Abstract
The numerous sigma (σ) factors present in Mycobacterium tuberculosis are indicative of the adaptability of this pathogen to different environmental conditions. In this report, we describe the M. tuberculosis σB regulon and the phenotypes of an M. tuberculosis sigB mutant strain exposed to cell envelope stress, oxidative stress, and hypoxia. The sigB mutant was especially defective in survival under hypoxic conditions in vitro, but it was not attenuated for growth in THP-1 cells or during mouse and guinea pig infection.
σ factors are components of RNA polymerases that bind to the enzyme's core subunits and give promoter specificity. The presence of 13 σ factors in Mycobacterium tuberculosis reflects the ability of this microorganism to adapt to various stress conditions that are likely encountered during host infection and that make M. tuberculosis a successful pathogen. σB is closely related to the primary sigma factor σA in terms of amino acid sequence (4). sigB, the structural gene for σB, is induced by different stresses (12) and is positively regulated by three extracytoplasmic function sigma factors, σE, σH, and σL (3, 13, 14). In vitro transcription studies showed that isolated mycobacterial RNA polymerases containing σE, σH, and σL can transcribe sigB using the same transcription start site (3). σF-containing RNA polymerase was also shown to transcribe sigB in these studies by using a different transcriptional start site. However, transcriptome studies with sigF mutants or strains overexpressing σF show no changes in sigB expression (7, 11), suggesting that the σF RNA polymerase transcription of sigB observed in biochemical experiments may not be physiological. The autoregulation of σB has also been recently determined by primer extension and reverse transcription-PCR (RT-PCR) (11).
In M. tuberculosis, the response to cell envelope is regulated by σE and the response to oxidative stress and heat shock is regulated by σH, and σB is a component of both regulons (13, 14). The fact that sigB expression is controlled by many regulatory pathways suggests that σB plays a central role in the M. tuberculosis stress response. In this report, we describe the in vitro phenotype of an M. tuberculosis sigB mutant exposed to stress conditions related to the activation of σE and σH. To analyze the extent to which the response to stress of σE and σH is transmitted through σB, we studied the σB regulon activated by cell envelope and oxidative stress in vitro. We also evaluated the growth of the M. tuberculosis sigB mutant strain in THP-1 macrophage-like cells and in vivo in the mouse and guinea pig models of infection.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Escherichia coli strains JM109 and GM161 were grown in Luria broth (LB) (Difco) at 37°C. Antibiotic concentrations used to isolate selectable markers in E. coli were as follows: kanamycin, 50 μg/ml; streptomycin, 20 μg/ml; and hygromycin, 50 μg/ml. The M. tuberculosis strains created in this study were derivatives of M. tuberculosis H37Rv. All M. tuberculosis culture conditions, unless otherwise specified, followed standard protocols (9). Bacteria were grown at 37°C on either Middlebrook 7H9 (liquid medium) or 7H10 (solid medium) (Difco) supplemented with 0.5% bovine serum albumin, fraction V (Boehringer Mannheim), 0.2% glucose-0.085% NaCl, 0.2% glycerol, and 0.1% Tween 80. Antibiotic concentrations used for selections in M. tuberculosis were as follows: kanamycin, 20 μg/ml; streptomycin, 20 μg/ml; and hygromycin, 150 μg/ml. Sucrose selection was performed on 7H10 plates with 10% sucrose. Bacterial stocks were maintained at −80°C in supplemented 7H9. Aliquots of bacteria were thawed and cultured on supplemented 7H10 solid medium. For preparation of liquid cultures, bacteria growing in 7H10 agar plates were suspended in 7H9 medium at an optical density at 540 nm (OD540) of 0.05 (5 × 106 CFU/ml). Liquid cultures were grown in plastic roller bottles in a roller apparatus or in 9-ml glass tubes in a rotating wheel.
Cloning and construction of M. tuberculosis sigB mutant and complemented strains.
