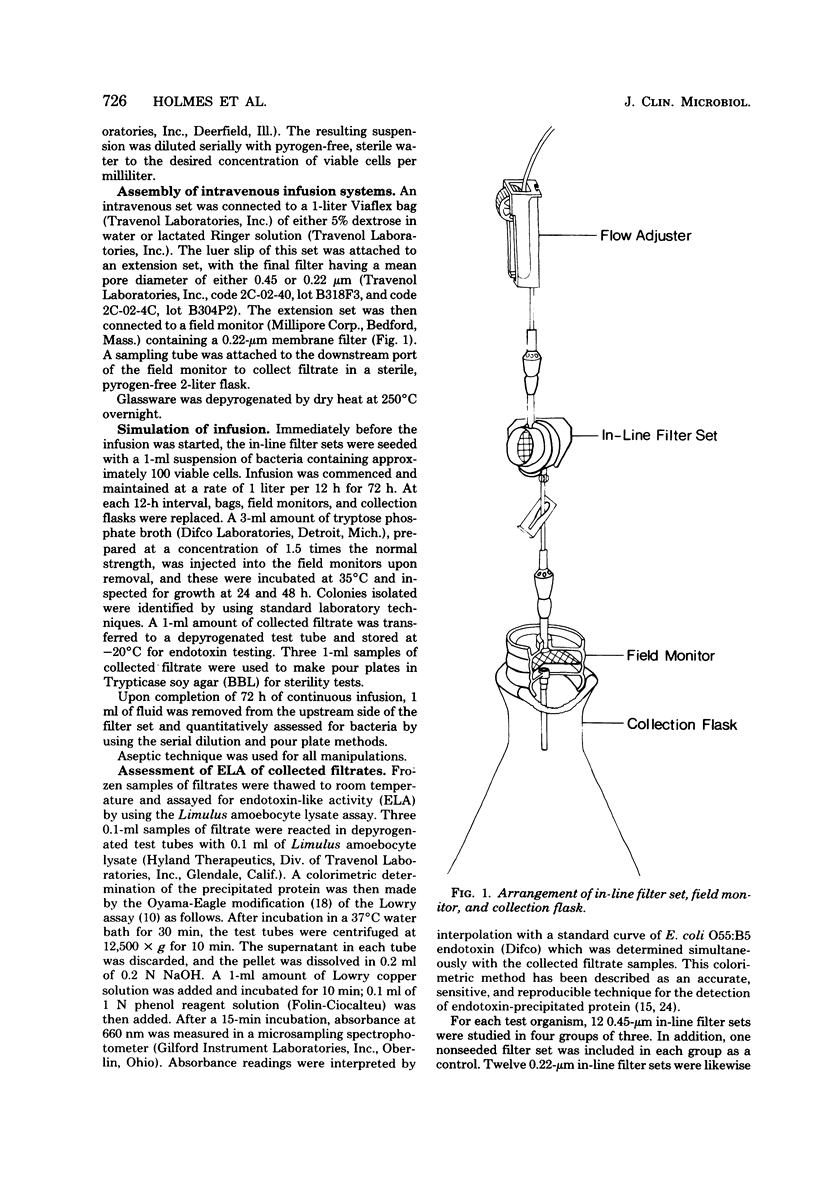
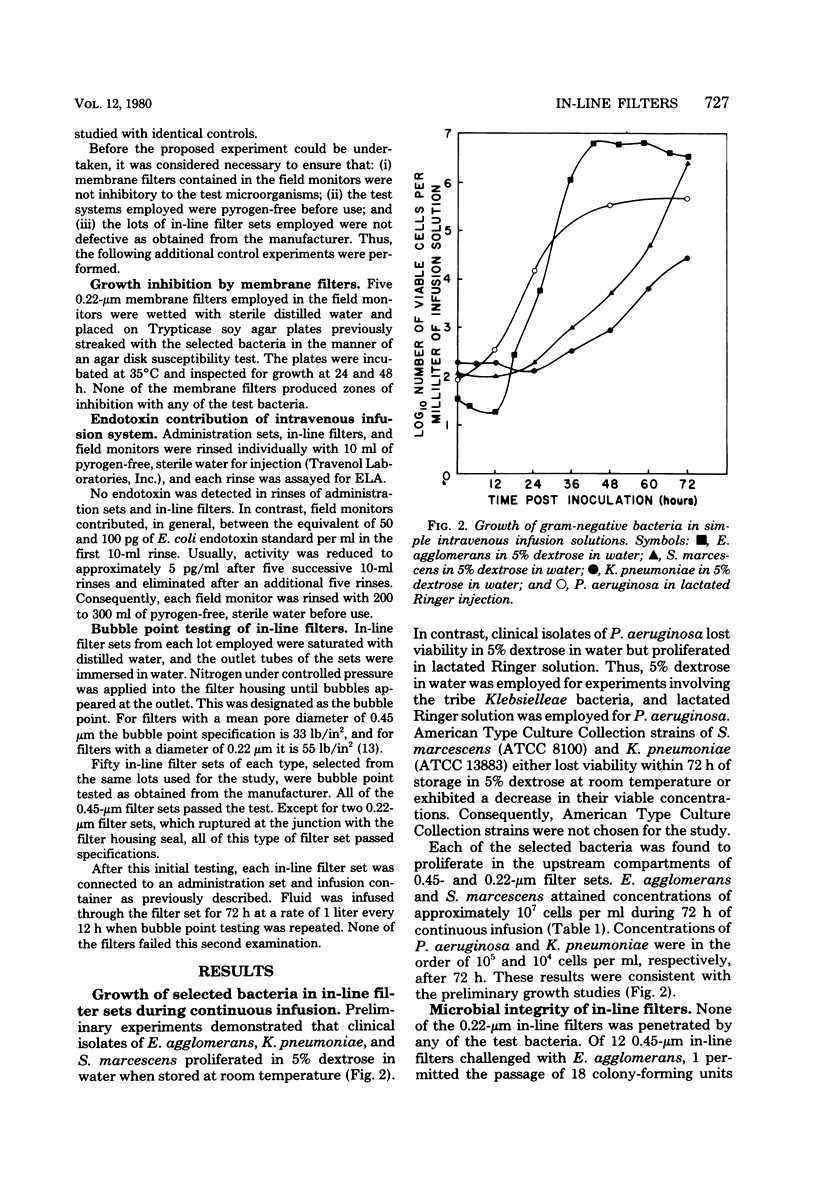
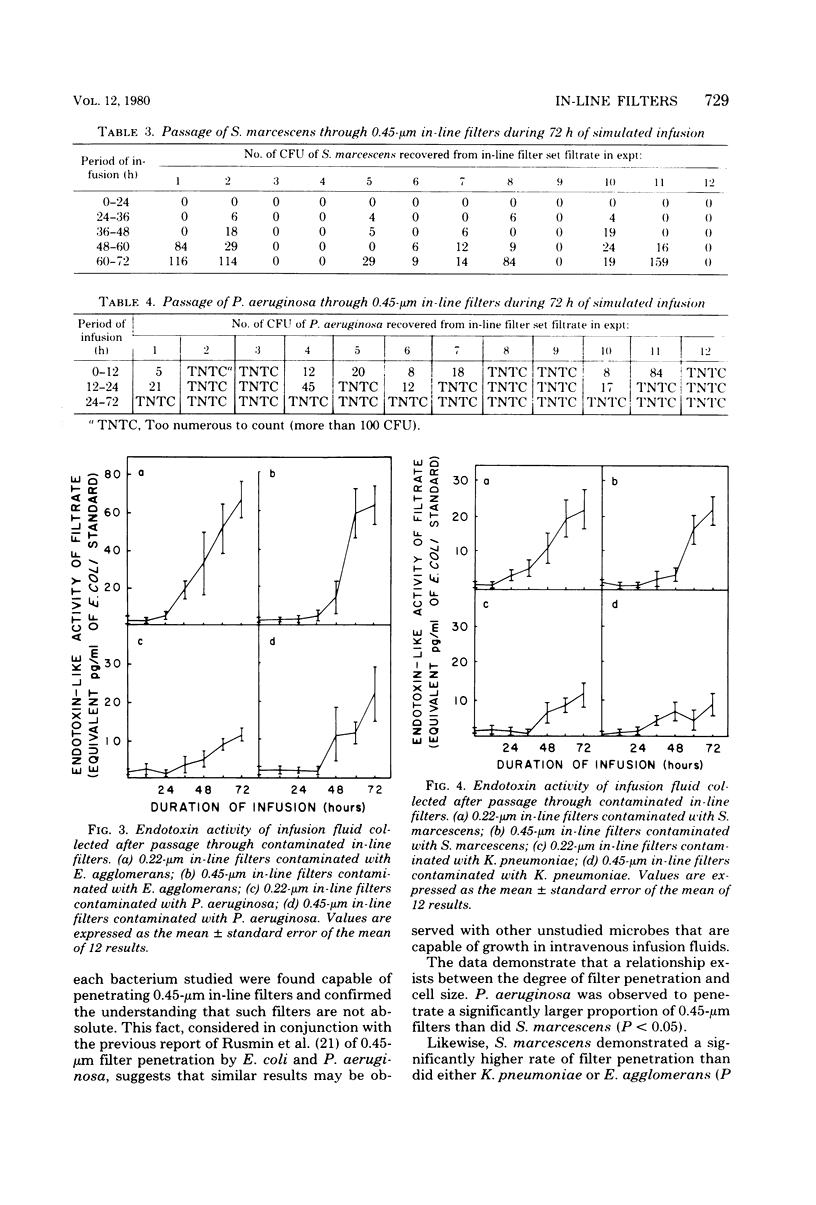
Abstract
The survival and multiplication of Enterobacter agglomerans, Klebsiella pneumoniae, Serratia marcescens, and Pseudomonas aeruginosa in 0.45- and 0.22-micrometer in-line filter sets during simulated infusions were studied to evaluate the ability of each filter type to prevent infusions of these bacteria into patients. Bacteria were found to proliferate in the upstream compartment of sets housing both filter porosities. None of the 0.22-micrometer in-line filters were penetrated by the test bacteria. In contrast, P. aeruginosa was observed to penetrate each 0.45-micrometer in-line filter examined within 12 h of continuous infusion. Tribe Klebsielleae organisms penetrated a proportion of the 0.45-micrometer filters usually between 48 and 72 h of infusion. In addition, the elution of endotoxin from gram-negative bacteria trapped in the filter set during infusion is reported. Collected infusion filtrate exhibited a trend of increasing endotoxin-like activity with an increasing duration of infusion. In the case of E. agglomerans, mean peak levels of approximately 65 pg of Escherichia coli endotoxin per ml were attained after 72 h. Other bacteria produced similar results, except mean peak levels ranged from 5 to 30 pg/ml. It was noted that endotoxin-like activity was not detected in filtrate eluted from contaminated filter sets during the initial 24 h of infusion. We conclude that to avoid potential hazards of bacterial penetration and endotoxin production during continuous use of in-line filter sets, the 0.22-micrometer filter type must be employed and replaced every 24 h.