Abstract
Clostridium difficile persists in hospitals by exploiting an infection cycle that is dependent on humans shedding highly resistant and infectious spores. Here we show that human virulent C. difficile can asymptomatically colonize the intestines of immunocompetent mice, establishing a carrier state that persists for many months. C. difficile carrier mice consistently shed low levels of spores but, surprisingly, do not transmit infection to cohabiting mice. However, antibiotic treatment of carriers triggers a highly contagious supershedder state, characterized by a dramatic reduction in the intestinal microbiota species diversity, C. difficile overgrowth, and excretion of high levels of spores. Stopping antibiotic treatment normally leads to recovery of the intestinal microbiota species diversity and suppresses C. difficile levels, although some mice persist in the supershedding state for extended periods. Spore-mediated transmission to immunocompetent mice treated with antibiotics results in self-limiting mucosal inflammation of the large intestine. In contrast, transmission to mice whose innate immune responses are compromised (Myd88−/−) leads to a severe intestinal disease that is often fatal. Thus, mice can be used to investigate distinct stages of the C. difficile infection cycle and can serve as a valuable surrogate for studying the spore-mediated transmission and interactions between C. difficile and the host and its microbiota, and the results obtained should guide infection control measures.
Clostridium difficile is a gram-positive, spore-forming, anaerobic bacterium that can reside asymptomatically in the intestinal tract of humans (6, 11, 42). The use of antibiotics that spare C. difficile but suppress the intestinal microbiota allows C. difficile to proliferate (23), potentially leading to intestinal damage, inflammation, and clinical disease (9). In most cases stopping antibiotic therapy is often sufficient to prevent or reverse disease symptoms in immunocompetent individuals (5, 32). However, in immunocompromised hospital patients, particularly the elderly, intestinal disease can quickly develop after antibiotic treatment, with clinical outcomes ranging from mild diarrhea to pseudomembraneous colitis to multiple-organ dysfunction syndrome (17, 31).
Unlike most pathogens, C. difficile produces a metabolically dormant spore form that is excreted by infected patients (43, 63). The infective spores persist in the environment and are highly resistant to commonly used disinfectants (24). Indeed, environmental spore contamination in a hospital results in a reservoir for transmission (21, 46) that can lead to a proportional increase in the percentage of the patient population colonized by C. difficile (33). As a result, C. difficile is endemic in many hospitals, and outbreaks are difficult to contain, highlighting the compelling need to understand the spore-mediated infection cycle and the factors that lead to C. difficile transmission (24, 59).
Most studies of C. difficile pathogenesis in animals have focused on the acute stage of infection (13, 38, 45, 57), so many aspects of the C. difficile infection cycle have not been investigated in detail, including intestinal carriage, interactions with the microbiota, the role of spores in transmission, and host susceptibility to severe disease. Recent reports that various virulent C. difficile ribotypes can colonize several mammalian hosts (25, 30, 48, 51, 52) led us to reinvestigate the mouse as a model for the C. difficile infection cycle. We found that C. difficile strain M68, a representative ribotype that frequently causes human disease (19), was very proficient at persisting in inbred mice. Here we describe the impact of antibiotics on the composition of the intestinal microbiota of murine C. difficile M68 carriers and reveal that antibiotics can inadvertently trigger high-level spore excretion and remarkably efficient host-to-host transmission of C. difficile. Further, we demonstrate that transmission of C. difficile to immunocompetent mice leads to self-limiting intestinal disease, whereas transmission to Myd88−/− mice, which are defective in a key signaling pathway in the innate immune response, leads to severe intestinal disease and multiple-organ dysfunction syndrome, potentially mimicking the situation in humans.
MATERIALS AND METHODS
C. difficile culture.
C. difficile M68 is a PCR ribotype 17, toxinotype VIII, tcdAB+ strain isolated from a multihospital outbreak of C. difficile disease in Dublin, Ireland (19). C. difficile M68 was routinely grown for 24 to 48 h at 37°C under anaerobic conditions in a Whitley DG250 workstation (Don Whitley, West Yorkshire, United Kingdom). For mouse infection, C. difficile was grown statically for 36 h in Wilson's broth (62). To enumerate the total C. difficile in fresh feces, samples (100 mg feces/ml phosphate-buffered saline [PBS]) were immediately serially diluted in PBS and plated on Brazier agar (Bioconnections, Whetherby, United Kingdom) plates (90 mm or 140 mm; Bibby Sterilin Ltd., Stone, Staffordshire, United Kingdom) supplemented with 0.5% taurocholate (Sigma, United Kingdom) to improve the C. difficile detection limit (62). Typically, fresh fecal samples were processed and plates were placed in the anaerobic cabinet within 45 min after excretion. To enumerate spores in feces, 0.1-ml fecal samples were mixed with 0.1 ml of 100% ethanol for 1 h at room temperature to kill the vegetative cells of C. difficile. Samples were pelleted, washed twice in PBS, resuspended in 0.1 ml of PBS, and cultured as described above.
