Abstract
The discovery of intrinsically photosensitive retinal ganglion cells (ipRGCs) has overthrown the long-held belief that rods and cones are the exclusive retinal photoreceptors1, 2. IpRGCs use melanopsin3 as the photopigment, and mediate non-image-forming visual functions such as circadian photoentrainment. In fish, melanopsin has been suggested by in situ hybridization studies to be in retinal horizontal cells (HCs)4-6– lateral association neurons critical for creating the center-surround receptive fields of visual neurons. Are fish HCs, then, possibly also intrinsically photosensitive? This iconoclastic notion was examined previously in flat-mount roach retina, but all HC light response disappeared after synaptic transmission was blocked6, making any conclusion difficult. To directly examine this question, we have now recorded from single, acutely dissociated fish HCs. We found that light induced a response in cone HCs but not rod HCs from catfish, consisting of a modulation of the nifedipine-sensitive, voltage-gated Ca current. The light response was extremely slow, lasting for many minutes. Similar light responses were observed in a high percentage of goldfish HCs. We have cloned from catfish two melanopsin genes and one vertebrate ancient (VA) opsin gene. In situ hybridization indicated that melanopsin, but less likely VA opsin, was expressed in the HC layer of catfish retina. This intrinsic light response may serve to modulate, over a long time scale, lateral inhibition mediated by these cells. Thus, at least in some vertebrates, there are retinal non-rod/non-cone photoreceptors involved primarily in image-forming vision.
We did whole-cell recordings from single, acutely dissociated HCs from catfish retina. This retina has rod-driven HCs and a single type of cone-driven HC7, 8. Rod HCs are larger, with >50-μm-long dendrites and a smooth somatic membrane (Fig. 1a, right)7, 8. Cone HCs are more compact, with short processes and a slender axon-like process often still attached after dissociation, and spine-like protrusions from the soma7, 8 (Fig. 1a, left). We examined first cone HCs because they have been studied much more extensively8-13. When held steadily at -20 to -30 mV (ca. in situ membrane potential in darkness) or more hyperpolarized/depolarized potentials, there was no obvious light-induced current. Catfish cone HCs have four reported voltage-gated currents9, 12: nifedipine-sensitive Ca current, tetrodotoxin (TTX)-sensitive Na current, delayed-rectifier K current, and inward-rectifier K current (Supplementary Information Fig. S1). Accordingly, to screen for a light response more closely, we isolated each current pharmacologically (Methods) and tested the effect of light. Indeed, we observed a modulation of the cone HC's Ca current by light. Rod HCs did not show this effect (see below).
Fig. 1.
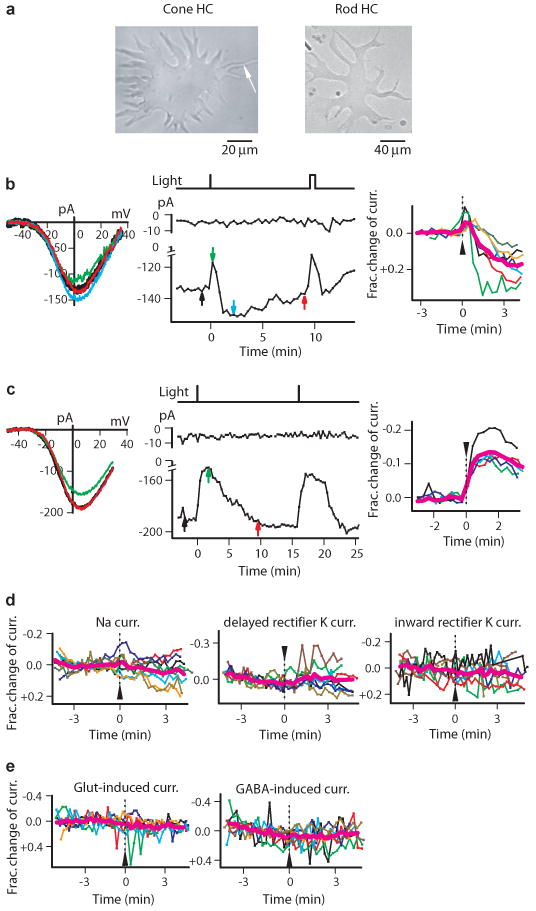
Light modulated the voltage-gated Ca current on catfish cone HCs. a, Dissociated cone HC (left; arrow indicates axon-like process) and rod HC (right). b, Biphasic light response of some cone HCs. Left, Ca current I-V relation before (black), shortly after (green), intermediately after (blue), and long after bright flash (red). Middle, time course of Ca-current modulation by light. Peak current plotted. Vertical bars at top indicate flashes. Colored arrows correspond to measurements on left panel. In this and subsequent figures, input resistance of recorded cell (to monitor recording stability) is indicated at top by the membrane current elicited by repetitive, 200-msec, -3 mV voltage steps. Time 0 designates onset of first light stimulus, as in other panels. 