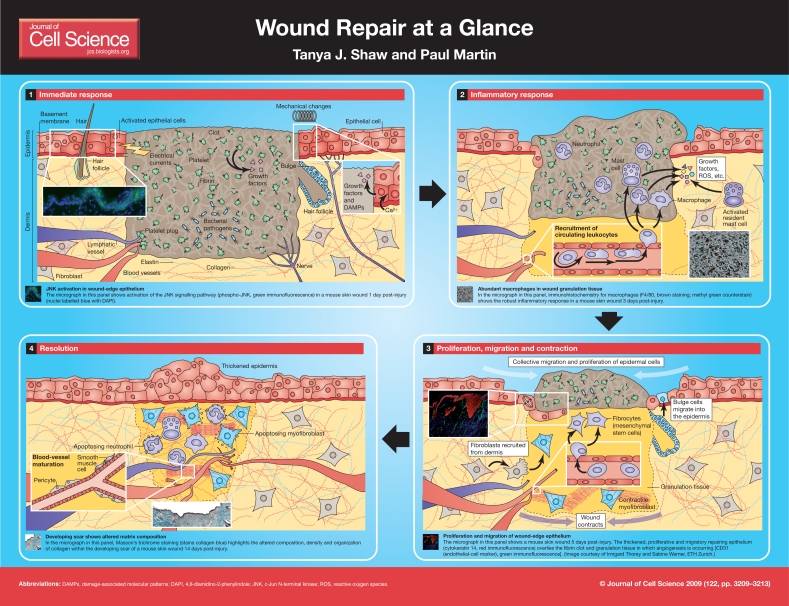
Wound repair is an essential physiological process that is important for tissue homeostasis, but it can be impaired in disease and contributes to numerous pathologies. The wound healing process, particularly in skin, has been well characterised histologically in studies extending back more than 100 years. Skin is a complex tissue (reviewed by Kanitakis, 2002), and thus a `full-thickness' wound results in damage to many structures, cell layers and lineages, including (from the outside in): the epidermal keratinocyte layer (the body's barrier to the outside world), together with associated epidermal appendages such as hair follicles and sweat glands; the basement membrane (BM) that underlies the epidermis; and the dermis, which is an intricate structure that consists of fibroblasts, extracellular matrix (ECM), nerves, and blood and lymphatic vessels. A wound also causes damage at the level of individual cells (McNeil and Kirchhausen, 2005).
Figure 1.
A considerable number of different cell lineages play key roles during the various overlapping phases of the repair process (the immediate response; the inflammatory response; the proliferation, migration and contraction phase; and the resolution phase). Here, we summarise the various cell behaviours and their contributions at the wound site. Significant headway has recently been made in our understanding of the genetic basis of wound healing, partly because of the vast amounts of information that have been obtained from microarray studies in which components of the process have been dissected. This article will refer you to these `gene signatures', and also to more focused reviews about the roles of individual cell types in the overall wound-healing process.
The immediate response
The immediate response to skin wounding (see poster, panel 1) is a consequence of a barrage of damage signals, which can be both mechanical and chemical. Cells at the edge of a wound are ruptured, as are vessels in the wound vicinity. Damaged and stressed cells respond by activating several `stress signal' pathways within minutes. For example, signaling via the SAPK/JNK and p38 pathways (Kobayashi et al., 2003; Yano et al., 2004) results in phosphorylation of a cascade of signaling molecules, which ultimately culminates in cellular changes including alterations in gene expression, cell survival and metabolism. The same cells leak endogenous molecules, including damage-associated molecular pattern molecules (DAMPs), which might act as activation cues and/or chemotactic factors for other cells in the area (Bianchi, 2007).
