Abstract
Recent genome-wide association studies have identified fibroblast growth factor receptor (FGFR)2 as one of a few candidate genes linked with breast cancer susceptibility. In particular, the disease-predisposing allele of FGFR2 is inherited as a 7.5-kb region within intron 2 that harbors eight single nucleotide polymorphisms. The relationship between these single nucleotide polymorphisms and FGFR2 gene expression remains unclear. Here we show the common occurrence of polymorphisms within the intron 2 region in a panel of 10 breast cancer cell lines. High FGFR2-expressing cell lines such as MCF-7 cells displayed polymorphic sequences with constitutive histone acetylation at multiple intron 2 sequences harboring putative transcription binding sites. Knockdown of Runx2 or CCAAT enhancer binding protein β in these cells resulted in diminished endogenous FGFR2 gene expression. In contrast FGFR2-negative MDA-231 cells were wild type and showed evidence of histone 3/4 deacetylation at the rs2981578, rs10736303, and rs7895676 disease-associated alleles that harbor binding sites for Runx2, estrogen receptor, and CCAAT enhancer binding protein β, respectively. Histone deacetylation inhibition with trichostatin A resulted in enhanced acetylation at these intron 2 sites, an effect associated with robust FGFR2 reexpression. Isoform analysis proved reexpression of the FGFR2-IIIc variant the splicing of which was positively influenced by trichostatin A-mediated recruitment of the Fas-activated serine/threonine phosphoprotein survival protein. Our findings highlight the potential role of histone acetylation in modulating access to selected polymorphic sites within intron 2 as well as downstream splicing sites in generating variable FGFR2 levels and isoforms in breast cancer.
We show that breast cancer intron 2 FGFR2 polymorphisms alter histone modifications modulating access to transcription factor binding sites that can drive gene expression.
Fibroblast growth factors (FGFs) have been widely implicated in cell differentiation and proliferation in many tissues. Signaling is mediated through a family of four transmembrane receptor tyrosine kinases, a number of which are dysregulated in developmental and neoplastic conditions (1). Each receptor is composed of three Ig-like extracellular domains, two of which are involved in ligand binding, a single transmembrane domain, a split tyrosine kinase, and a C-terminal tail with multiple autophosphorylation sites (1). Multiple forms of cell-bound or secreted forms of fibroblast growth factor receptor (FGFR)1, -2, and -3 are generated by alternative transcription, alternative initiation, alternative splicing, exon switching, or variable polyadenylation (2).
Recent genome-wide association studies have identified FGFR2 in particular as one of a few candidate genes linked with breast cancer (3,4). These studies identified the minor disease-predisposing allele of FGFR2 to be inherited as a region within intron 2 harboring eight single nucleotide polymorphisms (SNPs). The relationship between these SNPs and FGFR2 expression and function have naturally become of increasing interest. Earlier studies described variable FGFR2 expression including reduced levels in primary breast cancers compared with normal breast tissue (5). However, other studies have shown that FGFR2 is overexpressed and/or amplified in breast cancer cell lines (6). The basis for these discrepant findings remains unclear.
The FGFR2 gene is alternatively spliced at the third extracellular Ig-like domain to generate FGFR2-IIIb (also referred to as KGFR or Ksam-IIC1) that binds FGF1, FGF3, FGF7, and FGF10 with high affinity (7,8) or FGFR2-IIIc (also referred to as Bek) that binds FGF1 and FGF2 but not FGF7 or FGF10 (9,10). FGFR2-IIIb expression is characteristically noted in epithelial cells, whereas FGFR2-IIIc is typically expressed in mesenchymal cells (11). FGFR2 exon switching from the IIIb to the IIIc isoform has frequently been observed to accompany epithelial cell transformation (12). Indeed, higher expression of FGFR2-IIIc than FGFR2-IIIb in metastatic breast carcinomas has been reported (13). FGFR2 may also undergo splicing at the C terminus giving rise to the so-called C1/C2, and C3 subisoforms; the FGFR2-IIIb-C3 splice variant (also called Ksam-IIC3), in which the C terminus is shorter than that of wild-type FGFR2-IIIb and lacks the putative phospholipase Cγ binding site, has been described in the immortalized human mammary epithelial cell line H16N2 (14). In this report we examine the extent to which mechanisms by which the recently described intron 2 polymorphisms impact the expression of FGFR2 gene expression and isoform splicing.
Results
The FGFR2 intron 2 polymorphisms are frequent in human breast carcinoma cell lines
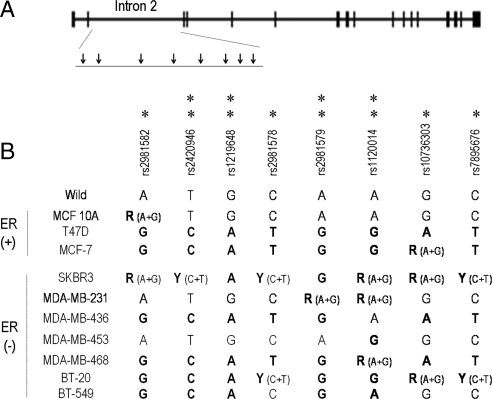
Thus far, eight SNPs situated within intron 2 of the human FGFR2 gene have been described to be associated with increased breast cancer susceptibility (3,4). Figure 1 depicts the FGFR2 gene locus and the intron 2 SNPs numbered as described in recent genome-wide studies (3,4). Characterization of the FGFR2 intron 2 SNPs in a panel of estrogen receptor (ER)-positive and ER-negative breast cancer cell lines is detailed in Fig. 1. This analysis revealed two or more SNPs in most cell lines. The near normal epithelial line MCF-10A showed only a single polymorphism. The MDA-231 cells were polymorphic at only a single intron 2 site, and MDA-453 cells were polymorphic at two intron 2 sites, but these SNP loci do not contain recognized putative transcription binding sites.
Figure 1.