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ERRINGTON F. P., POWELL E. O., THOMPSON N. GROWTH CHARACTERISITICS OF SOME GRAM-NEGATIVE BACTERIA. J Gen Microbiol. 1965 Apr;39:109–123. doi: 10.1099/00221287-39-1-109. [DOI] [PubMed] [Google Scholar]
- Freeman J. B., Litton A. A. Preponderance of gram-positive infections during parenteral alimentation. Surg Gynecol Obstet. 1974 Dec;139(6):905–908. [PubMed] [Google Scholar]
- Guynn J. B., Jr, Poretz D. M., Duma R. J. Growth of various bacteria in a variety of intravenous fluids. Am J Hosp Pharm. 1973 Apr;30(4):321–325. [PubMed] [Google Scholar]
- Kundsin R. B., Walter C. W. Detection of endotoxin on sterile catheters used for cardiac catheterization. J Clin Microbiol. 1980 Mar;11(3):209–212. doi: 10.1128/jcm.11.3.209-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maki D. G., Goldman D. A., Rhame F. S. Infection control in intravenous therapy. Ann Intern Med. 1973 Dec;79(6):867–887. doi: 10.7326/0003-4819-79-6-867. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Grogan J. B. Efficacy of inline bacterial filters in reducing contamination of intravenous nutritional solutions. Am J Surg. 1975 Nov;130(5):585–589. doi: 10.1016/0002-9610(75)90517-6. [DOI] [PubMed] [Google Scholar]