40-μm-diameter (480 nm) light spot centered on soma, delivering 3×108 photons μm-2 s-1 for 1 sec (first) and 30 sec (second). Right, collected results (n=7) showing fractional change in Ca current by light. Magenta shows average. Flash (arrowhead) was (1 to 3) × 108 photons μm-2 s-1 for 1 sec. Ca-Ringer and K-methanesulfonate pipette solution. c, Monophasic light response (decrease in Ca current) of other cone HCs. Same solutions and flash intensity as in b. 5 cells on right. d, Light had no effect on the other voltage-gated currents. Membrane potential stepped from -50 mV to 0 mV for Na current (n=8), to +60 mV for delayed-rectifier K current (n=7), and to -100 mV for inward-rectifier K current (n=8). Same intensity as above but for 2 to 30 sec. 400-, 440-, 540-, 600- and 680-nm light were also tried, but gave no responses either. K-methanesulfonate pipette solution. e, Light had no effect on glutamate- and GABA-induced currents. Same light as in d. Glutamate current recorded in Mg2+-free catfish Ringer and K-methanesulfonate pipette solution, n=6; GABA current recorded in normal catfish Ringer and KCl pipette solution, n=8. Both pipette solutions had 20 μM Cd2+ to block Ca current. Vhold = -50 mV.
In physiological concentrations of extracellular ions, the current-voltage (I-V) relation of the isolated Ca current of a cone HC showed modulation by light between ±30 mV as assayed by voltage-ramps. In darkness, the Ca current was 160±62 pA (mean±SD) at peak (∼0 mV) from 13 cells. For 7 cells, a bright flash (∼1.5×108 photons μm-2 at 480 nm; 40-μm-diameter spot centered on soma; see Methods) transiently reduced this current, then enhanced it for minutes (“biphasic” response, Fig. 1b; by a maximum of 10±4 pA and 28±12 pA respectively, or 9±8 % and 20±9 % of dark value, right panel). For 5 cells, the same flash produced only a slow decrease in the current (“monophasic” response, Fig. 1c; by 25±13 pA, or 14±4 % of dark value, right panel). One cell was not light-sensitive. For both response types, the amplitude but not voltage-dependence of the Ca current was light-sensitive (Fig. 1b and c, left). The biphasic response was likely the composite of a slow decrease and a slow increase of the Ca current, with the decrease occurring sooner. In the monophasic response, the current increase could still be present (see later) but was cancelled by a more delayed and superposing current decrease. The effect of light being on the Ca current can explain why no light response was detected at steady -30 mV or more negative, in that the current was not activated. At steady, more-positive potentials, on the other hand, the Ca current9 was likely inactivated. We attempted to measure a light-induced change in intracellular Ca2+ concentration with Fura-2 under current clamp, but, owing to spontaneous, wide swings in the membrane potential between positive and negative values (also observed by others 9, 12-14 in dissociated HCs) in darkness triggering spontaneous Ca2+-concentration changes, such measurements were not feasible.
The dark Ca current and its light modulation tended to run down with time, due possibly to washout by the whole-cell-pipette solution. A second, identical flash almost invariably elicited a smaller response (e.g., Supplementary Information Fig. S5a), and a third flash gave an even smaller, or no, response. This run-down and the response slowness precluded quantitative measurements such as the intensity-response relation. If flashes were delivered in rapid succession, before the cell's recovery from each, multiple responses could be observed (Supplementary Information Fig. S2). With a step of light, the response was more prolonged as expected (not shown). Light did not affect the voltage-gated Na current, the delayed-rectifier K current, and the inward-rectifier K current (Fig. 1d), or the ionotropic glutamate- and GABA-induced currents8, 10 (Fig. 1e; also Supplementary Information Fig. S3). Thus, light modulated specifically the Ca current. We found the same light effect when all methanesulfonate (standard anion in the pipette solution and impermeant through Cl channels) was replaced by Cl- to give a Cl- equilibrium potential of ∼0 mV, which argued against Cl- involvement in the light response. Finally, although larger than cone HCs, dissociated rod HCs had a smaller Ca current8 (30±11 pA, n=3) in darkness and showed no detectable photosensitivity (data in normal solution not shown, but see Fig. 2c).
Fig. 2.