One of the earliest responses to injury stems from the damage that is caused to local blood vessels. It is necessary to stop local hemorrhage immediately, and this is achieved by platelet activation and aggregation, which results in formation of a fibrin clot consisting of a network of insoluble fibrin fibers. As well as plugging vessels, the clot acts as a provisional matrix to which growth factors bind and through which cells can crawl (Nurden et al., 2008). Activated platelets are themselves an important source of growth factors [such as platelet-derived growth factor, CXCL4, basic fibroblast growth factor, transforming growth factor-β (TGFβ), vascular endothelial growth factor and RANTES (Bahou and Gnatenko, 2004)] and are known to promote various aspects of the repair process through this growth factor release, including angiogenesis, inflammation, and migration of both keratinocytes and fibroblasts.
In addition to the platelet-derived growth factors, cells are bathed in other serum factors. Serum, the fluid component of clotted blood, contains many interleukins, colony-stimulating factors, tumour necrosis factor-α, interferon-γ and other components that together lead to induction of serum response factor (SRF). SRF binds to and induces transcription of immediate early and other genes [such as fos, jun and early growth response genes (egr-1 and egr-2) (Chai and Tarnawski, 2002; Grose et al., 2002)]. Transcriptional profiling of in vivo wounds (Cole et al., 2001; Cooper et al., 2005; Deonarine et al., 2007; Roy et al., 2008), and of fibroblasts exposed to serum in vitro (Iyer et al., 1999), reveals an immediate response to wounding in which upwards of several hundred genes are up- or downregulated within an hour of damage.
Several other cues that might influence wound cells in vivo include mechanical signals such as `stretch', which occurs in response to changing tissue tensions (Kippenberger et al., 2000); electrical currents that result from membrane damage and breaks in the epithelial barrier (Nuccitelli et al., 2008); and exposure to various microorganisms whose epitopes are largely recognized by Toll-like receptors (TLRs) (Shaykhiev et al., 2008). Activation of epithelial cell TLRs triggers the expression and release of pro-inflammatory mediators and anti-microbial peptides.
The inflammatory response
The inflammatory response to wounding (see poster, panel 2) begins immediately with the passive leakage of circulating leukocytes (largely neutrophils) from damaged blood vessels into the wound. There is also rapid activation of immune cells that are already resident within the tissue [such as mast cells (Noli and Miolo, 2001), γδ T cells (Jameson et al., 2004) and Langerhans cells (Cumberbatch et al., 2000)], which in turn release a rapid pulse of chemokines and cytokines. The inflammatory response continues with active recruitment of neutrophils and then macrophages from nearby vessels, which is orchestrated by growth factor signals from the resident cells and serum, and by foreign epitopes such as the lipopolysaccharides (LPS) of invading microorganisms (Eming et al., 2007). Together, these signals trigger local endothelial cell `activation' and thus expression of selectins. Selectins control the rolling and then tethering of leukocytes to the vessel wall and subsequent crossing of the endothelial barrier (Yukami et al., 2007). This is enhanced by vessel dilation and an increase in vascular permeability that is triggered by inflammation-associated nitric oxide (NO), mast cell-derived histamine, tissue plasminogen activator and other factors (Eming et al., 2007).
The role of inflammatory cells
Neutrophils are activated and recruited to a wound within minutes (Kim et al., 2008). They have an important cleansing role and kill invading microorganisms through several strategies, including bursts of reactive oxygen species (ROS) (Dovi et al., 2004). Profiling of the genes whose expression is induced in neutrophils upon recruitment to a wound hints that these cells also influence many other aspects of repair, such as resolution of the fibrin clot and provisional ECM, promotion of angiogenesis, and re-epithelialization (Theilgaard-Monch et al., 2004).
Monocytes are drawn from the circulation somewhat later than neutrophils, and their numbers peak a day or so after injury (Mori et al., 2008). Monocytes mature into macrophages once they leave the circulation and `turn on' distinct gene expression and behavioural programs according to their surroundings and stimuli (Martinez et al., 2006). At the wound site, they are professional phagocytes and clear up matrix and cell debris, including spent neutrophils (Eming et al., 2007). Close analysis of the temporal profiles of macrophage-specific genes suggests that both classically activated (M1, pro-inflammatory) and alternatively activated (M2, anti-inflammatory and pro-angiogenic) macrophages are present early in repair, whereas M2 macrophages predominate later in repair (Deonarine et al., 2007).