The FGFR2 intron 2 polymorphisms are frequent in human breast carcinoma cell lines. Shown is the FGFR2 gene locus, and the intron 2 SNPs numbered as described in genome-wide studies (3,4). The SNP positions associated with increased breast cancer risk reported in Ref. 3 are denoted by a single asterisk; those reported in Ref. 4 are denoted by a double asterisk.
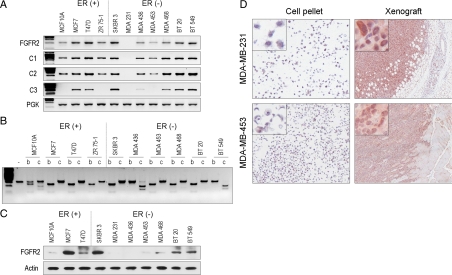
To determine the relationship between intron 2 SNPs and FGFR2 expression, we used RT-PCR, restriction isoform analysis, and western blotting. This examination confirmed the frequent expression of FGFR2 (Fig. 2A), mainly of the IIIb isoform (Fig. 2B), in most cell lines including ER (+) as well as ER (−) lines. MDA-436 and BT-549 cells expressed only the IIIc isoform whereas MCF-10A cells expressed the IIIb and IIIc isoforms. In general, cell lines with only a single polymorphism expressed lower levels of FGFR2 (Fig. 2C). This examination also revealed a relative abundance of the C1/C2 isoforms with less frequent expression of the FGFR2-IIIb-C3 isoform that lacks the putative phospholipase Cγ binding site (14). Significant up-regulation of FGFR2 in MDA-231 and MDA-453 was noted after xenografting in SCID mice (Fig. 2D). It should be noted that most FGFR2 antibodies recognize the C terminus, the region of least homology among the FGFRs. Thus, aberrant FGFR2 splicing within the cytoplasmic domain may partially explain the discrepant mRNA and protein expression in some breast carcinoma cell lines, such as MDA-436, that lack the C3 region of the C terminus (Fig. 2, A and C). It may also explain some of the conflicting data in earlier reports on FGFR2 down-regulation in breast carcinomas (5).
Figure 2.
FGFR2 mRNA expression, isoform splice variants, and protein expression in human breast carcinoma cell lines. A, Total RNA was examined by RT-PCR using primers that cover the FGFR2 coding region (FGFR2). Primers specific to the cytoplasmic C-terminal splice variants (C1, C2, C3) are shown immediately below. RNA integrity was verified by amplification of the phosphoglycerate kinase housekeeping gene. B, Unique restriction site permits distinction of the IIIb isoform which contains an AvaI restriction site (b) from the IIIc isoform that contains an EcoRV digestion site (c). Longer exposures of MCF-10A digests show two restricted products following AvaI or EcoRV enzymatic treatment consistent with dual isoform expression in this cell line. C, Western blotting of total cell lysates using an antibody that recognizes the C terminus of FGFR2. D, Two low-FGFR2-expressing cell lines as indicated show gene up-regulation upon xenografting into SCID mice as demonstrated by immunocytochemistry. Inset photomicrographs represent higher magnifications of corresponding images. PGK, Phosphoglycerate kinase.
Histone acetylation potently modifies FGFR2 expression in MDA-231 breast carcinoma cells
To examine potential mechanisms responsible for variable FGFR2 expression in breast carcinoma, we compared the DNA methylation status of the 5′-FGFR2 promoter in the different cell lines. This examination failed to show any degree of DNA methylation in any of the cell lines examined (data not shown).
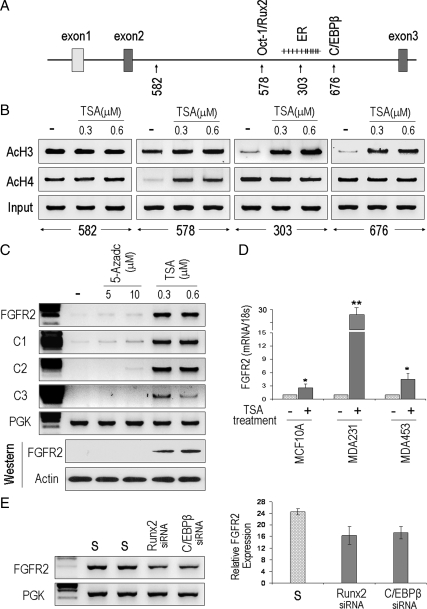
At least two cis-regulatory intron 2 SNPs have been described to alter affinity for the transcription factors Oct-1/Runx2 (site 676; Fig. 3A) and CCAAT enhancer binding protein (C/EBP)β (site 578; Fig. 3A), which may play a role in driving FGFR2 expression (15). This region of intron 2 also contains multiple potential CpG methylation sites particularly surrounding SNP 303 (Fig. 3A), which harbors a putative ER binding site (15). However, estrogen treatment alone was insufficient to induce endogenous FGFR2 expression (data not shown). Similarly, no difference in DNA methylation within this intronic region between the different cell lines was identified (data not shown). These findings prompted us to examine whether chromatin modifications could represent an alternative mechanism modulating accessibility to these putative transcription binding sites. Figure 3A depicts the SNP sites within intron 2 that were targeted for chromatin immunoprecipitation (ChIP) analysis. Interestingly, the sequence surrounding the 303 and 676 SNP sites, which harbor ER and C/EBPβ-binding sites, respectively (15), showed relative histone 3 deacetylation compared with the other SNP sites in MDA-231 cells (Fig. 3B). In contrast, site 578 revealed histone 4 deacetylation. These histone modifications were effectively reversed after histone deacetylase (HDAC) inhibition with trichostatin A (TSA) treatment (Fig. 3B). Note that site 582 (Fig. 3B) and site 014 (not shown) showed no histone 3 or histone 4 modifications in the absence or presence of TSA. Moreover, histone deacetylation was not observed in the other cell lines examined (data not shown). It should be emphasized that MDA-231 cells showed deficiency of FGFR2 expression (Fig. 2) and absence of polymorphisms at four of the eight intron 2 SNPs (Fig. 1). That these histone modifications are functionally related to endogenous gene expression was verified by the potent effect of TSA in driving FGFR2 reexpression (Fig. 3C). Similarly, TSA treatment of the two other low FGFR2-expressing lines (MCF-10A and MDA-453), which harbor wild-type sequences at the 578, 303, and 676 sites, also resulted in measurable up-regulation of endogenous gene expression (Fig. 3D). In contrast to these findings, other cell lines including MCF-7 and MDA-468, which were polymorphic at these sites (Fig. 1), showed uniform basal H3/H4 acetylation, a feature not further enhanced by TSA treatment or induction of gene expression (data not shown). Instead, down-regulation of Runx2 or C/EBPβ in the high-FGFR2-expressing MCF-7 cells resulted in reduction of FGFR2 expression (Fig. 3E). Taken together, these findings suggest an important contribution from histone 3/4 acetylation in modifying access to multiple intron 2 sites. In contrast, the polymorphic sites appear to be associated with constitutive histone acetylation and hence not amenable to further gene induction by HDAC inhibition.