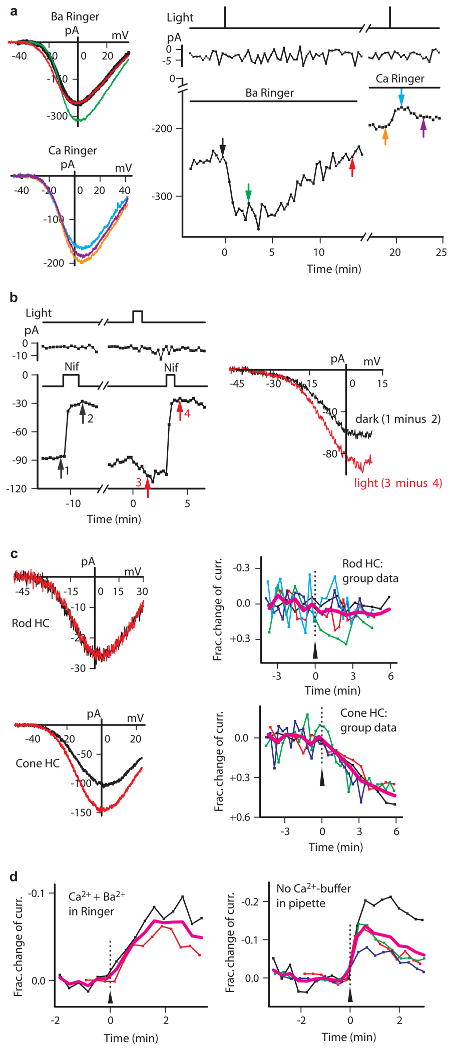
Characterization of catfish cone HC light response. a, Light only increased current in Ba solution. Time gap ∼2 min. Identical flashes. K-methanesulfonate pipette solution. b, Ba current and its light-induced increase were both sensitive to nifedipine (Nif, 10 μM). Time gap ∼5 min. c, Ba current of cone HCs (bottom), but not rod HCs (top), was light-sensitive. Left shows sample cell (black, before light; red, ∼3 min after light). n=5 (top) and n=4 (bottom) in group data. Simplified Ba-Ringer and Cs-methanesulfonate pipette solution in b and c. d, Ca2+ influx required for light-induced current decrease. Left, Ca2+ (3 mM) in Ba-Ringer restored light-induced current decrease. n=2 and average (magenta). K-methanesulfonate pipette solution. Right, omitting 10 mM BAPTA in pipette still gave light-induced current decrease (n=4). K-methanesulfonate pipette solution. Ca-Ringer. In all cases, light was 480 nm, (0.8 to 10)×108 photons μm-2 s-1 for 1-50 s.
Substitution of Ba2+ for Ca2+ extracellularly (Methods) increased the current through the Ca channels on cone HCs by ∼80 % (from 217±41 pA to 400±128 pA, n=5) and slowed its rundown13, 15. The associated light response was also larger. Interestingly, light produced only an increase but no initial decrease in Ba current, which lasted many minutes; again, the amplitude but not the voltage dependence was affected (Fig. 2a). Even a cone HC that gave only a light-induced decrease in Ca current in normal solution showed a light-induced increase in Ba-solution (Fig. 2a). Altogether, we observed a light response in Ba-solution in 108 of 132 recorded cone HCs, with a “threshold” flash intensity of ∼1 × 106 photons μm-2 (480 nm) for eliciting any detectable response. The dark Ba current and its modulation by light was blocked by 10-μM nifedipine (Fig. 2b, n=6), suggesting L-type Ca channels being involved and consistent with their presence in catfish cone HCs9, 13. Again, only cone HCs but not rod HCs (even with more intense light and at different wavelengths) showed modulation of the Ba current by light (Fig. 2c), although the Ba current on rod HCs was also nifedipine-sensitive (Supplementary Information Fig. S4).
A light-induced decrease in Ca current but not Ba current suggested that a Ca2+ influx through the Ca channel was necessary. Indeed, a light-induced current decrease reappeared when Ca2+ was added to the Ba-solution, (Fig. 2d, left). Also, the strong Ca2+ buffering in our typical pipette solution (10 mM BAPTA and no Ca2+; Methods) did not eliminate the light-induced decrease in Ca current, while removing all Ca2+ buffering in the pipette did not make much difference either, although the light-induced Ca-current decrease appeared somewhat faster and larger (Fig. 2d, right). Thus, Ca2+ might act very close to the Ca channel, where it entered the cell, to affect a light-sensitive target. The possibility that the light-induced current decrease originated instead from the opening of a Ca-activated K current16 is unlikely because this is inconsistent with the constant form of the I-V relation in light and darkness as we observed.
