrane-mimetic thin film on the surface of alginate/poly(L-lysine) microcapsules. (A) Alternating layers of poly(L-lysine) and alginate are first assembled on an alginate/Ca2+ hydrogel microsphere, followed by adsorption of an ampiphilic terpolymer with anionic anchoring groups. Following monolayer fusion of mono-acrylated phospholipids, photoinitiated polymerization is performed to crosslink the lipid layer. The film may be rendered anti-inflammatory by incorporating surface-bound heparin and thrombomodulin. (B, C) By doping mono-acrylate PC films with 0.1 mol% Texas Red acrylate PE, membrane-mimetic films can be readily observed on the surface of microcapsules (reprinted from [165] with permission from Elsevier). (D) Islets encapsulated in capsules coated with a membrane-mimetic film are viable as evidenced by ethidium homodimer and calcein AM staining. (E) Empty capsules retrieved from the peritoneal cavity of mice after 30 days remain free of cellular adhesion and capsular overgrowth (reprinted from [165] with permission from Elsevier).
Thrombin, invariably generated during device implantation [146], is an important conductor of cellular responses during inflammation [222]. Thrombin acts as a chemoattractant, directing neutrophils and monocytes to the injured site [222], can trigger expression of cell adhesion molecules necessary for inflammatory cell migration [223–225], and can mediate expression of proinflammatory cytokines, IL-6 and IL-8, and platelet activating factor, a potent neutrophil activator [223]. Thrombomodulin (TM) binds thrombin in a 1:1 ratio acting as a thrombin sink [222]. Therefore, TM immobilized on the pericapsular surface may sequester thrombin, preventing its participation in inflammatory processes. Perhaps more importantly, TM is able to redirect the catalytic activity of thrombin towards generation of activated protein C (APC), a potent antiinflammatory molecule [226,227]. Though the complete antiinflammatory mechanism of APC has yet to be fully elucidated, APC has been shown to block F-κB nuclear translocation, an important event in many inflammatory cascades [228]. Indeed, APC has been shown to inhibit macrophage activation, production of proinflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8) [229–233], and endothelial cell expression of E-selectin and ICAM-1 [234,235]. Notably, systemic administration of APC delayed xenograft rejection in a guinea pig to rat cardiac transplant [233], and significantly improved islet engraftment in a mouse autograft model of intraportal islet transplantation [186]. Given the capacity to incorporate such proteins and carbohydrates, membrane-mimetic films offer a route to generate robust, biocompatible, and actively anti-inflammatory immunoisolation membranes.
8. Graft failure due to activation of indirect antigen presentation pathways
The design features of an immunoisolation barrier are driven by the intent to limit the effects of rejection pathways initiated after implantation of donor cells. Recent advances in immunobiology have elucidated alternative mechanisms of immune system activation with important implications for the design of effective immunoisolation barriers. In particular, the role of indirect antigen presentation pathways in the rejection of xenografts, and to a lesser, but notable extent, allografts, has challenged the premise that an effective barrier can be created based solely on the selection of physical dimensions necessary to exclude cytotoxic molecules [236–238].
Allograft rejection is primarily mediated by cytotoxic CD8+ T cells activated by donor MHC-peptide complexes expressed on the surface of graft-derived antigen presenting cells;a process that has been referred to as direct antigen presentation [236,237]. Immunoisolation barriers that prevent cell–cell contact between donor cells and host immune cells are, therefore, expected to block the direct presentation pathway. By contrast, rejection of non-vascularized xenograft tissue is characterized by a response in which CD4+ helper T cells play a major role. In this pathway, termed indirect antigen presentation, host antigen presenting cells display peptides scavenged from free donor proteins to engage CD4+ T cells, which develop into Th2 cells. In turn, Th2 cells produce cytokines that stimulate maturation of B cells into plasma cells, which secrete xenoantigen-specific antibodies [236,237]. While many immunoisolation barriers prevent passage of antibodies, this may not be a prerequisite to protect cells from a humoral response [239], provided the required immunocellular and complement components, many of which are larger than antibodies, are excluded [146]. However, even if not in direct contact with the donor cell or tissue, immune complexes generated by the binding of newly formed antibodies to shed antigens may lead to activation of macrophages and recruitment of neutrophils through activation of the complement cascade or by direct binding of antigen–antibody complexes to leukocyte cell-surface Fc receptors [240,241]. Upon initiation of such a response, T cells and activated macrophages in the region of the graft secrete low molecular weight cytokines and free radicals that, as discussed previously, may be highly destructive to encapsulated cells (Fig. 7) [172,187,191]. Indeed, compelling evidence indicates that indirect antigen presentation is an operative mechanism in the destruction of immunoisolated xenogenic cells and tissues, as evidenced by production of xenoantibodes, CD4+ T cells, and macrophages at the graft site [242–249]. Recently, Orlowski et al. have shown that encapsulation of xenoislets in APA microcapsules does not prevent, but merely delays, provocation of the second set phenomenon, a clear indicator of host sensitization and a potential obstacle if subsequent cell transplantation is necessary [250].
Fig. 7.
Host recognition of immunoisolated cells initiated via the indirect antigen presentation pathway. AgX, shed foreign antigen; MHC, major histocompatibility complex; MHC+pep, MHC presenting foreign peptide; TCR, T-cell receptor; FcR, Fc receptor; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide (reprinted from [282] with permission from Birkhäuser Verlag AG).
The degree to which indirect antigen presentation plays a role in graft rejection is related to disparity between shed and host epitopes [237], which likely explains the common observation that immunoisolated xenogenic tissue is rejected more rapidly than allogenic tissue (Table 1). In principle, however, this mechanism is also operative in immunoisolated allograft tissue [237], which may explain several studies in which encapsulated allografts were rejected more quickly than autografted controls [251–253]. The significance of indirect antigen presentation in immunoisolated allograft rejection remains controversial and inadequately investigated. While it is unclear what, if any, shed alloantigens are responsible for initiating host responses to immunoisolated cells, some evidence indicates that peptides derived from MHC molecules themselves are the most likely candidates [237]. Clearly, the design of a barrier that prevents indirect antigen presentation by preventing the release of graft protein or peptide antigens, while simultaneously permitting passage of nutrients and therapeutic agents, remains a difficult hurdle to overcome.
9. Strategies to attenuate immune responses to immunoisolated cells
9.1. Adjunctive immunosuppressive therapy
While complete elimination of immunosuppression remains the paramount objective of immunoisolation, the immunoprotective capacities of many existing technologies may facilitate use of modified immunosuppressive therapies with important clinical benefits. Encapsulation has allowed several investigators to significantly reduce the dose of harmful immunosupressive agents necessary to protect allografted tissue [129,131,254,255]. For example, Lee et al. have reported combining covalent conjugation of PEG to allogenic islets and low dose cyclosporine (Fig. 8) [131]. Initial administration of cyclosporine for 2 weeks at 3 mg/kg/day followed by 1 mg/kg/day thereafter protected PEGylated rat islet allografts for more than 1 year. Non–modified islets receiving the same immunosuppressive treatment, as well as PEGylated islets transplanted in the absence of immunosupression were rejected at approximately 12 days.
Fig. 8.
Islet PEGylation and low dose immunosuppression act synergistically to protect pancreatic islets for one year in a rat allograft model. (A) Proposed mechanisms of immunological protection. (B) Maintenance of normoglycemia for one year post-transplant (reprinted from [131] with permission from Elsevier).
In a recent series of studies [256–258], Aebischer et al. have shown that combining cell encapsulation and transient immunosupression can induce host acceptance of xenogenic cells. Short-term administration (1–4 weeks) of tacrolimus significantly prolonged the survival of encapsulated genetically engineered myoblasts relative to non-encapsulated controls [256]. Importantly, this initial immunosuppressive treatment also prolonged survival of a subsequent encapsulated graft, indicating that cell immunoisolation and transient immunosupression may generate a state of host unresponsiveness to xenografts [257]. This finding has important implications for cell-based therapy, as encapsulated xenogenic cells could be replaced without the need for further immunosuppression. Interestingly, in a clinical trial of APA encapsulated islets by Amcyte, patients will receive a low dose, short term course of the immunosupressive agent Rapamune® [22].
Improved understanding of immune responses to encapsulated cells has helped elucidate targets for immune intervention, leading to the development of more specific and less harmful immunosuppressants. In particular, blocking antibodies and soluble antagonists targeted towards pathways necessary for T cell activation via the indirect antigen presentation pathway have emerged as promising adjuncts to cell encapsulation [153,248,249,259]. Safley et al. have observed that treatment with CTLA4-Ig, a CD28 antagonist, and anti-CD154, an inhibitor of CD40/CD40-ligand interactions, resulted in prolonged survival of encapsulated porcine xenografts in spontaneous diabetic mice, accompanied by reduced capsular overgrowth, inflammatory cell infiltration, and inflammatory cytokine production [248].
9.2. Bioactive immunoisolation barriers that locally attenuate immune responses
In addition to preventing the cell–cell interactions that underlie the initiation of the direct antigen presentation pathway, current immunoisolation strategies might be enhanced by incorporation of cells or molecules that actively limit immune responses to encapsulated cells. The rationale for a number of such barriers has emerged from the observation that co-transplantation of testicular Sertoli cells with allo- [260– 262] or xenogenic [263,264] tissue significantly abrogates graft rejection. In a controversial but noteworthy study, Valdes et al. co-transplanted isolated neonatal porcine islets and Sertoli cells in a vascularized subcutaneous chamber into diabetic human patients. At four year follow up, porcine insulin was present in many patients. Half of the patients showed significant reduction in exogenous insulin requirements, and two were insulin independent for several months [265].