DNA recombinant techniques were performed by following standard techniques. PCR DNA primers were commercially obtained (IDT), and Pfu DNA polymerase was used for all PCRs. M. tuberculosis genomic DNA from strain H37Rv was used as a template. PCR products were cloned into pCR-Blunt II-PCR-BLUNT II-TOPO and sequenced. Clones with error-free DNA sequences were used for further genetic manipulation. Transformation of plasmids into M. tuberculosis was done by electroporation, and DNA Southern blot analysis from the M. tuberculosis chromosome was performed as previously described (14). To construct the sigB mutant, a 4-kb PstI fragment of M. tuberculosis DNA containing the sigB gene was cloned into the vector pUC19 to construct plasmid pSM229. The sigB gene was disrupted by introducing a kanamycin cassette from pUC4K into a NruI deletion to construct pSM349. A 5.1-kb PstI DNA fragment from plasmid pSM349 was introduced into the SmaI site of the shuttle vector pSM270 (14), creating the plasmid pSM356. pSM356 was electroporated into wild-type M. tuberculosis H37Rv. Several M. tuberculosis clones were selected by kanamycin and sucrose resistance and streptomycin sensitivity. Southern blot analyses performed on chromosomal DNA isolated from a presumed M. tuberculosis mutant clone indicated that it had the disruption of the sigB gene, and this strain was named ST82. For complementation of the sigB mutant, a 1.3-kb DNA fragment containing the sigB gene and including a 274-bp upstream region from the translational start site of this gene (promoter region) was PCR amplified using primers sigB27U17 (5′CGCATCCCGCTGTTCCC3′) and sigB1360L17 (5′CTTGGCCAGCTGCGAAA3′). The DNA fragment was cloned into the PCR-BLUNT II-TOPO vector, creating plasmid pSM753. A HindIII/XbaI fragment from pSM753 was cloned into a HindIII/XbaI deletion site of the vector pMV306-Hyg, creating plasmid pSM754. Plasmid pSM754 was electroporated into strain ST82. The insertion by single crossover of this plasmid into the attB of strain ST82, to create the ST174 strain, was verified by Southern blot analysis.
Macrophage infections.
Infection of THP-1 human monocytic cells was performed as described before (5). Briefly, 105 THP-1 cells/well were differentiated with 40 nM phorbol myristate acetate in 96-well plates at a multiplicity of infection of 0.05 bacterium per macrophage. At various time points after infection, triplicate wells for each M. tuberculosis strain infection were treated with 0.05% sodium dodecyl sulfate (SDS) to lyse the macrophages, and numbers of CFU were determined by plating appropriate serial dilutions on 7H9 plates. Colonies were counted after 2 to 3 weeks of incubation at 37°C.
Mouse infections.
BALB/c mice were infected intravenously with the sigB mutant, the complemented mutant strain, or the wild-type parental strain. At selected times, three mice were killed and their lungs, spleens, and livers removed and processed for analysis of bacterial survival in these organs. Numbers of CFU were determined by plating appropriate serial dilutions on 7H9 plates. Colonies were counted after 2 to 3 weeks of incubation at 37°C.
Guinea pig infections.
Infections of guinea pigs were performed as previously described (10). Briefly, Hartley strain guinea pigs weighing 250 to 300 g (Charles River Laboratories) were infected via the aerosol route. Five guinea pigs per group were killed at days 1, 21, and 56 after infection, and the survival of the wild type, the mutant, and the complemented strain in the lungs was analyzed by counting CFU in lung homogenates.
In vitro stress treatments.
For DNA arrays, M. tuberculosis strains were cultured in roller bottles until early log phase (OD540, 0.2) at 37°C. Aliquots of 40 ml were treated with SDS or diamide at a final concentration of 0.05% or 5 mM, respectively. After 60 min of incubation at 37°C, bacteria were centrifuged at 3,000 × g and pellets were immediately frozen at −80°C until further processing.