In addition to the release of cytokines and growth factors, inflammatory cells exert their influence on the surrounding tissue by generating NO and large amounts of ROS (Schafer and Werner, 2008). NO and ROS are known to drive certain aspects of repair (Sen and Roy, 2008) but, in response to these stressors, affected wound cells must induce cytoprotective and detoxifying programs (Schafer and Werner, 2008). A number of microarray studies have compared gene signatures in wounds with and without inflammation (Cooper et al., 2005; Deonarine et al., 2007; Roy et al., 2008). These studies have been valuable for identifying genes expressed by the inflammatory cells themselves, as well as the downstream consequences of inflammation within a wound setting. Although the issue of whether inflammatory cells are an essential requirement for repair remains controversial (Martin and Leibovich, 2005), it is clear that these cell populations exert a profound influence on all other cells within the wound and in the surrounding tissue. For example, one of the important roles of inflammatory cytokines is to regulate angiogenesis, which they accomplish in concert with signals from other wound cells and from serum (Tonnesen et al., 2000).
Angiogenesis and lymphangiogenesis
Angiogenesis, which is integral to successful wound repair, involves sprouting of wound-edge capillaries followed by their invasion into the site of damage. After a few days, a microvascular network is apparent throughout the wound (Tonnesen et al., 2000), which provides nutrients and oxygen to the growing tissues and aids in the formation of the provisional wound matrix, also known as granulation tissue (described below). The complex response of blood vessels to damage has been studied using microarray analysis, with a comparison of gene expression profiles of blood vessels in intact and wounded skin (Roy et al., 2007).
Fluid accumulation is a common aspect of tissue damage and results from local vasodilation, increased vascular permeability and/or damage to lymphatic vessels (Adams and Alitalo, 2007). Lymphangiogenesis (repair of lymph vessels) has been studied much less than angiogenesis, but is likewise under tight regulation by a plethora of similar, as well as some unique, growth factors (Adams and Alitalo, 2007; Karkkainen et al., 2004).
Proliferation, migration and contraction
The clot, or scab, that forms during the immediate response to an insult is a temporary mechanism for restoring the function of the skin as a protective barrier. The next phase depicted in this poster (panel 3), which we have called `Proliferation, migration and contraction', involves the actions taken by cells within a wound to achieve permanent closure of the wound gap and replenishment of lost tissue. These processes initiate within hours, but the time required to heal is highly variable and depends on the size and location of the wound as well as on the age and health of the tissue. A small incisional skin wound on a neonatal mouse can heal completely in less than a day, whereas in aged or diabetic humans, the repair process can take weeks or months.
Epidermis
Re-epithelialization of a wound by keratinocytes is achieved by a combination of migration and proliferation of cells in the vicinity of the damage (Martin, 1997; Matoltsy and Viziam, 1970). Generally, the advancing cells of the wound epidermis migrate collectively (Ilina and Friedl, 2009) after undergoing a number of changes to facilitate their movement. Firstly, they alter cell-cell and cell-matrix adhesions to enable their progression from a laminin-V- and collagen-IV-rich BM onto and through provisional matrix substrates that constitute the clot, such as dermal collagen and fibronectin (Nguyen et al., 2000). Migrating cells also assemble actin-rich lamellar protrusions for crawling (Mitchison and Cramer, 1996), and upregulate the expression of proteolytic enzymes such as matrix metalloproteinases (Pilcher et al., 1999). This enables them to bore a pathway at the interface between the scab and viable tissue. In vitro scratch wound assays of human keratinocytes, although not a perfect model of in vivo re-epithelialization, reveal a gene profile from microarray analysis that progresses our understanding of this aspect of skin wound healing (Cheng et al., 2008; Dayem et al., 2003; Fitsialos et al., 2007).