Figure 3.
Histone acetylation modulates access to binding sites within intron 2 of FGFR2. A, Schematic representation of the four intron-2 SNPs that were targeted for ChIP analysis. The corresponding putative transcription factor binding sites are shown immediately above. B, Sites rs10736303 (303) (the ER binding site) and rs7895676 (676) (the C/EBPβ binding site) show relative deacetylation in FGFR2-deficient MDA-231 cells. Site rs2981578 (578) harboring a potential Oct/Runx2 binding site displays H4 deacetylation. C, Consistent with these features endogenous gene expression at the mRNA (upper panel) and protein levels (lower panel) are induced by the HDAC inhibitor TSA but not by the DNA methylation inhibitor AZC at the indicated doses in MDA-231 cells. D, Quantitative real-time PCR confirms the positive effect of TSA treatment (+) on FGFR2 mRNA expression in different cell lines as indicated. E, Down-regulation through siRNA directed at Runx2 or C/EBPβ in the FGFR2-expressing MCF-7 cells results in reduction of the endogenous receptor’s expression. Respective scrambled controls are represented by an “S” and the phosphoglycerate kinase housekeeping gene is represented by PGK. AcH3, Antiacetylhistone 3.
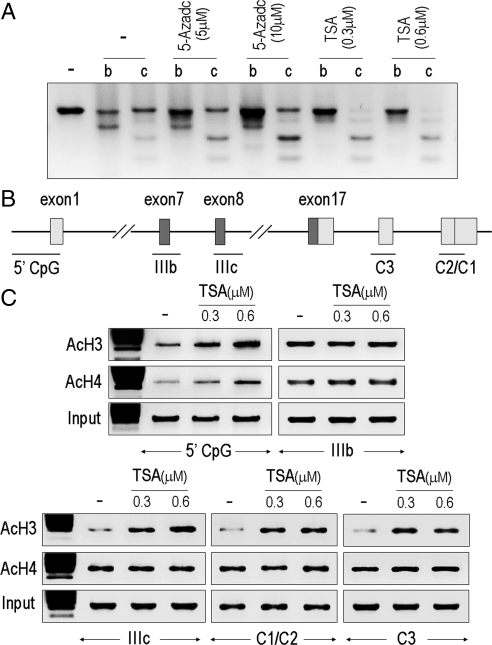
To gain further insight into the impact of histone modifications on FGFR2 expression, we characterized isoform splicing after HDAC inhibition with TSA. Figure 4A demonstrates the potent effect of TSA, but not the DNA methylation inhibitor 5-Aza-2′-deoxycytidine (AZC), on the re-expression of FGFR2 in MDA-231 cells mainly in the IIIc isoform with little impact on the IIIb isoforms. Consistent with this finding, ChIP analysis revealed that TSA results in selective histone acetylation surrounding the IIIc and the C1/C2 splice junctions, schematically shown in Fig. 4B, but not surrounding the IIIb intron/exon junctions (Fig. 4C).
Figure 4.
HDAC inhibition results in selective reexpression of the FGFR2-IIIc isoform. A, MDA-231 breast cells with very low FGFR2 expression (Fig. 2) were treated with AZC or TSA at the indicated doses. To detect the FGFR2-IIIb and IIIc isoforms before and after treatment in these cells, 10 μg of PCR product from five separate reactions was pooled for restriction analysis as shown. Only TSA treatment enhances reexpression mostly of the IIIc but not the IIIb isoform. B, Schematic representation of the intron/exon junctions that were examined by ChIP assays. C, TSA treatment enhances 5′-promoter region histone acetylation as well as the exon IIIc region. Little impact is observed surrounding the IIIb exon. AcH3, antiacetylhistone 3.