We probed the spectral sensitivity of the cone HC light response. We used only the Ba-solution because of the otherwise run-down of the Ca current, thus could examine only the light-induced current increase. By reiterated pair-wise comparisons between two wavelengths in a given cell (Supplementary Information and Fig. S5), we inferred a spectral-effectiveness ordering of 400 nm < 440 nm < 480 nm > 520 nm > 540 nm > 560 nm > 600 nm > 680 nm. Thus, the λmax of the underlying pigment is between 440 nm and 520 nm. This ordering is tentative because the method in principle applies only to bleachable pigments, which nonetheless include all known vertebrate pigments except possibly for melanopsin (see below), the bleachability of which remains unclear17.
Some teleosts have been reported to express melanopsin or vertebrate ancient (VA) opsin, or both, in their HCs4-6, 18, 19. VA opsin is a pigment of largely unknown function. By degenerate RT-PCR based on known melanopsin and VA opsins (Methods), we isolated from catfish retina the cDNA clones for two distinct melanopsin genes (catfish OPN4m1 and OPN4m2, based on sequence comparison with other fish melanopsins20) and two alternatively spliced cDNAs of a VA opsin gene (catfish VA and VAL, with VAL having a longer and different C-terminus19) (see Methods, Supplementary Information Fig. S6, and Tables S1 and S2). In situ hybridization labeling on flat-mount retina (Fig. 3a, left) indicated that, based on its depth of location in the retinal thickness and other landmarks, the OPN4m1- and OPN4m2-positive cells were situated near the outer margin of the inner nuclear layer (INL), where HCs are situated. The same labeling in retinal cross-sections supported this conclusion (Fig. 3a, right, and Supplementary Information Fig. S7). OPN4m1 was found also in cells near the inner margin of the INL, likely amacrine cells and/or displaced ganglion cells. This distribution pattern of melanopsin mRNA was found in most other fishes examined4-6. VA/VAL opsin transcripts were predominantly in the ganglion cell layer and less in the inner nuclear layer (Fig. 3b and Supplementary Information Fig. S7), therefore less likely in HCs6, 18, 19. Thus, the intrinsic light response of catfish cone HCs is possibly triggered by melanopsin, although VA/VAL's involvement cannot be completely excluded. There are other putative pigments in the retina, including teleost multiple tissue (TMT) opsin21 and neuropsin (OPN5)22, but their exact locations remain unknown.
Fig. 3.
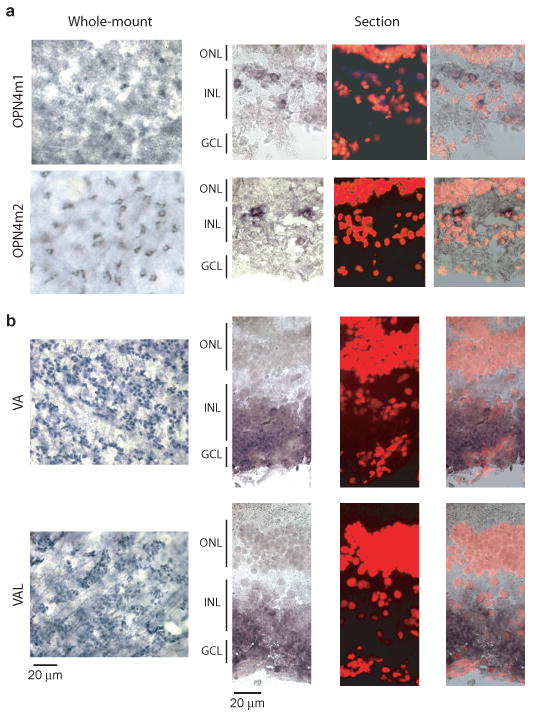
Melanopsin but perhaps not VA/VAL opsin was expressed in HC-layer of catfish retina. a, In situ hybridization of OPN4m1 and OPN4m2 mRNA. Left, digoxigenin (DIG) staining of flat-mount retina. Small, dark-blue areas surrounding hollow cores (presumably the nuclei) indicate stained cell cytoplasm. Right, Same experiments on retinal cross-sections showing DIG signal, propidium iodide (PI) nuclear staining to mark retinal layers, and the two merged. Catfish retinal layers are not very distinctive, with sparse nuclei in the inner nuclear layer7. b, In situ hybridization of VA/VAL opsin mRNA. Left, DIG staining of flat-mount retina. Dark-blue dots are the stained cells, situated close to the retina inner surface with blood vessels (arrows) evident in the same focal plane. Right, Same experiments on retinal cross-sections. In both a and b, cells appear smaller in the flat-mount retina than in retinal cross-sections owing to some tissue shrinkage during processing. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
We also examined goldfish retina. Its dissociated HCs are identifiable by their relatively large size and flat somata with stellar processes14 (Fig. 4a), albeit difficult to be further distinguished into the subtypes I through IV identified in whole retina23. In Ba-solution, the only solution tried, we observed a light-induced, similarly slow current increase in the majority of HCs recorded (Fig. 4b, 18 out of 24 cells; by 34±20 pA at peak, or 37±33 % over that in darkness, triggered by 5 ×108 photons μm-2 s-1 for 0.5-1s at 480 nm). The remaining HCs gave no light responses (Fig. 4c). This percentage of photoresponsive goldfish HCs was remarkably high, but could have resulted from the dissociation procedure biasing the yield of particular HC subtypes.