The regenerating epidermis also draws upon stem cells that are resident in the epidermis and the bulge region of hair follicles (Fuchs, 2008). In response to injury, bulge stem cells commit to an epidermal phenotype, and then migrate into the epidermis to participate in the repair process (see poster, panel 3) (Ito et al., 2005).
Dermis
In addition to the healing epidermis, the underlying dermis must also be reconstituted. The new stroma that replaces the fibrin clot is called granulation tissue and is contributed to by fibroblasts that are drawn from several sources: primarily the healthy dermis at the wound margins, from which fibroblasts can divide and migrate (Hinz, 2007); circulating fibrocytes; and bone marrow progenitor cells (Abe et al., 2001). Fibroblasts can also be recruited from multipotent cells that are resident in the dermis and have the potential to differentiate into dermal fibroblasts (Fernandes et al., 2004; Toma et al., 2001) and/or from pericytes that are associated with nearby blood vessels (Rajkumar et al., 2006). Fibroblasts in close proximity to the wound respond by forming stress fibres (weakly contractile actin bundles), which enable some connective tissue contraction. The contractility of the bundles is greatly enhanced, however, when the cells are driven to differentiate into α-smooth muscle actin-expressing myofibroblasts by the action of growth factors (such as TGFβ1), the ECM and/or mechanical stress (Hinz, 2007). Together, wound fibroblasts and myofibroblasts help to draw the wound closed, and contribute to the synthesis, bundling and alignment of collagen fibres (Hinz, 2007), the primary constituent of scar tissue. In the future, information about the gene signatures of these cells might be obtained by mining gene expression analyses of other fibrotic conditions in which wound fibroblasts and myofibroblasts are significant players, such as pulmonary fibrosis (Brass et al., 2008).
Resolution
The resolution phase of wound repair (see poster, panel 4) is essential for restoration of full functionality and a `normal' appearance to the injured tissue. Migrating and proliferating keratinocytes at the wound edge confront one another as the wound seals, and must then stop and subsequently restratify. Our group (Cooper et al., 2005) temporally dissected the wound transcriptome in order to gain insight into how this `stop phase' is regulated; however, more work is needed to understand the molecules and signalling pathways involved in the contact inhibition and epithelial fusion that occurs as cells from the two epidermal wound fronts confront one another.
Simply reforming a continuous epidermal sheet does not, however, return the tissue to its pre-wound state, because epidermal appendages such as hair follicles and sebaceous glands also need to regenerate. Appendages do not readily develop within a scar, but it has been reported that inflammation can promote appendage re-growth (Osaka et al., 2007), and this is thought to require re-enactment of the epidermal developmental program, in which Wnt signalling has a pivotal role (Ito et al., 2007).
During wound resolution, many changes also occur in the dermis. The blood vessels within the scar are refined, and mature to form a functional network (Adams and Alitalo, 2007). The dense ECM that was haphazardly deposited early in repair is remodelled. This remodeling, which aims to restore normal architecture to the dermis, is accomplished by a delicate balance of collagen synthesis, bundling and degradation (for example, by matrix metalloproteinases). Moreover, most myofibroblasts undergo apoptosis at this stage (Hinz, 2007; Ulrich et al., 2007).
The inflammatory response to wounding also resolves once healing is complete. Neutrophils are cleared from the wound site, at least in part by apoptosis and subsequent phagocytosis by macrophages (Haslett, 1992). Some neutrophils and macrophages return to the vasculature, as observed in zebrafish larval wounds (Mathias et al., 2006), and/or emigrate via lymphatic vessels (Cao et al., 2005; Schwab et al., 2007). Macrophages are thought to be deactivated by anti-inflammatory cytokines, glucocorticosteroids, cell-cell contact or phagocytosis (Ma et al., 2003). Other strategies in place to dampen inflammation include sequestration of pro-inflammatory chemokines by non-functional `decoy' receptors on the inflammatory cells themselves (D'Amico et al., 2000) and production of endogenous anti-inflammatory molecules such as resolvin E (Schwab et al., 2007) and annexin I (Perretti and Gavins, 2003).
