Abstract
Contextual fear conditioning was tested in infant, adolescent, and adult rats in terms of Pavlovian conditioned suppression. When a discrete auditory conditioned stimulus (CS) was paired with footshock (unconditioned stimulus, US) within the largely olfactory context, infants and adolescents conditioned to the context with substantial effectiveness but adult rats did not. When unpaired presentations of the CS and US occurred within the context, contextual fear conditioning was strong for adults, weak for infants, but about as strong for adolescents as when pairings of CS and US occurred in the context. Nonreinforced presentations of either the CS or context markedly reduced contextual fear conditioning in infants, but, in adolescents, CS extinction had no effect on contextual fear conditioning, although context extinction significantly reduced it. Neither CS extinction nor context extinction affected responding to the CS-context compound in infants, suggesting striking discrimination between the compound and its components. Female adolescents showed the same lack of effect of component extinction on response to the compound as infants, but CS extinction reduced responding to the compound in adolescent males, a sex difference seen also in adults. Theoretical implications are discussed for the development of perceptual-cognitive processing and hippocampus role.
Keywords: contextual fear conditioning, fear extinction, learning, olfactory context, hippocampus, ontogeny
Contextual fear conditioning has received a lot of attention from behavioral neuroscientists during the last decade (e.g., Anagnostaras, Gale, & Fanselow, 2001; Fanselow & Poulos, 2004; Phillips & LeDoux, 1992). The purpose of this report is to assess the effectiveness of contextual fear conditioning experiments by rats at each of three disparate periods of ontogeny: late infancy, adolescence and early adulthood. The ontogeny of contextual fear conditioning is of interest because --in relation to conditioning of other events within the context-- it can provide a window to the ontogeny of encoding and memory organization (e.g., Brasser & Spear, 2004; Lariviere, Chen, & Spear, 1990), and because it has been used as a marker for the development of brain function important for memory (e.g., Pugh & Rudy, 1996; Rudy & Morledge, 1994).
Several experiments led to the conclusion that acquisition of an aversion to a particular context paired with footshock is less effective in rats during late infancy (postnatal days 17–20, P17–20) than in weaned juvenile rats just a few days older, and much less effective than in adults. This pattern of results has been observed frequently when the context to be paired with footshock is the common aversive conditioning chamber used for most contextual fear conditioning in adults, consisting basically of a grid floor, clear or opaque walls, and distinguished primarily by visual and tactile attributes (e.g., Rudy, 1993; Rudy & Morledge, 1994; many unpublished experiments from our own laboratory). These results have been observed whether or not the footshock (unconditioned stimulus, US) is signaled on discrete occasions within the context by a brief auditory signal, a tone or noise (conditioned stimulus, CS).
Quite different conclusions from similar experiments have emerged, however, when the context is distinguished instead by novel olfactory stimuli. In these cases, infants have seemed equally or more effective in contextual fear conditioning than older animals, including adults (e.g., Brasser & Spear, 2004; Carew & Rudy, 1991; Lariviere et al., 1990). And when a discrete auditory CS predicts the footshock within a salient olfactory context, contextual fear conditioning in the infant exceeds that in the adult, even when conditioning to the CS is held constant (Brasser & Spear, 2004; Mckinzie & Spear, 1995).
It should not be a surprise that infant rats --in some cases-- show more contextual fear conditioning than adults, given other instances in which infants have seemed more sensitive to context than older animals: CS conditioning is disrupted by a change in olfactory context more extremely for infants than adults (Solheim, Hensler, & Spear, 1980); Pavlovian trace conditioning is regularly facilitated in infant rats by a salient olfactory and visual context but unaffected by the same manipulations in adults (Brasser & Spear, 1998; McKinzie & Spear, 1995); the presence of a familiar context such as odor of the rat’s home is more likely to affect infant learning than adult learning (Spear, Kucharski, & Hoffmann, 1985); and infants are more likely than older animals to show enhanced unconditioned responding to visual, auditory and tactile stimuli in the presence of a context rich in novel odor (Brasser & Spear, 1998; Kraebel, Vizvary, & Spear, 1998).
Thus, the goal of the present series of experiments was to examine fear conditioning to an olfactory context, the type of situation where infant rats seem to show the strongest disposition for contextual conditioning. They were intended to replicate and extend previous experiments (viz., Brasser & Spear, 2004) suggesting that contextual fear conditioning in infants may exceed that of adults, particularly when an auditory CS within an olfactory context also predicts the US. Experiments 1–3 tested rats from the same ontogenetic periods as Brasser and Spear, but the specific ages differed slightly in the two studies. The present study differed also in adding specific tests of the effects of sex and, most importantly, consequences of context and CS extinction treatments on subsequent expression of contextual fear conditioning at each of the ages.
EXPERIMENT 1
The main goal was to assess, in infant rats, contextual fear conditioning after receiving CS-US pairings. The experimental design (see Table 1) comprised five treatments (groups). The group intended to reflect primarily the consequences of conditioning (No Extinction group, NOEXT, which also served as a control group to assess the effects of the extinction treatments) remained in an isolation cage for 1 hr, instead of nonreinforced exposure either to the training context or to the CS after CS-US pairings. This condition also controlled the influence of isolation (experienced by animals in all the other experimental conditions during the extinction phase) on pup behavior (Hofer, 1980). Comparison of this group with the Context Control (CXTCTL) or UNPAIRED groups allowed estimates of conditioning to context and CS-context compound, respectively.
Table 1.
