Abstract
Objectives
Previous studies demonstrated increased levels of cysteine proteases cathepsins in serum and adipose tissues from obese patients. We now provide evidence from a mouse model of obesity to suggest a direct participation of cathepsin K (CatK) in mouse body weight gain and glucose metabolism.
Methods and Results
Using real-time PCR, we detected 12-fold increase in CatK transcripts after adipogenesis of human preadipocytes. Using an immunohistology analysis, we consistently observed high levels of CatK expression in adipose tissues from obese humans and mice. Selective inhibition of CatK activity blocked the lipid accumulation in human and mouse preadipocytes. In mice, CatK deficiency reduced significantly diet-induced body weight gain and serum glucose and insulin levels. Similar results were obtained in diet-induced and genetically created (ob/ob) obese mice after animals were treated with a CatK-selective inhibitor. Mechanistic study demonstrated a role for CatK in degrading fibronectin, a matrix protein that controls adipogenesis. Deficiency or inhibition of CatK leads to fibronectin accumulation in muscle and adipose tissues.
Conclusion
This study demonstrates an essential role of CatK in adipogenesis and mouse body weight gain, possibly via degradation of fibronectin, thus suggesting a novel therapeutic strategy for the control of obesity by regulating CatK activity.
Keywords: cysteine protease, cathepsin K, adipogenesis, obesity, fibronectin
INTRODUCTION
Cysteine protease cathepsins play important roles in cancer, autoimmune diseases, infectious diseases, and cardiovascular diseases. Recent observations indicate that expression of cathepsin S (CatS) is associated with adipogenesis and obesity in humans. Increased expression of CatS, both at RNA and protein levels, has been reported in subcutaneous adipose tissues of obese patients,1 where CatS mRNA levels are significantly associated with body mass index. In humans, surgery-induced weight loss is associated with significant reduction of CatS levels in serum and white adipose tissues.2 In cultured human preadipocytes, inhibition of CatS reduced cell adipogenesis.3 It has been suggested that CatS participates in adipogenesis by degrading fibronectin, an important extracellular matrix protein that regulates preadipocyte differentiation4 via down-regulation of lipogenic gene expression and interference with cytoskeletal and morphological changes necessary for new gene expression.5 Similar to CatS, increased CatK expression was also detected in differentiated preadipocyte 3T3-L1 and in adipose tissues from db/db obese mice, especially in lysosome-enriched fractions.6,7 However, it remains unknown whether increased levels of cathepsins in human/murine adipose tissue or serum merely serve as a hallmark of inflammation, and more importantly, whether cathepsins offer a potential drug target to control human obesity. 1,2,6,7
In this study, we demonstrate that CatK is highly expressed in adipose tissues from obese humans and mice. Deficiency or selective inhibition of CatK activity reduces preadipocyte differentiation and impairs mouse body weight gain in diet-induced and genetically created obese mice.
METHODS
Preadipocyte culture and differentiation
Human subcutaneous preadipocytes (Cambrex Corporation) and murine 3T3-L1 were differentiated with or without a non-selective cathepsin inhibitor E64d (20 µM, Sigma), a CatK-selective inhibitor-II (0.5~1 µM, Calbiochem), or a CatS-selective inhibitor N-morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS)8 as we described previously.9 Differentiated human and mouse adipocytes were fixed and stained with oil-red O. To quantify adipogenesis, we extracted intracellular oil-red O with 100% isopropanol and quantified OD510nm. Data were presented as percentage of OD510nm reading relative to cells without protease inhibitors.
Real-time PCR
Real-time PCR and data analysis were performed as described elsewhere.10 Five human housekeeping genes, peptidylprolyl isomerase A (PPIA), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), eukaryotic elongation factor 1A (EEF1A), ribosomal protein L13a (RPL13A), and ubiquitin, were used as experimental controls.
Mice
CatK knockout mice (CatK−/−) (C57BL/6/129S background)11 and their littermates (CatK+/+, CatK+/−) began a high-fat diet (HFD, Research Diet, New Brunswick, NJ) at 6 weeks of age for 16 weeks. Body weight was monitored biweekly. To examine the effect of CatK inhibitor in mouse body weight gain, we started feeding female wild-type mice (C57BL/6, 6 weeks old) a HFD while also giving mice a CatK-selective inhibitor K4b (1 mg/kg/day) or DMSO for 14 weeks. Mouse body weight was monitored biweekly. To examine the ability of K4b to control body weight gain in ob/ob mice, we treated 4-week-old female ob/ob mice (C57BL/6, Jackson Laboratory) with K4b (1 mg/kg/day) for 8 weeks. Due to fast body weight gain of ob/ob mice, we monitored their body weight weekly. Energy expenditure, serum insulin level, and glucose tolerance were determined as we previously reported.9
Immunohistology
Paraffin sections of human white adipose tissue and normal muscle (n=9/group with unknown gender and age) were obtained from the Department of Pathology, Brigham and Women’s Hospital under a pre-approved human subject research protocol. Mouse visceral fat and muscle tissues were fixed in 3% paraformaldehyde and paraffin sections were prepared for immunostaining with antibodies against human fibronectin (1:10,000, Dako), mouse fibronectin (1:10,000, NeoMarkers), mouse CatK (1:75, Calbiochem), and mouse Mac-2 (1:1200, Cedarlane Laboratories, Ontario, Canada).