Imperfect regulation of wound resolution can result in hyperproliferation, persistence of an inflammatory reaction and, consequently, fibrosis and excessive scar formation, all of which can contribute to numerous pathologies (Coussens and Werb, 2002; Wynn, 2008). We and others suggest that epigenetic mechanisms (such as histone modifications and microRNA-based regulation) contribute both to the concerted induction of the `repair transcriptome' by wound-edge cells and to the subsequent repression that is so clinically important (Shaw and Martin, 2009; Shilo et al., 2007).
Conclusion
This poster article briefly illustrates and describes the complex process of wound repair. We refer you to a number of recent microarray studies that provide transcriptional information about specific aspects of the process. On the basis of these publications, as well as the first reported proteomic analyses of wound exudates (Fernandez et al., 2008; Huang et al., 2006), it is evident that the wound milieu contains many potentially novel proteins that influence wound repair. Studies using transgenic and knockout mice, as well as other strategies such as topical treatment of wounds with ligands and/or inhibitors, have already confirmed that a plethora of factors influence the migration and proliferation of epidermal and dermal cells, and wound contraction. Validation and substantiation of the array findings should bring us one step closer to fully understanding the molecular intricacies of each phase of the repair process. Such an understanding will be clinically valuable, and could lead to treatments that increase the rate and quality of normal tissue repair, and that prevent or reverse the adverse consequences of improper wound resolution.
Supplementary Material
The authors thank Ryoichi Mori (Nagasaki University, Japan), Eva Polk (University of Bristol, UK), Irmgard Thorey and Sabine Werner (ETH Zurich, Switzerland) for images. Funding for work performed in our laboratory is from Cancer Research UK, the Wellcome Trust and the MRC. Deposited in PMC for release after 6 months.
References
- Abe, R., Donnelly, S. C., Peng, T., Bucala, R. and Metz, C. N. (2001). Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 166, 7556-7562. [DOI] [PubMed] [Google Scholar]
- Adams, R. H. and Alitalo, K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464-478. [DOI] [PubMed] [Google Scholar]
- Bahou, W. F. and Gnatenko, D. V. (2004). Platelet transcriptome: the application of microarray analysis to platelets. Semin. Thromb. Hemost. 30, 473-484. [DOI] [PubMed] [Google Scholar]
- Bianchi, M. E. (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1-5. [DOI] [PubMed] [Google Scholar]
- Brass, D. M., Yang, I. V., Kennedy, M. P., Whitehead, G. S., Rutledge, H., Burch, L. H. and Schwartz, D. A. (2008). Fibroproliferation in LPS-induced airway remodeling and bleomycin-induced fibrosis share common patterns of gene expression. Immunogenetics 60, 353-369. [DOI] [PubMed] [Google Scholar]
- Cao, C., Lawrence, D. A., Strickland, D. K. and Zhang, L. (2005). A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106, 3234-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, J. and Tarnawski, A. S. (2002). Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J. Physiol. Pharmacol. 53, 147-157. [PubMed] [Google Scholar]
- Cheng, C. F., Fan, J., Bandyopahdhay, B., Mock, D., Guan, S., Chen, M., Woodley, D. T. and Li, W. (2008). Profiling motility signal-specific genes in primary human keratinocytes. J. Invest. Dermatol. 128, 1981-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J., Tsou, R., Wallace, K., Gibran, N. and Isik, F. (2001). Early gene expression profile of human skin to injury using high-density cDNA microarrays. Wound Repair Regen. 9, 360-370. [DOI] [PubMed] [Google Scholar]