Fig. 4.
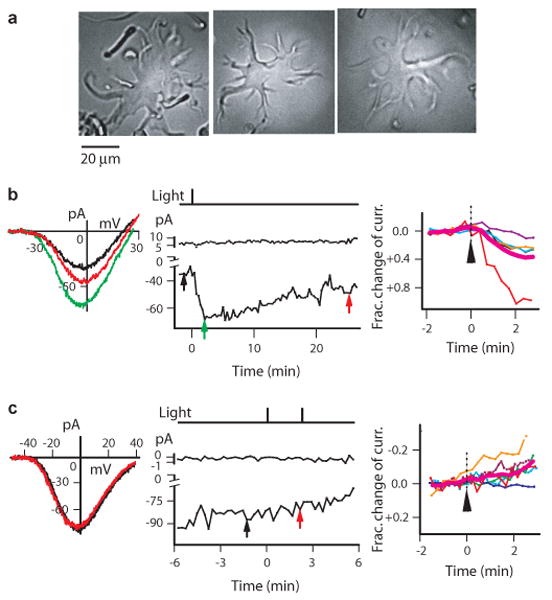
Many goldfish HCs also showed light responses. a, Dissociated goldfish HCs. Left two responded to light, but right one did not. b, Light increased Ba current in some HCs. Left and middle, representative cell (left cell in a). Right, collected data and average (magenta) from 5 cells. c, Non-photoresponsive HCs under same conditions as in b. Left and middle, representative cell (right cell in a). Right, collected data and average (magenta) from 6 cells. In both b and c, flash was 5 ×108 photons μm-2 (480 nm), simplified Ba-Ringer and Cs-methanesulfonate pipette solution.
In summary, we have established the presence of intrinsically photosensitive horizontal cells (ipHCs) in the fish retina. In catfish, these are specifically cone HCs, with a flash threshold of perhaps ∼106 photons μm-2 (480 nm), or 104 – 105 fold less sensitive than fish cones (see Supplementary Information), not greatly different from that of intrinsically photosensitive retinal ganglion cells (ipRGCs) in mouse17. With this low sensitivity, it makes sense at least that the intrinsically photosensitive HCs are associated with cone rather than rod circuitry. In compensation, the prolonged response (lasting for minutes) should make temporal summation extremely effective, especially for sensing steady ambient light. If the intrinsic photosensitivity exists all over the cell, the large surface area of HCs will also capture ambient light quite effectively. The HC light response in this study appears distinct from the supposed intrinsic light response previously reported by others6 for in situ HCs in the roach flat-mount retina. This other response6, although having an action-spectrum λmax (∼477 nm) distinct from those of roach rods and cones, was observed in only 43 out of more than 1000 cells, was found in rod HCs, and, finally, disappeared in the presence of synaptic blockers, which made a definitive conclusion of intrinsic photosensitivity difficult.
Unlike mammalian ipRGCs, which mediate predominantly non-image-forming visual functions2 albeit not exclusively24, 25, the ipHCs are almost certainly involved primarily in image vision because HCs are principal elements in the retinal circuitry generating the antagonistic surround of the bipolar cell's receptive field. With the operating range of HC membrane potential under in situ conditions being typically -20 mV to -65 mV, the corresponding range for light modulation would be ∼-20 mV to -30 mV, within which the Ca current is active. This narrow range means that the effect of light is likely subtle. Because of the extremely slow intrinsic HC light response, ambient light may alter, on a slow time scale, the bipolar-cell receptive-field surround, such as by modulating the HCs' electrical coupling or synaptic properties. The relation of intracellular Ca2+ concentration to HC electrical coupling and to HC synaptic-transmitter release in fish has been studied, but the details are nonetheless unsettled especially on a slow time scale11, 26-28. Many questions remain, such as by what mechanism light affects the HC Ca current, what specific function this intrinsic photosensitivity serves in the fish retina, and whether light has additional (and possibly electrically silent) effects in these HCs. What is clear from this work on ipHCs and previous work on ipRGCs1, 2, however, is that the types of intrinsically-photosensitive retinal neurons can be much more complex than previously thought.