Summary of Experimental Procedure
| Group | Phase 1 | Phase 2 | Interval | Phase 3 | Test |
|---|---|---|---|---|---|
| CXTEXT | cxt 1 | cxt 2 | 23 hr | cxt 2 | cxt 2 |
| 8 CS-US | cxt ?CR | ||||
| CS-cxt ?CR | |||||
| NOEXT | cxt 1 | cxt 2 | 23 hr | Isolation cage | cxt 2 |
| 8 CS-US | cxt ?CR | ||||
| CS-cxt ?CR | |||||
| CXTCTL | cxt 1 | cxt 2 | 23 hr | cxt 2 | cxt 2 |
| 8 US | 8 CS | cxt ?CR | |||
| CS-cxt ?CR | |||||
| UNPAIRED | cxt 1 | cxt 2 | 23 hr | cxt 2 | cxt 2 |
| 8 CS/US | cxt ?CR | ||||
| CS-cxt ?CR | |||||
| CSEXT | cxt 1 | cxt 2 | 23 hr | cxt 1 | cxt 2 |
| 8 CS-US | CS- | cxt ?CR | |||
| CS-cxt ?CR | |||||
Note. CS = conditioned stimulus (tone); US = unconditioned stimulus (mild electric footshock); CS-US = CS and US are paired; CS/US = CS and US are unpaired; ?CR = measurement of the conditioned response; cxt = context.
The CXTCTL group was a control condition to determine strength of contextual fear conditioning in groups NOEXT and Context Extinction (CXTEXT). CXTCTL animals received tones in the same context in which subjects from the other two groups received tones paired with shock (i.e., Context 2), but they did not receive shock in this context. Equivalent shock was delivered in a different context, to which they had been exposed before exposure to the tones (i.e., Context 1). UNPAIRED animals received the CS and US presented in a pseudo-random way in Context 2, with the constraint that the US could never appear during the 60-second period immediately following the CS.
To assess the effect of extinction of context, the CXTEXT group was given the context extinction treatment (Phase 3; 1 hour of exposure to the training context) after Pavlovian conditioning training (Phase 2; 8 pairings of tone and electric shock). To assess the effect of CS extinction, the CS Extinction group (CSEXT) was given 8 CS-US pairings of Pavlovian training during Phase 2, followed by 1 hour of nonreinforced exposure to the CS in a context different from the training context (Phase 3).
To equate the groups in experience with the additional context employed in CXTCTL (i.e., Context 1), all experimental conditions included exposure during Phase 1 to Context 1, in which no shock was delivered. Strength of conditioning was assessed by the effectiveness with which context alone or the CS-context compound suppressed spontaneous activity (Moye & Rudy, 1985).
Method
Subjects
The animals were 73 Sprague-Dawley rats (with a maximum in each group of two rats --one male and one female -- coming from the same litter; see Holson & Pearce, 1992), 17 days old on the first day of the experiment. They were bred in our breeding colony at the Center for Developmental Psychobiology (SUNY-Binghamton), where ambient temperature was 22 +/− 2° C, and a 16 h light/8 h dark cycle began with light onset at 0600 h. Rats had free access to food and water. Experimental manipulations were done at the late portion of the luminous phase of the cycle (1500–2100h).
Apparatus
Training context (Context 2) included a chamber with dimensions of 54 × 40 × 46 cm (long × wide × high) and an electric grid floor (38 × 30.5 × 7 cm). The bars of the grid were 3 mm of diameter and were separated 1.3 cm center to center. An electric shock generator (Grason-Stadler) provided a 1.0 mA scrambled shock through the grid, with a duration of .5 s. The CS, according to measures done from the grid floor, was a 79 dB (scale-A), 2000 Hz tone, with a duration of 15 sec. The tone was generated by three speakers located 34 cm above the grid floor. A ventilation fan provided a background noise of 72 dB. A 1.5 W light bulb (centered on the right wall and 34 cm above the floor of the chamber) provided ambient illumination. Within the chamber, and located on the electric grid, was a box with black Plexiglas walls, overall dimensions of 28 × 20.5 × 25.5, and divided into two equal size compartments (each of them held one rat). A metallic grid was used to cover the box, as a ceiling. Three 7.5 W bulbs located 29 cm above the grid floor were on for .5 s and off .5 s throughout the experimental session. Newspapers located under the grid floor were renewed before each experimental session. The cleaning of the grid bars was done with a piece of wet cotton. Lemon odorant (1.0 cc) was distributed evenly on a 20 × 10 cm piece of cotton situated on the newspapers. Cotton and odorant were also renewed before each session.
Control context (Context 1) was located in another experimental room. It included, primarily, an isolation chamber with the same dimensions as the chamber of the training context, and an electric grid floor (38 × 30 × 7 cm) located on the floor of the chamber. The grids were 3 mm of diameter, and the distance among them was .7 cm center to center. An electric shock generator, similar to the one used in Context 2, allowed administration of scrambled shocks to rats in the CXTCTL group. A 28 × 20.5 × 25.5 cm transparent Plexiglas box was situated on the grid floor. This box was different from the one employed in Context 2, not only by being transparent (instead of black), but also by being divided into three identical compartments (instead of two). Rats were allocated into the two extreme compartments, and pink color paper on the middle walls prevented the animals from seeing each other. The door of the chamber was kept opened, exposing animals to the environmental light of the room. A metallic grid covered the box, as a ceiling. Also, fresh newspapers were located under the grid floor. No lemon odor was delivered.
Conditioning measurement took place in the training context (Context 2). The compartment of the black Plexiglas box employed for testing was identical to that of training, except that two circular disks (2.5 cm in diameter) were located on one wall 1.5 cm above the grid floor and separated by 4.0 cm of distance. These disks were the emisor and receptor of an ultrasonic mechanism designed to measure general activity. Any movement inside the box led to a discrepancy in the ultrasound frequency that, once detected by the receptor, closed an electromechanic device, constituting an activity count. Sensitivity and reliability of the mechanism were assessed periodically with a metronome.
Stimulus presentation (tones and shocks) in both contexts was controlled by a computer program. Another program was employed to record the latencies to reach successive blocks of 25 activity counts.
Procedure
The rats were randomly assigned to the 5 groups (see Table 1). There were 3 training phases, followed by a test phase in which the strengths of the fear responses to the training context and to the CS-context compound were assessed. During Phase 1, all rats were located in Context 1 for 30 min. CXTCTL rats received 8 US presentations according to a VT 3 min schedule. Almost immediately after completion of Phase 1, animals were moved to Context 2, where Phase 2 took place.