- Nandan R., Brown D. R. An improved in vitro pyrogen test: to detect picograms of endotoxin contamination in intravenous fluids using limulus amoebocyte lysate. J Lab Clin Med. 1977 Apr;89(4):910–918. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Pall D. B. Quality control of absolute bacteria removal filters. Bull Parenter Drug Assoc. 1975 Jul-Aug;29(4):192–204. [PubMed] [Google Scholar]
- Reti A. R., Leahy T. J. Filter validation. III. Validation of bacterially retentive filters by bacterial passage testing. J Parenter Drug Assoc. 1979 Sep-Oct;33(5):257–272. [PubMed] [Google Scholar]
- Rusmin S., Althauser M. B., DeLuca P. P. Consequences of microbial contamination during extended intravenous therapy using inline filters. Am J Hosp Pharm. 1975 Apr;32(4):373–377. [PubMed] [Google Scholar]
- Rusmin S., DeLuca P. P. Effect of antibiotics and osmotic change on the release of endotoxin by bacteria retained on intravenous inline filters. Am J Hosp Pharm. 1975 Apr;32(4):378–380. [PubMed] [Google Scholar]
- Wallhäusser K. H. Is the removal of microorganisms by filtration really a sterilization method? J Parenter Drug Assoc. 1979 May-Jun;33(3):156–170. [PubMed] [Google Scholar]
- Weary M., Baker B. Utilization of the limulus amebocyte lysate test for pyrogen testing large volume parenterals, administration sets, and medical devices. Bull Parenter Drug Assoc. 1977 May-Jun;31(3):127–133. [PubMed] [Google Scholar]
- Wilmore D. W., Dudrick S. J. An in-line filter for intravenous solutions. Arch Surg. 1969 Oct;99(4):462–463. doi: 10.1001/archsurg.1969.01340160042009. [DOI] [PubMed] [Google Scholar]