While the mechanisms through which Sertoli cells confer protection to foreign tissue is not well understood, recent data suggests that they may locally modulate immune responses through secretion of bioactive factors such as TGF-β and clusterin [266]. Hence, cell–cell contact between Sertoli and immune cells may not be necessary to confer immunoprotection, though close proximity to grafted tissue appears to be required [260]. Korbutt et al. co-encapsulated Sertoli cells with allogenic pancreatic islets in alginate microcapsules, and reported improved encapsulated graft survival in the peritoneum, extending normoglycemia from 10.4±0.6 to 85±4.9 days [267]. Yang et al. have extended this concept to xenogenic immunoisolation, co-encapsulating fish islets and syngeneic or xenogenic Sertoli cells [268]. In accord with Korbutt et al., microcapsules containing Sertoli cells prolonged survival of intraperitoneal islets grafts (46±6.3 days) relative to capsules containing islets alone (21±6.7 days). It has recently been hypothesized that graft function might be prolonged through replenishment of Sertoli cells at the site of transplantation. In this regard, Luca et al. [269] have produced microcapsules for optimized Sertoli cell viability that may be transplanted along side immunoisolated cells. It is unclear whether or not this approach will confer the same benefit as co-encapsulation of both cell types.
Cells of immunoprivileged tissues express a type-II membrane receptor known as Fas ligand (FasL), which induces apoptosis of autoreactive lymphocytes or infiltrating inflammatory cells through its interaction with Fas (CD95) [270]. The efficacy of the Fas/FasL pathway in conferring immunoprotection at ectopic sites is unclear and reports are conflicting [271– 274]. Lau et al. found that co-transplantation of islets with myoblasts genetically engineered to express FasL dramatically improved allograft survival [271]. Yolcu et al. observed similar results using splenocytes chemically modified to present a novel chimeric streptavidin-FasL molecule [272]. By contrast, islets engineered to express FasL on their beta cells did not protect grafts from rejection and were more susceptible to inflammation-mediated destruction [274]. Nonetheless, presentation of immunoregulatory molecules on the surface of immunoisolation devices may provide a mechanism to specifically and/or locally prevent immune responses to immunoisolated cells. Cheung et al. have recently functionalized PEG-diacrylate hydrogels with anti-Fas antibodies (FasAb) using cell compatible chemistry [275]. FasAbs can induce T cell apoptosis in a manner similar to FasL via crosslinking of Fas receptors on T cells. FasAb functionalized hydrogels were shown to induce apoptosis of Fas sensitive Jurkat T cells, whereas non-modified barriers did not. Though the efficacy of this approach remains to be seen, this work establishes an important step towards the design of stable, biologically active immunoisolation barriers that address operative mechanisms of immunoisolated graft rejection.
10. Conclusions
Protection of transplanted cells from deleterious host immune responses will be necessary to exploit the full clinical potential of cell-based therapeutics, and immunoisolation stands to play a pivotal role towards this end. Over thirty years of intensive research directed at developing and optimizing the performance of immunoisolation devices has yielded both promise and inherent challenges, several of which have been discussed in detail within this review. Maintenance of adequate oxygen and nutrient transport is of paramount importance for success of any transplant, but particular attention must be given to immunoisolated cells due to often limited vascularization of the device or at the intended site of transplantation. Early inflammatory responses are intrinsic to any implanted cell-material composite, and such responses, if not inherently detrimental, can initiate chronic inflammation or otherwise intensify immune responses to transplanted cells and tissues. Finally, recent advances in immunobiology have identified fissures in the prevailing dogma of immunoisolation, particularly as applied to xenogenic tissue. Though advances in membrane technology may ultimately make it possible to prevent such host responses through proper control of membrane transport properties, the possibility that indirect antigen presentation may be operative should be recognized and new paradigms in the development of immunoisolation barriers must be explored (Fig. 9). With these limitations in mind, barrier design must ultimately be governed by clinical objectives and the inherent pathophysiologic mechanisms associated with the underlying disease process. Continued collaboration between engineers, biological and physical scientists, and clinician-scientists will be essential for defining realistic objectives and overcoming existing obstacles.
Fig. 9.
Emerging paradigms in the immunoisolation of cells and tissues include implantation of encapsulated cells into prevascularized sites or native tissue microvasculature, minimization of transplant volume through use of conformal coatings or nanoencapsulation, and local attenuation of host inflammatory and immune responses to encapsulated cells using bioactive membranes
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Emerging Trends in Cell-Based Therapeutics”.
References
- 1.Lohr M, Hoffmeyer A, Kroger J, Freund M, Hain J, Holle A, Karle P, Knofel WT, Liebe S, Muller P, Nizze H, Renner M, Saller RM, Wagner T, Hauenstein K, Gunzburg WH, Salmons B. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591–1592. doi: 10.1016/s0140-6736(00)04749-8. [DOI] [PubMed] [Google Scholar]
- 2.Wollert KC, Drexler H. Cell-based therapy for heart failure. Curr. Opin. Cardiol. 2006;21:234–239. doi: 10.1097/01.hco.0000221586.94490.d2. [DOI] [PubMed] [Google Scholar]
- 3.Visted T, Bjerkvig R, Enger PO. Cell encapsulation technology as a therapeutic strategy for CNS malignancies. Neuro. Oncol. 2001;3:201–210. doi: 10.1093/neuonc/3.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash S, Chang TM. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nat. Med. 1996;2:883–887. doi: 10.1038/nm0896-883. [DOI] [PubMed] [Google Scholar]
- 5.Shoichet MS, Winn SR. Cell delivery to the central nervous system. Adv. Drug Deliv. Rev. 2000;42:81–102. doi: 10.1016/s0169-409x(00)00055-7. [DOI] [PubMed] [Google Scholar]
- 6.Emerich DF, Winn SR. Immunoisolation cell therapy for CNS diseases. Crit. Rev. Ther. Drug Carrier. Syst. 2001;18:265–298. [PubMed] [Google Scholar]
- 7.Hao S, Su L, Guo X, Moyana T, Xiang J. A novel approach to tumor suppression using microencapsulated engineered J558/TNF-alpha cells. Exp. Oncol. 2005;27:56–60. [PubMed] [Google Scholar]
- 8.Lee MK, Bae YH. Cell transplantation for endocrine disorders. Adv. Drug Deliv. Rev. 2000;42:103–120. doi: 10.1016/s0169-409x(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 9.Algire GH, Weaver JM, Prehn RT. Growth of cells in vivo in diffusion chambers. I. Survival of homografts in immunized mice. J. Natl. Cancer Inst. 1954;15:493–507. [PubMed] [Google Scholar]
- 10.Chick WL, Like AA, Lauris V. Beta cell culture on synthetic capillaries: an artificial endocrine pancreas. Science. 1975;187:847–849. doi: 10.1126/science.187.4179.847. [DOI] [PubMed] [Google Scholar]
- 11.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 12.Li RH. Materials for immunoisolated cell transplantation. Adv. Drug Deliv. Rev. 1998;33:87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 13.Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat. Biotechnol. 1996;14:1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 14.Chaikof EL. Engineering and material considerations in islet cell transplantation. Annu. Rev. Biomed. Eng. 1999;1:103–127. doi: 10.1146/annurev.bioeng.1.1.103. [DOI] [PubMed] [Google Scholar]
- 15.Tibell A, Rafael E, Wennberg L, Nordenstrom J, Bergstrom M, Geller RL, Loudovaris T, Johnson RC, Brauker JH, Neuenfeldt S, Wernerson A. Survival of macroencapsulated allogeneic parathyroid tissue one year after transplantation in nonimmunosuppressed humans. Cell Transplant. 2001;10:591–599. [PubMed] [Google Scholar]
- 16.Leoni L, Desai TA. Micromachined biocapsules for cell-based sensing and delivery. Adv. Drug Deliv. Rev. 2004;56:211–229. doi: 10.1016/j.addr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Chang PL, Bowie KM. Development of engineered cells for implantation in gene therapy. Adv. Drug Deliv. Rev. 1998;33:31–43. doi: 10.1016/s0169-409x(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 18.Efrat S. Development of engineered pancreatic beta-cell lines for cell therapy of diabetes. Adv. Drug Deliv. Rev. 1998;33:45–52. doi: 10.1016/s0169-409x(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 19.Parikh SA, Edelman ER. Endothelial cell delivery for cardiovascular therapy. Adv. Drug Deliv. Rev. 2000;42:139–161. doi: 10.1016/s0169-409x(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 20.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 21.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29:137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 22. www.amcyte.com. [Google Scholar]
- 23.Aebischer P, Schluep M, Deglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat. Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 24.Buchser E, Goddard M, Heyd B, Joseph JM, Favre J, Tribolet Nde, Lysaght M, Aebischer P. Immunoisolated xenogenic chromaffin cell therapy for chronic pain. Initial clinical experience. Anesthesiology. 1996;85:1005–1012. doi: 10.1097/00000542-199611000-00007. (discussion 1029A–1030A). [DOI] [PubMed] [Google Scholar]