M. tuberculosis RNA from in vitro cultures.
Bacterial cell pellets were suspended in 1 ml TRI reagent (Molecular Research Center, OH) and immediately transferred to a 2-ml screw-cap microcentrifuge tube containing 0.5 ml zirconia beads (0.1-mm diameter; Biospec Products, Inc., OH). Samples were disrupted by two 1-min pulses in a BeadBeater, with the samples kept on ice for 2 min between pulses. RNA from the samples was purified as previously described (5) using RNeasy columns (Qiagen). The purified RNA was kept at −80°C until further use.
cDNA labeling and microarray hybridization.
70-mer oligonucleotides obtained from Qiagen (now Operon) were printed on poly-l-lysine-coated glass (29). Microarray procedures were performed essentially as previously described (25).
Microarray data analysis.
Microarrays were scanned using a GenePix 4000A imaging device (Axon Instruments). Intensities of the two dyes at each spot were quantified using ScanAlyze (Michael Eisen, http://rana.lbl.gov/EisenSoftware.htm). All gene-specific spots on the microarray other than those whose induction ratio was in the top or bottom 5% were used to normalize the intensities of Cy3 and Cy5 from each spot. After Cy3 and Cy5 channel normalization, large-percentage fluctuations in low-background spot values were eliminated by adjusting low-signal-intensity spots to a minimum noise value. The noise value for each channel was determined by calculating the average intensity value for the spots with the lowest intensity (the bottom 20%), and then every value below this average noise value was raised to the noise value. Microarray-determined ratios were calculated from three biological replicates and two microarrays for each biological replicate. A false-discovery rate of <2% and a regulation of at least 1.8-fold were used as criteria to consider a gene differentially regulated. False-discovery rates were determined using the Significance Analysis of Microarray (SAM) program (23).
Quantitative RT and real-time PCR with SYBR green.
RT-PCRs were previously described (6). Primers were designed by using the software OLIGO 6.6 (Molecular Biology Insights, Cascade, CO) and purchased from IDT. For RT, 50 ng of RNA and Transcriptor RT polymerase (Roche) were used. PCRs were carried out in sealed tubes in an Mx4000 spectrofluorometric thermal cycler (Stratagene).
Killing curves after hypoxic treatment.
Cultures of M. tuberculosis strains were inoculated at an OD540 of 0.05 (5 × 106 CFU/ml) in 6 ml of medium, using tubes with a capacity of 9 ml in order to maintain an air/medium ratio of 1:2 (vol/vol) (28). Methylene blue was used as an indicator of oxygen consumption (1). Tubes were sealed and incubated at 37°C with rotation. After 4 days, the cultures reached an OD540 of 0.3, and the loss of blue color indicated that the oxygen had been consumed. Several tubes were prepared in order to be able to open three tubes for each time point to determine the number of CFU/ml in each culture. Tubes were discarded after being opened.
Determination of growth inhibition by zone diffusion assay.
M. tuberculosis strains were grown to exponential phase. Aliquots of 1 ml of culture were spread on 7H10 plates. Paper discs (6.5 mm in diameter; Schleicher and Schuell) containing 20 μl of 10% SDS were placed on top of the agar. The diameters of the zones of inhibition were measured after 10 days of incubation at 37°C.
DNA array data.
DNA array data were deposited in the TB database (http://www.tbdb.org/).