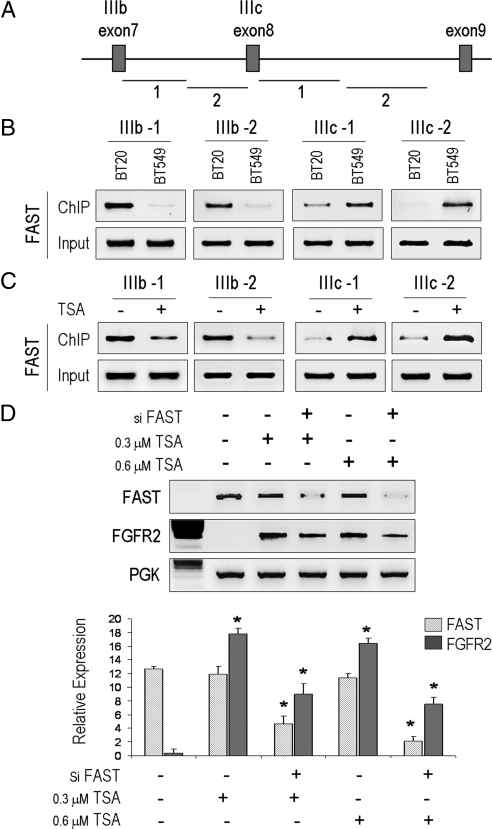
Fas-activated serine/threonine phosphoprotein (FAST) binding to FGFR2 mRNA splicing
Having identified the reexpression of the FGFR2-IIIc isoform after HDAC inhibition, we next asked whether FAST, a survival protein that responds to environmental stress by moving to stress granules, is responsible for favoring FGFR2-IIIc splicing. In reconstitution systems, FAST promotes the inclusion of exons flanked by weak splice-recognition sites (16). To this end, we used a ChIP assay to compare the ability of FAST to bind to the FGFR2-IIIb vs. the IIIc intron/exon junctions in different cell types as depicted in Fig. 5A. This revealed a distinct intron/exon-specific pattern for constitutive FAST binding in BT-20 cells that express the FGFR2-IIIb isoforms (Fig. 5B). In contrast, no appreciable binding to the IIIb junction was observed in BT-549 cells that do not express this FGFR2 isoform. Instead, FAST binding was more evident at the IIIc junction consistent with the predominant expression of this isoform in these cells. Furthermore, given the ability of TSA to potently induce FGFR2 in MDA-231 cells, we used these cells as a model to examine the impact of HDAC inhibition on FGFR2 isoform splicing. Here, TSA treatment resulted in enhanced FAST binding to the IIIc region with minimal, if not diminished, binding to the IIIb region (Fig. 5C). Moreover, down-regulation using a small interfering RNA (siRNA) against FAST abrogated the effect of TSA treatment on FGFR2 expression (Fig. 5D). These findings provide further support for a selective role for FAST in directing FGFR2 splicing.
Figure 5.
HDAC inhibition promotes FAST binding to FGFR2 mRNA splicing. A, Schematic representation of the overlapping regions including exon IIIb/IIIc junctions that were examined by ChIP. B, ChIP analysis demonstrates constitutive FAST binding to the IIIb regions in BT-20 cells, which express the receptor isoform, compared with BT-549, which recognize FAST binding at the IIIc isoform, that they exclusively express (Fig. 2). C, TSA treatment enhances FAST binding selectively to the IIIc region favoring its splicing inclusion in MDA-231 cells. D, FAST down-regulation using an siRNA (si-FAST) in MDA-231 cells abrogates the effect of TSA on FGFR2 induction. PGK, Phosphoglycerate kinase.
Discussion
In this report we perform a detailed examination of FGFR2 expression and isoform splicing as they relate to the recently described germ-line polymorphisms associated with increased breast cancer risk (3,4). Given the previously conflicting data on the expression and action of FGFR2 in different systems, a deeper understanding of FGFR2 isoform expression and regulation in breast cancer has proven essential.
Targeted disruption of FGFR2-IIIb causes agenesis of the lungs, pituitary, thyroid, teeth, and limbs without impact on breast development (17). Variable degrees of FGFR2 mRNA expression, however, have been reported in neoplastic tissues. Down-regulation of FGFR2 has been reported in brain astrocytomas, bladder and prostate carcinomas, thyroid carcinomas, and pituitary tumors (17,18,19,20). Further, forced FGFR2-IIIb expression significantly retards cancer cell proliferation (20,21,22). These actions have been ascribed to several mechanisms including the ability of FGFR2-IIIb to impose on the RAS/BRAF/MAPK pathway to modulate cancer progression (23). Thus, at least one line of evidence supports a tumor-suppressive role for FGFR2 in cancer cells.
More recent studies, however, have suggested that FGFR2 may be an oncogene that is significantly up-regulated in breast cancer (15). In particular, four of the eight recognized intron 2 polymorphisms associated with increased breast cancer risk have been putatively linked with enhanced FGFR2 expression (24,25). Indeed, it has been proposed that four of these sites harbor transcription binding regions serving to drive endogenous gene overexpression. Of these SNPs, sites rs2981578 and rs7895676 have been shown to enhance binding to Oct1/Runx2 and C/EBPβ, respectively (15). Further, we show here that knockdown of Runx2 or C/EBPβ results in diminished endogenous FGFR2 gene expression. More significantly, we show the importance of epigenetic modifications in modulating access to these intron 2 sites. In their wild-type states, we found sites rs2981578, rs10736303 (harboring a putative ER-binding site), and rs7895676 to be relatively histone underacetylated. HDAC inhibition revealed the amenability of these sites to pharmacological manipulation. Such HDAC inhibition results in FGFR2 gene induction in MDA-231, MCF-10A, and MDA-453 cells but not in cells in which these sites are polymorphic. Indeed, other cell lines in which these three sites were polymorphic showed constitutive expression of FGFR2 with little response to TSA-mediated gene induction.
We also examined evidence supporting the significance of FAST in FGFR2 mRNA splicing. FAST is a survival protein that, upon environmental stress, moves to stress granules, where it interacts with TIA1 to modulate stress-induced translational silencing. Both FAST and TIA1 are found in the nucleus, where TIA1 promotes the inclusion of exons flanked by weak splice-recognition sites such as those surrounding the FGFR2-IIIb. A two-hybrid interaction screen recently revealed that FAST binds to several alternative and constitutive splicing regulators. In fact, both FAST and TIA1 promote the inclusion of exon 7 of the FGFR2 mRNA and target a U-rich intronic sequence (IAS1) adjacent to the 5′-splice site of exon 7 to generate the FGFR2-IIIb isoform. Knockdown experiments showed that FAST and TIA1 act independently to promote the inclusion of exon 7 (16). The extent to which such a mechanism is involved in neoplastic cell determination of FGFR2 splicing, however, is unknown. Here we show the first evidence that FAST binds to the exons flanking the major FGFR2 splicing sites. Constitutive FAST binding correlated with IIIb vs. IIIc isoform predominance in BT-20 and BT-549 cells, respectively. Moreover, TSA-mediated up-regulation of FGFR2-IIIc in MDA-231 cells was associated with selective FAST binding to the IIIc, but not IIIb, splicing sites. FAST knockdown in this system resulted in abrogation of the effect of TSA on FGFR2-IIIc induction. Taken together, these findings provide evidence for FAST involvement in directing FGFR2 isoform splicing, a feature with potentially important functional implications given the recognized differences between the actions of FGFR2-IIIb and FGFR2-IIIc (23).
