Abstract
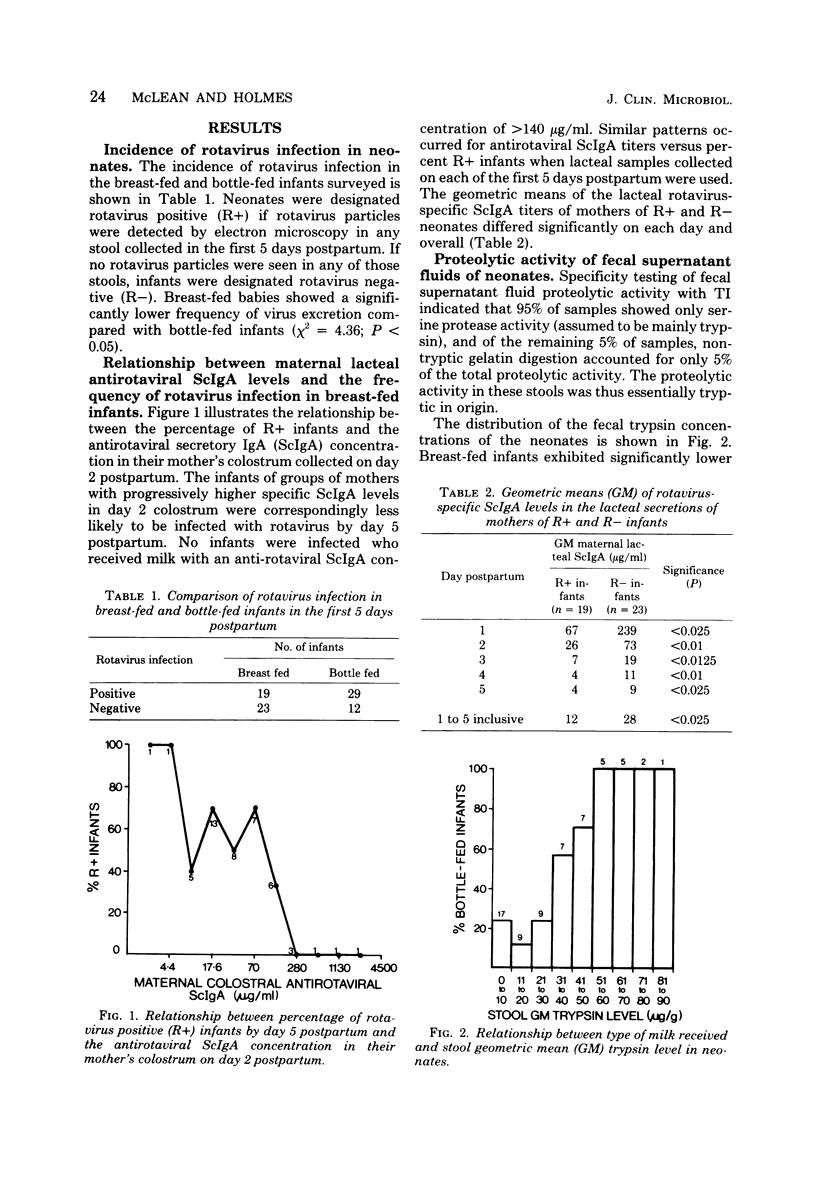
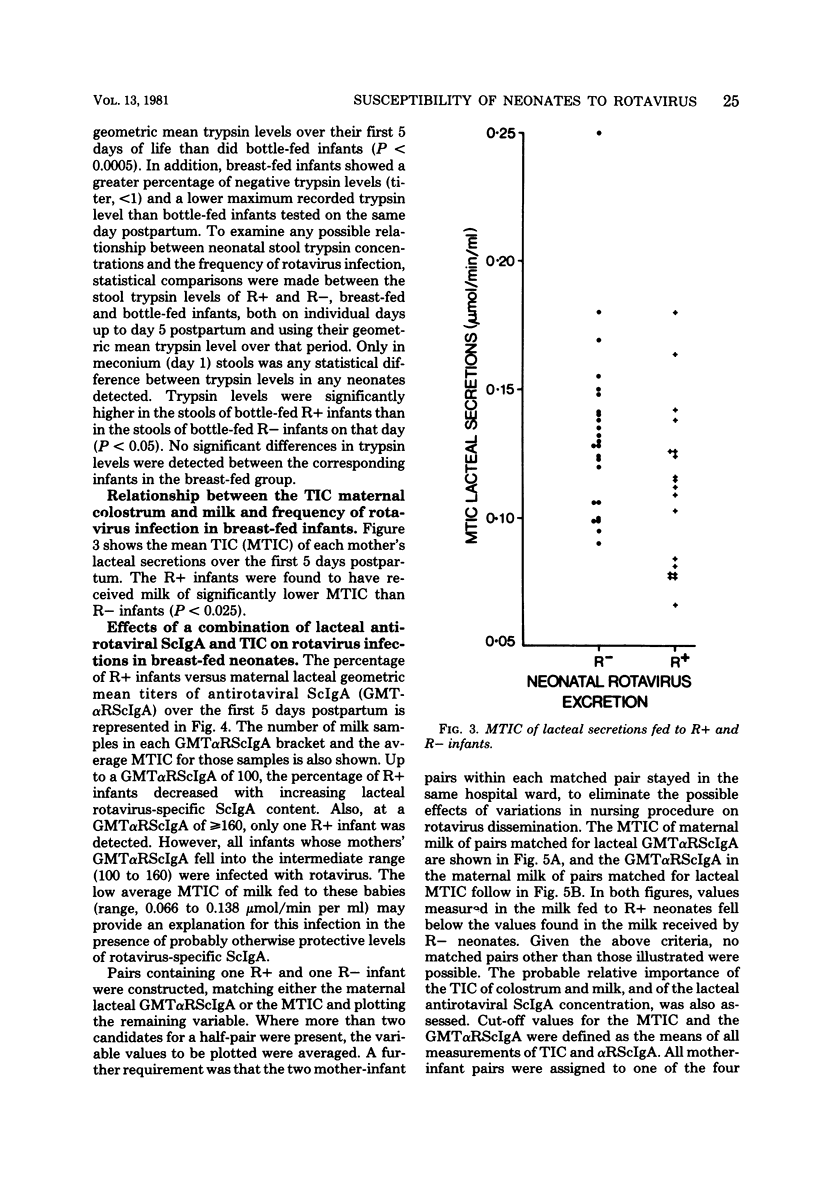
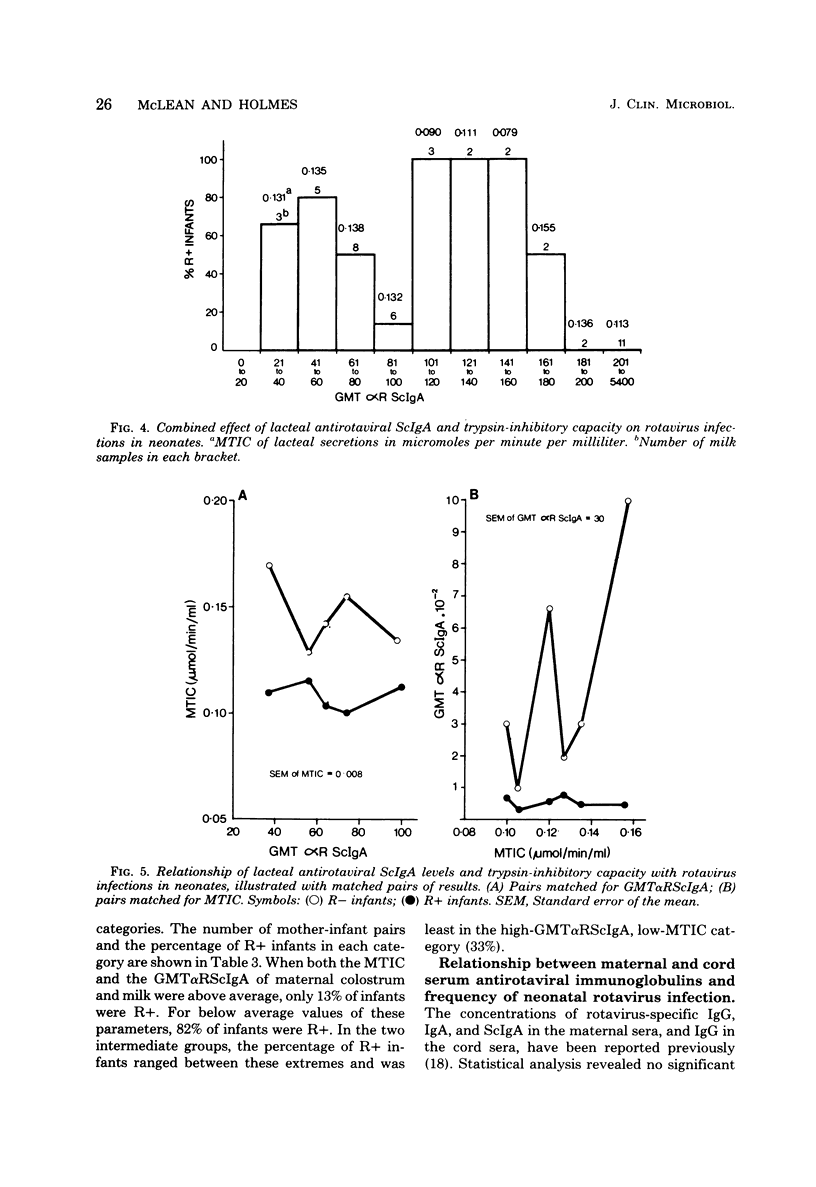
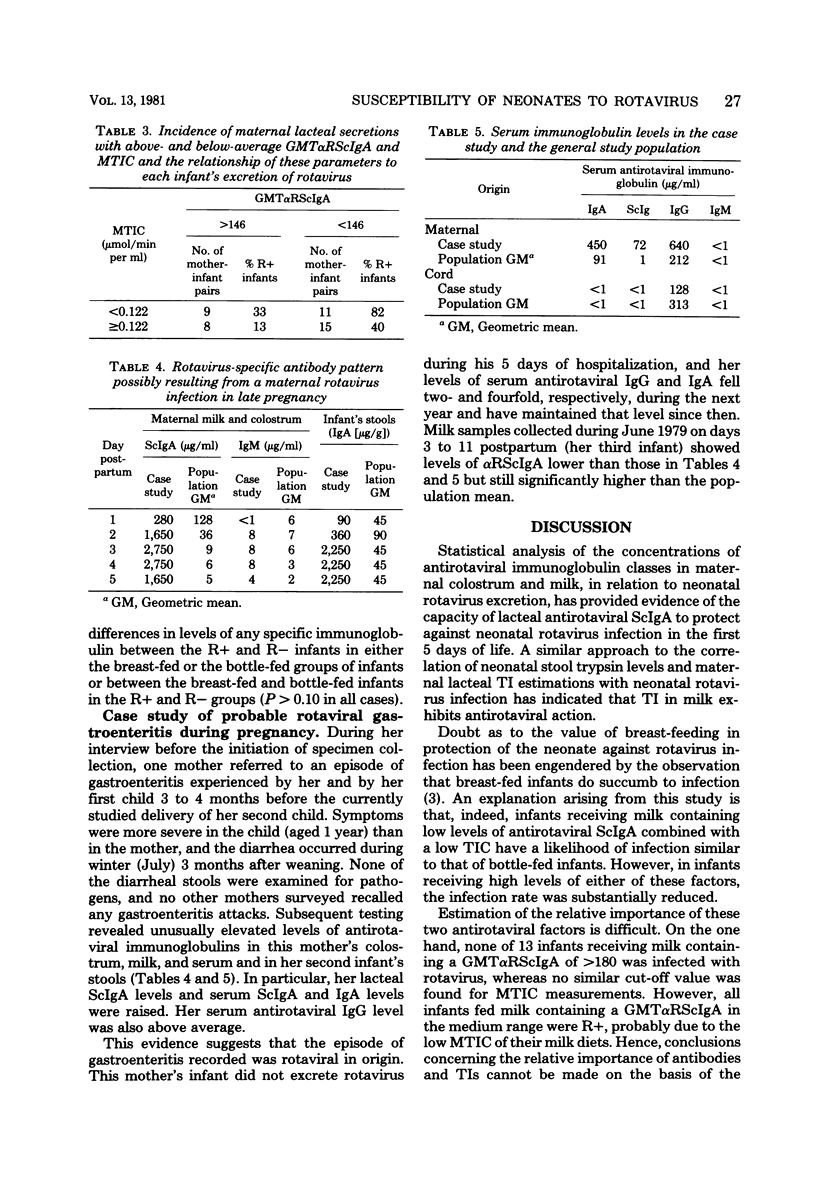
Levels of antirotaviral secretory immunoglobulin A were measured by enzyme-linked immunosorbent assay in colostrum and milk samples collected daily for the first 5 days postpartum from 49 mothers breast-feeding their infants. The trypsin-inhibitory capacity of these lacteal secretion samples was assessed by their ability to inhibit the hydrolysis of alpha-N-benzoyl-DL-arginine-p-nitroanilide by trypsin. Stools passed by these breast-fed infants and by an additional 43 bottle-fed infants were pooled by individual and examined by electron microscopy for rotavirus. Stool trypsin levels were estimated with the gelatin hydrolysis test. Breast-fed infants were significantly less likely to become infected with rotavirus and showed significantly lower stool tryptic activity than did bottle-fed infants. Breast-fed infants who did not excrete rotavirus over the 5-day period received milk of significantly higher antirotaviral secretory immunoglobulin A or trypsin-inhibitory capacity or both than breast-fed infants who were infected with rotavirus. A case of probable maternal rotavirus infection during pregnancy, producing greatly elevated lacteal antirotaviral secretory immunoglobulin A levels lasting for 2 years, was detected. Results of this study suggest that both antibodies and trypsin inhibitors in human milk can be associated with the protection of neonates against rotavirus infection in the first 5 days of life.