Abstract
A novel plasmon-coupled quantum dot (QD) nanocomposite via covalently interfacing the QD surfaces with silver nanoparticles was developed with greatly reduced blinking and enhanced emission fluorescence.
The emission properties of quantum dots (QDs), especially high-absorption cross section, exceptional photostability, wide excitation spectra and narrow emission bands, are important to biological imaging.1,2 As quantum dots have low photobleaching quantum yields and very strong absorption, they possess a number of advantages over organic fluorophores as single molecule probes. Still they present several issues when applied to biological systems, in particular strong fluorescence intermittency on all time scales at the single molecule level. While an exact mechanism has yet to be generally accepted, blinking is usually considered to arise from a charging process in which an electron is temporarily lost to the surrounding matrix or captured to surface related trap states. Surrounding the QDs with oligo ligands or thiol groups suppresses the blinking.3–7 This suppression is most likely due to the passivation of surface traps by ligand groups. Enhancements or quenching of photoluminescence and reduction of blinking of QDs deposited on rough metallic surfaces have also been reported recently.8–13
Noble metallic interface or structured surfaces can significantly impact the radiative decay rates of nearby fluorescence emitters.14 In recent years we have studied the e3ects of metallic nanoparticles and surfaces with fluorophores.15–17 Metal induced surface plasmons give rise to local electromagnetic field enhancement. Coupling between the surface plasmon resonance e3ect and the quantum size e3ect of the semiconductor QD may develop new aspects of nanocomposite material systems and also widen applications for noble metal nanodevices. Here we report a simple method to incorporate single QDs into metallic nanoparticles. Plasmoncoupled emitters provide an opportunity to create ultra-bright probes based on complexes of metal nanoparticles with fluorophores, in particular when photostability and brightness are required and relatively large size of probes is not a limitation. Such advances would help to better implement QDs in biological and solid-state device applications.
Biotin-coated silver nanoparticles were prepared in aqueous solution.‡ TEM images revealed these spherical monodisperse colloids appeared in the range of 20 nm (ESI†). Commercially available strepavidin functionalized CdSe/ZnS QDs (Qdot655 streptavidin conjugate, Invitrogen, ~15–20 nm) were covalently linked to biotin-coated silver nanoparticles under biologically relevant conditions. During the coupling reaction, QDs were in 5-fold excess. Free QDs were removed by ethanol precipitation as QDs are soluble in ethanol. Diluted solutions of QD/Ag nanocomposites were spun-cast on clean glass coverslips, which were used to visualize single emitters. Single-molecule fluorescence measurements were performed using a time-resolved confocal microscope with an excitation line at 470 nm. Images were recorded by raster scanning the sample over the focused spot of the incident laser. The fluorescence was filtered through a 655/20 band-pass filter, centered approximately at the peak emission wavelength of the QDs used in the experiments.
Coupling single QDs to individual biotin-coated silver nanoparticles was verified from TEM images (Fig. 1). The particle sizes vary in the range of 40–50 nm, identifying them as isolated nanocomposites consisting of a single QD and a silver colloid. We assume most of the hybrid nanostructures consist of a single QD and a single Ag nanoparticle. Aggregation of QDs and Ag nanoparticles was minimized under the selected concentrations. Fig. 1 also displays typical fluorescence images taken from the individual QDs and nanocomposites on glass coverslips. An increase in the brightness from QD/Ag nanoparticles was clearly observed. The noticeable di3erences in brightness of fluorescent spots indicate preliminary information of enhanced fluorescence emission in the presence of silver nanostructure.
Fig. 1.