Pharmacological methylation inhibition with AZC has been reported to result in FGFR2-IIIb reexpression in epithelial cancer cells with minimal impact on the IIIc isoform (26). Similarly, FGFR2-IIIb isoform silencing has been described in prostate cancer (27) and in a subset of transitional cell carcinomas of the bladder (20,28). Reexpression of FGFR2-IIIb into T24 bladder cancer cells results in tumor growth inhibition. These data support the notion that FGFR2-IIIb gene inactivation may be implicated in tumor progression rather than a consequence of it. There is also evidence, at least in osteosarcomas and in glioblastomas, that the FGFR2 locus on chromosome 10q26 may be the subject of loss of heterozygosity (29). However, an extensive genome-wide amplification and allelotyping of several human carcinomas has failed to show FGFR2 down-regulation due to such losses (30). Here we show that DNA methylation plays a limited role in silencing FGFR2 gene expression at least in breast carcinoma cell lines.
In summary, our findings highlight the potential role of histone 3/4 acetylation in modulating selected regions within intron 2 as well as downstream splicing sites in generating variable FGFR2 levels and isoforms in breast cancer. Our data provide the first epigenetic explanation toward understanding the functional significance of the recently identified FGFR2 intronic polymorphisms associated with increased breast cancer risk.
Materials and Methods
Cell lines and cultures
Human breast cancer cell lines examined included the ERα-positive T47D, Zr-75-1, MCF7 cells and the ERα-negative SKBR3, MDA-231, MDA-436, MDA-453, MDA-468, BT-20, and BT-549 breast cancer cell lines as well as the near-normal mammary epithelial cell line MCF-10A. MCF-10A were maintained in mammary epithelial cell basal medium (Clonetics Corp., San Diego, CA) supplemented with insulin 5 mg/ml, EGF (10 μg/liter), and cholera toxin (100 ng/ml). MCF-7 cells were maintained in Eagle’s MEM supplemented with 10% fetal bovine serum. All other cell lines were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mmol/liter l-glutamine, 1 mmol/liter sodium pyruvate, and 1× nonessential amino acid (Sigma-Aldrich Co. Ltd., Irvine, UK). Cells were cultured in 10-cm plates at 37 C in 5% CO2 and 95% O2. Cell viability was monitored using trypan blue exclusion before and after all experiments.
For growth in vivo, 5 × 106 cells were injected sc in 6-wk-old female SCID mice for 6–7 wk as described previously (18). Cell pellets were fixed in 10% neutral-buffered formalin, dehydrated, and embedded in paraffin. Sections (4-μm thick) from cell pellets, harvested xenografted tumors, or paraffin-embedded tissue were dewaxed in xylene and rehydrated through graded alcohols into water for immunocytochemistry.
Cell genotyping
Genomic DNA was isolated from all cell lines by standard methods of proteinase K digestion and phenol-chloroform extraction. The eight FGFR2 intron 2 SNP regions (Fig. 1) were amplified by PCR using primers as listed Table 1. PCR was carried out for 10 min at 95 C followed by 40 cycles of 30 sec at 95 C, 30 sec at temperature (Tm) and 30 sec at 72 C, followed by a 10-min extension at 72 C, in a 30 μl reaction mixture containing 1.5 mm MgCl2, 0.2 mm deoxynucleotide triphosphate (dNTP), 0.2 mm of each primer, and 0.25 U/10 μl of AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA). Of the PCR products, 5 μl was loaded on 2.0% agarose gels and visualized with ethidium staining to confirm successful amplification. The remaining 25 μl of the reaction were purified by a PCR kit (QIAGEN, Chatsworth, CA) for automated sequencing.
Table 1.