RESULTS
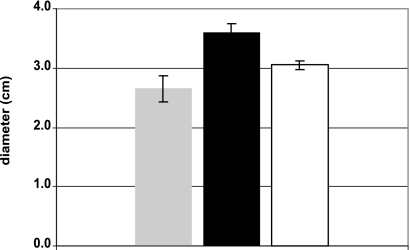
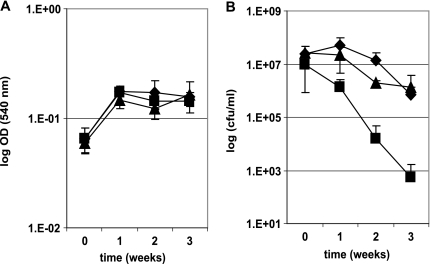
To analyze the σB regulon and the role of this sigma factor on the pathogenicity of M. tuberculosis, we constructed an M. tuberculosis mutant strain by disrupting the sigB gene with a kanamycin cassette as previously described (26). A complemented strain was constructed by reintroducing the sigB gene in the mutant strain by using an integrative plasmid. We compared the survival of the mutant strain to that of the wild-type strain H37Rv and the complemented strain under different conditions. We observed that the sigB mutant was more sensitive to the detergent SDS than the wild-type parental strain in disc assays (Fig. 1). Treatment of M. tuberculosis broth cultures with high temperature (45°C) for short periods (up to 24 h) decreased the survival of the sigB mutant strain in standing but not in rolling cultures (data not shown). This result suggested that the loss of viability of the sigB mutant observed in these experiments was due principally to low levels of oxygen in the standing cultures. To verify this hypothesis, we analyzed the effect of gradual loss of oxygen on the sigB mutant, as wild-type M. tuberculosis is known to survive but not grow under those conditions (27). M. tuberculosis rolling cultures prepared in 9-ml glass tubes were sealed and incubated with rotation at 37°C over long periods of time (up to 3 weeks). In these experiments, we observed that the survival of the sigB mutant strain was highly sensitive to this condition compared to that of the wild type or the complemented strain, showing approximately 3-log-order-lower survival (Fig. 2).
FIG. 1.
Effect of SDS stress on the growth of the M. tuberculosis sigB mutant strain. The effect of the SDS treatment was determined by a disc assay for the H37Rv strain (gray bar), the sigB mutant strain (black bar), and the complemented strain (white bar). The figure shows the arithmetic means ± standard deviations from three biological replicates.
FIG. 2.
Effect of hypoxia treatment on the growth of the M. tuberculosis sigB mutant strain. M. tuberculosis rolling broth cultures were grown at 37°C in sealed tubes. The figure shows the arithmetic means ± standard deviations for three biological replicates represented as ODs (A) or numbers of CFU/ml (B) of the cultures. Diamonds, H37Rv; squares, sigB mutant strain; triangles, complemented strain.
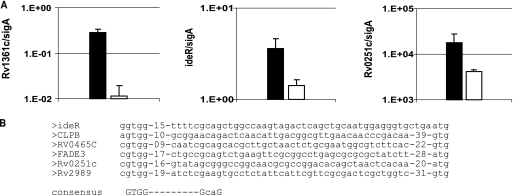
Since both the response to cell envelope stress by σE and the response to oxidative stress by σH converge in the activation of σB, we speculated that the analysis of the σB regulon under these stress conditions may reveal the identities of the σB target genes relevant for M. tuberculosis pathogenicity. Therefore, to analyze the σB regulon, we compared the transcriptional profiles of the sigB mutant strain and the wild-type strain under conditions of cell envelope or oxidative stress. The distribution in functional categories of genes whose expression is regulated by σB when the bacteria are under cell envelope or oxidative stress is summarized in Table 1. Transcriptional comparison of the wild type and the sigB mutant, growing logarithmically in broth cultures, indicated that only eight genes were downregulated in the mutant strain relative to levels of regulation in the wild-type strain (see Table S1 in the supplemental material). However, 28 genes were upregulated in the mutant strain, particularly sigE and several genes known to be part of the σE regulon (14). The upregulation of sigE in the sigB mutant may be due to a competitive regulation in which the absence of one sigma factor, σB, allows more access to core RNA polymerase by other sigma factors that transcribe sigE promoters. Another possibility is that the absence of σB may cause a stress that induces the expression of sigE. Comparison of the transcriptional profiles of the sigB mutant and the wild-type strain after treatment with SDS revealed 72 genes downregulated in the sigB mutant (see Table S2 in the supplemental material). Since sigB is under the regulation of σE, we expected a significant overlap between the σE and the σB regulons. However, the