Western blot
Equal amount of proteins (40 µg/lane) from fat, muscle, or 3T3-L1 cells were separated on 8% SDS-PAGE for immunoblot analysis with anti-mouse fibronectin (1:200, NeoMarkers), Glut4 (1:100, R&D Systems), insulin receptor (IR) β-subunit (1:200, Calbiochem), CatK (1:1000, Santa Cruz), and tubulin (1:1000, Santa Cruz) monoclonal antibodies, and anti-GAPDH (1:1000, Abcam) and CatK (1:1000) polyclonal antibodies.
In vitro fibronectin digestion with CatK
Human plasma fibronectin (10 µg/reaction, Chemicon) was incubated with different amounts of recombinant human CatK (Calbiochem) in a pH5.5 buffer.12 After 45 min of incubation at 37 °C, samples were separated on a 8% SDS-PAGE.
Cysteine protease active site labeling and immunoprecipitation
Active cathepsins in mouse splenocytes, peritoneal macrophages, fat and muscle tissues were detected by incubating protein lysate (50 µg/sample) with [125I]-JPM as we previously described.12 To examine the inhibitory specificities of cathepsin inhibitors in mouse adipocytes, differentiated 3T3-L1 cells were incubated with E64d (20 µM) or CatK-selective inhibitor-II (0.5~1 µM) for 6 hrs followed by labeling the cell lysate (200 µg/sample) with [125I]-JPM at 37 °C for 1 hour. Labeled cell lysate was neutralized with 1M Tris.HCl, pH10.0, boiled for 5~10 min, and then incubated with mouse CatK monoclonal antibody (Santa Cruz)-coated protein A agarose beads at 4 °C overnight. Affinity bound CatK proteins were boiled and separated on a 12% SDS-PAGE.
Statistics
Due to the relative small sample sizes and data distribution abnormality, we selected the non-parametric Mann-Whitney test to examine the statistical significances throughout this study. P<0.05 were considered significant.
RESULTS
Expression of cathepsins and cathepsin inhibitors in human adipocytes
Increased expression of CatS and CatK have been detected in human adipocytes,3,6 but a broad view of cathepsin expression profile is still unavailable. Following the process of human primary adipocyte differentiation, we measured the mRNA levels of a list of selected cathepsins and their endogenous inhibitors cystatins with real-time PCR. As shown in Supplemental Table I, among all tested cathepsins, CatS and CatK demonstrated the highest induction after preadipocyte differentiation (~12-fold). In human adipocytes, CatB, CatK, and CatL transcripts were 42~100-fold greater than those of CatS. In contrast, among all tested cystatins, cystatin C is the most abundant cathepsin inhibitor, >30~2000-fold more transcripts than other cystatins. Importantly, while all tested cathepsins are increased after preadipocyte differentiation, cystatin C expression remains unchanged.
CatK inhibition reduces human preadipocyte adipogenesis
Increased expression of CatK in human adipocytes suggests its high expression in human adipose tissues. Using human CatK antibody-mediated immunohistology analysis, we detected CatK expression in human adipose tissues (Supplemental Fig. IB), but negligibly in human skeletal muscles (Supplemental Fig. IA). Although prior experiments from Xiao et al.6 demonstrated that the non-selective cathepsin inhibitor E64 reduces human preadipocyte differentiation, it is unknown whether their observations were due to the inhibition of CatK or other cathepsins. To test this possibility, we differentiated human preadipocytes in the presence of Boc-Phe-Leu-NHNH-CO-NHNH-Leu-Z (Calbiochem), a CatK inhibitor that is 50-fold more sensitive to CatK than CatL and 2~4 orders of magnitude more selective to CatK than to CatB or papain.13 Oil-red O staining showed adipogenesis or lipid accumulation after the cells were induced for differentiation. When human preadipocytes were incubated with E64d (20 µM), we observed strong inhibition of adipogenesis. When we used 0.5~1 µM of CatK-selective inhibitor, concentrations that inhibited CatK (Supplemental Fig. IIB) but not other major cathepsins such as cathepsins B and L (Supplemental Fig. IIA),13 we also detected significant reduction of adipogenesis (Supplemental Fig. IC–G). A quantitative analysis of adipogenesis in response to different cathepsin inhibitors is presented in Supplemental Fig. IH by determining the relative content of intracellular oil-red O staining.