- Cooper, L., Johnson, C., Burslem, F. and Martin, P. (2005). Wound healing and inflammation genes revealed by array analysis of `macrophageless' PU.1 null mice. Genome Biol. 6, R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens, L. M. and Werb, Z. (2002). Inflammation and cancer. Nature 420, 860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch, M., Dearman, R. J., Griffiths, C. E. and Kimber, I. (2000). Langerhans cell migration. Clin. Exp. Dermatol. 25, 413-418. [DOI] [PubMed] [Google Scholar]
- D'Amico, G., Frascaroli, G., Bianchi, G., Transidico, P., Doni, A., Vecchi, A., Sozzani, S., Allavena, P. and Mantovani, A. (2000). Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat. Immunol. 1, 387-391. [DOI] [PubMed] [Google Scholar]
- Dayem, M. A., Moreilhon, C., Turchi, L., Magnone, V., Christen, R., Ponzio, G. and Barbry, P. (2003). Early gene expression in wounded human keratinocytes revealed by DNA microarray analysis. Comp. Funct. Genomics 4, 47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonarine, K., Panelli, M. C., Stashower, M. E., Jin, P., Smith, K., Slade, H. B., Norwood, C., Wang, E., Marincola, F. M. and Stroncek, D. F. (2007). Gene expression profiling of cutaneous wound healing. J. Transl. Med. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovi, J. V., Szpaderska, A. M. and DiPietro, L. A. (2004). Neutrophil function in the healing wound: adding insult to injury? Thromb. Haemost. 92, 275-280. [DOI] [PubMed] [Google Scholar]
- Eming, S. A., Krieg, T. and Davidson, J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514-525. [DOI] [PubMed] [Google Scholar]
- Fernandes, K. J., McKenzie, I. A., Mill, P., Smith, K. M., Akhavan, M., Barnabe-Heider, F., Biernaskie, J., Junek, A., Kobayashi, N. R., Toma, J. G. et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 6, 1082-1093. [DOI] [PubMed] [Google Scholar]
- Fernandez, M. L., Broadbent, J. A., Shooter, G. K., Malda, J. and Upton, Z. (2008). Development of an enhanced proteomic method to detect prognostic and diagnostic markers of healing in chronic wound fluid. Br. J. Dermatol. 158, 281-290. [DOI] [PubMed] [Google Scholar]
- Fitsialos, G., Chassot, A. A., Turchi, L., Dayem, M. A., LeBrigand, K., Moreilhon, C., Meneguzzi, G., Busca, R., Mari, B., Barbry, P. et al. (2007). Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J. Biol. Chem. 282, 15090-15102. [DOI] [PubMed] [Google Scholar]
- Fuchs, E. (2008). Skin stem cells: rising to the surface. J. Cell Biol. 180, 273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose, R., Harris, B., Cooper, L., Topilko, P. and Martin, P. (2002). The immediate early genes krox-24 and krox-20 are rapidly upregulated following wounding in the embryonic and adult mouse. Dev. Dynamics 223, 371-378. [DOI] [PubMed] [Google Scholar]
- Haslett, C. (1992). Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin. Sci. (Lond.) 83, 639-648. [DOI] [PubMed] [Google Scholar]
- Hinz, B. (2007). Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127, 526-537. [DOI] [PubMed] [Google Scholar]
- Huang, C. M., Wang, C. C., Barnes, S. and Elmets, C. A. (2006). In vivo detection of secreted proteins from wounded skin using capillary ultrafiltration probes and mass spectrometric proteomics. Proteomics 6, 5805-5814. [DOI] [PubMed] [Google Scholar]
- Ilina, O. and Friedl, P. (2009). Mechanisms of collective cell migration at a glance. J. Cell Sci. 122, 3203-3208. [DOI] [PubMed] [Google Scholar]
- Ito, M., Liu, Y., Yang, Z., Nguyen, J., Liang, F., Morris, R. J. and Cotsarelis, G. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351-1354. [DOI] [PubMed] [Google Scholar]