- 25.Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugieres P, Boisse MF, Baudic S, Cesaro P, Hantraye P, Aebischer P, Peschanski M. Neuroprotective gene therapy for Huntington’s disease, using polymerencapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum. Gene. Ther. 2004;15:968–975. doi: 10.1089/hum.2004.15.968. [DOI] [PubMed] [Google Scholar]
- 26.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol. Med. 2002;8:363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 27.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann. N. Y. Acad. Sci. 1997;831:145–167. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 28.Dulong JL, Legallais C. A theoretical study of oxygen transfer including cell necrosis for the design of a bioartificial pancreas. Biotechnol. Bioeng. 2007;96:990–998. doi: 10.1002/bit.21140. [DOI] [PubMed] [Google Scholar]
- 29.Gross JD, Constantinidis I, Sambanis A. Modeling of encapsulated cell systems. J. Theor. Biol. 2007;244:500–510. doi: 10.1016/j.jtbi.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tziampazis E, Sambanis A. Tissue engineering of a bioartificial pancreas: modeling the cell environment and device function. Biotechnol. Prog. 1995;11:115–126. doi: 10.1021/bp00032a001. [DOI] [PubMed] [Google Scholar]
- 31.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 32.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin. Exp. Immunol. 2006;144:179–187. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vos P, De Haan B, Pater J, Van Schilfgaarde R. Association between capsule diameter, adequacy of encapsulation, and survival of microencapsulated rat islet allografts. Transplantation. 1996;62:893–899. doi: 10.1097/00007890-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Orive G, Hernandez RM, Rodriguez Gascon A, Calafiore R, Chang TM, de Vos P, Hortelano G, Hunkeler D, Lacik I, Pedraz JL. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004;22:87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Welty JR, Wicks CE, Wilson RE, Rorrer GL. Fundamentals of momentum, heat and mass transfer. New York, NY: John Wiley; 2001. [Google Scholar]
- 36.Dionne KE, Cain BM, Li RH, Bell WJ, Doherty EJ, Rein DH, Lysaght MJ, Gentile FT. Transport characterization of membranes for immunoisolation. Biomaterials. 1996;17:257–266. doi: 10.1016/0142-9612(96)85563-3. [DOI] [PubMed] [Google Scholar]
- 37.Hiemstra H, Dijkhuizen L, Harder W. Diffusion of oxygen in alginate gels related to the kinetics of methanol oxidation by immobilized hansenula-polymorpha cells. Eur. J. Appl. Microbiol. 1983;18:189–196. [Google Scholar]
- 38.Li RH, Altreuter DH, Gentile FT. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol. Bioeng. 1996;50:365–373. doi: 10.1002/(SICI)1097-0290(19960520)50:4<365::AID-BIT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Mehmetoglu U, Ates S, Berber R. Oxygen diffusivity in calcium alginate gel beads containing Gluconobacter suboxydans. Artif. Cells. Blood. Substit. Immobil. Biotechnol. 1996;24:91–106. doi: 10.3109/10731199609118877. [DOI] [PubMed] [Google Scholar]
- 40.Haan BJde, Faas MM, de Vos P. Factors influencing insulin secretion from encapsulated islets. Cell Transplant. 2003;12:617–625. doi: 10.3727/000000003108747226. [DOI] [PubMed] [Google Scholar]
- 41.Tatarkiewicz K, Garcia M, Omer A, Van Schilfgaarde R, Weir GC, Vos PDe. C-peptide responses after meal challenge in mice transplanted with microencapsulated rat islets. Diabetologia. 2001;44:646–653. doi: 10.1007/s001250051672. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi N, Keegan M, Steil GM, Hollister-Lock J, Hasenkamp WM, Colton CK, Bonner-Weir S, Weir GC. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses. Transplantation. 2001;71:203–211. doi: 10.1097/00007890-200101270-00006. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 44.Canaple L, Rehor A, Hunkeler D. Improving cell encapsulation through size control. J. Biomater. Sci. Polym. Ed. 2002;13:783–796. doi: 10.1163/156856202760197410. [DOI] [PubMed] [Google Scholar]
- 45.Chicheportiche D, Reach G. In vitro kinetics of insulin release by microencapsulated rat islets: effect of the size of the microcapsules. Diabetologia. 1988;31:54–57. doi: 10.1007/BF00279134. [DOI] [PubMed] [Google Scholar]
- 46.Lum ZP, Tai IT, Krestow M, Norton J, Vacek I, Sun AM. Prolonged reversal of diabetic state in NOD mice by xenografts of microencapsulated rat islets. Diabetes. 1991;40:1511–1516. doi: 10.2337/diab.40.11.1511. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 48.Babensee JE, Sefton MV. Viability of HEMA-MMA microencapsulated model hepatoma cells in rats and the host response. Tissue Eng. 2000;6:165–182. doi: 10.1089/107632700320784. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, Ko S, Nagao M, Harb G, Nakajima Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006;15:359–365. doi: 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- 50.Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, Vasconcellos AV, Emerich DF, Thanos C, Bambra C. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant. Proc. 2005;37:3505–3508. doi: 10.1016/j.transproceed.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of micro-encapsulated porcine islets without immunosuppression. J. Clin. Invest. 1996;98:1417–1422. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polonsky K, Jaspan J, Emmanouel D, Holmes K, Moossa AR. Differences in the hepatic and renal extraction of insulin and glucagon in the dog: evidence for saturability of insulin metabolism. Acta Endocrinol. (Copenh) 1983;102:420–427. doi: 10.1530/acta.0.1020420. [DOI] [PubMed] [Google Scholar]
- 53.Selam JL, Bergman RN, Raccah D, Jean-Didier N, Lozano J, Charles MA. Determination of portal insulin absorption from peritoneum via novel nonisotopic method. Diabetes. 1990;39:1361–1365. doi: 10.2337/diab.39.11.1361. [DOI] [PubMed] [Google Scholar]
- 54.De Vos P, Vegter D, De Haan BJ, Strubbe JH, Bruggink JE, Van Schilfgaarde R. Kinetics of intraperitoneally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes. 1996;45:1102–1107. doi: 10.2337/diab.45.8.1102. [DOI] [PubMed] [Google Scholar]
- 55.de Vos P, Vegter D, Strubbe JH, de Haan BJ, van Schilfgaarde R. Impaired glucose tolerance in recipients of an intraperitoneally implanted microencapsulated islet allograft is caused by the slow diffusion of insulin through the peritoneal membrane. Transplant. Proc. 1997;29:756–757. doi: 10.1016/s0041-1345(96)00456-3. [DOI] [PubMed] [Google Scholar]
- 56.Duvivier-Kali VF, Omer A, Parent RJ, O’Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium–alginate membrane. Diabetes. 2001;50:1698–1705. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 57.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 58.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 59.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vos P, Haan BJde, de Haan A, van Zanten J, Faas MM. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplant. 2004;13:515–524. doi: 10.3727/000000004783983738. [DOI] [PubMed] [Google Scholar]
- 61.Fritschy WM, van Straaten JF, de Vos P, Strubbe JH, Wolters GH, van Schilfgaarde R. The efficacy of intraperitoneal pancreatic islet isografts in the reversal of diabetes in rats. Transplantation. 1991;52:777–783. doi: 10.1097/00007890-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Okugawa K, Fukuda Y, Sakimoto H, Nishihara M, Tashiro H, Urushihara T, Ikeda M, Iwata H, Dohi K. Intermittent intraperitoneal implantation of islets in rat islet transplantation. Cell Transplant. 1996;5:S51–S53. doi: 10.1016/0963-6897(96)00040-1. [DOI] [PubMed] [Google Scholar]
- 63.Siebers U, Horcher A, Bretzel RG, Klock G, Zimmermann U, Federlin K, Zekorn T. Transplantation of free and microencapsulated islets in rats: evidence for the requirement of an increased islet mass for transplantation into the peritoneal site. Int. J. Artif. Organs. 1993;16:96–99. [PubMed] [Google Scholar]
- 64.Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. Transplantation of micro- and macroencapsu-lated piglet islets into mice and monkeys. Transplant. Proc. 2005;37:466–469. doi: 10.1016/j.transproceed.2004.12.198. [DOI] [PubMed] [Google Scholar]
- 65.Brendel MD, BJH, Schultz AO, Bretzel RG. Insulin independence following islet transplantation: a comparison of different recipient categories. Int. Islet. Transpl. Regist. 1999;8:5–18. [Google Scholar]
- 66.Robertson RP. Islet transplantation as a treatment for diabetes—a work in progress. N. Engl. J. Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362:1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 68.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 69.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 70.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson Ekdahl K, Korsgren O, Nilsson B. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 71.Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: progress and challenge. Arch. Med. Res. 2005;36:273–280. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Giustozzi GM, Moggi L, Brunetti P. Alginate/polyaminoacidic coherent microcapsules for pancreatic islet graft immunoisolation in diabetic recipients. Ann. N. Y. Acad. Sci. 1997;831:313–322. doi: 10.1111/j.1749-6632.1997.tb52206.x. [DOI] [PubMed] [Google Scholar]