CatK expression in mouse adipose tissues
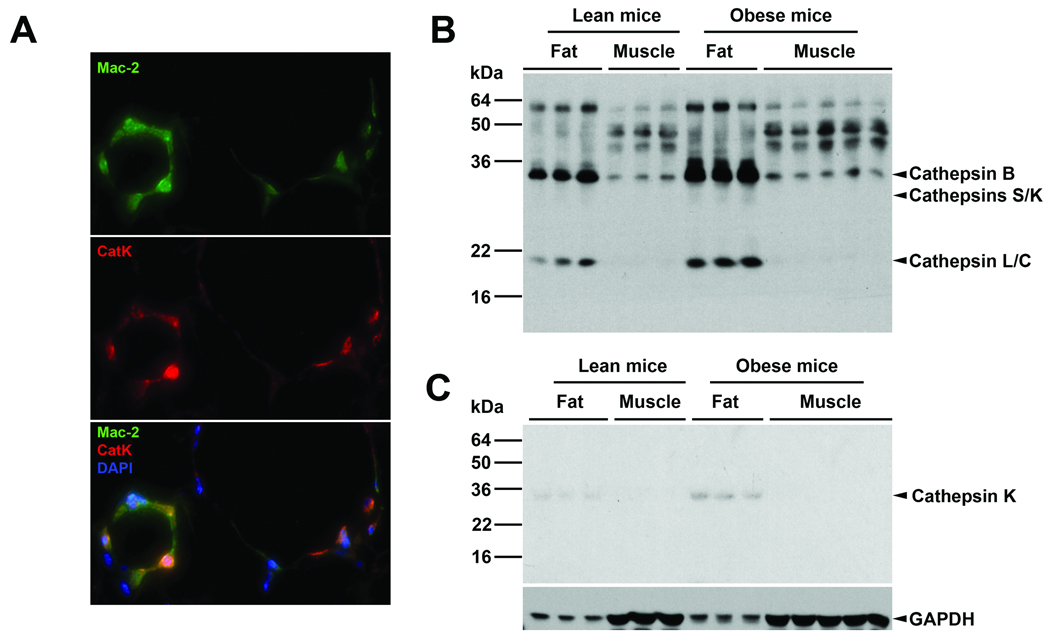
Visceral fat from HFD-induced obese mice contains CatK. By co-immunostaining mouse adipose tissue section with CatK polyclonal antibody (red) and Mac-2 (macrophages, green) monoclonal antibody, we detected clusters of CatK-positive macrophages (orange) in the adipose tissues (Fig. 1A), indicating an infiltration of CatK-positive macrophages to the adipose tissues. When we labeled the fat and muscle tissue extracts with a cysteine protease active site probe [125I]-JPM, which detects only active cathepsins, we revealed much stronger total cathepsin activities in the fat than the muscle. Importantly, fat from obese mice contained even more cathepsin activities than fat from lean mice (Fig. 1B). These cathepsins may come in part from infiltrated macrophages. Immunoblot analysis using an anti-mouse CatK monoclonal antibody we demonstrated higher amount of the 28-kDa active form of CatK proteins in fat tissues from obese mice than those of lean mice. In contrast, we detected negligible CatK signals in muscles from either obese or lean mice under this experimental condition (Fig. 1C).
Figure 1. CatK expression in mouse adipose and muscle tissues.
A. Mouse visceral fat section co-immunostaining for macrophages (Mac-2) and CatK. Orange cells on the bottom panel were CatK-positive macrophages. B. Fat and muscle tissue lysate cathepsin active site [125I]-JPM labeling. Arrowheads indicate active cathepsins. C. CatK immunoblot analysis in mouse fat and muscle tissue extracts. Arrowhead indicates the 28-kDa mature CatK. Immunoblot for GAPDH was used for protein loading control.
CatK inhibition reduces 3T3-L1 cell adipogenesis
Increased CatK expression in mouse adipose tissues (Fig. 1A/C) suggests its participation in mouse preadipocyte differentiation. To examine this hypothesis, we induced 3T3-L1 cells in the presence of non-selective cathepsin inhibitor E64d and CatK-selective inhibitor. Insulin-induced 3T3-L1 cell differentiation is clearly blocked by E64d (20 µM) and CatK-selective inhibitor (0.5~1 µM). Inhibition of CatS with 10 nM LHVS, which demonstrated limited inhibitory effect on CatL or CatB in live macrophages (Supplemental Fig. IIC), showed minimal inhibition on 3T3-L1 cell differentiation (Supplemental Fig. IIIA–F). A role for CatK in 3T3-L1 cell differentiation was further proven by enhanced adipogenesis in cells over-expressing CatK. Using a Fugene-mediated gene transfection, we over-expressed the full-length human CatK in 3T3-L1 cells followed by stimulation of adipogenesis with insulin. As shown in Supplemental Fig. IIIH, a 28-kDa active human CatK and its precursor were detected by CatK immunoblot analysis in CatK-transfected cells, but not in non-transfected or vector-transfected 3T3-L1 cells. Importantly, CatK-transfected 3T3-L1 cells demonstrate significantly more advanced adipogenesis, as reflected by increased accumulation of intracellular oil-red O-positive lipid deposition (Supplemental Fig. IIIG/I), supporting a role of CatK in preadipocyte differentiation.