During Phase 2, infants from the UNPAIRED group received 8 unpaired presentations of the CS and the US over a 30-min duration. The animals from the CXTCTL group received 8 CS presentations, and those from the remaining conditions (CSEXT, CXTEXT, and NOEXT) received 8 CS-US pairings during this same period. After Phase 2 training, all the pups were moved to the home cages with their mothers and littermates, where they remained for 23 hr.
During Phase 3, animals from the CSEXT group were exposed to the CS in Context 1 for 1 hr. Animals from all the other experimental conditions (but NOEXT) remained in the training context (Context 2) for 1 hr. NOEXT pups spent the 1 hr period in the isolation cage (identical to the home cage, but with a white and opaque structure that divided the cage into 8 compartments). The animals were moved from their Phase 3 situation to their home cages, and then to Test, almost immediately, except for the very short time needed to change Context 2 from an extinction to a testing arrangement.
During Test Phase, there were four steps. The goal of the first one was to promote the animal’s adaptation to the testing situation. The rat was placed into the testing box for a 1-min period. No manipulation (or behavior recording) of the animal was done during this time. The goal of the second step was to assess contextual conditioning. Using the ultrasound device formerly described (see Apparatus section), latencies to reach 100 activity counts (through 4 blocks of 25 counts each) were recorded. It was assumed that, the longer the latency time, the stronger the freezing (and, therefore, the stronger the conditioning). The goal of the third step was to establish a baseline of animal’s activity before CS onset (Annau & Kamin, 1961). Therefore, latencies to reach 4 blocks of 25 activity counts (i.e., 100 activity counts) were recorded (Hurwitz & Davis, 1983). If during the latency to complete the last block of 25 counts, a difference was detected of more than 2 standard deviations from the average of the 3 previous baseline blocks latencies, latency to complete another 25 activity counts block was registered, and compared again with the average of the latencies of all the former blocks, and so forth, until the last block registered no more than 2 standard deviations from the previous average. Once the baseline was established, the fourth phase was started. The purpose of this phase was to assess the amount of CS-context compound conditioning. Therefore, the auditory CS was presented, and latency to register 100 activity counts was measured. A maximum of 25 min was allowed for testing, so data from animals that did not reach 100 activity counts during those 25 min of CS onset were removed from the statistical analysis. As a consequence of applying this criterion, the data from 6 animals were eliminated (1 CXTEXT, 2 NOEXT, 2 CXTCTL, and 1 UNPAIRED).
A logarithmic transformation of the latencies was completed to promote the normal distribution of the scores and justify using parametric statistical tests. The scores of the different groups were compared through an Analysis of Variance (ANOVA). Planned comparisons were made when appropriate. The criterion for statistical significance was α = .05.
Results and Discussion
The results, detailed below, showed strong contextual fear conditioning only when the CS and US were paired during training and no extinction treatment followed (i.e., NOEXT group). Additionally, they showed that the extinction treatment applied to the training context extinguished contextual fear conditioning, but did not alter conditioned suppression to the CS-context compound. The CS extinction treatment (in a context different from the training context) had no effect on suppression of activity in the presence of the CS-context compound, but it did tend to reduce contextual fear conditioning. The following statistical analysis confirmed these impressions.
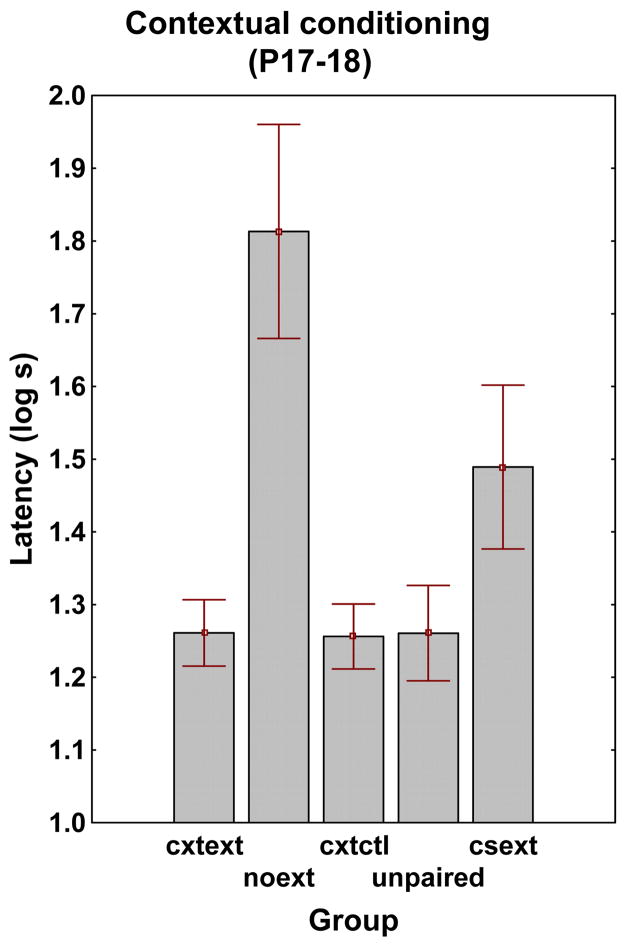
Figure 1 represents the results for contextual fear conditioning, obtained when the pups were exposed to the context alone during the Test Phase. A two-way ANOVA, 5 (Group) × 2 (Sex) run on the latencies to reach the first 50 activity counts, showed a main effect of the Group factor [F (4, 57) = 5.61], but effects of neither Sex, nor Group × Sex interaction, were found. Therefore, data were clustered across the Group factor, and Fisher least significant difference (LSD) test conducted. The test showed that the differences in conditioned suppression between NOEXT and all other groups (which did not differ) were significant. A significant difference between NOEXT and CXTCTL reflects contextual fear conditioning in the NOEXT group. The difference between CXTEXT and NOEXT, taken together with the lack of difference between CXTEXT and CXTCTL, indicates that the context extinction treatment was effective in extinguishing conditioned suppression to the training context in the CXTEXT group. Finally, in view of the lack of difference between CSEXT and CXTCTL, the difference between CSEXT and NOEXT verifies the effectiveness of the CS extinction treatment in reducing conditioned suppression to the training context in the CSEXT group.