comparison of the transcriptional profiles of both sigma factors after SDS treatment indicated only one gene in common, Rv0465c, annotated to code for a putative transcriptional regulator (http://genolist.pasteur.fr/TubercuList/). Among the genes downregulated in the sigB mutant were many genes related to the cell envelope stress response and also several encoding transcriptional regulators. For example, the expression of ideR was upregulated in bacteria treated with SDS, and this regulation depended on σB, in agreement with other reports (11, 19). As expected, the downregulation of ideR in the sigB mutant caused the concomitant increased expression of genes from the mbt cluster. It has been previously shown that the mbt genes, which encode enzymes for the biosynthesis of siderophores, are repressed by IdeR (8, 20). The analysis of the transcriptional profile of the sigB mutant under oxidative stress induced by diamide treatment (13) indicated 40 genes under σB regulation (see Table S3 in the supplemental material). Again, we expected an overlap between σB and σH regulons, but we found that only two genes, Rv0251c and Rv0384c, encoding heat shock proteins were previously observed to be under σH control. The DNA array under diamide treatment indicated that furA (Rv1909), encoding a peroxidase, is under the control of σB. Interestingly, in a report by Mulder et al. (16), it was shown that there was an increased expression of catalase and peroxidase mRNA after overexpression of σB in Mycobacterium bovis BCG. Moreover, katG is downstream of the furA gene in the same transcriptional unit (15). These observations and our DNA array results suggest that σB regulates the expression of furA and katG in M. tuberculosis. The regulation of ideR (Rv2711), PPE19 (Rv1361c), and hsp20 (Rv0251c) by σB under different stress conditions observed by DNA array analyses was confirmed by quantitative RT-PCR (Fig. 3A). The transcription of ideR by RNA polymerase with σB has also been reported in a previous study that evaluated the effect of σB overproduction on M. tuberculosis gene expression during exponential growth (11). We chose to confirm by RT-PCR that the expression of gene PPE19 (Rv1361c) depends on σB because Rv1361c is one of the few genes whose expression is under σB regulation in nonstressed bacteria, according to the DNA array data. The gene hsp20 was selected for confirmation by RT-PCR since this is a gene whose expression was found to be highly regulated by σB when M. tuberculosis was exposed to oxidative stress. The putative promoter consensus sequence recognized by σB that was defined in a previous study (11) could be identified in some genes whose expression is regulated by σB under stress conditions (Fig. 3B).
TABLE 1.
Distribution in functional categories of σB-regulated genes
| Type of stress | Category | No. of regulated genes |
|---|---|---|
| Cell envelope | Hypothetical proteins | 25 |
| Cell wall-associated genes | 20 | |
| Regulatory proteins | 13 | |
| Intermediate metabolism | 5 | |
| Virulence, detoxification, adaptation | 4 | |
| Information pathways | 4 | |
| Lipid metabolism | 1 | |
| Insertion sequences and phages | 1 | |
| Oxidative | Hypothetical proteins | 15 |
| Intermediate metabolism | 12 | |
| Regulatory proteins | 7 | |
| Virulence, detoxification, adaptation | 4 | |
| Cell wall-associated genes | 3 |
FIG. 3.
Expression of M. tuberculosis genes under the control of σB. (A) The expression of some M. tuberculosis genes found to be differentially regulated by σB in the DNA microarray analyses was confirmed by quantitative RT-PCR with SYBR green. The figure shows the arithmetic means ± standard deviations from three biological replicates. Results are expressed in logarithmic scale. The expression of each gene in strain H37Rv (black bars) or in the sigB mutant (white bars) was normalized to the expression of sigA. Expression levels of Rv1361c in nonstressed broth cultures, ideR in SDS-treated broth cultures, and Rv0251c in diamide-treated broth cultures are indicated. (B) Alignment of sequences of the promoter regions of σB-regulated genes. The previously proposed −35 and −10 σB consensus regions are at the bottom (11).