- 73.Leblond FA, Simard G, Henley N, Rocheleau B, Huet PM, Halle JP. Studies on smaller (approximately 315 microM) microcapsules: IV. Feasibility and safety of intrahepatic implantations of small alginate poly-l-lysine microcapsules. Cell Transplant. 1999;8:327–337. doi: 10.1177/096368979900800303. [DOI] [PubMed] [Google Scholar]
- 74.Schneider S, Avon Mach M, Kraus O, Kann P, Feilen PJ. Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats. Artif. Organs. 2003;27:1053–1056. doi: 10.1046/j.1525-1594.2003.07159.x. [DOI] [PubMed] [Google Scholar]
- 75.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 76.Orive G, Tam SK, Pedraz JL, Halle JP. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691–3700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 77.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 78.Geller RL, Loudovaris T, Neuenfeldt S, Johnson RC, Brauker JH. Use of an immunoisolation device for cell transplantation and tumor immunotherapy. Ann. N. Y. Acad. Sci. 1997;831:438–451. doi: 10.1111/j.1749-6632.1997.tb52216.x. [DOI] [PubMed] [Google Scholar]
- 79.Lembert N, Wesche J, Petersen P, Doser M, Zschocke P, Becker HD, Ammon HP. Ammon, Encapsulation of islets in rough surface, hydroxymethy-lated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant. 2005;14:97–108. [PubMed] [Google Scholar]
- 80.Padera RF, Colton CK. Time course of membrane microarchitecture-driven neovascularization. Biomaterials. 1996;17:277–284. doi: 10.1016/0142-9612(96)85565-7. [DOI] [PubMed] [Google Scholar]
- 81.Orive G, Ponce S, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials. 2002;23:3825–3831. doi: 10.1016/s0142-9612(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 82.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, Bellocq JP, Pinget M, Kessler L. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12:627–635. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 83.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Legeay G, Bellocq JP, Pinget M, Kessler L. Induction of angiogenesis in omentum with vascular endothelial growth factor: influence on the viability of encapsulated rat pancreatic islets during transplantation. J. Vasc. Res. 2003;40:359–367. doi: 10.1159/000072700. [DOI] [PubMed] [Google Scholar]
- 84.Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant. 2000;9:115–124. doi: 10.1177/096368970000900114. [DOI] [PubMed] [Google Scholar]
- 85.Miki A, Rivas-Carrillo JD, Navarro-Alvarez N, Soto-Gutierrez A, Chen Y, Tanaka K, Narushima M, Tabata Y, Okitsu T, Noguchi H, Matsumoto S, Tanaka N, Kobayashi N. Maintenance of neovascularization at the implantation site of an artificial device by bFGF and endothelial cell transplant. Cell Transplant. 2006;15:893–901. doi: 10.3727/000000006783981378. [DOI] [PubMed] [Google Scholar]
- 86.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Inverardi, Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 87.Balamurugan AN, Gu Y, Tabata Y, Miyamoto M, Cui W, Hori H, Satake A, Nagata N, Wang W, Inoue K. Bioartificial pancreas transplantation at prevascularized intermuscular space: effect of angio-genesis induction on islet survival. Pancreas. 2003;26:279–285. doi: 10.1097/00006676-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 88.De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997;63:824–830. doi: 10.1097/00007890-199703270-00006. [DOI] [PubMed] [Google Scholar]
- 89.de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45:159–173. doi: 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- 90.Sugiura S, Oda T, Aoyagi Y, Matsuo R, Enomoto T, Matsumoto K, Nakamura T, Satake M, Ochiai A, Ohkohchi N, Nakajima M. Microfabricated airflow nozzle for microencapsulation of living cells into 150 micrometer microcapsules. Biomed. Microdev. 2007;9:91–99. doi: 10.1007/s10544-006-9011-9. [DOI] [PubMed] [Google Scholar]
- 91.Ross CJ, Chang PL. Development of small alginate microcapsules for recombinant gene product delivery to the rodent brain. J. Biomater. Sci. Polym. Ed. 2002;13:953–962. doi: 10.1163/156856202320401988. [DOI] [PubMed] [Google Scholar]
- 92.Aoki T, Hui HX, Umehara Y, LiCalzi S, Demetriou AA, Rozga J, Perfetti R. Intrasplenic transplantation of encapsulated genetically engineered mouse insulinoma cells reverses streptozotocin-induced diabetes in rats. Cell Transplant. 2005;14:411–421. doi: 10.3727/000000005783982990. [DOI] [PubMed] [Google Scholar]
- 93.Black SP, Constantinidis I, Cui H, Tucker-Burden C, Weber CJ, Safley SA. Immune responses to an encapsulated allogeneic islet beta-cell line in diabetic NOD mice. Biochem. Biophys. Res. Commun. 2006;340:236–243. doi: 10.1016/j.bbrc.2005.11.180. [DOI] [PubMed] [Google Scholar]
- 94.Robitaille R, Leblond FA, Bourgeois Y, Henley N, Loignon M, Halle JP. Studies on small (<350 microm) alginate-poly-L-lysine micro-capsules.V. Determination of carbohydrate and protein permeation through microcapsules by reverse-size exclusion chromatography. J. Biomed. Mater. Res. 2000;50:420–427. doi: 10.1002/(sici)1097-4636(20000605)50:3<420::aid-jbm17>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 95.Robitaille R, Pariseau JF, Leblond FA, Lamoureux M, Lepage Y, Halle JP. Studies on small (<350 microm) alginate-poly-L-lysine micro-capsules. III. Biocompatibility of smaller versus standard microcapsules. J. Biomed. Mater. Res. 1999;44:116–120. doi: 10.1002/(sici)1097-4636(199901)44:1<116::aid-jbm13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 96.Toso C, Oberholzer J, Ceausoglu I, Ris F, Rochat B, Rehor A, Bucher P, Wandrey C, Schuldt U, Belenger J, Bosco D, Morel P, Hunkeler D. Intra-portal injection of 400-microm microcapsules in a large-animal model. Transpl. Int. 2003;16:405–410. doi: 10.1007/s00147-003-0555-9. [DOI] [PubMed] [Google Scholar]
- 97.Toso C, Mathe Z, Morel P, Oberholzer J, Bosco D, Sainz-Vidal D, Hunkeler D, Buhler LH, Wandrey C, Berney T. Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplant. 2005;14:159–167. doi: 10.3727/000000005783983223. [DOI] [PubMed] [Google Scholar]
- 98.De Vos P, De Haan B, Wolters GH, Van Schilfgaarde R. Factors influencing the adequacy of microencapsulation of rat pancreatic islets. Transplantation. 1996;62:888–893. doi: 10.1097/00007890-199610150-00003. [DOI] [PubMed] [Google Scholar]
- 99.De Vos P, Van Straaten JF, Nieuwenhuizen AG, de Groot M, Ploeg RJ, De Haan BJ, Van Schilfgaarde R. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes. 1999;48:1381–1388. doi: 10.2337/diabetes.48.7.1381. [DOI] [PubMed] [Google Scholar]
- 100.Zekorn T, Siebers U, Horcher A, Schnettler R, Zimmermann U, Bretzel RG, Federlin K. Alginate coating of islets of Langerhans: in vitro studies on a new method for microencapsulation for immuno-isolated transplantation. Acta Diabetol. 1992;29:41–45. doi: 10.1007/BF00572829. [DOI] [PubMed] [Google Scholar]
- 101.Park YG, Iwata H, Ikada Y. Microencapsulation of islets and model beads with a thin alginate–Ba2+ gel layer using centrifugation. Polym. Adv. Technol. 1998;9:734–739. [Google Scholar]
- 102.May MH, Sefton MV. Conformal coating of small particles and cell aggregates at a liquid–liquid interface. Ann. N. Y. Acad. Sci. 1999;875:126–134. doi: 10.1111/j.1749-6632.1999.tb08498.x. [DOI] [PubMed] [Google Scholar]
- 103.Sefton MV, May MH, Lahooti S, Babensee JE. Making microencapsulation work: conformal coating, immobilization gels and in vivo performance. J. Control. Release. 2000;65:173–186. doi: 10.1016/s0168-3659(99)00234-5. [DOI] [PubMed] [Google Scholar]
- 104.Khademhosseini A, May MH, Sefton MV. Conformal coating of mammalian cells immobilized onto magnetically driven beads. Tissue Eng. 2005;11:1797–1806. doi: 10.1089/ten.2005.11.1797. [DOI] [PubMed] [Google Scholar]
- 105.Basta G, Osticioli L, Rossodivita ME, Sarchielli P, Tortoioli C, Brunetti P, Calafiore R. Method for fabrication of coherent microcapsules — a new, potential immunoisolatory barrier for pancreatic-islet transplantation. Diabetes Nutr. Metab. 1995;8:105–112. [Google Scholar]
- 106.Leung A, Ramaswamy Y, Munro P, Lawrie G, Nielsen L, Trau M. Emulsion strategies in the microencapsulation of cells: pathways to thin coherent membranes. Biotechnol. Bioeng. 2005;92:45–53. doi: 10.1002/bit.20597. [DOI] [PubMed] [Google Scholar]
- 107.Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Bufalari A, Cassarani MP, Giustozzi GM, Brunetti P. Transplantation of pancreatic islets contained in minimal volume microcapsules in diabetic high mammalians. Ann. N. Y. Acad. Sci. 1999;875:219–232. doi: 10.1111/j.1749-6632.1999.tb08506.x. [DOI] [PubMed] [Google Scholar]
- 108.Wyman JL, Kizilel S, Skarbek R, Zhao X, Connors M, Dillmore WS, Murphy WL, Mrksich M, Nagel SR, Garfinkel MR. Immunoisolating pancreatic islets by encapsulation with selective withdrawal. Small. 2007;3:683–690. doi: 10.1002/smll.200600231. [DOI] [PubMed] [Google Scholar]
- 109.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8:293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 110.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly (ethylene glycol) diacrylate upon porcine islets. Biotechnol. Bioeng. 1998;57:655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 111.Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, Hegre OD, Scharp DW. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann. N. Y. Acad. Sci. 1997;831:332–343. doi: 10.1111/j.1749-6632.1997.tb52208.x. [DOI] [PubMed] [Google Scholar]
- 112. www.novocell.com.
- 113. www.clinicaltrails.gov.