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Cameron D. J., Veenstra A. A., Barnes G. L. Diarrhea and rotavirus infection associated with differing regimens for postnatal care of newborn babies. J Clin Microbiol. 1979 Apr;9(4):525–529. doi: 10.1128/jcm.9.4.525-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R. K. Prospective studies of the effect of breast feeding on incidence of infection and allergy. Acta Paediatr Scand. 1979 Sep;68(5):691–694. doi: 10.1111/j.1651-2227.1979.tb18439.x. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Wyatt R. G., Kapikian A. Z. Immunization of infants and young children against rotaviral gastroenteritis--prospects and problems. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):570–572. [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R., Capozza F. E., Panjvani Z. F., Bednarek F. Persistence of antibodies to rotavirus in human milk. J Clin Microbiol. 1979 Jan;9(1):93–96. doi: 10.1128/jcm.9.1.93-96.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz A. A., Hodges L. K., Rubinstein H. M., Briney R. R. Estimation of the antitrypsin activity of serum. Clin Chem. 1967 Mar;13(3):242–254. [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose L. H., Schnagl R. D., Holmes I. H. Comparison of an enzyme-linked immunosorbent assay for quantitation of rotavirus antibodies with complement fixation in an epidemiological survey. J Clin Microbiol. 1978 Sep;8(3):268–276. doi: 10.1128/jcm.8.3.268-276.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Keller R., Dwyer J. E. Neutralization of poliovirus by IgA coproantibodies. J Immunol. 