List of primers and PCR conditions
| Primer | Forward (5′–3′) | Reverse (5′–3′) | Tm (C) |
|---|---|---|---|
| Intron 2-SNP | |||
| rs2981582 | cagcttaccccagacaccactc | tgcactgaaatctgtcatcagtagg | 58 |
| rs2981579 | actcaagtccctgcttgtaggttc | tcagtggtgtgactcccttcatc | 58 |
| rs11200014 | ggctgttcatgacagtgttctcc | ttcaatacctgcgtcttagtggtc | 58 |
| rs1219648 | aagcacgcctattttacttgacac | ttcctcactgtgatttgtatgtgg | 58 |
| rs2420946 | ccagaacttcatcgacctccttc | gaagttccccgagtgagtctttg | 58 |
| rs10736303 | aagggacaaatactccgcacaa | taggaacatgagccagtctggag | 58 |
| rs7895676 | acaaaaattagatgggcatggtg | gcaaaggtggttggttctgac | 58 |
| rs2981578 | ttttctcctgtgttttgcagagg | ctaaagcttccctctgaatgctg | 58 |
| RT-PCR | |||
| FGFR2 (exon 6-9) | ggatcaagcacgtggaaaagaac | ggcgattaagaagacccctatgc | 60 |
| C1 | acactcatcagagtgatgtctggtc | ccatatatacaagtggagacaacaagc | 58 |
| C2 | acactcatcagagtgatgtctggtc | ctgaaagaagggaagagagacg | 58 |
| C3 | acactcatcagagtgatgtctggtc | tctcaatgaagccataaactttcag | 58 |
| FAST | tactgcacagacttcctgctgtg | acaccagcaccaccctctgg | 59 |
| Human PGK | gctgacaagtttgatgagaat | aggactttaccttccaggagc | 58 |
| FAST siRNA | gcagcaaggugguacagatt | uucuguaccaccuugcugctg | |
| Runx2 siRNA | ccaaauuugccuaaccagatt | ucugguuaggcaaauuuggat | |
| C/EBPβ siRNA | ccgccugccuuuaaauccatt | uggauuuaaaggcaggcggcg | |
| Real-time PCR | |||
| Human FGFR2 IIIb | agtgctggctctgttcaatgtg | ggcgattaagaagacccctatg | |
| 18s | aggaattgacggaagggcac | ggacatctaagggcatcaca | |
| Bisulfite sequencing (BS) | |||
| 5′-Promoter | gagtaaagtttggtggaggtaa | caaaaaaataatccttaaatcttc | 51 |
| SNP303 (outer) | gatttgtaagaagattaaattttttatatt | tcccatctaataactaaaaacataaacc | 54 |
| SNP303 (inner) | atattaaatagagattttataaggtatagg | ctaaaatttctaaaaaaatccaactacc | 53 |
| IIIc-CpG (outer) | ggtttgtggaggtgagttggttag | acaatatatacctaacaaaactatcaacc | 56 |
| IIIc-CpG (inner) | tgtggaggtgagttggttagtgttg | ataaaaaaatatcccaataaaattaccc | 58 |
| ChIP | |||
| 5′-Promoter | tcctagtagccagctgtgtggtc | acaaatgaccgaaggctctaacc | 58 |
| IIIb-ChIP-1 | actgtcctgccaaaacagcaag | gcaacaaggtcaagaacagcaac | 58 |
| IIIb-ChIP-2 | tggacgctactaaaggcaaaagg | gagagacacggagcaacactgac | 58 |
| IIIc-ChIP-1 | ctctgcatggttgacagttctgc | aggtaggcaacacactgcttctg | 58 |
| IIIc-ChIP-2 | gataggatggactttcggtttgc | caggcaaactggttaagaagtgc | 58 |
| IIIc-ChIP-3 | tggatcatcgaggactaagaagaag | aaaatgaataaccaggggtccatc | 58 |
PGK, Phosphoglycerate kinase.
Cell treatments
For assessment of DNA methylation, cells were treated with freshly prepared 5 μm or 10 μm of the DNA methyltransferase inhibitor AZC (Sigma, St. Louis, MO) for 5 d. At 48-h intervals, fresh medium containing the drug was added. For assessment of chromatin histone acetylation, cells were treated with 0.3 or 0.6 μm of the HDAC inhibitor, TSA (Sigma) for 24 h. For estrogen stimulation, cells were serum starved overnight and then treated with 17β-estradiol (10−9 to 10−7 m) in the absence or presence of TSA (0.15 μm) for 72 h. For FAST down-regulation, MDA-231 cells were treated with an siRNA (Ambion, Inc., Austin, TX) (60 nm; Table 1) or scrambled sequence control oligonucleotide in the absence or presence of TSA for 48 h. For Runx 2 and C/EBPβ knockdown, MCF-7 cells were treated with validated siRNAs (Ambion; s2456 and s2891, respectively) at 60 nm (Table 1). Each experiment was independently performed in three separate dishes on three different occasions.
Western blotting
Cells were lysed with RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulfonyl fluoride, aprotinin and sodium orthovanadate in PBS). Total cell lysates were quantified by the Bio-Rad method (Bio-Rad Laboratories, Hercules, CA). Whole lysates (50 μg) were separated on 10% sodium dodecyl sulfate denaturing polyacrylamide gels, and transferred onto a nylon membrane (Millipore Corp., Bedford, MA) at 100 v for 1.5 h at room temperature. Blots were incubated with a rabbit polyclonal IgG antibody, which recognizes the C-terminal fragment of FGFR2 (c-17; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:1000 dilution in PBS-5% nonfat milk with 0.1% Tween at 4 C overnight, followed by washing with PBS-Tween 20 four times of 10 min each at room temperature, and then incubated with peroxidase-conjugated goat antirabbit IgG horseradish peroxidase (1:2000) for 1 h at room temperature with agitation. Protein bands were visualized using chemiluminescence (Amersham, Oakville, Ontario, Canada). Experiments were performed on three independent occasions.
RNA extraction and FGFR2 isoform examination
Total RNA was isolated from untreated or cells treated with different concentrations of AZC or TSA using TriZol reagents (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Approximately 1.0 μg of total RNA from each sample was reverse transcribed in a 20-μl vol using the TaqMan reverse transcription reagents kit (Applied Biosystems, Inc., Branchburg, NJ, and Foster City, CA). The reaction mixture was incubated at 25 C for 10 min, 42 C for 30 min, and 95 C for 5 min. The synthesized cDNA was used for PCR amplification or stored at −20 C for further analysis. RT-PCR primers were designed to span exons 6 to 9 of FGFR2, the region that contains exon 7 (encoding the FGFR2-IIIb isoform) and exon 8 (encoding the FGFR2-IIIc isoform) and those spanning the C terminus splice variants (C1, 2, and 3) (Table 1). PCR was carried out for 10 min at 95 C followed by 35 cycles of 30 sec at 95 C, 30 sec at Tm, and 30 sec at 72 C, followed by a 10-min extension at 72 C, in a reaction mixture containing 1.5 mm MgCl2, 0.2 mm dNTP, 0.2 mm of each primer, and 0.375 U of AmpliTaq Gold polymerase (Applied Biosystems). PCR products were separated on 2.0% agarose gels and visualized with ethidium staining.