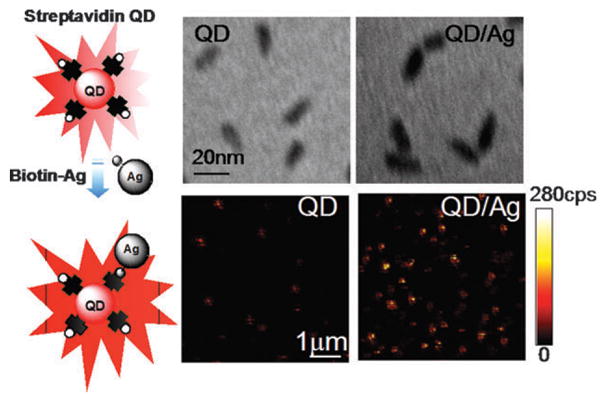
(Top) TEM images of free QDs and QD/Ag nanocomposites showing the coupling of streptavidin-QDs to biotin-coated silver nanoparticles. (Bottom) Typical 10 × 10 μm fluorescence images of free QDs and QD/Ag nanocomposites. These two-dimensional images are 150 × 150 pixels and were acquired in less than one minute. Each pixel has a 0.6 ms dwell time and the fluorescence intensity is displayed on a colorized scale, ranging from dark to light. The excitation power in the microscope was maintained at less than 0.1 μW.
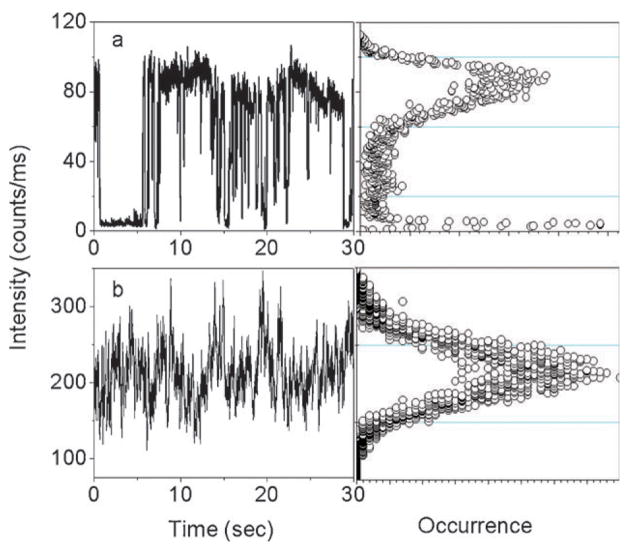
For free QDs under continuous illumination, the single QD emits abruptly between a fluorescent ‘‘on’’ state and a non-fluorescent ‘‘off’’ or weak fluorescent ‘‘dim’’ state (Fig. 2a). The photocount distribution is displayed to the right of the intensity trajectory. Two intensity levels are obviously distinguishable beyond those expected from shot-noise limited fluctuations, demonstrating that the nanocrystal remains for a significant fraction of the total time in the dark state. In contrast, it is clear that a much higher emission rate is observed from the time transient in the presence of silver nanoparticle (Fig. 2b), which is approximately 3-fold greater than that observed in the absence of silver nanostructure. In addition, many time traces exhibit relatively continuous emission rather than discrete intensity fluctuations. The interval of ‘‘on’’ times increases to a point where the blinking events nearly disappear during the illumination. As shown in Fig. 2, we do not observe dark states during the 30 second observation time. Surprisingly, the fluorescence intensity of the nanocomposite is well above the background noise level.
Fig. 2.
(a) A typical intensity trajectory and its corresponding photo-count histogram from a single QD on a glass substrate. (b) A typical intensity trajectory and its corresponding photocount histogram from a single QD/Ag nanocomposite on a glass substrate.
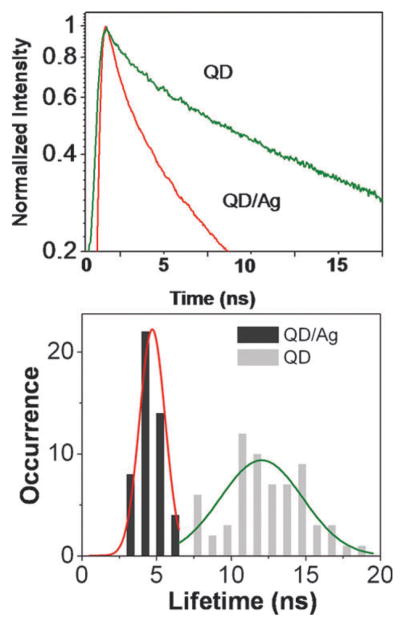
In Fig. 3, the fluorescence decay profile of a single QD is compared with that of a single QD/Ag nanocomposite. The single molecule fluorescence lifetime measurement is implemented using time-correlated single photon counting by plotting a histogram of time lags between the excitation pulses and the detected fluorescence photons. Clearly, the fluorescence decay of the QD/Ag nanocomposite is faster than that of the free QD on glass. This suggests that the fluorescence decay rates are also enhanced by the surface plasmon coupling. The analysis of the fluorescence signal of QD on glass yields an averaged lifetime τ = 12 ns. In the contrast, we observed a considerable decrease in lifetime of single QD/Ag nanocomposites (τ = 4.7 ns).