1968 Aug;101(2):192–202. [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Mock R. Reovirus-like agent associated with fatal diarrhea in neonatal pigs. Infect Immun. 1976 Sep;14(3):816–825. doi: 10.1128/iai.14.3.816-825.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. H., Nair C. D., Lawrence M. K., Tyrrell D. A. Antiviral activity in milk of possible clinical importance. Lancet. 1976 Dec 25;2(8000):1387–1389. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- McClelland D. B., McGrath J., Samson R. R. Antimicrobial factors in human milk. Studies of concentration and transfer to the infant during the early stages of lactation. Acta Paediatr Scand Suppl. 1978;(271):1–20. [PubMed] [Google Scholar]
- McLean B., Holmes I. H. Transfer of antirotaviral antibodies from mothers to their infants. J Clin Microbiol. 1980 Sep;12(3):320–325. doi: 10.1128/jcm.12.3.320-325.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean B., Sonza S., Holmes I. H. Measurement of immunoglobulin A, G, and M class rotavirus antibodies in serum and mucosal secretions. J Clin Microbiol. 1980 Sep;12(3):314–319. doi: 10.1128/jcm.12.3.314-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measurement of alpha 1-antitrypsin in serum, by immunodiffusion and by enzymatic assay. Clin Chem. 1974 Mar;20(3):396–399. [PubMed] [Google Scholar]
- Moosai R. B., Gardner P. S., Almeida J. D., Greenaway M. A. A simple immunofluorescent technique for the detection of human rotavirus. J Med Virol. 1979;3(3):189–194. doi: 10.1002/jmv.1890030304. [DOI] [PubMed] [Google Scholar]