Methods Summary
Retina dissociation and electrophysiology
The fish was euthanized and the eyes removed in dim red light. Subsequent procedures were performed under infra-red light. The retina-dissociation procedure for obtaining HCs followed published methods12, 29. After visual identification, an HC was voltage-clamped with whole-cell, patch-clamp recording at 23°C. The access resistance (generally 20-40MΩ) was not compensated but should introduce an error of under a few millivolts because the measured Ca current was small. Liquid-junction potentials were measured and corrected. Voltage ramps (45-100 mV s-1) were used to elicit Ca/Ba currents in most experiments (Supplementary Information Fig. S8). Light stimuli were delivered via an optical fiber to the microscope, with strength varied by changing the intensity and/or duration of the flash. Perforated-patch recording (with nystatin, amphotericin B or gramicidin) was also attempted, but the membrane under the pipette invariably ruptured to become whole-cell recording in < 30 min, as indicated by Lucifer Yellow entering the cell from the pipette solution. With the light response being extremely slow, this time duration would allow at most one or two light responses. Accordingly, this method was not used.
Cloning of melanopsins and VA/VAL opsin from catfish retina
cDNA was synthesized from catfish-retina total RNA, and used as template for nested degenerate PCR. Primers were designed based on the conserved regions in known melanopsin and VA opsin amino-acid sequences. Conventional 5′- and 3′- RACE were performed to obtain full-length cDNA sequences. Products were cloned into a commercial vector and sequenced. Sequences with more than three identical clones were considered true.
In situ hybridization of melanopsins and VA/VAL opsin mRNA in catfish retina
RNA probes were made against cloned catfish OPN4m1 and OPN4m2 sequences, and against the C-termini of catfish VA and VAL opsins. Conventional in situ hybridization method for whole-mount embryos was adapted for whole-mount staining of catfish retina. In situ hybridization of fresh-frozen retinal sections was also performed (see Methods in PDF file).
Methods
Retina dissociation and electrophysiology
Channel catfish (Ictalurus punctatus) about 1-ft long were purchased from Osage Catfisheries (Missouri) and maintained in 14/10 light/dark cycles (light on 7 AM – 9 PM) at the Johns Hopkins School of Medicine animal facility. Experiments were done in daytime hours of noon – 8 PM. The procedure for dissociating the catfish retina was largely as published12. Before experiments, a catfish dark-adapted for ∼ 60 min was euthanized in dim-red light with an institution-approved protocol, the eyes were excised and the cornea, iris and lens removed. The posterior eyecup was incubated in catfish low-Ca2+ dissociation Ringer (in mM: 123 NaCl, 0.3 CaCl2, 4 KCl, 2 MgCl2, 15 glucose, 10 HEPES, 1 sodium pyruvate, pH 7.4) with 0.2-0.3 mg/ml hyaluronidase (Sigma type I-S) for 4 min, then in the same base dissociation solution with 20-28 U/ml papain (Fluka) for 4-6 min. Afterwards, the retina was peeled from the eyecup, cut into 6-8 pieces and incubated further in base dissociation solution with 4-20 U/ml papain for 3-12 min. The retina pieces were rinsed four times in base dissociation Ringer with 0.1 mg/ml BSA and transferred into normal catfish Ringer (in mM: 118 NaCl, 3 CaCl2, 4 KCl, 2 MgCl2, 15 glucose, 10 HEPES, pH 7.4) and kept on ice until use. When needed, HCs were dissociated from pieces of tissue by mechanical trituration with a fire-polished Pasteur pipette.
The dissociation procedure for goldfish (Carassius auratus) retina also followed a published procedure29. Goldfish 4-6 inch long were purchased from local pet stores and maintained in 12/12 light/dark cycles. Experimental procedures were largely similar to above. Goldfish low-Ca2+ dissociation Ringer contained (in mM): 117 NaCl, 0.5 CaCl2, 2.5 KCl, 5 MgCl2, 16 glucose, 10 HEPES, 1 sodium pyruvate, 1 NaHCO3, 1 NaH2PO4, pH 7.3). Eyecups were made, and the retina was peeled off and incubated in hyaluronidase solution (Sigma type I-S, 1 mg/ml in base dissociation Ringer) for 10 min. The retina was rinsed in base dissociation Ringer, cut into 4-6 pieces, transferred into papain solution (Fluka, 1 mg/ml in base dissociation Ringer) for 30 min, and rinsed four times in base dissociation Ringer containing 0.1 mg/ml BSA. The retinal pieces were transferred to normal goldfish Ringer (in mM: 122 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, 16 glucose, pH 7.3) and kept on ice until use as above.