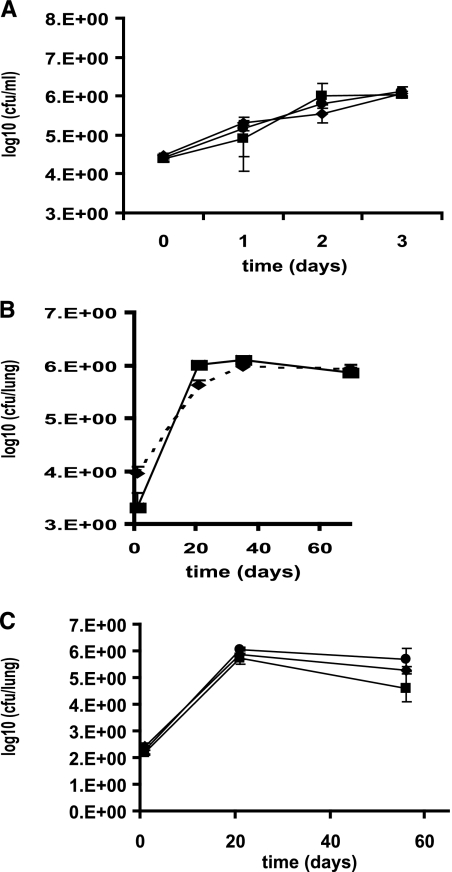
The attenuated phenotype of the sigB mutant strain in broth cultures under cell envelope stress and hypoxia prompted us to evaluate the relevance of σB for M. tuberculosis pathogenicity. The mutant strain did not show differences in growth in human macrophages (Fig. 4A) or survival in the mouse model of lung infection from the wild-type strain (Fig. 4B). The attenuated growth of the mutant in broth cultures subjected to hypoxia suggested that σB probably controls the expression of M. tuberculosis components necessary to tolerate this stress. For that reason, we evaluated the survival of the sigB mutant in the guinea pig model of lung infection, since it has been previously demonstrated that the lung granulomas of guinea pigs infected with M. tuberculosis are a low-oxygen environment (24). However, no differences were observed when the growth of the wild-type strain and that of the sigB mutant were compared (Fig. 4C).
FIG. 4.
Growth of the M. tuberculosis sigB mutant in various models of infection. The survival of the M. tuberculosis wild-type H37Rv strain (diamonds), the sigB mutant strain (squares), and the complemented strain (circles) was analyzed in THP-1 macrophage-like cells (A), intravenously inoculated BALB/c mice (B), and aerosol-infected guinea pigs (C).
DISCUSSION
In this article we have defined the σB regulon activated by cell envelope stress and oxidative stress. Moreover, we have demonstrated that a sigB mutant strain is sensitive to SDS stress and hypoxic treatment in vitro, although the inactivation of the sigB gene did not affect the survival of M. tuberculosis during infection.
σB appears to be a central regulator in the M. tuberculosis response to stress, since its structural gene, sigB, is positively regulated by three other sigma factors. We have identified by DNA microarrays two different sets of genes that were under σB regulation depending on the stress to which M. tuberculosis was exposed (cell envelope stress or oxidative stress). This observation indicates that other regulatory mechanisms should be activated simultaneously in response to a particular stress condition. For example, we reported here that genes previously shown to be important for the pathogenicity of M. tuberculosis, like Rv1130 and Rv1131 (17) or hsp20 (22), are under σB regulation; however, it has been demonstrated that these genes are also under the regulation of σE (14), MprAB (18, 19), and PhoP (26). Surprisingly, we observed only a small overlap between the regulons of σB and those of σE and σH. This observation could be explained because in sigE or sigH mutants, the expression of sigB is not totally abolished, as it is in the sigB mutant. Also, these dissimilar results may be related to the type of DNA arrays used to analyze the samples: more-specific oligonucleotide arrays in this work (25) versus amplicon arrays in previous works (13, 14). We also observed a very small overlap in genes of the M. tuberculosis σB regulon between a previous report (11) and ours, probably due to the different methodologies used for both analyses. In the experiments reported here, the expression of genes in the wild-type strain was compared to the expression of genes in the sigB mutant strain, while in the other study, the transcription of the wild-type strain was compared to the transcription of the strain overproducing σB. Also, evaluation of the regulon under stress, rather than the overexpression of the protein, may be a better representation of the physiological conditions under which activation of the sigma factor occurs. For example, other regulatory mechanisms (i.e., anti-sigma and anti-anti-sigma factors) that may be activated by stress and affect the expression of the regulon might not be activated during overexpression of the sigma factor.