- 114.Chen AM, Scott MD. Immunocamouflage: prevention of transfusioninduced graft-versus-host disease via polymer grafting of donor cells. J. Biomed. Mater. Res., A. 2003;67:626–636. doi: 10.1002/jbm.a.10146. [DOI] [PubMed] [Google Scholar]
- 115.Murad KL, Gosselin EJ, Eaton JW, Scott MD. Stealth cells: prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood. 1999;94:2135–2141. [PubMed] [Google Scholar]
- 116.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee DY, Yang K, Lee S, Chae SY, Kim KW, Lee MK, Han DJ, Byun Y. Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules. J. Biomed. Mater. Res. 2002;62:372–377. doi: 10.1002/jbm.10246. [DOI] [PubMed] [Google Scholar]
- 118.Panza JL, Wagner WR, Role HLR, Rao RH, Beckman EJ, Russell AJ. Treatment of rat pancreatic islets with reactive PEG. Biomaterials. 2000;21:1155–1164. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 119.Kellam B, Bank PADe, Shakesheff KM. Chemical modification of mammalian cell surfaces. Chem. Soc. Rev. 2003;32:327–337. doi: 10.1039/b211643j. [DOI] [PubMed] [Google Scholar]
- 120.Contreras JL, Xie D, Mays J, Smyth CA, Eckstein C, Rahemtulla FG, Young CJ, Thompson JAnthony, Bilbao G, Curiel DT, Eckhoff DE. A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets. Surgery. 2004;136:537–547. doi: 10.1016/j.surg.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 121.Chen AM, Scott MD. Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs. 2001;15:833–847. doi: 10.2165/00063030-200115120-00005. [DOI] [PubMed] [Google Scholar]
- 122.Scott MD, Chen AM. Beyond the red cell: pegylation of other blood cells and tissues. Transfus. Clin. Biol. 2004;11:40–46. doi: 10.1016/j.tracli.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, Rasaee MJ, Armstrong JK, Moin M, Pourpak Z. A method to optimize PEG-coating of red blood cells. Bioconjug. Chem. 2006;17:1288–1293. doi: 10.1021/bc060057w. [DOI] [PubMed] [Google Scholar]
- 124.Nacharaju P, Boctor FN, Manjula BN, Acharya SA. Surface decoration of red blood cells with maleimidophenyl-polyethylene glycol facilitated by thiolation with iminothiolane: an approach to mask A, B, and D antigens to generate universal red blood cells. Transfusion. 2005;45:374–383. doi: 10.1111/j.1537-2995.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- 125.Xie D, Smyth CA, Eckstein C, Bilbao G, Mays J, Eckhoff DE, Contreras JL. Cytoprotection of PEG-modified adult porcine pancreatic islets for improved xenotransplantation. Biomaterials. 2005;26:403–412. doi: 10.1016/j.biomaterials.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 126.Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25:3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 127.Lee DY, Nam JH, Byun Y. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J. Biomater. Sci. Polym. Ed. 2004;15:753–766. doi: 10.1163/156856204774196144. [DOI] [PubMed] [Google Scholar]
- 128.Lee DY, Park SJ, Lee S, Nam JH, Byun Y. Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication. Tissue Eng. 2007;13:2133–2141. doi: 10.1089/ten.2006.0009. [DOI] [PubMed] [Google Scholar]
- 129.Lee DY, Park SJ, Nam JH, Byun Y. A combination therapy of PEGylation and immunosuppressive agent for successful islet transplantation. J. Control. Release. 2006;110:290–295. doi: 10.1016/j.jconrel.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 130.Lee DY, Park SJ, Nam JH, Byun Y. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: long-functioning PEGylated islets in vivo. Tissue. Eng. 2006;12:615–623. doi: 10.1089/ten.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 131.Yun Lee D, Hee Nam J, Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials. 2007;28:1957–1966. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 132.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 133.Elbert DL, Herbert CB, Hubbell JA. Thin polymer layers formed by polyelectrolyte multilayer techniques on biological surfaces. Langmuir. 1999;15:5355–5362. [Google Scholar]
- 134.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto arteries via layer-by-layer deposition: toward the in vivo repair of damaged blood vessels. J. Am. Chem. Soc. 2003;125:7494–7495. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 135.Georgieva R, Moya S, Donath E, Baumler H. Permeability and conductivity of red blood cell templated polyelectrolyte capsules coated with supplementary layers. Langmuir. 2004;20:1895–1900. doi: 10.1021/la035779f. [DOI] [PubMed] [Google Scholar]
- 136.Diaspro A, Silvano D, Krol S, Cavalleri O, Gliozzi A. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir. 2002;18:5047–5050. [Google Scholar]
- 137.Krol S, Diaspro A, Magrassi R, Ballario P, Grimaldi B, Filetici P, Ornaghi P, Ramoino P, Gliozzi A. Nanocapsules: coating for living cells. IEEE. Trans. Nanobiosci. 2004;3:32–38. doi: 10.1109/tnb.2004.824279. [DOI] [PubMed] [Google Scholar]
- 138.Hillberg AL, Tabrizian M. Biorecognition through layer-by-layer polyelectrolyte assembly: In-situ hybridization on living cells. Biomacromolecules. 2006;7:2742–2750. doi: 10.1021/bm060266j. [DOI] [PubMed] [Google Scholar]
- 139.Chanana M, Gliozzi A, Diaspro A, Chodnevskaja I, Huewel S, Moskalenko V, Ulrichs K, Galla HJ, Krol S. Interaction of polyelectrolytes and their composites with living cells. Nano. Lett. 2005;5:2605–2612. doi: 10.1021/nl0521219. [DOI] [PubMed] [Google Scholar]
- 140.Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Jr, Banaszak Holl MM. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 141.Prokop A, Hunkeler D, DiMari S, Haralson MA, Wang TG. Advances in Polymer Science. vol. 136. Berlin: Springer; 1998. Water soluble polymers for immunoisolation I: complex coacervation and cytotoxicity; pp. 1–51. [Google Scholar]
- 142.Germain M, Balaguer P, Nicolas JC, Lopez F, Esteve JP, Sukhorukov GB, Winterhalter M, Richard-Foy H, Fournier D. Protection of mammalian cell used in biosensors by coating with a polyelectrolyte shell. Biosens. Bioelectron. 2005;21:1566–1573. doi: 10.1016/j.bios.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 143.Veerabadran NG, Goli PL, Stewart-Clark SS, Lvov YM, Mills DK. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 2007;7:877–882. doi: 10.1002/mabi.200700061. [DOI] [PubMed] [Google Scholar]
- 144.Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27:5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 145.Stuhlmeier KM, Lin Y. Camouflaging endothelial cells: does it prolong graft survival? Biochim. Biophys. Acta. 1999;1428:177–190. doi: 10.1016/s0304-4165(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 146.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 147.Rihova B. Immunocompatibility and biocompatibility of cell delivery systems. Adv. Drug Deliv. Rev. 2000;42:65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 148.Tang L, Hu W. Molecular determinants of biocompatibility. Expert. Rev. Med. Dev. 2005;2:493–500. doi: 10.1586/17434440.2.4.493. [DOI] [PubMed] [Google Scholar]
- 149.Ziats NP, Miller KM, Anderson JM. In vitro and in vivo interactions of cells with biomaterials. Biomaterials. 1988;9:5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 150.Anderson JM. Inflammatory response to implants. ASAIO. Trans. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 151.de Vos P, van Hoogmoed CG, de Haan BJ, Busscher HJ. Tissue responses against immunoisolating alginate–PLL capsules in the immediate posttransplant period. J. Biomed. Mater. Res. 2002;62:430–437. doi: 10.1002/jbm.10345. [DOI] [PubMed] [Google Scholar]
- 152.Robitaille R, Dusseault J, Henley N, Desbiens K, Labrecque N, Halle JP. Inflammatory response to peritoneal implantation of alginate- poly-L-lysine microcapsules. Biomaterials. 2005;26:4119–4127. doi: 10.1016/j.biomaterials.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 153.McKenzie AW, Georgiou HM, Zhan Y, Brady JL, Lew AM. Protection of xenografts by a combination of immunoisolation and a single dose of anti-CD4 antibody. Cell Transplant. 2001;10:183–193. [PubMed] [Google Scholar]
- 154.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 155.Shoichet MS, Winn SR, Athavale S, Harris JM, Gentile FT. Poly (ethylene oxide) grafted thermoplastic membranes for use as cellular hybrid bioartificial organs in the central-nervous-system. Biotechnol. Bioeng. 1994;43:563–572. doi: 10.1002/bit.260430705. [DOI] [PubMed] [Google Scholar]
- 156.Flamme KELa, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials. 2007;28:2638–2645. doi: 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sawhney AS, Hubbell JA. Poly(ethylene oxide)-graft-poly(L-lysine) copolymers to enhance the biocompatibility of poly(l-lysine)-alginate microcapsule membranes. Biomaterials. 1992;13:863–870. doi: 10.1016/0142-9612(92)90180-v. [DOI] [PubMed] [Google Scholar]
- 158.King A, Sandler S, Andersson A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. J. Biomed. Mater. Res. 2001;57:374–383. doi: 10.1002/1097-4636(20011205)57:3<374::aid-jbm1180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 159.Strand BL, Ryan TL, In’t Veld P, Kulseng B, Rokstad AM, Skjak-Brek G, Espevik T. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10:263–275. [PubMed] [Google Scholar]