Fig. 3.
(Top) Fluorescence decay profiles of QD and QD/Ag nanocomposites. (Bottom) Histograms of averaged lifetimes of QD (gray bars) and QD/Ag nanocomposites (black bars). The fitting parameters are provided in ESI.‡
The lifetime histograms of QDs in the presence and in the absence of Ag nanoparticles show distinctive di3erences: dramatic shift (τ = 6–20 ns) to short time scale (3–6 ns) and narrowing of the lifetime distribution. Strongly shortened lifetimes measured for QD/Ag nanocomposites are consistent with resonance energy transfer with the metal. The strong energy transfer from the excited molecules to a nearby metallic nano-object results an increase in the radiative decay rate of the emitter, which can dramatically shorten the lifetime of the excited state, leading to fast de-excitation.
In conclusion, a novel plasmon-coupled QD nanocomposite was developed via covalently interfacing QD surfaces with silver nanoparticles. We observed about 3-fold enhancement in fluorescence intensity, ~3-fold decrease in lifetime accompanied by dramatic changes in the dynamic behavior. The fluorescence decay time is often given by τ = 1/(Γ + knr), where Γ and knr are the radiative and non-radiative decay rates, respectively. Accordingly, the quantum yield of a natural emitter is given by Q = Γ /(Γ + knr). The lifetime and the quantum yield near the metal then become τm = 1/(Γ + Γ m + knr) and Qm = (Γ + Γ m)/(Γ + Γ m + knr), respectively. The total radiative rate is given by Γ + Γ m, where Γ m is the part due to the metal. Proximity of quantum dots to metals can result in an increase in the total radiative decay rate. This change in radiative rate would result in remarkable e3ects such as an increase in quantum yield and decrease in lifetime, which is consistent with our observations. From the point of view of fluorescence detection, a higher quantum yield suggests more detectable emission photons. A dark state of the QD is ascribed to a photo-induced Auger process. The ‘‘off’’ state happens when one of the electrons in the photo-excited exciton either becomes trapped at the QD surface or tunnels into the matrix. The ‘‘off’’ intervals are associated with the recombination rate of the ejected electrons with the ionized dot. When the emitter is localized near the metal particle, the noble metallic nanostructure usually displays a plasmon resonance arising from the collective oscillation of migrated electrons on its surfaces. The radiating energy from the emitter is dramatically altered through coupling with the metal plasmon resonance to cause a change of the emission properties. We propose that the plasmon interaction could alter the energy gap between the exciton hole and trapped hole, and thus suppresses the hole-trapping process, leading to a reduction of the frequency of blinking.
Supplementary Material
Acknowledgments
The present work was supported by grants from the National Institutes of Health NHGRI (HG-002655) and NIBIB (EB-006521).
Footnotes
Electronic supplementary information (ESI) available: Synthesis scheme and experiment details of biotin-coated silver nanoparticles; a TEM image of silver nanoparticles, single-molecule experiments, measurement and data analysis for single molecule lifetimes.