Restriction digestion
RT-PCR products were purified using Ultrafree-MC centrifugal filter units (Millipore Corp.). Purified PCR products (10 μg) were incubated in a 15 μl volume reaction with 5 U of restriction endonuclease AvaI or EcoRV (Roche, Penzberg, Germany) overnight, at 37 C. Restriction digestion products were separated on 2.5% agarose gels, followed by a 5-min immersion in ethidium bromide for UV exposure. Experiments were performed on three independent occasions.
Quantitative real-time PCR
Total RNA (50 ng) was reverse transcribed in 20 μl reaction mixture containing 250 μm of each deoxynucleotide triphosphate, 20 U ribonuclease inhibitor, and reverse transcriptase (Applied Biosystems). The reaction mixture was incubated at 25 C for 10 min, 48 C for 30 min, and 95 C for 5 min. PCR was performed on cDNA samples in triplicate using an ABI PRISM 7700 sequence detection system (Applied Biosystems). Primers (Table 1) were designed using the Primer Express 1.0 software (Applied Biosystems). As an endogenous control, the human 18 S (Applied Biosytems) was used to normalize for variations in RNA. After optimization, PCRs were performed in a 15-μl volume containing 2 μl cDNA, 200 nm of each primer, and 7.5 μl of Power Sybr Green Master Mix (Applied Biosystems) using the following conditions: 50 C for 2 min, 95 C for 10 min, 40 cycles of 95 C for 15 sec, and 60 C for 1 min. The results were analyzed using a comparative method similar to a standard curve model, except it uses a mathematical formula to derive the same result for relative quantitation as suggested by the manufacturer. The amount of target, normalized to the endogenous reference and relative to a calibrator, is given by the formula 2−ΔΔCt, where Ct represents the threshold cycle, indicating the fractional cycle number at which the amount of amplified target reaches a fixed threshold.
Bisulfite sequencing
Genomic DNA (1 μg) was bisulfite modified according to the manufacturer’s protocol (Chemicon International, Temecula, CA) diluted in 25 μl volume. Modified DNA (1 μl) was used for bisulfite sequencing. Primer sequence and PCR conditions are indicated in Table 1. Two rounds of PCRs were carried out for bisulfite sequencing, both for 10 min at 95 C followed by 40 cycles of 30 sec at 95 C, 45 sec at Tm and 45 sec at 72 C, followed by a 10-min extension at 72 C in a reaction mixture containing 1.5 mm MgCl2, 0.2 mm dNTP, 0.2 mm of each primer, and 0.25 U/10 μl of AmpliTaq Gold polymerase (Applied Biosystems). Final PCR products were cut from 2.0% agarose gels, extracted, and cloned into the TA cloning system (Invitrogen) for automated sequencing. At least 10 positive clones from each sample were sequenced.
ChIP assays
The ChIP assay was performed in accordance with the manufacturer’s recommendations (Upstate Biotechnology, Inc., Lake Placid, NY) and as previously described (31). Briefly, histone was cross-linked to DNA by the direct addition of 37% formaldehyde, and cells were washed with cold PBS containing protease inhibitors before lysis. The lysates were sonicated to shear DNA lengths between 200 and 1000 bp. After centrifugation, cell suspensions were further diluted, and 20 μl of lysate from each sample was used to monitor the amount of DNA present (input DNA) for PCR detection. The rest of the lysate was cleared with salmon sperm DNA/protein G-agarose beads. Immunoprecipitation was performed using rabbit antiserum antiacetylhistone 3 (Lys9), rabbit polyclonal antiacetylhistone 4 antibody (all from Upstate Biotechnology) or goat polyclonal anti-FAST antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); overnight at 4 C with agitation. Negative controls included omission of antibody or use of an anti-IgG antibody. For PCR analysis, the histone-DNA cross-links of eluates were reversed at 65 C, and the immunocomplexes were digested with proteinase-K for 1 h at 50 C, and DNA was finally purified by phenol extraction and used for PCR amplification. PCR primers and conditions are shown in Table 1. PCRs were carried out in a volume of 15 μl containing 1.5 mm MgCl2, 0.2 mm dNTP, 0.2 mm of each primer, and 0.375 U of AmpliTaq Gold polymerase (Applied Biosystems). Experiments were performed on three independent occasions.
Footnotes
This work was supported by the Canadian Institutes of Health Research (Grant MOP-86493).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 4, 2009
Abbreviations: AZC, 5-Aza-2′-deoxycytidine; C/EBP, CCAAT enhancer binding protein; ChIP, chromatin immunoprecipitation; dNTP, deoxynucleotide triphosphate; ER, estrogen receptor; FAST, Fas-activated serine/threonine phosphoprotein; FGFR, fibroblast growth factor receptor; HDAC, histone deacetylase; siRNA, small interfering RNA; SNP, single nucleotide polymorphism; Tm, temperature; TSA, trichostatin A.