- Ito, M., Yang, Z., Andl, T., Cui, C., Kim, N., Millar, S. E. and Cotsarelis, G. (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316-320. [DOI] [PubMed] [Google Scholar]
- Iyer, V. R., Eisen, M. B., Ross, D. T., Schuler, G., Moore, T., Lee, J. C., Trent, J. M., Staudt, L. M., Hudson, J., Jr, Boguski, M. S. et al. (1999). The transcriptional program in the response of human fibroblasts to serum. Science 283, 83-87. [DOI] [PubMed] [Google Scholar]
- Jameson, J. M., Sharp, L. L., Witherden, D. A. and Havran, W. L. (2004). Regulation of skin cell homeostasis by gamma delta T cells. Front. Biosci. 9, 2640-2651. [DOI] [PubMed] [Google Scholar]
- Kanitakis, J. (2002). Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 12, 390-399. [PubMed] [Google Scholar]
- Karkkainen, M. J., Haiko, P., Sainio, K., Partanen, J., Taipale, J., Petrova, T. V., Jeltsch, M., Jackson, D. G., Talikka, M., Rauvala, H. et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74-80. [DOI] [PubMed] [Google Scholar]
- Kim, M. H., Liu, W., Borjesson, D. L., Curry, F. R., Miller, L. S., Cheung, A. L., Liu, F. T., Isseroff, R. R. and Simon, S. I. (2008). Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Invest. Dermatol. 128, 1812-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenberger, S., Bernd, A., Loitsch, S., Guschel, M., Muller, J., Bereiter-Hahn, J. and Kaufmann, R. (2000). Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Invest. Dermatol. 114, 408-412. [DOI] [PubMed] [Google Scholar]
- Kobayashi, H., Aiba, S., Yoshino, Y. and Tagami, H. (2003). Acute cutaneous barrier disruption activates epidermal p44/42 and p38 mitogen-activated protein kinases in human and hairless guinea pig skin. Exp. Dermatol. 12, 734-746. [DOI] [PubMed] [Google Scholar]
- Ma, J., Chen, T., Mandelin, J., Ceponis, A., Miller, N. E., Hukkanen, M., Ma, G. F. and Konttinen, Y. T. (2003). Regulation of macrophage activation. Cell Mol. Life Sci. 60, 2334-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P. (1997). Wound healing-aiming for perfect skin regeneration. Science 276, 75-81. [DOI] [PubMed] [Google Scholar]
- Martin, P. and Leibovich, S. J. (2005). Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599-607. [DOI] [PubMed] [Google Scholar]
- Martinez, F. O., Gordon, S., Locati, M. and Mantovani, A. (2006). Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303-7311. [DOI] [PubMed] [Google Scholar]
- Mathias, J. R., Perrin, B. J., Liu, T. X., Kanki, J., Look, A. T. and Huttenlocher, A. (2006). Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281-1288. [DOI] [PubMed] [Google Scholar]
- Matoltsy, A. G. and Viziam, C. B. (1970). Further observations on epithelialization of small wounds: an autoradiographic study of incorporation and distribution of 3H-thymidine in the epithelium covering skin wounds. J. Invest. Dermatol. 55, 20-25. [DOI] [PubMed] [Google Scholar]
- McNeil, P. L. and Kirchhausen, T. (2005). An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6, 499-505. [DOI] [PubMed] [Google Scholar]
- Mitchison, T. J. and Cramer, L. P. (1996). Actin-based cell motility and cell locomotion. Cell 84, 371-379. [DOI] [PubMed] [Google Scholar]
- Mori, R., Shaw, T. J. and Martin, P. (2008). Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 205, 43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, B. P., Gil, S. G. and Carter, W. G. (2000). Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J. Biol. Chem. 275, 31896-31907. [DOI] [PubMed] [Google Scholar]
- Noli, C. and Miolo, A. (2001). The mast cell in wound healing. Vet. Dermatol. 12, 303-313. [DOI] [PubMed] [Google Scholar]