Figure 1.
Experiment 1. Infant rats (P17–18). Mean time in log seconds to reach 50 activity counts in the presence of the contextual cues (with no target CS). Freezing was measured through suppression of general activity; hence, the longer the latency time, the stronger the freezing. Error brackets represent standard errors of means.
Figure 2 represents responding to the CS-context compound. The difference in conditioned suppression between NOEXT and UNPAIRED (and also between NOEXT and CXTCTL, insofar as the latter group can be considered an extreme case of explicitly unpaired condition) verifies conditioning to the CS-context compound in the NOEXT group. The lack of differences between CXTEXT and NOEXT suggests that the context extinction treatment did not affect conditioned responding to the CS-context compound in the CXTEXT group. In the same vein, the lack of differences between CSEXT and NOEXT shows that the CS extinction treatment did not alter conditioned responding to the CS-context compound in the CSEXT group. An ANOVA run on the last baseline period of 50 activity counts confirmed that there were no significant effects of Group on baseline behavior, simplifying the statistical comparison of the latencies to register 50 activity counts once the CS was present. A two-way ANOVA, 5 (Group) × 2 (Sex), showed an effect of the Group factor [F (4, 57) = 7.01], but no effect of either Sex, or the Group × Sex interaction. So data were clustered across Group and LSD tests conducted. In accord with visual inspection of Figure 2, they showed that the difference between NOEXT and UNPAIRED (and CXTCTL) was significant, and that neither CSEXT nor CXTEXT differed from NOEXT, but both differed from UNPAIRED (and CXTCTL).
Figure 2.
Experiment 1. Infant rats (P17–18). Mean time in log seconds to reach 50 activity counts in the presence of the CS-context compound (i.e., the CS in the training context). Freezing was measured through suppression of general activity; hence, the longer the latency time, the stronger the freezing reaction. Error brackets represent standard errors of means.
In summary, neither extinction of the training context, nor that of the CS, altered expression of conditioning to the CS-context compound, in consonance with previous data gathered on infant rats (e.g., Kucharski & Spear, 1985; Lariviere et al., 1990; Mellon, Kraemer, & Spear, 1991).
It should be noted that responding to the CS-context compound after CS exposure might simply reflect the failure of such exposure as an effective extinction treatment. However, because the CS extinction treatment led to a diminished fear response to the context alone, it seems reasonable to assume that this extinction treatment was, somehow, able to weaken some underlying association with the US.
Some studies with adult rats on the effects of CS extinction treatment delivered outside the training context upon later response to the CS in the original context (i.e., to the CS-context compound) have found similar results to those we have just reported for infants (renewal effect; e.g., Bouton & Ricker, 1994; Rosas & Bouton, 1997). There is some evidence suggesting that --for adult rats-- the context may work as an occasion setter, modulating the first order CS-US associations, but establishing no direct associations with neither the CS nor the US (Bouton & Swartzentruber, 1986). It has been argued that the hippocampus is the neuroanatomical structure underlying this phenomenon (Myers & Gluck, 1994). It is usually assumed that infant rats have an inmature hippocampus (e.g., Rudy & Morledge, 1994). Therefore, different mechanisms --both neurobiological and psychological-- may be at work when the renewal effect occurs in either infant or adult rats.
EXPERIMENT 2
The main goal was to assess, in adolescent rats, contextual fear conditioning after exposure to CS-US pairings. In order to do so, animals received the same treatment as infants in Experiment 1.
Method
Animals and Apparatus
The animals were 59 Sprague-Dawley derived rats. In each experimental condition there was a maximum of two rats (one male and one female) coming from the same litter. They were 28–31 days old and bred in our animal colony. The conditions for temperature, light, and access to water and food were identical to the conditions in Experiment 1. The experimental manipulations were completed during the latter portion of the light phase of the cycle, as in Experiment 1. The apparatus employed was the same as in Experiment 1.
Procedure
Design and procedure were identical to those employed in Experiment 1. Due to their exceeding the ceiling time of 25 min to reach 100 activity counts during CS onset, data from 8 animals were eliminated from the statistical analysis (4 CXTEXT, 1 NOEXT, 1 CXTCTL, 2 CSEXT).
Results
As with infants, adolescent rats showed strong contextual fear conditioning when the CS and the US were paired, but contextual conditioning was also observed in the UNPAIRED group (despite the extinction treatment). Additionally, contextual fear conditioning in these adolescents was decreased by context extinction, but --unlike infants-- it was not decreased by CS extinction. Also as with infants, the context extinction treatment had no effect on conditioned suppression to the CS-context compound. Unlike infants, CS extinction treatment decreased conditioned suppression to the CS-context compound for male adolescents, although like infants, it had no effect for females. These conclusions emerged from the following statistical analysis.
The results of contextual conditioning are shown in Figure 3. A two-way ANOVA, 5 (Group) × 2 (Sex), revealed a main effect of Group [F (4, 41) = 4.47], but no effect of Sex, nor Group × Sex interaction. Therefore, the data were clustered through the Group factor and LSD test conducted. This test confirmed that the apparent differences between NOEXT and CXTCTL were statistically significant, implying contextual conditioning in NOEXT. The differences between CXTEXT and NOEXT (greater suppression in the second group) also were statistically significant. The same test did not show significant differences between CXTEXT and CXTCTL. Both observations, as a whole, show that the context extinction treatment was effective in extinguishing the contextual activity suppression otherwise expected (and confirmed), as indicated by greater response to context by CSEXT and NOEXT than by CXTCTL. In contrast with our observation in Experiment 1, the differences between NOEXT and CSEXT were not quite statistically significant.
Figure 3.
Experiment 2. Adolescent rats (P28–31). Mean time in log seconds to reach 50 activity counts in the presence of the contextual cues (with no target CS). Freezing was measured through suppression of general activity; hence, the longer the latency time, the stronger the freezing reaction. Error brackets represent standard errors of means.