References
- Givol D, Yayon A 1992 Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J 6:3362–3369 [PubMed] [Google Scholar]
- Yan G, Wang F, Fukabori Y, Sussman D, Hou J, McKeehan WL 1992 Expression and transformation of a variant of the heparin-binding fibroblast growth factor receptor (flg) gene resulting from splicing of the exon at alternate 3′-acceptor site. Biochem Biophys Res Commun 183:423–430 [DOI] [PubMed] [Google Scholar]
- Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey CS, Bowman R, Meyer KB, Haiman CA, Kolonel LK, Henderson BE, Le Marchand L, Brennan P, Sangrajrang S, Gaborieau V, Odefrey F, Shen CY, Wu PE, et al. 2007 Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447:1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, et al. 2007 A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani YA, Graham M, Coombes RC 1992 Expression of basic fibroblast growth factor, FGFR1 and FGFR2 in normal and malignant human breast, and comparison with other normal tissues. Br J Cancer 66:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannheimer SL, Rehemtulla A, Ethier SP 2000 Characterization of fibroblast growth factor receptor 2 overexpression in the human breast cancer cell line SUM-52PE. Breast Cancer Res 2:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M 1996 Receptor specificity of the fibroblast growth factor family. J Biol Chem 271:15292–15297 [DOI] [PubMed] [Google Scholar]
- Luo Y, Ye S, Kan M, McKeehan WL 2006 Structural specificity in a FGF7-affinity purified heparin octasaccharide required for formation of a complex with FGF7 and FGFR2IIIb. J Cell Biochem 97:1241–1258 [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C, Weston JA 1995 Novel FGF receptor (Z-FGFR4) is dynamically expressed in mesoderm and neurectoderm during early zebrafish embryogenesis. Dev Dyn 203:377–391 [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C 2005 Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol 287:390–402 [DOI] [PubMed] [Google Scholar]
- Baraniak AP, Lasda EL, Wagner EJ, Garcia-Blanco MA 2003 A stem structure in fibroblast growth factor receptor 2 transcripts mediates cell-type-specific splicing by approximating intronic control elements. Mol Cell Biol 23:9327–9337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL 1993 Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol 13:4513–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani YA, Bansal GS, Mortimer C, Buluwela L, Coombes RC 1996 Expression of FGFR2 BEK and K-SAM mRNA variants in normal and malignant human breast. Eur J Cancer 32A:518–524 [DOI] [PubMed] [Google Scholar]
- Moffa AB, Tannheimer SL, Ethier SP 2004 Transforming potential of alternatively spliced variants of fibroblast growth factor receptor 2 in human mammary epithelial cells. Mol Cancer Res 2:643–652 [PubMed] [Google Scholar]
- Meyer KB, Maia AT, O'Reilly M, Teschendorff AE, Chin SF, Caldas C, Ponder BA 2008 Allele-specific up-regulation of FGFR2 increases susceptibility to breast cancer. PLoS Biol 6:e108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro M, Mauger D, Rhee K, Pujana MA, Kedersha NL, Yamasaki S, Cusick ME, Vidal M, Garcia-Blanco MA, Anderson P 2007 Fas-activated serine/threonine phosphoprotein (FAST) is a regulator of alternative splicing. Proc Natl Acad Sci USA 104:11370–11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C 2001 Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231:47–62 [DOI] [PubMed] [Google Scholar]
- St Bernard R, Zheng L, Liu W, Winer D, Asa SL, Ezzat S 2005 Fibroblast growth factor receptors as molecular targets in thyroid carcinoma. Endocrinology 146:1145–1153 [DOI] [PubMed] [Google Scholar]
- Grose R, Dickson C 2005 Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev 16:179–186 [DOI] [PubMed] [Google Scholar]
- Ricol D, Cappellen D, El Marjou A, Gil-Diez-de-Medina S, Girault JM, Yoshida T, Ferry G, Tucker G, Poupon MF, Chopin D, Thiery JP, Radvanyi F 1999 Tumour suppressive properties of fibroblast growth factor receptor 2-IIIb in human bladder cancer. Oncogene 18:7234–7243 [DOI] [PubMed] [Google Scholar]
- Feng S, Wang F, Matsubara A, Kan M, McKeehan WL 1997 Fibroblast growth factor receptor 2 limits and receptor 1 accelerates tumorigenicity of prostate epithelial cells. Cancer Res 57:5369–5378 [PubMed] [Google Scholar]
- Zhang Y, Wang H, Toratani S, Sato JD, Kan M, McKeehan WL, Okamoto T 2001 Growth inhibition by keratinocyte growth factor receptor of human salivary adenocarcinoma cells through induction of differentiation and apoptosis. Proc Natl Acad Sci USA 98:11336–11340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Zheng L, Liu W, Kurebayashi J, Asa SL, Ezzat S 2007 Epigenetically controlled fibroblast growth factor receptor 2 signaling imposes on the RAS/BRAF/mitogen-activated protein kinase pathway to modulate thyroid cancer progression. Cancer Res 67:5461–5470 [DOI] [PubMed] [Google Scholar]
- Naderi A, Teschendorff AE, Barbosa-Morais NL, Pinder SE, Green AR, Powe DG, Robertson JF, Aparicio S, Ellis IO, Brenton JD, Caldas C 2007 A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene 26:1507–1516 [DOI] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavaré S, Caldas C, Miska EA 2007 MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 8:R214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lee K, Asa SL, Ezzat S 2007 Epigenetic silencing through DNA and histone methylation of fibroblast growth factor receptor 2 in neoplastic pituitary cells. Am J Pathol 170:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi B, Latil A, Fournier G, Mangin P, Cussenot O, Berthon P 2002 Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. Prostate 52:245–252 [DOI] [PubMed] [Google Scholar]
- Bernard-Pierrot I, Ricol D, Cassidy A, Graham A, Elvin P, Caillault A, Lair S, Broët P, Thiery JP, Radvanyi F 2004 Inhibition of human bladder tumour cell growth by fibroblast growth factor receptor 2b is independent of its kinase activity. Involvement of the carboxy-terminal region of the receptor. Oncogene 23:9201–9211 [DOI] [PubMed] [Google Scholar]
- Mendoza S, David H, Gaylord GM, Miller CW 2005 Allelic loss at 10q26 in osteosarcoma in the region of the BUB3 and FGFR2 genes. Cancer Genet Cytogenet 158:142–147 [DOI] [PubMed] [Google Scholar]
- Simpson DJ, Bicknell EJ, Buch HN, Cutty SJ, Clayton RN, Farrell WE 2003 Genome-wide amplification and allelotyping of sporadic pituitary adenomas identify novel regions of genetic loss. Genes Chromosomes Cancer 37:225–236 [DOI] [PubMed] [Google Scholar]
- Ezzat S, Yu S, Asa SL 2003 Ikaros isoforms in human pituitary tumors: distinct localization, histone acetylation, and activation of the 5′ fibroblast growth factor receptor-4 promoter. Am J Pathol 163:1177–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]