CatK deficiency reduces body weight gain in mice
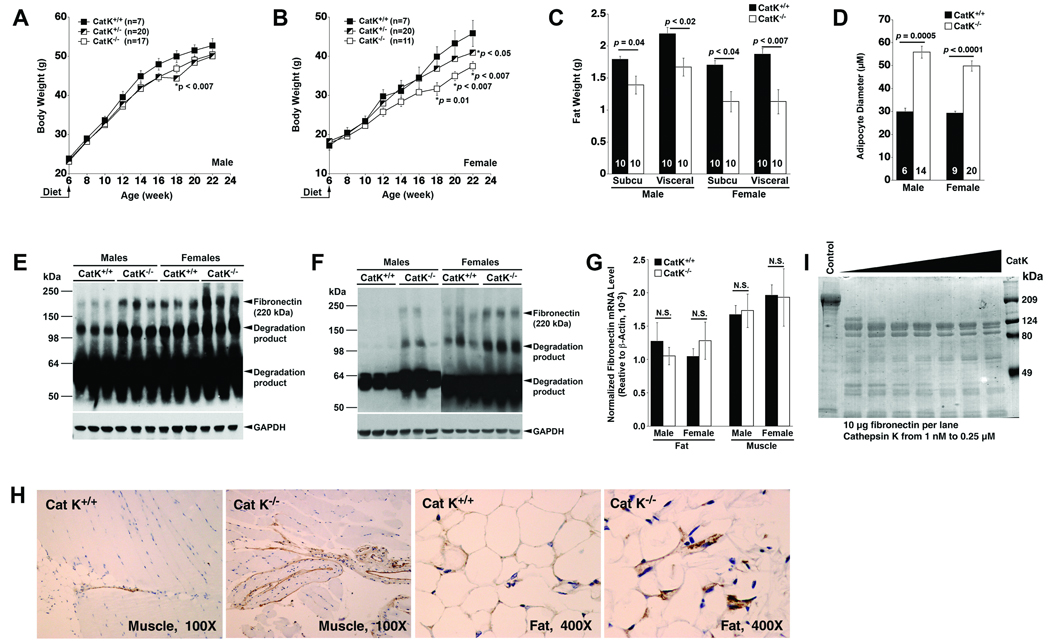
Increased CatK expression in human and mouse adipose tissue could be one of the hallmarks of obesity. To examine the direct participation of CatK in obesity, we fed CatK-deficient (CatK−/−) and heterozygous (CatK+/−) mice a HFD for 16 weeks to induce obesity. Compared with wild-type littermate control (CatK+/+) mice, overall body weight was reduced in male CatK−/− and CatK+/− mice (Fig. 2A), but most data points did not reach statistical significance. However, body weight gain in female CatK−/− mice was significantly reduced compared with that of female CatK+/+ mice at later time points (Fig. 2B). To test whether reduced body weight gain in female CatK−/− mice was due to possible alterations of food/water consumption or metabolic rate, we performed energy expenditure analysis in mice that consumed a HFD by monitoring mouse food consumption (gram/24 hrs), water uptake (ml/24 hrs), O2 consumption (ml/min), CO2 production (ml/min), and metabolic rate (cal/min), and found no significant differences in these parameters between CatK+/+ and CatK−/− mice (Supplemental Table II). Importantly, CatK contributes to body weight gain in a gene dose-dependent manner. CatK+/− mice gained more weight than CatK−/− mice but less than the CatK+/+ mice (Fig. 2B). Consistent with reduced body weight gain, both male and female CatK−/− mice demonstrated significant reduction of total visceral and subcutaneous fat weight compared with those of the CatK+/+ mice, and such reductions were more prominent in females (Fig. 2C). Interestingly, adipocytes in CatK−/− mice visceral fat are bigger than those from CatK+/+ mice (Fig. 2D), suggesting reduced adipocyte contents in CatK−/− mouse adipose tissues. Therefore, lean fat does not necessarily contain small adipocytes. The same is true in collagen VI-null mice, which also gained less body weight but had larger adipocytes than the wild-type controls, although mechanisms behind these phenotypes remain unknown.14
Figure 2. CatK-deficiency reduces mouse body weight gain.
Male (A) and female (B) mouse body weight gain. Visceral and subcutaneous fat weight and adipocyte diameter are presented in C and D. Mouse numbers are indicated in the bars. Fibronectin immunoblots with fat (E) and muscle (F) tissue extracts. GAPDH immunoblot was used for protein loading controls. G. Fibronectin RT-PCR analysis in fat and muscle tissues (NS: not significant). H. Fibronectin immunostaining of mouse muscle and fat paraffin sections. I. In vitro digestion of human fibronectin with CatK.
Prior in vitro studies suggest that CatS promotes adipogenesis by degrading fibronectin,3 an extracellular matrix protein that regulates preadipocyte differentiation.5 When 3T3-L1 cells were cultured on fibronectin-precoated plates, adipogenesis was impaired.4 Therefore, we examined whether reduced body weight gain in CatK−/− mice correlated with higher tissue fibronectin levels. Using immunoblot analysis, we did detect fibronectin accumulation in fat (Fig. 2E) and muscle (Fig. 2F) from CatK−/− mice. Female mice had lower body weight (Fig. 2A/B) and less adipose tissues (Fig. 2C) and higher fibronectin proteins in the fat (Fig. 2E) and muscle (Fig. 2F) compared with those in male mice (Fig. 2A/B/C/E/F). Increased tissue fibronectin levels in CatK−/− mice or female mice were not due to altered gene transcription. RT-PCR analysis did not reveal significant differences of fibronectin transcripts among different genotypes or genders (Fig. 2G). Similar results were obtained from immunohistology analysis. Strong immunogenic fibronectin signals were detected in fat and muscle from CatK−/− mice, but were almost negligible in tissues from the CatK+/+ mice (Fig. 2H). These observations suggest a role of CatK in adipogenesis in part by reducing tissue fibronectin protein levels. To examine this hypothesis in vitro, we digested human plasma fibronectin with recombinant human CatK. Fibronectin digestion with 1~250 nM of CatK yielded 90~110 kDa degradation fragments (Fig. 2I).