- 160.Lanza RP, Kuhtreiber WM, Ecker D, Staruk JE, Chick WL. Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres. Transplantation. 1995;59:1377–1384. doi: 10.1097/00007890-199505270-00003. [DOI] [PubMed] [Google Scholar]
- 161.Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81:1345–1353. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 162.Schneider S, Feilen PJ, Brunnenmeier F, Minnemann T, Zimmermann H, Zimmermann U, Weber MM. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes. 2005;54:687–693. doi: 10.2337/diabetes.54.3.687. [DOI] [PubMed] [Google Scholar]
- 163.de Vos P, van Hoogmoed CG, van Zanten J, Netter S, Strubbe JH, Busscher HJ. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24:305–312. doi: 10.1016/s0142-9612(02)00319-8. [DOI] [PubMed] [Google Scholar]
- 164.Tatarkiewicz K. New membrane for cell encapsulation. Artif. Organs. 1988;12:446–448. doi: 10.1111/j.1525-1594.1988.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 165.Wilson JT, Cui W, Sun XL, Tucker-Burden C, Weber CJ, Chaikof EL. In vivo biocompatibility and stability of a substrate-supported polymerizable membrane-mimetic film. Biomaterials. 2007;28:609–617. doi: 10.1016/j.biomaterials.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 166.Roth DJ, Jansen ED, Powers AC, Wang TG. A novel method of monitoring response to islet transplantation: bioluminescent imaging of an NF-kB transgenic mouse model. Transplantation. 2006;81:1185–1190. doi: 10.1097/01.tp.0000203808.84963.13. [DOI] [PubMed] [Google Scholar]
- 167.Kessler L, Jesser C, Lombard Y, Karsten V, Belcourt A, Pinget M, Poindron P. Cytotoxicity of peritoneal murine macrophages against encapsulated pancreatic rat islets: in vivo and in vitro studies. J. Leukoc. Biol. 1996;60:729–736. doi: 10.1002/jlb.60.6.729. [DOI] [PubMed] [Google Scholar]
- 168.King A, Andersson A, Sandler S. Cytokine-induced functional suppression of microencapsulated rat pancreatic islets in vitro. Transplantation. 2000;70:380–383. doi: 10.1097/00007890-200007270-00025. [DOI] [PubMed] [Google Scholar]
- 169.Kulseng B, Thu B, Espevik T, Skjak-Braek G. Alginate polylysine microcapsules as immune barrier: permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant. 1997;6:387–394. doi: 10.1177/096368979700600405. [DOI] [PubMed] [Google Scholar]
- 170.Cole DR, Waterfall M, McIntyre M, Baird JD. Microencapsulated islet grafts in the BB/E rat: a possible role for cytokines in graft failure. Diabetologia. 1992;35:231–237. doi: 10.1007/BF00400922. [DOI] [PubMed] [Google Scholar]
- 171.de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12:867–875. [PubMed] [Google Scholar]
- 172.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J. Leukoc. Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 173.Gysemans CA, Waer M, Valckx D, Laureys JM, Mihkalsky D, Bouillon R, Mathieu C. Early graft failure of xenogeneic islets in NOD mice is accompanied by high levels of interleukin-1 and low levels of transforming growth factor-beta mRNA in the grafts. Diabetes. 2000;49:1992–1997. doi: 10.2337/diabetes.49.12.1992. [DOI] [PubMed] [Google Scholar]
- 174.Ozasa T, Newton MR, Dallman MJ, Shimizu S, Gray DW, Morris PJ. Cytokine gene expression in pancreatic islet grafts in the rat. Transplantation. 1997;64:1152–1159. doi: 10.1097/00007890-199710270-00013. [DOI] [PubMed] [Google Scholar]
- 175.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem. Biophys. Res. Commun. 2003;308:474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 176.Berney T, Molano RD, Cattan P, Pileggi A, Vizzardelli C, Oliver R, Ricordi C, Inverardi L. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation. 2001;71:125–132. doi: 10.1097/00007890-200101150-00020. [DOI] [PubMed] [Google Scholar]
- 177.Matsuda T, Omori K, Vuong T, Pascual M, Valiente L, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am. J. Transplant. 2005;5:484–493. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 178.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004;11:184–194. doi: 10.1046/j.1399-3089.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 180.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 181.Schroppel B, Zhang N, Chen P, Chen D, Bromberg JS, Murphy B. Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation. J. Am. Soc. Nephrol. 2005;16:444–451. doi: 10.1681/ASN.2004090743. [DOI] [PubMed] [Google Scholar]
- 182.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L, Secchi A, Di Carlo V, Allavena P, Bertuzzi F. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 183.Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia. 2001;44:325–332. doi: 10.1007/s001250051622. [DOI] [PubMed] [Google Scholar]
- 184.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51:311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 185.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE, Differential roles of Mac-1+ cells. and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J. Exp. Med. 1990;172:291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernandez JA, Griffin JH, Eckhoff DE. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between activation, endothelial cell thrombosis, inflammation, and islet cell death. Diabetes. 2004;53:2804–2814. doi: 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- 187.Montolio M, Biarnes M, Tellez N, Escoriza J, Soler J, Montanya E. Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J. Endocrinol. 2007;192:169–177. doi: 10.1677/joe.1.06968. [DOI] [PubMed] [Google Scholar]
- 188.Bertuzzi F, Marzorati S, Maffi P, Piemonti L, Melzi R, de Taddeo F, Valtolina V, D’Angelo A, di Carlo V, Bonifacio E, Secchi A. Tissue factor and CCL2/monocyte chemoattractant protein-1 released by human islets affect islet engraftment in type 1 diabetic recipients. J. Clin. Endocrinol. Metab. 2004;89:5724–5728. doi: 10.1210/jc.2004-0659. [DOI] [PubMed] [Google Scholar]
- 189.de Vos P, Smedema I, van Goor H, Moes H, van Zanten J, Netters S, de Leij LF, de Haan A, de Haan BJ. Association between macrophage activation and function of micro-encapsulated rat islets. Diabetologia. 2003;46:666–673. doi: 10.1007/s00125-003-1087-7. [DOI] [PubMed] [Google Scholar]
- 190.Basta G, Sarchielli P, Luca G, Racanicchi L, Nastruzzi C, Guido L, Mancuso F, Macchiarulo G, Calabrese G, Brunetti P, Calafiore R. Optimized parameters for microencapsulation of pancreatic islet cells: an in vitro study clueing on islet graft immunoprotection in type 1 diabetes mellitus. Transplant. Immunol. 2004;13:289–296. doi: 10.1016/j.trim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 191.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 192.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 193.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40:262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 194.de Groot M, Schuurs TA, Leuvenink HG, van Schilfgaarde R. Macrophage overgrowth affects neighboring nonovergrown encapsulated islets. J. Surg. Res. 2003;115:235–241. doi: 10.1016/j.jss.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 195.Chae SY, Lee M, Kim SW, Bae YH. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials. 2004;25:843–850. doi: 10.1016/s0142-9612(03)00605-7. [DOI] [PubMed] [Google Scholar]
- 196.Wiegand F, Kroncke KD, Kolb-Bachofen V. Macrophage-generated nitric oxide as cytotoxic factor in destruction of alginate-encapsulated islets. Protection by arginine analogs and/or coencapsulated erythrocytes. Transplantation. 1993;56:1206–1212. doi: 10.1097/00007890-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 197.Kavdia M, Lewis RS. Free radical profiles in an encapsulated pancreatic cell matrix model. Ann. Biomed. Eng. 2002;30:721–730. doi: 10.1114/1.1481054. [DOI] [PubMed] [Google Scholar]
- 198.Omer A, Keegan M, Czismadia E, De Vos P, Van Rooijen N, Bonner-Weir S, Weir GC. Macrophage depletion improves survival of porcine neonatal pancreatic cell clusters contained in alginate macrocapsules transplanted into rats. Xenotransplantation. 2003;10:240–251. doi: 10.1034/j.1399-3089.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 199.Gysemans C, Stoffels K, Giulietti A, Overbergh L, Waer M, Lannoo M, Feige U, Mathieu C. Prevention of primary non-function of islet xenografts in autoimmune diabetic NOD mice by anti-inflammatory agents. Diabetologia. 2003;46:1115–1123. doi: 10.1007/s00125-003-1154-0. [DOI] [PubMed] [Google Scholar]
- 200.Arita S, Nagai T, Ochiai M, Sakamoto Y, Smith CV, Shevlin L, Mullen Y. Pravastatin prevents primary nonfunction of canine islet autografts. Transplant. Proc. 1998;30:411. doi: 10.1016/s0041-1345(97)01331-6. [DOI] [PubMed] [Google Scholar]
- 201.Lee I, Wang L, Wells AD, Ye Q, Han R, Dorf ME, Kuziel WA, Rollins BJ, Chen L, Hancock WW. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J. Immunol. 2003;171:6929–6935. doi: 10.4049/jimmunol.171.12.6929. [DOI] [PubMed] [Google Scholar]