To pharmacologically isolate each of the four known voltage-gated ion currents in catfish cone HCs, the following blockers or their combinations were added, or equimolar-substituted, to the normal fish Ringer9, 12. 10 μM TTX was added to block the voltage-gated Na current. 20 μM Cd2+ was added to block the voltage-gated Ca current. 20 mM TEA-Cl and 10 mM 4-AP were equimolar-substituted for NaCl to block the delayed-rectifier K current, and 1 mM Ba2+ was added to block the inward-rectifier K current. For example, the Ringer (“Ca-Ringer”) for isolating the Ca current contained (in mM): 94 NaCl, 3 CaCl2, 4 KCl, 2 MgCl2, 15 glucose, 10 HEPES, 10 μM TTX, 20 TEA-Cl, 10 4-AP, 1 BaCl2, pH 7.4. “Ba-Ringer” had 3 mM Ba2+ in place of the 3 mM Ca2+, in addition to the 1 mM Ba2+ to block the inward-rectifier K current. The substitution of Ba2+ for Ca2+ shifted the I-V relation by a few millivolts in the hyperpolarized direction30 (see Fig. 2a). Normal internal (i.e., pipette-) solution had (in mM): 105 K-methanesulfonate, 10 BAPTA, 10 HEPES, 10 NaCl, 4 ATP-Mg, 0.3 GTP-Na, pH 7.3 (“K-methanesulfonate-based pipette solution”). In later experiments, to record a stable Ba current for a longer period, we replaced all external Na+ and K+ with TEA+ (in mM): 4 BaCl2, 120 TEA-Cl, 3 MgCl2, 15 glucose, 10 HEPES, pH 7.4 (referred to as “simplified Ba-Ringer”), and substituted Cs+ for K+ in the pipette solution (in mM): 95 Cs-methanesulfonate, 10 BAPTA, 10 HEPES, 20 TEA-Cl, 4 ATP-Mg, 0.3 GTP-Na, pH 7.3 (“Cs-methanesulfonate-based pipette solution”)15. Pipette solution without Ca2+ buffer contained (in mM): 120 K-methanesulfonate, 10 HEPES, 10 NaCl, 4 ATP-Mg, 0.3 GTP-Na, pH 7.3. “KCl-based pipette solution” contained (in mM): 100 KCl, 10 BAPTA, 10 HEPES, 10 NaCl, 4 ATP-Mg, 0.3 GTP-Na, pH 7.3. All solutions had 260-265 mOsm.
Membrane currents elicited with voltage ramps gave similar results as those with voltage steps (Supplementary Information Fig. S8). The Ca current was measured from recordings as shown in Supplementary Information Fig. S9.
The light stimuli were generally a 40-μm-diameter spot centered on the soma of the cell. The light-spot size was chosen to illuminate enough membrane area yet to avoid other dissociated cells and debris. Larger light spots (with twice the diameter) were occasionally used but gave qualitatively similar results. 200-μm-diameter light spots were used for catfish rod HCs because these cells were larger. The microscope did not allow changing the light-spot size or moving it to different parts of the cell during the experiment without disturbing the recording.
Cloning melanopsins and VA/VAL opsin from catfish retina
Total RNA was isolated from catfish retina using TRIzol Reagent (Invitrogen), and reverse-transcribed into cDNA with SuperScript II reverse transcriptase (Invitrogen). Degenerate PCR primers were manually designed from a highly conserved region of 8-10 amino acids. Degeneracy was generally kept below 100-fold and idenosine was used when necessary in the 5′-region of the primers. A second set of primers were designed using the program CODEHOP (consensus-degenerate hybrid oligonucleotide primers: http://blocks.fhcrc.org/help/CODEHOP/CODEHOP_help.html). Nested degenerate PCR was performed with Taq polymerase. Phusion DNA polymerase (Finnzymes) was used for PCR in 5′- and 3′- RACE.
The DNA sequences of degenerate PCR products were translated into amino-acid sequences and aligned with those of known melanopsins and VA opsins using Vector NTI (Invitrogen). The translated sequences were also used as templates to search for similar sequences in the genome database using NCBI Blast.
The accession numbers for catfish OPN4m1 and OPN4m2 are, respectively, FJ839437 and FJ839438. The accession numbers for VA and VAL opsins are FJ839435 and FJ839436, respectively.