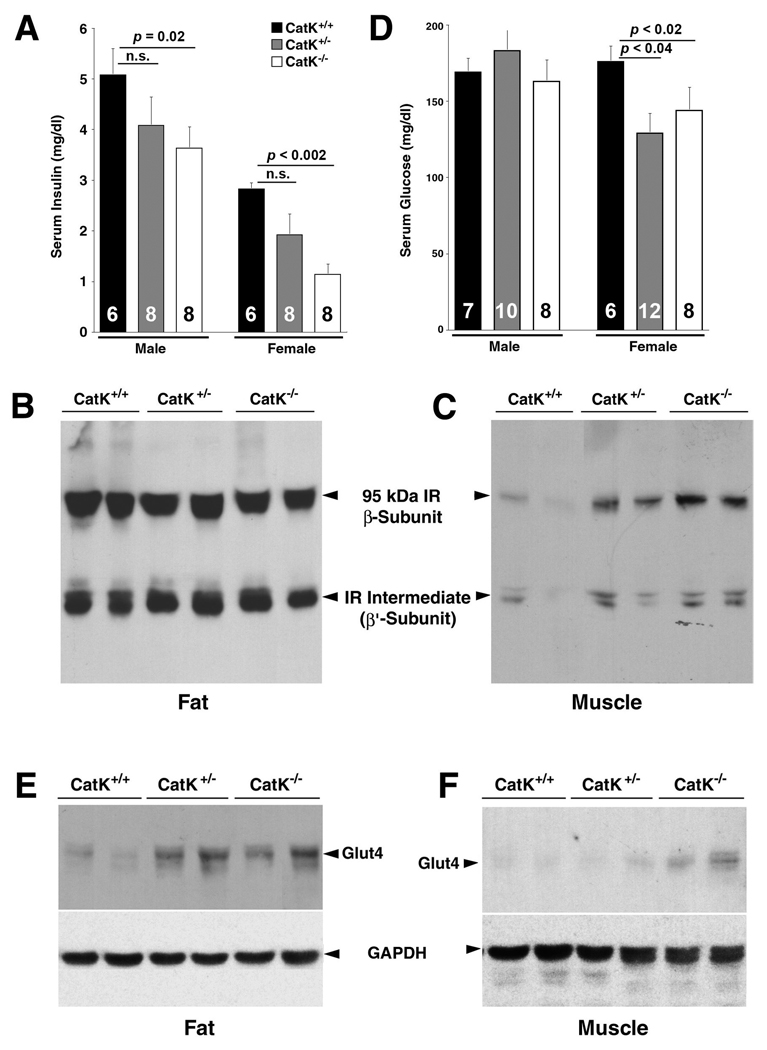
CatK deficiency reduces mouse serum insulin and glucose levels
Visceral obesity is often associated with increased serum glucose and insulin levels.15 Therefore, it is possible that reduced body weight gain in female CatK−/− mice associates with a reduction of serum insulin levels. To examine this possibility, we detected a significant reduction of serum insulin in CatK−/− mice (both males and females) with an insulin ELISA kit. Although lower serum insulin levels were also observed in CatK+/− mice, this reduction did not reach statistical significance (Fig. 3A). It is possible that reduced serum insulin in CatK−/− mice could result from enhanced insulin metabolism, mediated by IR. To test this hypothesis, we performed immunoblot analysis in visceral fat and skeletal muscle from female mice. Although we did not detect differences in IR protein levels in fat from different mice (Fig. 3B), increased levels of IR (95-kDa β-subunit) were detected in muscles from CatK−/− and CatK+/− mice (Fig. 3C), suggesting that IR turnover in the endosomal compartment requires CatK.16
Figure 3. CatK-deficiency reduces serum insulin and glucose and increases muscle and/or fat IR and Glut4.
A. Serum insulin levels. Immunoblot analysis for IR β-subunit in fat (B) and muscle (C) from female mice. D. Serum glucose levels. Immunoblot analysis for Glut4 in fat (E) and muscle (F) from female mice. GAPDH immunoblots were used for protein loading controls.
By measuring fasted mouse serum glucose levels, we detected significant reduction in female CatK−/− (p<0.02) and CatK+/− (p<0.04) mice but not in males (Fig. 3D), indicating improved insulin sensitivity in CatK−/− mice. Muscle has long been considered the major site of insulin-induced glucose uptake in vivo,17 mediated by a family of glucose transporter proteins, which are expressed in specific tissues. Among these proteins, Glut4 is the major glucose transporter isoform in tissues that exhibit insulin-stimulated glucose uptake, such as adipose tissue and skeletal muscle.18 Consistently, both fat and muscle tissues from CatK−/− and CatK+/− mice also contain higher amounts of Glut4 than tissues from CatK+/+ mice (Fig. 3E/F).