Synthesis and characterization of biotin-coated silver nanoparticles: Tiopronin-coated silver nanoparticles were prepared using a modified Brust reaction with a mole ratio of tiopronin:silver nitrate = 1:6 in methanol, an excess of sodium borohydride was used as reducing agent. (2-Mercapto-propionylamino)acetic acid 2,5-dioxo-pyrrolidin-1-yl ester (2 × 10−6 M) and silver particles (2 × 10−7 M) were co-dissolved in a mixed solvent of water/ethanol (v:v = 1:1) and stirred for 72 h at room temperature. The ligand displacements were expected to occur on the metal cores in a mole ratio of 1:1. Unbound compounds were removed by centrifuging at 6,000 rpm for 30 min. The residuals were dispersed in water and then further purified by dialysis against water (MWCO 50000). The succinimidylated ligand on the metal particle was lengthened and aminated by two step surface reactions. The succinimidylated metal particles (1 × 10−7 M) were co-dispersed in water with an excess of polybis(ethylene glycol) (3-aminopropyl) (MW 1500, 5 × 10−6 M). The solution was stirred for 2 h, and then a drop ammonium was added dropwise to block non-reacted terminal succinimidyl esters. The solution was removed by centrifugation at 6000 rpm. The residue was washed with water, and then dispersed in a water/methanol mixture (1:1, vol ratio). The metal particles (1 × 10−7 M) were biotinylated by co-dissolving an excess of biotin N-succinimidyl ester (2 × 10−6 M). The solution was stirred for 4 h. The suspension was removed by centrifugation at 6000 rpm. The residue was washed by methanol and water, respectively, and then dispersed in 10 mM PBS bu3er solution at pH = 7.2. The metal particles were purified by dialysis against 10 mM PBS. Streptavidin QD was dissolved in 10 mM PBS bu3er solutions (pH = 7.2) and the concentration was controlled to be 1 × 10−7 M. The biotin-metal particles and QDs were mixed at a fixed molar ratio (1:5) to incubate in bu3er solution under stirring and the conjugation occurred at room temperature for at least 8 h. The product was collected after centrifuging. Excess QDs were removed by ethanol precipitation. TEM measurements: TEM images were obtained with a Tecnai T12 (FEI Inc). Samples were prepared on standard Formvar/carbon-coated copper grids (200–300 Å).
References
- 1.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 2.Chan W, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 3.Early KT, McCarthy KD, Hammer NI, Odoi MY, Tangirala R, Emrick T, Barnes MD. Nanotechnology. 2007;18:424027. doi: 10.1088/0957-4484/18/42/424027. [DOI] [PubMed] [Google Scholar]
- 4.Hammer NI, Early KT, Sill K, Odoi MY, Emrick T, Barnes MD. Journal of Physical Chemistry B. 2006;110:14167–14171. doi: 10.1021/jp062065f. [DOI] [PubMed] [Google Scholar]
- 5.Hohng S, Ha T. J Am Chem Soc. 2004;126:1324–1325. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]
- 6.Odoi MY, Hammer NI, Early KT, McCarthy KD, Tangirala R, Emrick T, Barnes MD. Nano Lett. 2007;7:2769–2773. doi: 10.1021/nl0713068. [DOI] [PubMed] [Google Scholar]
- 7.Fomenko V, Nesbitt DJ. Nano Lett. 2008;8:287–293. doi: 10.1021/nl0726609. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Zhang J, Lakowicz JR. Chem Phys Lett. 2007;447:96–100. doi: 10.1016/j.cplett.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulakovich O, Strekal N, Yaroshevich A, Maskevich S, Gaponenko S, Nabiev I, Woggon U, Artemyev M. Nano Lett. 2002;2:1449–1452. [Google Scholar]
- 10.Okamoto K, Vyawahare S, Scherer A. J Opt Soc Am B. 2006;23:1674–1677. [Google Scholar]
- 11.Ray K, Badugu R, Lakowicz JR. J Am Chem Soc. 2006;128:8998–8999. doi: 10.1021/ja061762i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu KT, Woo WK, Fisher BR, Eisler HJ, Bawendi MG. Physical Review Letters. 2002;89:117401. doi: 10.1103/PhysRevLett.89.117401. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Atray T, Shi S, Urabe H, Nurmikko AV. Nano Lett. 2005;5:1557–1561. doi: 10.1021/nl050813r. [DOI] [PubMed] [Google Scholar]
- 14.Lakowicz JR. Anal Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Zhang J, Lakowicz JR. Langmuir. 2008;24:3429–3433. doi: 10.1021/la702673p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakowicz JR. Anal Biochem. 2005;337:171–194. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Fu Y, Chowdhury MH, Lakowicz JR. Nano Lett. 2007;7:2101–2107. doi: 10.1021/nl071084d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.