The results for CS-context compound conditioning are shown in Figure 4. An ANOVA run on the last baseline period of 50 activity counts revealed that the baseline levels were homogeneous before the CS presentation in the training context, justifying statistical comparisons among latencies during CS presentation. The two-way ANOVA, 5 (Group) × 2 (Sex), run on the latencies to reach 50 activity counts once the CS was on, showed a significant effect of the Group factor [F (4, 41) = 20.80], and of the interaction Group × Sex [F (4, 41) = 5.01]. LSD tests were then conducted to isolate the source of the interaction with Sex.
Figure 4.
Experiment 2. Adolescent rats (P28–31). Mean time in log seconds to reach 50 activity counts in the presence of the CS-context compound (i.e., the CS in the training context). Freezing was measured through suppression of general activity; hence, the longer the latency time the stronger the freezing reaction. Error brackects represent standard errors of means.
For males, but not for females, UNPAIRED animals showed conditioning to the CS-context compound: their latencies were significantly greater than those of animals from CXTCTL. Thereafter, CXTCTL was employed as a control condition to assess conditioning to the CS-context compound.
Male rats in CXTEXT, NOEXT, and CSEXT showed greater conditioned suppression to the CS-context compound than those in CXTCTL, verifying maintained expression of conditioning with or without extinction of either component of this compound. This conditioned suppression to the CS-context compound did not diminish as a consequence of the context extinction treatment (we found similar degrees of suppression in animals in CXTEXT and NOEXT). However, and differently than observed in females (and infants), the CS extinction treatment led to a statistically significant decrement in responding to the CS-context compound (the conditioned suppression was significantly less in CSEXT than NOEXT), although the decrement was not sufficient to produce complete extinction of the response (responding in CSEXT was significantly greater than in CXTCTL).
Females in CXTEXT, NOEXT, and CSEXT also had significantly greater conditioned suppression than those in CXTCTL, verifying that, like males, they expressed conditioning to the CS-context compound despite extinction of the components. Unlike the males, but like infants, conditioned suppression to the CS-context compound did not diminish as a consequence of either the context extinction treatment or the CS extinction treatment.
EXPERIMENT 3
The main goal was to assess, in young adult rats, contextual fear conditioning after exposure to CS-US pairings. Therefore, the animals received the same treatment as infants in Experiment 1 and adolescents in Experiment 2.
Method
Animals and Apparatus
The animals were 55 Sprague-Dawley rats, 50–70 days old. They came from the breeding colony at SUNY-Binghamton, and also from an industrial provider (Harlan), with experimental conditions balanced among sources. The conditions of temperature, light, and access to food and water were identical to those in the previous experiments.
The apparatus was essentially the same as in the previous experiments. The only difference was in the thickness and spacing of the grids used in the chamber floor from Context 1. They were thicker than in former experiments (to accommodate the larger, adult rats): 4 mm in diameter and 1.4 cm from center to center.
Procedure
Design and procedure were essentially identical to those employed in Experiments 1 and 2. For these adults it was necessary to establish a new ceiling during the test phase, such that the data of those animals that did not reach 50 (instead of 100) activity counts during 25 min of CS onset were eliminated. The reason for this change was the adults’ greater incidence of freezing, and hence greater latency to movement. As a consequence of this criterion, data from 7 animals were eliminated from the statistical analysis (2 CXTEXT, 2 NOEXT, and 3 CSEXT).
Results
Effects on contextual fear conditioning were different in these adults than in the younger rats. Contextual fear conditioning itself occurred only in the UNPAIRED condition (despite the extinction treatment), and was not statistically significant in any group exposed to pairings of CS and US. In terms of conditioning to the CS-context compound, the sex differences were again more likely than for contextual fear conditioning and, as one would expect, seemed more apparent in adults than adolescents. Conditioned suppression by females appeared to be increased by the context extinction treatment (an effect primarily due to their unexpectedly low fear conditioning in the NOEXT condition) but unaffected by CS extinction. In males, conditioned suppression was decreased by CS extinction and unaffected by context extinction.
The results for contextual conditioning are shown in Figure 5. A two-way ANOVA, 5 (Group) × 2 (Sex), revealed a significant effect of the Group factor [F (4, 38) = 4. 34], but not for Sex or the interaction Group × Sex. After data were clustered through the Group factor, LSD tests showed that the UNPAIRED group differed from all others, and no other significant difference was found, showing that contextual conditioning was found only in the UNPAIRED animals. Because there was no evidence for contextual conditioning in the NOEXT group, we could not determine the basic effect of the context extinction treatment on contextual fear conditioning.
Figure 5.
Experiment 3. Young adult rats (P50–70). Mean time in log seconds to reach 50 activity counts in the presence of the contextual cues (with no target CS). Freezing was measured through suppression of general activity; hence, the longer the latency time, the stronger the freezing reaction. Error brackets represent standard errors of means.
The results for CS-context compound conditioning are shown in Figure 6. An ANOVA run on the last baseline period of 50 activity counts confirmed that the baseline levels were homogeneous before CS presentation in the training context. The two-way ANOVA, 5 (Group) × 2 (Sex), run on the latencies to reach 50 activity counts once the CS was on, showed a significant effect of Group [F (4, 38) = 44.32] and of the interaction Sex × Group [F (4, 38) = 2.64]. We used LSD tests to make the relevant comparisons.
Figure 6.
Experiment 3. Young adult rats (P50–70). Mean time in log seconds to reach 50 activity counts in the presence of the CS-context compound (i.e., the CS in the training context). Freezing was measured through suppression of general activity; hence, the longer the latency time, the stronger the freezing reaction. Error brackets represent standard errors of means.
The male rats from CXTEXT, NOEXT, and CSEXT showed greater conditioned suppression than males from UNPAIRED, confirming conditioning to the CS-context compound. The context extinction treatment did not affect conditioned suppression of males to the CS-context compound (no differences between CXTEXT and NOEXT), but the CS extinction treatment did decrease conditioned suppression to the CS-context compound (although responding remained significantly greater for CSEXT than for UNPAIRED).