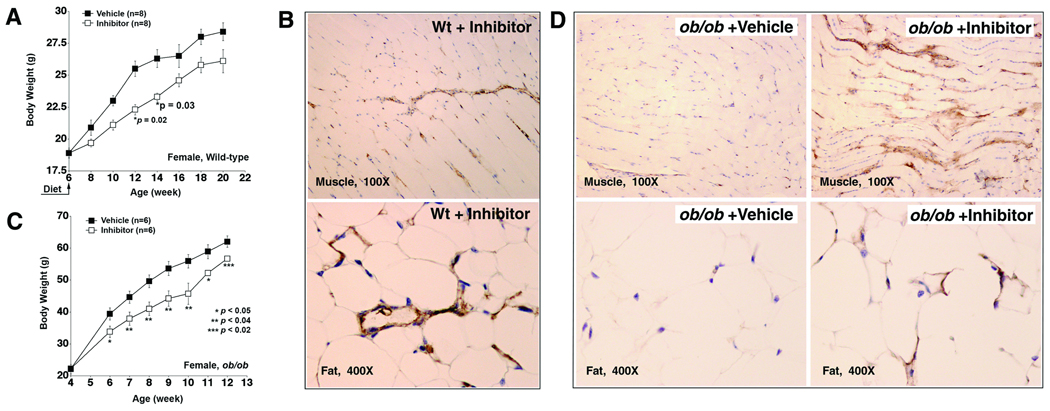
CatK inhibition reduces body weight gain in mice
We tested whether we could reduce mouse body weight gain by selectively inhibiting CatK activity. A recently discovered CatK-selective inhibitor Nα-phenoxybenzyloxycarbonyl-L-leucine(2-phenylaminoethyl)amide (K4b) was prepared according to an established method.19 K4b inhibits preferentially CatK with IC50 <0.006 µM, whereas it inhibits cathepsins S and L with IC50 >10 µM.19 Since significantly reduced body weight gain was observed mainly in female CatK−/− mice (Fig. 2B), we injected female wild-type C57BL/6 mice intraperitoneally with K4b (1 mg/kg/day) and detected reduction of HFD-induced body weight gain, although some time points did not reach statistical significance (Fig. 4A). To examine whether K4b inhibited other major tissue cathepsins under this concentration, we performed cathepsin active site labeling using splenocyte, one of the best tissue cell types expressing spectrum of cathepsins, harvested 6, 12, and 24 hours after K4b injection, which did not reveal obvious inhibition of CatB, CatS, or CatL (not shown). CatK inhibition with K4b also increased the fibronectin levels in skeletal muscle and visceral fat (Fig. 4B). In contrast, fat or muscle from obese wild-type C57BL/6 mice that were injected with vehicle (DMSO) contained negligible fibronectin (not shown).
Figure 4. Pharmacological inhibition of CatK reduces mouse body weight gain and increases tissue fibronectin.
A. Female C57BL/6 mice started consuming a HFD and receiving K4b (1 mg/kg/day) or DMSO at age 6 weeks (indicated). B. Fibronectin immunostaining in muscle and fat paraffin sections from K4b-treated C57BL/6 mice. C. Inhibition of CatK reduces body weight gain in ob/ob mice. D. Fibronectin immunostaining in fat and muscle from vehicle- and K4b-treated ob/ob mice.
Genetically altered ob/ob mice on a chow diet gain body weight consistently. Mice received K4b (1 mg/kg/day) at 4 weeks of age, when wild-type mice and ob/ob mice did not show significant body weight differences. Female ob/ob mice that received K4b demonstrated significant reduction of body weight gain at all time points tested compared with those that received only vehicle (Fig. 4C). In ob/ob mice, inhibition of CatK with K4b also led to accumulations of both muscle and fat fibronectin (Fig. 4D). However, all tested energy expenditure parameters, including food/water intake, O2 consumption, CO2 production, and metabolic rate were not affected in C57BL/6 or ob/ob mice by this compound (not shown).
DISCUSSION
Both CatS and CatK are expressed in adipose tissues from humans and mice.1,2,7 However, increased expression of these proteases can be the result of inflammatory infiltrates, including macrophages and lymphocytes. It is now well accepted that macrophages, rich in cathepsin expression, are recruited to the adipose tissues20 where they further polarize to adipose tissue-specific macrophages.21 In diet-induced obese mice, consumption of a HFD also stimulates adipose tissue chemokine expression for the recruitment of macrophages, lymphocytes, and other inflammatory cells. Recent studies suggest that CatS promotes human preadipocyte differentiation.3 Inhibition of CatS activity delayed adipogenesis. These data are consistent with our finding that CatS and CatK had the greatest induction among all tested cathepsins after human preadipocyte differentiation (Supplemental Table I). Although we cannot exclude the possibility that CatS controls human adipogenesis, we did not see reduced body weight gain from a HFD-fed CatS/low-density lipoprotein receptor (LDLr)12 or CatS/apolipoprotein E (ApoE) (Shi, unpublished data) double mutant mice compared with their wild-type controls. We do not know whether these phenotypes were due to the deficiency of LDLr or ApoE or because CatS expression levels were too low relative to other cathepsins (42~100-fold less than CatB, CatK, and CatL) (Supplemental Table I), or perhaps human CatS acts differently from mouse CatS. These theories remain to be examined.