In situ hybridization of melanopsins and VA/VAL opsin mRNAs in catfish retina
T7 or Sp6 RNA polymerase (New England Biolabs) was used to synthesize both sense and antisense RNA probes tagged with digoxigenin. For whole-mount staining, retinas were fixed in 4% paraformaldehyde, digested by proteinase K, post-fixed, washed with PBS, pre-hybridized and then hybridized overnight at 68°C, followed by post-hybridization steps. Anti-digoxigenin alkaline phosphatase was used for color development. Cross-sections (25-μm thickness) of fresh-frozen eyecups were made using a cryostat and processed to examine more clearly in which layer(s) the DIG signal was located.
Supplementary Material
Acknowledgments
This work was supported by an NIH grant and the António Champalimaud Vision Award (Portugal) to K.-W.Y. We thank Richard Johnson, Chunqiao Liu and Dan Liu for advice on molecular cloning and in situ hybridization staining, Ying Shen, Cindy Linn and Gary Matthews for suggestions on retinal dissociation. Dwight Bergles, Paul Fuchs and Jeremy Nathans offered excellent comments and suggestions throughout the work. Leonardo Belluscio was extremely generous by letting N.C. take a brief leave of absence from her postdoctoral work to return to Hopkins in order to complete some experiments. Members of the Yau laboratory, especially Michael Do and Donggen Luo, as well as Kartikeya Murari, have kindly provided comments on the manuscript.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions: N.C., T.T. and K.-W.Y. discussed and designed the experiments. N.C. performed all of the experiments, with T.T. offering technical advice. N.C. and K.-W.Y. wrote the paper together.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position. Brain Res Mol Brain Res. 2002;107:128–136. doi: 10.1016/s0169-328x(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 5.Drivenes O, et al. Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J Comp Neurol. 2003;456:84–93. doi: 10.1002/cne.10523. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins A, et al. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr Biol. 2003;13:1269–1278. doi: 10.1016/s0960-9822(03)00509-8. [DOI] [PubMed] [Google Scholar]
- 7.Naka K, Carraway NR. Morphological and functional identifications of catfish retinal neurons. I. Classical morphology. J Neurophysiol. 1975;38:53–71. doi: 10.1152/jn.1975.38.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Dong C, Picaud S, Werblin F. GABA transporters and GABAC-like receptors on catfish cone- but not rod- driven horizontal cells. J Neurosci. 1994;14:2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingai R, Christensen BN. Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol. 1986;56:32–49. doi: 10.1152/jn.1986.56.1.32. [DOI] [PubMed] [Google Scholar]
- 10.O'Dell TJ, Christensen BN. Horizontal cells isolated from catfish retina contain two types of excitatory amino acid receptors. J Neurophysiol. 1989;61:1097–1109. doi: 10.1152/jn.1989.61.6.1097. [DOI] [PubMed] [Google Scholar]
- 11.DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SF, Linn CL. Activation of NMDA receptors linked to modulation of voltage-gated ion channels and functional implications. Am J Physiol Cell Physiol. 2003;284:C757–68. doi: 10.1152/ajpcell.00252.2002. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Dixon DB, Copenhagen DR. Modulation of a sustained calcium current by intracellular pH in horizontal cells of fish retina. J Gen Physiol. 1993;101:695–714. doi: 10.1085/jgp.101.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean BP. Whole-cell recording of calcium channel currents. Methods Enzymol. 1992;207:181–193. doi: 10.1016/0076-6879(92)07013-e. [DOI] [PubMed] [Google Scholar]
- 16.Heyer CB, Lux HD. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976;262:349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do MTH, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signaling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photoreceptors. Nature. 1998;394:27–28. doi: 10.1038/27794. [DOI] [PubMed] [Google Scholar]
- 19.Kojima D, Mano H, Fukada Y. Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J Neurosci. 2000;20:2845–2851. doi: 10.1523/JNEUROSCI.20-08-02845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellingham J, et al. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:1334–1343. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moutsaki P, et al. Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res Mol Brain Res. 2003;112:135–145. doi: 10.1016/s0169-328x(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 22.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 23.Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975;159:473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- 24.Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 26.McMahon DG, Mattson MP. Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain Res. 1996;718:89–96. doi: 10.1016/0006-8993(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 27.Ayoub GS, Lam DM. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- 29.Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24:9752–9759. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linn CL, Gafka AC. Activation of metabotropic glutamate receptors modulates the voltage-gated sustained calcium current in a teleost horizontal cell. J Neurophysiol. 1999;81:425–434. doi: 10.1152/jn.1999.81.2.425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