Females in CXTEXT, NOEXT, and CSEXT also had significantly greater conditioned suppression than those in UNPAIRED, confirming conditioning to the CS-context compound. But due --apparently-- to weak conditioning in the NOEXT group, conditioned suppression to the CS-context compound appeared to increase for females as a consequence of the context extinction treatment in the CXTEXT group. In the absence of further clear evidence, it seems wise to ignore --or, at least, limit-- the significance of the difference between females in NOEXT and those in CXTEXT.
GENERAL DISCUSSION
Regarding contextual fear conditioning as a function of age, the results nicely replicated those of Brasser and Spear (2004) in terms of the three major findings. First, contextual fear conditioning occurred for infants only when CS conditioning was imbedded in it by pairings of the CS and US; when CS and US were unpaired, there was no significant contextual fear conditioning (Mckinzie & Spear, 1995). Second, adolescents again were shown to have a particularly strong disposition to learn about context (e.g., Barrett, Rizzo, N. E. Spear, & Spear, 1984), in that contextual fear conditioning was significant whether it included paired or unpaired presentations of the CS and US. Finally, adults were again shown to be relatively limited in contextual fear conditioning: such conditioning occurred when unpaired presentations of CS and US were presented within that context (Marlin, 1981; McKinzie & Spear, 1995; Odling-Smee, 1975), and not with paired CS and US-- opposite the infants.
In a general sense, these results are consistent with the idea that preweanling rats are less likely than adults to be selective in their learning and more likely to acquire memory attributes redundant to the particular learning task at hand (Spear, 1979, 1984; Spear, McKinzie, & Arnold, 1994; Spear, Kraemer, Molina, & Smoller, 1988). The apparently special effectiveness of infants in learning redundant aspects of an episode is exemplified by their strong conditioning to context in a circumstance in which a CS is markedly more predictive of the US, whereas adults exhibit their weakest conditioning to context in this circumstance (Brasser & Spear, 2004; McKinzie & Spear, 1995). One possible reason is that infants are more likely to process the CS and context, for example, as a “single intense event” -- an undifferentiated configural representation that is conditioned more effectively because of its greater perceived intensity. When only the context is presented for testing, the infant’s broader generalization gradients protect against the generalization decrement likely experienced by adults, thus contributing to the age-related differences. Such amodal configural processing of the CS-context compound would be expected to be greater when the CS and context are simultaneously reinforced (paired condition) than when the context is reinforced apart from the CS (unpaired condition). When stimulus competition between CS and context emerge later in ontogeny, the greater conditioning to context in the paired condition shifts to the opposite relationship in older animals: greater contextual fear conditioning in the unpaired than the paired condition. This way, adult rats always show best responding to the cues that best predict US occurrence. In the paired condition, the best predictor was the CS, so animals showed fear to it during testing, but not to context. In the unpaired condition, the best predictor of US occurrence were the contextual cues, so rats showed fear to these cues during testing, but not to the CS. It should be noted that this pattern of behavior is not an unusual one for adult rats, that is, one that could be seen as particularly linked to the presence of an olfactory context. Indeed, such pattern of responding by adults is a clear instance of the well documented contingency effect (e.g., Hallam, Grahame, & Miller, 1992; Rescorla, 1968), and is at consonance with the contemporary theories of Pavlovian conditioning that have explicitly addressed the relation of contextual versus target cues learning and responding (e.g., Denniston, Savastano, & Miller, 2001; Miller, Barnet, & Grahame, 1995; Rescorla & Wagner, 1972).
Effects of extinguishing the nominal components of the CS-context compound on responding to the contextual component also differed systematically with ontogeny. Expression of contextual fear conditioning was reduced by context extinction for both infants and adolescents, but for infants it was also reduced by CS extinction, whereas it was not for adolescents. Obviously, and unfortunately, effects of the extinction treatments on expression of contextual fear conditioning could not be determined fully for adults, because their contextual fear conditioning occurred only in the unpaired condition. Similar age-related differences in responding to components of a compound stimulus previously have been reported for analogous circumstances (e.g., Lariviere et al., 1990; Kucharski & Spear, 1985; Mellon et al., 1991).
Effects of extinction of components on expression of conditioning to the entire CS-context compound were complicated by emergence of sex differences in the older animals. For infants, fear to the CS-context compound was unaffected by extinction of either context alone or the CS alone, implying the same strong differentiation between a compound and its individual components observed for infants in other paradigms (e.g., Lariviere et al., 1990; Mellon et al., 1991; Spear & Kucharski, 1984). Responding to the CS-context compound by adolescent males and females also was unaffected by context extinction, and the females were unaffected by CS extinction, as occurred for infants. But unlike infants (and more like adults) expression of the CS-context compound conditioning was decreased by CS extinction in male adolescents. In adults, sex differences were more marked and the conclusions correspondingly more complex. Expression of conditioning by males was decreased by CS extinction (as occurred for male adolescents, but not infants) but unaffected by context extinction (as occurred for infants and both male and female adolescents). Like adolescent females, expression of CS-context compound conditioning by adult females was unaffected by CS extinction; and, although there appeared to be an increase in expression of this conditioning due to context extinction, this was at most a weak effect, possibly aberrant.
The observed conservation of responding to the CS-context compound after context or CS extinction treatments is perfectly consistent with the idea that infant rats have acquired a configural representation of the CS-context compound, which apparently is not weakened by generalization (during the extinction treatment) neither from context, nor from the CS. Although we have already assumed a generalization process to explain contextual responding in those infants exposed to CS-US pairings during the acquisition phase, it seems reasonable to expect a diminished role of generalization as a consequence of the extinction treatment. During the extinction phase, the main effect of presenting any nominal component alone (i.e., context or CS) should be an acquired representation of the negative relation between the component and the reinforcer. This new representation would be responsible for the decrement observed in responding to the extinguished component (as we have found for context) and would also facilitate the discrimination (and, therefore, diminish the generalization) between the component and the compound (Spence, 1937). Such a discrimination should protect the configural representation of the CS-context compound from extinction. Empirical evidence about improved discrimination as a consequence of extinction may be already found in the classic papers of Friedman and Guttman (1965) or Kalish and Haber (1963).