- 202.Katz SM, Bennett F, Stecker K, Clark JH, Pham T, Wang ME, Kahan BD, Stepkowski SM. ICAM-1 antisense oligodeoxynucleotide improves islet allograft survival and function. Cell Transplant. 2000;9:817–828. doi: 10.1177/096368970000900608. [DOI] [PubMed] [Google Scholar]
- 203.Kenmochi T, Miyamoto M, Mullen Y. Protection of mouse islet isografts from nonspecific inflammatory damage by recipient treatment with nicotinamide and 15-deoxyspergualin. Cell Transplant. 1996;5:41–47. doi: 10.1177/096368979600500108. [DOI] [PubMed] [Google Scholar]
- 204.Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, Anzai K, Ono J, Taniguchi M, Ikeda S. Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice. Transplantation. 2007;83:1085–1092. doi: 10.1097/01.tp.0000260161.81775.58. [DOI] [PubMed] [Google Scholar]
- 205.Hsu BR, Chang FH, Juang JH, Huang YY, Fu SH. The rescue effect of 15-deoxyspergualin on intraperitoneal microencapsulated xenoislets. Cell Transplant. 1999;8:307–315. doi: 10.1177/096368979900800311. [DOI] [PubMed] [Google Scholar]
- 206.Bunger CM, Tiefenbach B, Jahnke A, Gerlach C, Freier T, Schmitz KP, Hopt UT, Schareck W, Klar E, Vos Pde. Deletion of the tissue response against alginate-PLL capsules by temporary release of co-encapsulated steroids. Biomaterials. 2005;26:2353–2360. doi: 10.1016/j.biomaterials.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 207.Luca G, Basta G, Calafiore R, Rossi C, Giovagnoli S, Esposito E, Nastruzzi C. Multifunctional microcapsules for pancreatic islet cell entrapment: design, preparation and in vitro characterization. Biomaterials. 2003;24:3101–3114. doi: 10.1016/s0142-9612(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 208.Ricci M, Blasi P, Giovagnoli S, Rossi C, Macchiarulo G, Luca G, Basta G, Calafiore R. Ketoprofen controlled release from composite microcapsules for cell encapsulation: effect on post-transplant acute inflammation. J. Control. Release. 2005;107:395–407. doi: 10.1016/j.jconrel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 209.Chae SY, Kim YY, Kim SW, Bae YH. Prolonged glucose normalization of streptozotocin-induced diabetic mice by transplantation of rat islets coencapsulated with crosslinked hemoglobin. Transplantation. 2004;78:392–397. doi: 10.1097/01.tp.0000128617.14309.26. [DOI] [PubMed] [Google Scholar]
- 210.Chae SY, Kim SW, Bae YH. Effect of cross-linked hemoglobin on functionality and viability of microencapsulated pancreatic islets. Tissue. Eng. 2002;8:379–394. doi: 10.1089/107632702760184655. [DOI] [PubMed] [Google Scholar]
- 211.Cui W, Barr G, Faucher KM, Sun XL, Safley SA, Weber CJ, Chaikof EL. A membrane-mimetic barrier for islet encapsulation. Transplant. Proc. 2004;36:1206–1208. doi: 10.1016/j.transproceed.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 212.Liu H, Faucher KM, Sun XL, Feng J, Johnson TL, Orban JM, Apkarian RP, Dluhy RA, Chaikof EL. A membrane-mimetic barrier for cell encapsulation. Langmuir. 2002;18:1332–1339. [Google Scholar]
- 213.Faucher KM, Sun XL, Chaikof EL. Fabrication and characterization of glycocalyx-mimetic surfaces. Langmuir. 2003;19:1664–1670. [Google Scholar]
- 214.Feng J, Tseng PY, Faucher KM, Orban JM, Sun XL, Chaikof EL. Functional reconstitution of thrombomodulin within a substrate-supported membrane-mimetic polymer film. Langmuir. 2002;18:9907–9913. [Google Scholar]
- 215.Tseng PY, Jordan SW, Sun XL, Chaikof EL. Catalytic efficiency of a thrombomodulin-functionalized membrane-mimetic film in a flow model. Biomaterials. 2006;27:2768–2775. doi: 10.1016/j.biomaterials.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 216.Tseng PY, Rele SM, Sun XL, Chaikof EL. Fabrication and characterization of heparin functionalized membrane-mimetic assemblies. Biomaterials. 2006;27:2627–2636. doi: 10.1016/j.biomaterials.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 217.Tseng PY, Rele SS, Sun XL, Chaikof EL. Membrane-mimetic films containing thrombomodulin and heparin inhibit tissue factor-induced thrombin generation in a flow model. Biomaterials. 2006;27:2637–2650. doi: 10.1016/j.biomaterials.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 218.Tibell LA, Sethson I, Buevich AV. Characterization of the heparinbinding domain of human extracellular superoxide dismutase. Biochim. Biophys. Acta. 1997;1340:21–32. doi: 10.1016/s0167-4838(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 219.Edens RE, Linhardt RJ, Bell CS, Weiler JM. Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacology. 1994;27:145–153. doi: 10.1016/0162-3109(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 220.Maillet F, Petitou M, Choay J, Kazatchkine MD. Structure-function relationships in the inhibitory effect of heparin on complement activation: independency of the anti-coagulant and anti-complementary sites on the heparin molecule. Mol. Immunol. 1988;25:917–923. doi: 10.1016/0161-5890(88)90130-7. [DOI] [PubMed] [Google Scholar]
- 221.Sahu A, Pangburn MK. Identification of multiple sites of interaction between heparin and the complement system. Mol. Immunol. 1993;30:679–684. doi: 10.1016/0161-5890(93)90079-q. [DOI] [PubMed] [Google Scholar]
- 222.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25:536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 224.Rabiet MJ, Plantier JL, Dejana E. Thrombin-induced endothelial cell dysfunction. Br. Med. Bull. 1994;50:936–945. doi: 10.1093/oxfordjournals.bmb.a072935. [DOI] [PubMed] [Google Scholar]
- 225.Dugina TN, Kiseleva EV, Chistov IV, Umarova BA, Strukova SM. Receptors of the PAR family as a link between blood coagulation and inflammation. Biochemistry. (Mosc) 2002;67:65–74. doi: 10.1023/a:1013952114485. [DOI] [PubMed] [Google Scholar]
- 226.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 227.Esmon CT. Coagulation inhibitors in inflammation. Biochem. Soc. Trans. 2005;33:401–405. doi: 10.1042/BST0330401. [DOI] [PubMed] [Google Scholar]
- 228.Esmon CT. Inflammation and thrombosis. J. Thromb. Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 229.Hancock WW, Grey ST, Hau L, Akalin E, Orthner C, Sayegh MH, Salem HH. Binding of activated protein C to a specific receptor on human mononuclear phagocytes inhibits intracellular calcium signaling and monocyte-dependent proliferative responses. Transplantation. 1995;60:1525–1532. doi: 10.1097/00007890-199560120-00026. [DOI] [PubMed] [Google Scholar]
- 230.Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein-C on the responses of human mononuclear phagocytes to Lps, Ifn-gamma, or phorbol ester. J. Immunol. 1994;153:3664–3672. [PubMed] [Google Scholar]
- 231.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87:642–647. [PubMed] [Google Scholar]
- 232.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am. J. Physiol. -Lung. Cell. Molec. Physiol. 1997;16:L197–L202. doi: 10.1152/ajplung.1997.272.2.L197. [DOI] [PubMed] [Google Scholar]
- 233.Hancock WW, Bach FH. Immunobiology and therapeutic applications of protein C protein S thrombomodulin in human and experimental allotransplantation and xenotransplantation. Trends Cardiovasc. Med. 1997;7:174–183. doi: 10.1016/S1050-1738(97)00032-7. [DOI] [PubMed] [Google Scholar]
- 234.Grinnell BW, Hermann RB, Yan SB. Human protein-C inhibits selectin-mediated cell-adhesion— role of unique fucosylated oligosaccharide. Glycobiology. 1994;4:221–225. doi: 10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- 235.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 236.Gill RG. Antigen presentation pathways for immunity to islet transplants. Relevance to immunoisolation. Ann. N. Y. Acad. Sci. 1999;875:255–260. doi: 10.1111/j.1749-6632.1999.tb08508.x. [DOI] [PubMed] [Google Scholar]
- 237.Gray DW. Encapsulated islet cells: the role of direct and indirect presentation and the relevance to xenotransplantation and autoimmune recurrence. Br. Med. Bull. 1997;53:777–788. doi: 10.1093/oxfordjournals.bmb.a011647. [DOI] [PubMed] [Google Scholar]
- 238.Gray DW. An overview of the immune system with specific reference to membrane encapsulation and islet transplantation. Ann. N. Y. Acad. Sci. 2001;944:226–239. doi: 10.1111/j.1749-6632.2001.tb03835.x. [DOI] [PubMed] [Google Scholar]
- 239.Duvivier-Kali VF, Omer A, Lopez-Avalos MD, O’Neil JJ, Weir GC. Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am. J. Transplant. 2004;4:1991–2000. doi: 10.1111/j.1600-6143.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 240.III WMBaldwin, Pruitt SK, Brauer RB, Daha MR, Sanfilippo F. Complement in organ transplantation. Contributions to inflammation, injury, and rejection. Transplantation. 1995;59:797–808. [PubMed] [Google Scholar]
- 241.Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol. Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 242.Loudovaris T, Mandel TE, Charlton B. CD4+ Tcell mediated destruction of xenografts within cell-impermeable membranes in the absence of CD8+ T cells and B cells. Transplantation. 1996;61:1678–1684. doi: 10.1097/00007890-199606270-00003. [DOI] [PubMed] [Google Scholar]
- 243.Lanza RP, Beyer AM, Chick WL. Xenogenic humoral responses to islets transplanted in biohybrid diffusion chambers. Transplantation. 1994;57:1371–1375. doi: 10.1097/00007890-199405150-00015. [DOI] [PubMed] [Google Scholar]