- Ogra S. S., Ogra P. L. Immunologic aspects of human colostrum and milk. I. Distribution characteristics and concentrations of immunoglobulins at different times after the onset of lactation. J Pediatr. 1978 Apr;92(4):546–549. doi: 10.1016/s0022-3476(78)80285-6. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnagl R. D., Holmes I. H., Mackay-Scollay E. M. A survey of rotavirus associated with gastroenteritis in Aboriginal children in Western Australia. Med J Aust. 1978 Mar 25;1(6):304–307. doi: 10.5694/j.1326-5377.1978.tb107863.x. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Rotavirus infection in lambs: studies on passive protection. Arch Virol. 1976;52(3):201–205. doi: 10.1007/BF01348017. [DOI] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H. Rotavirus neutralisation by human milk. Br Med J. 1977 Nov 26;2(6099):1390–1390. doi: 10.1136/bmj.2.6099.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell B. M., Chrystie I. L., Banatvala J. E. Rotavirus infections in a maternity unit. Arch Dis Child. 1976 Dec;51(12):924–928. doi: 10.1136/adc.51.12.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN R. J., LEPOW M. L., BARTSCH G. E., ROBBINS F. C. THE RELATIONSHIP OF MATERNAL ANTIBODY, BREAST FEEDING, AND AGE TO THE SUSCEPTIBILITY OF NEWBORN INFANTS TO INFECTION WITH ATTENUATED POLIOVIRUSES. Pediatrics. 1964 Jul;34:4–13. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Effects of pancreatin on the growth of reovirus. J Bacteriol. 1966 Jul;92(1):155–160. doi: 10.1128/jb.92.1.155-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