In this study, we used an in vitro human and murine preadipocyte culture system, genetically altered mice, and pharmacologically treated obese mice to test a hypothesis that CatK participates directly in obesity. Although the exact mechanisms by which CatK participates in adipogenesis or obesity require further investigation, our data suggest that CatK affects at least two molecules that are essential to adipogenesis: fibronectin and IR β-subunit. Participation of fibronectin in adipogenesis has been proposed since the early 1980s.5 Although mice lacking fibronectin are lethal,22 which makes it difficult to test a direct role of fibronectin in obesity, human and murine preadipocytes fail to differentiate on fibronectin pre-coated culture dishes.4 Significant inhibition of adipogenesis was also observed when mouse 3T3-L1 cells were cultured in 5~15 µg/ml of fibronectin (data not shown). In this study, we demonstrated in vitro that CatK degraded 220-kDa fibronectin into 90~110-kDa fragments. Similar size fragments were also detected from fat and muscle tissues from CatK+/+ mice (Fig. 2E/F), suggesting an in situ CatK activity in fibronectin degradation. However, this hypothesis is challenged by the observations of 90~110-kDa fibronectin fragment production in tissues from CatK−/− mice (Fig. 2E/F). Therefore, proteases other than CatK are also involved in native fibronectin proteolysis. Nevertheless, we detected accumulation of fibronectin in fat and muscle from both CatK−/− mice and CatK inhibitor-treated mice (Fig. 2E/F/H, Fig. 4B/D), which may account at least in part for the reduced body weight gain in these mice.
Internalization of insulin-IR complex contributes to insulin clearance23 However, the exact intracellular enzyme(s) responsible for this processing pathway remains unidentified. Prior studies suggested a role of thio-proteases in IR catabolism.24 Despite the observation that the rate of insulin-IR internalization and degradation is reduced in cells from obese patients, the pathological implication of altered IR processing remains unknown. It is anticipated that alteration of IR processing influences insulin action and glucose metabolism. Indeed, obese Zucker fatty rats treated with E64 demonstrated reduced proteolysis of cell membrane IR β-subunits25 and improved the insulin resistance.24 Our observations demonstrated that CatK was one of these thio-proteases responsible for IR β-chain processing, and absence of CatK resulted in accumulation of IR β-chain (Fig. 3C). We also showed that CatK-deficiency reduced insulin and glucose circulating levels (Fig. 3A/D). These changes could be secondary to reduced weight gain in CatK−/− mice. Alternatively, CatK-deficiency could improve insulin action per se, by undefined cellular and molecular mechanisms. Among the potential candidates, increased Glut4 expression in muscles and fat of CatK−/− mice (Fig. 3E/F) could contribute in part to improve insulin effect on glucose utilization.18
Although body weight gain showed no significant differences between male CatK−/− and CatK+/+ mice (Fig. 2A/B), serum insulin levels did (Fig. 3A). In contrast, both body weight gain and serum insulin levels are reduced in female CatK−/− mice, compared with female CatK+/+ mice. Therefore, at least two questions remain. First, why are female mice more sensitive to CatK than male mice in body weight gain? Cathepsins play a role in hormone activation or degradation. For examples, cathepsins produce active forms of thyroid hormones,26 which regulate body weight gain.27 It is possible that obesity-associated hormone activation by CatK is differentially regulated in different genders. Second, why did male CatK−/− and CatK+/+ mice show differences in serum insulin levels (p=0.02) (Fig. 3A) but less so in body weight gain (Fig. 2A)? These observations suggest different mechanisms of body weight gain and insulin secretion and/or clearance, although how CatK involves in these regulations remains unknown.
In conclusion, our data suggest an important participation of cysteine protease CatK in obesity by degrading matrix protein fibronectin. Deficiency and pharmacological inhibition of CatK reduces mouse body weight gain, indicating a potential means of controlling human obesity by targeting CatK activity.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study is partially supported by the grants from the NIH (HL67283, HL60942, HL81090, and HL88547 G.P.S.). INSERM U872 received a grant from the French National Agency of Research (Obcat N° ANR-05-PCOD-026-01) and from the Fondation pour la Recherche Médicale and Danone Institute.
ABBREVIATIONS
- CatK
cathepsin K
- LHVS
N-morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl
- PPIA
peptidylprolyl isomerase A
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- EEF1A
eukaryotic elongation factor 1A
- RPL13A
ribosomal protein L13a
- HFD
high-fat diet
- K4b
Nα-phenoxybenzyloxycarbonyl-L-leucine(2-phenylaminoethyl)amide
- IR
insulin receptor
- Glut4
glucose transporter-4
- LDLr
low-density lipoprotein receptor
- ApoE
apolipoprotein E
Footnotes
DISCLOSURES
G-P. Shi declares a competing interest in NMPI, LLC. All other authors declare no competing financial interests.