The lack essentially of σB in vivo despite its apparent central role for the M. tuberculosis response to stress could reflect a functional redundancy of M. tuberculosis sigma factors and other regulators in vivo. On the other hand, the differences between the in vivo and in vitro phenotypes of the sigB mutant may indicate a poor representation of M. tuberculosis virulence by the available animal models of M. tuberculosis infection. Similar disparities have been obtained with other M. tuberculosis regulators, like DosR, whose regulon is necessary to sustain the survival of M. tuberculosis in broth cultures under anaerobiosis (25). However, a dosR mutant strain appears dispensable for M. tuberculosis survival in vivo under some conditions (21), although in other in vivo environments, the DosR regulon may be required (2). These observations indicate the complexity of the regulatory network that allows M. tuberculosis to establish intriguing and still-unresolved host-pathogen relationships.
Supplementary Material
Acknowledgments
We acknowledge Target (Tuberculosis Animal Research and Gene Evaluation Taskforce; NIH/NIAID NO1-A/30036) for testing the sigB mutant in the guinea pig model of lung infection and Kevin Visconti and Yevgeniy Senin for technical assistance.
This work was supported by NIH grants RO1 AI-44856 (awarded to I.S.), AI-061505 (awarded to M.I.V.), and RO1 AI44826 (awarded to G.K.S.); European Project StopLATENTTB grant agreement no. 200999 (awarded to L.F.); and European Project Mycomancy contract number LSHO-CT-2006-037566 (awarded to R.M.).
Footnotes
Published ahead of print on 10 July 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43717-731. [DOI] [PubMed] [Google Scholar]
- 2.Converse, P. J., P. C. Karakousis, L. G. Klinkenberg, A. K. Kesavan, L. H. Ly, S. S. Allen, J. H. Grosset, S. K. Jain, G. Lamichhane, Y. C. Manabe, D. N. McMurray, E. L. Nuermberger, and W. R. Bishai. 2009. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect. Immun. 771230-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dainese, E., S. Rodrigue, G. Delogu, R. Provvedi, L. Laflamme, R. Brzezinski, G. Fadda, I. Smith, L. Gaudreau, G. Palù, and R. Manganelli. 2006. Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor σL and roles in virulence and in global regulation of gene expression. Infect. Immun. 742457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doukhan, L., M. Predich, G. Nair, O. Dussurget, I. Mandic-Mulec, S. T. Cole, D. R. Smith, and I. Smith. 1995. Genomic organization of the mycobacterial sigma gene cluster. Gene 16567-70. [DOI] [PubMed] [Google Scholar]
- 5.Dubnau, E., P. Fontán, R. Manganelli, S. Soares-Appel, and I. Smith. 2002. Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect. Immun. 702787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontán, P., V. Aris, S. Ghanny, P. Soteropoulos, and I. Smith. 2008. Global transcriptional profile of Mycobacterium tuberculosis during THP-1 human macrophage infection. Infect. Immun. 76717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiman, D. E., D. Kaushal, C. Ko, S. Tyagi, Y. C. Manabe, B. G. Schroeder, R. D. Fleischmann, N. E. Morrison, P. J. Converse, P. Chen, and W. R. Bishai. 2004. Attenuation of late-stage disease in mice infected by the Mycobacterium tuberculosis mutant lacking the SigF alternate sigma factor and identification of SigF-dependent genes by microarray analysis. Infect. Immun. 721733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold, B., G. M. Rodriguez, S. A. Marras, M. Pentecost, and I. Smith. 2001. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol. Microbiol. 42851-865. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204537-555. [DOI] [PubMed] [Google Scholar]