An interesting question is raised by the observation that --only for infant rats-- the CS extinction treatment led to extinction of responding to the training context but not to the CS-context compound. As just discussed, our data suggest that -because we did not observe the decrement in responding to the CS-context compound expected if generalization from the CS to the compound had occurred-- generalization from the component to the compound did not play a relevant role as a consequence of the CS extinction treatment, which would have been an obvious way to explain the decrement in response to context, once the CS-context configural representation had weakened through generalization of CS extinction. A second possibility would be the occurrence of some degree of generalization from the context where the CS extinction was delivered (i.e., Context 1) to the training context (i.e., Context 2). However, the lack of contextual generalization shown in the CXTCTL group (whose animals behaved similarly to many others from our own laboratory, who received the same treatment, but without the contextual extinction of Phase 3; e.g., Mckinzie & Spear, 1995) suggests that this one was not the cause. Given the former considerations, the decrement in responding to the contextual cues as a consequence of the CS extinction treatment becomes somewhat puzzling. A tentative explanation would be to consider that the infants also acquired an amodal representation (in terms of intensity) of the context alone during the intertrial intervals of the acquisition phase, and another amodal representation of the CS alone during the CS extinction treatment. Because any component alone should be encoded as a “less intense” stimulus than the compound (Kraebel & Spear, 2000), the CS extinction treatment would teach the animal that the CS (or, in amodal terms, a stimulus of less intensity than the CS-context compound) is not being followed by the US. Therefore, a decrement in response to other “less intense” stimuli --like the contextual cues presented at testing-- should be expected.
It should be noted that the present study was not primarily intended as a rigorous test of competing theories (e.g., Bahrick & Lickliter, 2000, 2002; Rescorla & Durlach, 1981). It is clear that there are more definitive tests of a perceptual configuration theory than postconditioning extinction of one of the nominal components of the compound. One such test is negative patterning (e.g., Kehoe & Graham, 1988), but we have attempted for several years to develop a negative patterning paradigm consistent with the present procedures, without success. It is well known that, to establish negative patterning, a substantial number of training trials are required for rats and --in our hands, at least-- we could not do so without employing too many footshocks and such a long training period that the rats were no longer infants at the point of the critical tests. We nevertheless believe that the empirical relationship between ontogeny and the efficacy of contextual fear conditioning, and --to some extent-- compound conditioning in general, is made more clear by the present experiments.
The present results have some straightforward implications for understanding the role of hippocampus in Pavlovian fear conditioning. The hippocampus is considered by many as a neuroanatomical structure necessary for stimulus competition in mammals (Anagnostaras et al., 2001; Fanselow & Poulos, 2004; Winocur, Rawlins, & Gray, 1987). Expression of conditioning to context when the CS is the best predictor of US occurrence --as shown by these infants--, may be seen as an instance of lack of stimulus competition. Because infant rats are assumed to have an inmature hippocampus (Pugh & Rudy, 1996; Rudy & Morledge, 1994), a lack of stimulus competition between the CS and the context is predicted from this perspective. Thus, the data from our infants --suggesting such a lack of stimulus competition-- provide further support for this widely accepted view of the hippocampus role.
As already discussed, only our adolescent and adult male rats showed a decrement in responding to the CS-context compound as a consequence of the CS extinction treatment. This finding is consistent with the idea of males (but not females) acquiring an elemental representation of the CS, that is, the best predictor of US occurrence in their experimental condition. Thus, they were showing evidence of greater stimulus competition than females from the same age periods. Such observation seems at consonance with evidence showing greater hippocampal development in males from some rodent species (e.g., Jacobs, 1996; Jacobs, Gaulin, Sherry, & Hoffman, 1990). Although quite a speculative idea at present, the sex differences we have documented may eventually provide further support for the view of the hippocampus as the neuroanatomical structure underlying stimulus competition in the rat brain.
Acknowledgments
This research was funded by a Dirección General de Investigación Científica y Técnica Fellowship (Spain) to Francisco J. Esmorís-Arranz, and by a National Institute of Mental Health Grant (5R37 MH35219) to Norman E. Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Francisco J. Esmorís-Arranz, Universidad de Santiago de Compostela
Cástor Méndez, Universidad de Santiago de Compostela.