REFERENCES
- 1.Taleb S, Lacasa D, Bastard JP, Poitou C, Cancello R, Pelloux V, Viguerie N, Benis A, Zucker JD, Bouillot JL, Coussieu C, Basdevant A, Langin D, Clement K. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J. 2005;19:1540–1542. doi: 10.1096/fj.05-3673fje. [DOI] [PubMed] [Google Scholar]
- 2.Taleb S, Cancello R, Poitou C, Rouault C, Sellam P, Levy P, Bouillot JL, Coussieu C, Basdevant A, Guerre-Millo M, Lacasa D, Clement K. Weight loss reduces adipose tissue cathepsin S and its circulating levels in morbidly obese women. J Clin Endocrinol Metab. 2006;91:1042–1047. doi: 10.1210/jc.2005-1601. [DOI] [PubMed] [Google Scholar]
- 3.Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor KC, Song H, Rosenzweig N, Jansen DA. Extracellular matrix substrata alter adipocyte yield and lipogenesis in primary cultures of stromal-vascular cells from human adipose. Biotechnol Lett. 2003;25:1967–1972. doi: 10.1023/b:bile.0000004386.08923.ab. [DOI] [PubMed] [Google Scholar]
- 5.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Y, Junfeng H, Tianhong L, Lu W, Shulin C, Yu Z, Xiaohua L, Weixia J, Sheng Z, Yanyun G, Guo L, Min L. Cathepsin K in adipocyte differentiation and its potential role in the pathogenesis of obesity. J Clin Endocrinol Metab. 2006;91:4520–4527. doi: 10.1210/jc.2005-2486. [DOI] [PubMed] [Google Scholar]
- 7.Chiellini C, Costa M, Novelli SE, Amri EZ, Benzi L, Bertacca A, Cohen P, Del Prato S, Friedman JM, Maffei M. Identification of cathepsin K as a novel marker of adiposity in white adipose tissue. J Cell Physiol. 2003;195:309–321. doi: 10.1002/jcp.10253. [DOI] [PubMed] [Google Scholar]
- 8.Villadangos JA, Riese RJ, Peters C, Chapman HA, Ploegh HL. Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J Exp Med. 1997;186:549–560. doi: 10.1084/jem.186.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi GP, Dolganov GM. Comprehensive transcriptome of proteases and protease inhibitors in vascular cells. Stroke. 2006;37:537–541. doi: 10.1161/01.STR.0000198816.62266.e9. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, Sukhova GK, Doria A, Katunuma N, Peroni OD, Guerre-Millo M, Kahn BB, Clement K, Shi GP. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 11.Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Pechar M, Li W, Kopeckova P, Bromme D, Kopecek J. Inhibition of cathepsin K with lysosomotropic macromolecular inhibitors. Biochemistry. 2002;41:8849–8859. doi: 10.1021/bi0257080. [DOI] [PubMed] [Google Scholar]
- 14.Scherer PE. Adipose tissue and acute phase response. Molecular control of adipogenesis and obesity. Keystone Symposia, Banff, Alberta, Canada. 2008 Feb 19–24; [Google Scholar]
- 15.Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:3–10. doi: 10.1111/j.1365-2036.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 16.Authier F, Metioui M, Fabrega S, Kouach M, Briand G. Endosomal proteolysis of internalized insulin at the C-terminal region of the B chain by cathepsin D. J Biol Chem. 2002;277:9437–9446. doi: 10.1074/jbc.M110188200. [DOI] [PubMed] [Google Scholar]
- 17.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248:E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 18.Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- 19.Altmann E, Renaud J, Green J, Farley D, Cutting B, Jahnke W. Arylaminoethyl amides as novel non-covalent cathepsin K inhibitors. J Med Chem. 2002;45:2352–2354. doi: 10.1021/jm010801s. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 23.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 24.Knutson VP, Donnelly PV, Balba Y, Lopez-Reyes M. Insulin resistance is mediated by a proteolytic fragment of the insulin receptor. J Biol Chem. 1995;270:24972–24981. doi: 10.1074/jbc.270.42.24972. [DOI] [PubMed] [Google Scholar]
- 25.Trischitta V, Brunetti A, Chiavetta A, Benzi L, Papa V, Vigneri R. Defects in insulin-receptor internalization and processing in monocytes of obese subjects and obese NIDDM patients. Diabetes. 1989;38:1579–1584. doi: 10.2337/diab.38.12.1579. [DOI] [PubMed] [Google Scholar]
- 26.Tepel C, Brömme D, Herzog V, Brix K. Cathepsin K in thyroid epithelial cells: sequence, localization and possible function in extracellular proteolysis of thyroglobulin. J Cell Sci. 2000;113:4487–4498. doi: 10.1242/jcs.113.24.4487. [DOI] [PubMed] [Google Scholar]
- 27.Roti E, Minelli R, Salvi M. Thyroid hormone metabolism in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S113–S115. doi: 10.1038/sj.ijo.0801293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.