- 10.Jain, S. K., S. M. Hernandez-Abanto, Q. J. Cheng, P. Singh, L. H. Ly, L. G. Klinkenberg, N. E. Morrison, P. J. Converse, E. Nuermberger, J. Grosset, D. N. McMurray, P. C. Karakousis, G. Lamichhane, and W. R. Bishai. 2007. Accelerated detection of Mycobacterium tuberculosis genes essential for bacterial survival in guinea pigs, compared with mice. J. Infect. Dis. 1951634-1642. [DOI] [PubMed] [Google Scholar]
- 11.Lee, J.-H., P. C. Karakousis, and W. R. Bishai. 2008. Roles of SigB and SigF in the Mycobacterium tuberculosis sigma factor network. J. Bacteriol. 190699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31715-724. [DOI] [PubMed] [Google Scholar]
- 13.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, E. Dubnau, M. Gomez, and I. Smith. 2002. Role of the extracytoplasmic-function sigma factor sigma (H) in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 45365-374. [DOI] [PubMed] [Google Scholar]
- 14.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41423-437. [DOI] [PubMed] [Google Scholar]
- 15.Master, S., T. C. Zahrt, J. Song, and V. Deretic. 2001. Mapping of Mycobacterium tuberculosis katG promoters and their differential expression in infected macrophages. J. Bacteriol. 1834033-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulder, N. J., R. E. Powles, H. Zappe, and L. M. Steyn. 1999. The Mycobacterium tuberculosis mysB gene product is a functional equivalent of the Escherichia coli sigma factor, KatF. Gene 240361-370. [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Elias, E. J., A. M. Upton, J. Cherian, and J. D. McKinney. 2006. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol. Microbiol. 601109-1122. [DOI] [PubMed] [Google Scholar]
- 18.Pang, X., and S. T. Howard. 2007. Regulation of the α-crystallin gene acr2 by the MprAB two-component system of Mycobacterium tuberculosis. J. Bacteriol. 1896213-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang, X., P. Vu, T. F. Byrd, S. Ghanny, P. Soteropoulos, G. V. Mukamolova, S. Wu, B. Samten, and S. T. Howard. 2007. Evidence for complex interactions of stress-associated regulons in an mprAB deletion mutant of Mycobacterium tuberculosis. Microbiology 1531229-1242. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez, G. M., and I. Smith. 2003. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 471485-1494. [DOI] [PubMed] [Google Scholar]
- 21.Rustad, T. R., M. I. Harrell, R. Liao, and D. R. Sherman. 2008. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart, G. R., S. M. Newton, K. A. Wilkinson, I. R. Humphreys, H. N. Murphy, B. D. Robertson, R. J. Wilkinson, and D. B. Young. 2005. The stress-responsive chaperone alpha-crystallin 2 is required for pathogenesis of Mycobacterium tuberculosis. Mol. Microbiol. 551127-1137. [DOI] [PubMed] [Google Scholar]
- 23.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Via, L. E., P. L. Lin, S. M. Ray, J. Carrillo, S. S. Allen, S. Y. Eum, K. Taylor, E. Klein, U. Manjunatha, J. Gonzales, E. G. Lee, S. K. Park, J. A. Raleigh, S. N. Cho, D. N. McMurray, J. L. Flynn, and C. E. Barry III. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 762333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters, S. B., E. Dubnau, I. Kolesnikova, F. Laval, M. Daffe, and I. Smith. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60312-330. [DOI] [PubMed] [Google Scholar]
- 27.Wayne, L., and C. Sohaskey. 2001. Nonreplicating persistence of mycobacterium tuberculosis. Annu. Rev. Microbiol. 55139-163. [DOI] [PubMed] [Google Scholar]
- 28.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 642062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson, M. A., M. I. Voskuil, D. Schnappinger, and G. K. Schoolnik. 2001. Functional genomics of Mycobacterium tuberculosis using DNA microarrays. Methods Mol. Med. 54335-357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.