Norman E. Spear, Binghamton University (SUNY)
References
- Anagnostaras SC, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. Journal of Comparative and Physiological Psychology. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Bahrick LE, Lickliter R. Intersensory redundancy guides attentional selectivity and perceptual learning in infancy. Developmental Psychology. 2000;36:190–201. doi: 10.1037//0012-1649.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrick LE, Lickliter R. Intersensory redundancy guides early perceptual and cognitive development. Advances in Child Development and Behavior. 2002;30:153–187. doi: 10.1016/s0065-2407(02)80041-6. [DOI] [PubMed] [Google Scholar]
- Barrett BA, Rizzo T, Spear NE, Spear LP. Stimulus selection in passive avoidance learning and retention: Weanling, periadolescent and young adult rats. Behavioral and Neural Biology. 1984;42:23–32. doi: 10.1016/s0163-1047(84)90388-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Ricker ST. Renewal of extinguished responding in a second context. Animal Learning & Behavior. 1994;22:317–324. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:333–350. [Google Scholar]
- Brasser SM, Spear NE. A sensory-enhanced context facilitates learning and multiple measures of unconditioned stimulus processing in the preweanling rat. Behavioral Neuroscience. 1998;112:126–140. doi: 10.1037//0735-7044.112.1.126. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiology of Learning and Memory. 2004;81:46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Carew MB, Rudy JW. Multiple functions of context during conditioning: A developmental analysis. Developmental Psychobiology. 1991;24:191–209. doi: 10.1002/dev.420240305. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2004;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Friedman H, Guttman N. Further analysis of the various effects of discrimination training upon stimulus generalization gradients. In: Mostosfsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. pp. 255–267. [Google Scholar]
- Hallam SC, Grahame NJ, Miller RR. Exploring the edges of Pavlovian contingency space: An assessment of contingency theory and its various metrics. Learning and Motivation. 1992;23:225–249. [Google Scholar]
- Hofer MA. Toward a developmental basis for disease predisposition: The effect of early maternal separation on brain, behavior, and cardiovascular system. In: Weinder H, Hofer MA, Stunkard AJ, editors. Brain, behavior, and bodily disease. New York: Raven Press; 1980. pp. 209–227. [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology & Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hurwitz HMB, Davis H. The description and analysis of conditioned suppression: A critique of the conventional suppression ratio. Animal Learning & Behavior. 1983;11:383–390. [Google Scholar]
- Jacobs LF. Sexual selection and the brain. Trends in Ecology and Evolution. 1996;11:82–86. doi: 10.1016/0169-5347(96)81048-2. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Gaulin SJC, Sherry DF, Hoffman GE. Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Sciences. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish HI, Haber A. Generalization: I. Generalization gradients from single and multiple stimulus points. II. Generalization of inhibition. Journal of Experimental Psychology. 1963;65:176–181. doi: 10.1037/h0046826. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Graham P. Summation and configuration: Stimulus compounding and negative patterning in the rabbit. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:320–333. [Google Scholar]
- Kraebel KS, Spear NE. Infant rats are more likely than adolescents to orient differentially to amodal (intensity-based) features of single-element and compound stimuli. Developmental Psychobiology. 2000;36:49–66. [PubMed] [Google Scholar]
- Kraebel KS, Vizvary L, Spear NE. Stimulus intensity modulates associative and nonassociative responding in preweanling rats. Developmental Psychobiology. 1998;32:199–214. doi: 10.1002/(sici)1098-2302(199804)32:3<199::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Spear NE. Potentiation and overshadowing in preweanling and adult rats. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:15–34. doi: 10.1037//0097-7403.11.1.15. [DOI] [PubMed] [Google Scholar]
- Lariviere NA, Chen W, Spear NE. The influence of olfactory context on Pavlovian conditioning and its expression in preweanling (16-day-old) and adult rats. Animal Learning & Behavior. 1990;18:179–190. [Google Scholar]
- Marlin NA. Contextual associations in trace conditioning. Animal Learning & Behavior. 1981;9:519–523. [Google Scholar]
- McKinzie D, Spear NE. Ontogenetic differences in conditioning to context and CS as a function of context saliency and CS-US interval. Animal Learning & Behavior. 1995;23:304–313. [Google Scholar]
- Mellon RC, Kraemer PJ, Spear NE. Development of intersensory function: Age-related differences in stimulus selection of multimodal compounds in rats as revealed by Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:448–464. doi: 10.1037//0097-7403.17.4.448. [DOI] [PubMed] [Google Scholar]
- Miller RR, Barnet RC, Grahame NJ. Assessment of the Rescorla-Wagner model. Psychological Bulletin. 1995;117:363–386. doi: 10.1037/0033-2909.117.3.363. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning: VI. Learned and unlearned responses to visual stimulation in the infant hooded rat. Developmental Pychobiology. 1985;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Myers CE, Gluck MA. Context, conditioning, and hippocampal rerepresentation in animal learning. Behavioral Neuroscience. 1994;198:835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. Background stimuli and the inter-stimulus interval during Pavlovian conditioning. Quarterly Journal of Experimental Psychology. 1975;27:387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Developmental Psychobiology. 1996;29:87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Durlach PJ. Within-event learning in Pavlovian conditioning. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 81–112. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rosas JM, Bouton ME. Renewal of a conditioned taste aversion upon return to the conditioning context after extinction in another one. Learning and Motivation. 1997;28:216–229. [Google Scholar]
- Rudy J. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy J, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;198:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Solheim GS, Hensler JG, Spear NE. Age-dependent contextual effects on short-term active avoidance retention in rats. Behavioral and Neural Biology. 1980;30:250–259. doi: 10.1016/s0163-1047(80)91138-3. [DOI] [PubMed] [Google Scholar]
- Spear NE. Memory storage factors in infantile amnesia. In: Bower G, editor. The psychology of learning and motivation. Vol. 13. New York: Academic Press; 1979. pp. 91–154. [Google Scholar]
- Spear NE. Ecologically determined dispositions control the ontogeny of learning and memory. In: Kail R, Spear NE, editors. Comparative perspectives on the development of memory. Hillsdale, NJ: Erlbaum; 1984. pp. 325–358. [Google Scholar]
- Spear NE, Kucharski D. Ontogenetic differences in the processing of multi-element stimuli: Potentiation and overshadowing. In: Roitblatt H, Bever T, Terrace H, editors. Animal cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 545–567. [Google Scholar]
- Spear NE, Kucharski D, Hoffmann H. Contextual influences on conditioned taste aversion in the developing rat. In: Braverman N, Bronstein P, editors. Experimental assessments and clinical applications of conditioned food aversions. New York: New York Academy of Sciences; 1985. pp. 42–53. [DOI] [PubMed] [Google Scholar]
- Spear NE, McKinzie DL, Arnold HM. Suggestions from the infant rat about brain dysfunction and memory. In: Delacour J, editor. The memory system of the brain. Singapore: World Scientific Publishing; 1994. pp. 278–315. [Google Scholar]
- Spear NE, Kraemer PJ, Molina JC, Smoller DE. Developmental change in learning and memory: Infantile disposition for unitization. In: Delacour J, Levy JCS, editors. Systems with learning and memory abilities. Amsterdam: Elsevier/North-Holland Press; 1988. pp. 27–52. [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44:430–444. [Google Scholar]
- Winocur G, Rawlins JNP, Gray JA. The hippocampus and conditioning to contextual cues. Behavioral Neuroscience. 1987;101:617–625. doi: 10.1037//0735-7044.101.5.617. [DOI] [PubMed] [Google Scholar]