- 244.Weber CJ, Safley S, Hagler M, Kapp J. Evaluation of graft-host response for various tissue sources and animal models. Ann. N. Y. Acad. Sci. 1999;875:233–254. doi: 10.1111/j.1749-6632.1999.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 245.Weber CJ, Zabinski S, Koschitzky T, Wicker L, Rajotte R, D’Agati V, Peterson L, Norton J, Reemtsma K. The role of CD4+ helper T cells in the destruction of microencapsulated islet xenografts in nod mice. Transplantation. 1990;49:396–404. doi: 10.1097/00007890-199002000-00034. [DOI] [PubMed] [Google Scholar]
- 246.Jones KS, Sefton MV, Gorczynski RM. In vivo recognition by the host adaptive immune system of microencapsulated xenogeneic cells. Transplantation. 2004;78:1454–1462. doi: 10.1097/01.tp.0000142094.63083.fb. [DOI] [PubMed] [Google Scholar]
- 247.Brauker J, Martinson LA, Young SK, Johnson RC. Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation. 1996;61:1671–1677. doi: 10.1097/00007890-199606270-00002. [DOI] [PubMed] [Google Scholar]
- 248.Safley SA, Kapp LM, Tucker-Burden C, Hering B, Kapp JA, Weber CJ. Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation. 2005;79:409–418. doi: 10.1097/01.tp.0000150021.06027.dc. [DOI] [PubMed] [Google Scholar]
- 249.Safley SA, Kapp JA, Weber CJ. Proliferative and cytokine responses in CTLA4-Ig-treated diabetic NOD mice transplanted with microencap-sulated neonatal porcine ICCs. Cell Transplant. 2002;11:695–705. doi: 10.3727/000000002783985413. [DOI] [PubMed] [Google Scholar]
- 250.Orlowski T, Godlewska E, Moscicka M, Sitarek E. The influence of intraperitoneal transplantation of free and encapsulated Langerhans islets on the second set phenomenon. Artif. Organs. 2003;27:1062–1067. doi: 10.1111/j.1525-1594.2003.07046.x. [DOI] [PubMed] [Google Scholar]
- 251.Siebers U, Horcher A, Bretzel RG, Federlin K, Zekorn T. Alginate-based microcapsules for immunoprotected islet transplantation. Ann. N. Y. Acad. Sci. 1997;831:304–312. doi: 10.1111/j.1749-6632.1997.tb52205.x. [DOI] [PubMed] [Google Scholar]
- 252.Horcher A, Zekorn T, Siebers U, Klock G, Frank H, Houben R, Bretzel RG, Zimmermann U, Federlin K. Transplantation of microencapsulated islets in rats: evidence for induction of fibrotic overgrowth by islet alloantigens released from microcapsules. Transplant. Proc. 1994;26:784–786. [PubMed] [Google Scholar]
- 253.Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79:52–58. doi: 10.1097/01.tp.0000149340.37865.46. [DOI] [PubMed] [Google Scholar]
- 254.Lanza RP, Ecker DM, Kuhtreiber WM, Marsh JP, Ringeling J, Chick WL. Transplantation of islets using microencapsulation: studies in diabetic rodents and dogs. J. Mol. Med. 1999;77:206–210. doi: 10.1007/s001090050337. [DOI] [PubMed] [Google Scholar]
- 255.Figliuzzi M, Plati T, Cornolti R, Adobati F, Fagiani A, Rossi L, Remuzzi G, Remuzzi A. Biocompatibility and function of microencapsulated pancreatic islets. Acta. Biomater. 2006;2:221–227. doi: 10.1016/j.actbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 256.Peduto G, Rinsch C, Schneider BL, Rolland E, Aebischer P. Longterm host unresponsiveness to encapsulated xenogeneic myoblasts after transient immunosuppression. Transplantation. 2000;70:78–85. [PubMed] [Google Scholar]
- 257.Rinsch C, Peduto G, Schneider BL, Aebischer P. Inducing host acceptance to encapsulated xenogeneic myoblasts. Transplantation. 2001;71:345–351. doi: 10.1097/00007890-200102150-00002. [DOI] [PubMed] [Google Scholar]
- 258.Schneider BL, Schwenter F, Pralong WF, Aebischer P. Prevention of the initial host immuno-inflammatory response determines the long-term survival of encapsulated myoblasts genetically engineered for erythropoietin delivery. Mol. Ther. 2003;7:506–514. doi: 10.1016/s1525-0016(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 259.Weber CJ, Hagler MK, Chryssochoos JT, Kapp JA, Korbutt GS, Rajotte RV, Linsley PS. CTLA4-Ig prolongs survival of microencapsulated neonatal porcine islet xenografts in diabetic NOD mice. Cell Transplant. 1997;6:505–508. doi: 10.1177/096368979700600510. [DOI] [PubMed] [Google Scholar]
- 260.Takeda Y, Gotoh M, Dono K, Nishihara M, Grochowiecki T, Kimura F, Yoshida T, Ohta Y, Ota H, Ohzato H, Umeshita K, Takeda T, Matsuura N, Sakon M, Kayagaki N, Yagita H, Okumura K, Miyasaka M, Monden M. Protection of islet allografts transplanted together with Fas ligand expressing testicular allografts. Diabetologia. 1998;41:315–321. doi: 10.1007/s001250050909. [DOI] [PubMed] [Google Scholar]
- 261.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 262.Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 263.Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat. Biotechnol. 1996;14:1692–1695. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- 264.Dufour JM, Rajotte RV, Kin T, Korbutt GS. Immunoprotection of rat islet xenografts by cotransplantation with sertoli cells and a single injection of antilymphocyte serum. Transplantation. 2003;75:1594–1596. doi: 10.1097/01.TP.0000058748.00707.88. [DOI] [PubMed] [Google Scholar]
- 265.Valdes-Gonzalez RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Davila-Perez R, Elliott RB, Teran L, White DJ. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur. J. Endocrinol. 2005;153:419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 266.Dufour JM, Rajotte RV, Korbutt GS, Emerich DF. Harnessing the immunomodulatory properties of Sertoli cells to enable xenotransplantation in type I diabetes. Immunol. Invest. 2003;32:275–297. doi: 10.1081/imm-120025106. [DOI] [PubMed] [Google Scholar]
- 267.Korbutt GS, Ao Z, Flashner M, Elliott JF, Rajotte RV. Coencapsulation of allogeneic islets with allogeneic Sertoli cells prolongs graft survival without systemic immunosuppression. Transplant. Proc. 1998;30:419. doi: 10.1016/s0041-1345(97)01335-3. [DOI] [PubMed] [Google Scholar]
- 268.Yang H, Wright JR., Jr Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation. 1999;67:815–820. doi: 10.1097/00007890-199903270-00006. [DOI] [PubMed] [Google Scholar]
- 269.Luca G, Calvitti M, Nastruzzi C, Bilancetti L, Becchetti E, Angeletti G, Mancuso F, Calafiore R, Encapsulation, characterization invitro. and in vivo biocompatibility of Sertoli cells in alginate-based microcapsules. Tissue. Eng. 2007;13:641–648. doi: 10.1089/ten.2006.0137. [DOI] [PubMed] [Google Scholar]
- 270.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr. Opin. Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 271.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 272.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 273.Allison J, Georgiou HM, Strasser A, Vaux DL. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat. Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 275.Cheung CY, Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconjug. Chem. 2006;17:1036–1042. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 276.Tashiro H, Iwata H, Warnock GL, Takagi T, Machida H, Ikada Y, Tsuji T. Characterization and transplantation of agarose microencapsulated canine islets of Langerhans. Ann. Transplant. 1997;2:33–39. [PubMed] [Google Scholar]
- 277.Kin T, Iwata H, Aomatsu Y, Ohyama T, Kanehiro H, Hisanaga M, Nakajima Y. Xenotransplantation of pig islets in diabetic dogs with use of a microcapsule composed of agarose and polystyrene sulfonic acid mixed gel. Pancreas. 2002;25:94–100. doi: 10.1097/00006676-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 278.Ohyama T, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Kin T, Nishio K, Ohashi K, Sho M, Nagao M, Tatekawa Y, Ikeda N, Kanokogi H, Yamada T, Iwata H, Nakano H. Long-term normalization of diabetes by xenotransplantation of newly developed encapsulated pancreatic islets. Transplant. Proc. 1998;30:3433–3435. doi: 10.1016/s0041-1345(98)01091-4. [DOI] [PubMed] [Google Scholar]
- 279.Omer A, Duvivier-Kali VF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes. 2003;52:69–75. doi: 10.2337/diabetes.52.1.69. [DOI] [PubMed] [Google Scholar]
- 280.Lum ZP, Krestow M, Tai IT, Vacek I, Sun AM. Xenografts of rat islets into diabetic mice. An evaluation of new smaller capsules. Transplantation. 1992;53:1180–1183. doi: 10.1097/00007890-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 281.Tun T, Inoue K, Hayashi H, Aung T, Gu YJ, Doi R, Kaji H, Echigo Y, Wang WJ, Setoyama H, Imamura M, Maetani S, Morikawa N, Iwata H, Ikada Y. A newly developed three-layer agarose microcapsule for a promising biohybrid artificial pancreas: rat to mouse xenotransplantation. Cell Transplant. 1996;5:S59–S63. doi: 10.1016/0963-6897(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 282.Weber CJ, Kapp JA, Hagler MK, Safley S, Chryssochoos JT, Chaikof EL. Long-term survival of poly-l-lysine-alginate microencapsulated islet xenografts in spontaneously diabetic NOD mice. In: Kuhtreiber WM, Lanza RP, Chick WL, editors. Cell Encapsulation Technology and Therapeutics. Boston: Birkhauser; 1999. pp. 117–137. [Google Scholar]
- 283.Krol S, Guerra Sdel, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6:1933–1939. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]