- Nuccitelli, R., Nuccitelli, P., Ramlatchan, S., Sanger, R. and Smith, P. J. (2008). Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 16, 432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden, A. T., Nurden, P., Sanchez, M., Andia, I. and Anitua, E. (2008). Platelets and wound healing. Front. Biosci. 13, 3532-3548. [DOI] [PubMed] [Google Scholar]
- Osaka, N., Takahashi, T., Murakami, S., Matsuzawa, A., Noguchi, T., Fujiwara, T., Aburatani, H., Moriyama, K., Takeda, K. and Ichijo, H. (2007). ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J. Cell Biol. 176, 903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti, M. and Gavins, F. N. (2003). Annexin 1, an endogenous anti-inflammatory protein. News Physiol. Sci. 18, 60-64. [DOI] [PubMed] [Google Scholar]
- Pilcher, B. K., Wang, M., Qin, X. J., Parks, W. C., Senior, R. M. and Welgus, H. G. (1999). Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. NY Acad. Sci. 878, 12-24. [DOI] [PubMed] [Google Scholar]
- Rajkumar, V. S., Shiwen, X., Bostrom, M., Leoni, P., Muddle, J., Ivarsson, M., Gerdin, B., Denton, C. P., Bou-Gharios, G., Black, C. M. et al. (2006). Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 169, 2254-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., Patel, D., Khanna, S., Gordillo, G. M., Biswas, S., Friedman, A. and Sen, C. K. (2007). Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc. Natl. Acad. Sci. USA 104, 14472-14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., Khanna, S., Rink, C., Biswas, S. and Sen, C. K. (2008). Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol. Genomics 34, 162-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, M. and Werner, S. (2008). Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 58, 165-171. [DOI] [PubMed] [Google Scholar]
- Schwab, J. M., Chiang, N., Arita, M. and Serhan, C. N. (2007). Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, C. K. and Roy, S. (2008). Redox signals in wound healing. Biochim. Biophys. Acta 1780, 1348-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, T. and Martin, P. (2009). Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep. 10, 881-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev, R., Behr, J. and Bals, R. (2008). Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS ONE 3, e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo, S., Roy, S., Khanna, S. and Sen, C. K. (2007). MicroRNA in cutaneous wound healing: a new paradigm. DNA Cell Biol. 26, 227-237. [DOI] [PubMed] [Google Scholar]
- Theilgaard-Monch, K., Knudsen, S., Follin, P. and Borregaard, N. (2004). The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 172, 7684-7693. [DOI] [PubMed] [Google Scholar]
- Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. and Miller, F. D. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778-784. [DOI] [PubMed] [Google Scholar]
- Tonnesen, M. G., Feng, X. and Clark, R. A. (2000). Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5, 40-46. [DOI] [PubMed] [Google Scholar]
- Ulrich, M. M., Verkerk, M., Reijnen, L., Vlig, M., van den Bogaerdt, A. J. and Middelkoop, E. (2007). Expression profile of proteins involved in scar formation in the healing process of full-thickness excisional wounds in the porcine model. Wound Repair Regen. 15, 482-490. [DOI] [PubMed] [Google Scholar]
- Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, S., Komine, M., Fujimoto, M., Okochi, H. and Tamaki, K. (2004). Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J. Invest. Dermatol. 122, 783-790. [DOI] [PubMed] [Google Scholar]
- Yukami, T., Hasegawa, M., Matsushita, Y., Fujita, T., Matsushita, T., Horikawa, M., Komura, K., Yanaba, K., Hamaguchi, Y., Nagaoka, T. et al. (2007). Endothelial selectins regulate skin wound healing in cooperation with L-selectin and ICAM-1. J. Leukoc. Biol. 82, 519-531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.