Mouse infection and transmission experiments.
Specific-pathogen-free female wild-type, Igh6−/−, or Myd88−/− mice with a C57BL/6 genetic background that were 5 to 7 weeks old (from mouse colonies maintained at Wellcome Trust Sanger Institute) were infected as indicated below by either oral gavage or transmission. Mice were housed in sterile cages containing wood shavings, food pellets, and water. Once a week, mice were aseptically moved to a sterile cage. Cage changing and mouse handling were performed in a sterile biosafety hood by a worker wearing a clean smock and gloves that were disinfected with 2% Virkon (potassium peroxymonosulfate). To establish the carrier state in the experiment whose results are shown in Fig. 1, mice were pretreated with 1 mg of neomycin 24 h prior to oral gavage with 107 CFU of C. difficile in 0.2 ml of PBS, although neomycin pretreatment is not necessary to establish the carrier state. For example, the carrier state in mice used for the experiment whose results are shown in Fig. 2 was established without the antibiotic pretreatment. To induce the supershedder state, carrier mice were treated with 1 mg clindamycin in 200 μl PBS via gavage (see Fig. 1 and 2) or clindamycin (250 mg/liter) was added to the drinking water (see Fig. 5).
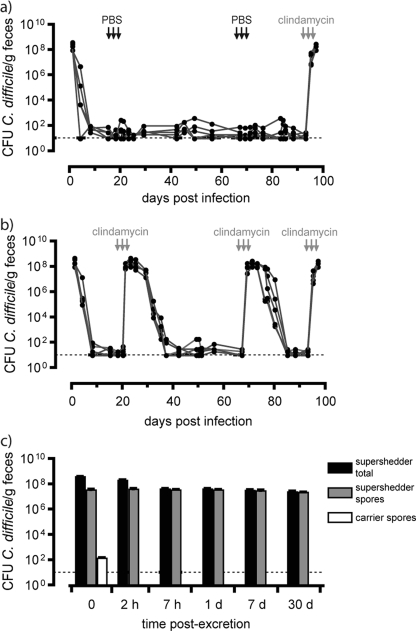
FIG. 1.
Induction of high-level fecal shedding of C. difficile spores by clindamycin treatment of carrier mice shedding low levels of spores. (a) Long-term fecal shedding from individual mice (five mice per cage) infected with C. difficile M68. The horizontal dashed line indicates the detection limit, 101 CFU/g. (b) Clindamycin treatment (1 mg/day via oral gavage) (arrows) of carriers (five mice per cage) reproducibly resulted in rapid induction of high-level shedding of C. difficile that gradually returned to low-level shedding 12 to 16 days after treatment was discontinued. In contrast, PBS treatment of carrier mice (five mice per cage) (arrows) did not affect the levels of C. difficile cultured from feces, although these mice were prone to high-level shedding of C. difficile after clindamycin treatment (see panel a). In this experiment mice were pretreated with neomycin, although prior antibiotic treatment is not necessary to establish the carrier state, as shown in Fig. 2. (c) Levels of C. difficile vegetative and spore forms (supershedder total) and spores (supershedder spores) excreted by supershedders and of spores excreted by carrier mice. Five mice were used for each sample.
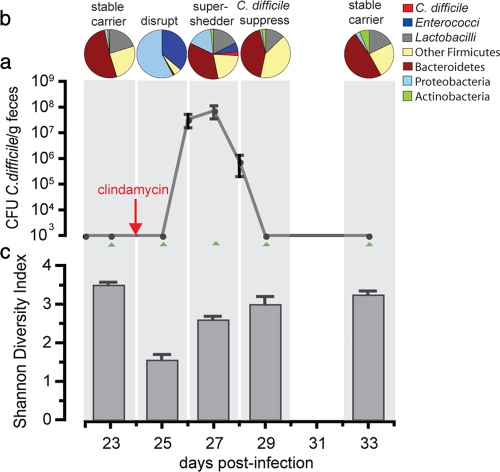
FIG. 2.
Antibiotic-induced C. difficile supershedding state is associated with reduced intestinal microbiota diversity. (a) Average fecal shedding of C. difficile by carrier mice (five mice per cage) treated with clindamycin to induce a transient supershedder state. The error bars indicate standard deviations. DNA was extracted from fresh feces from each mouse at the indicated time points (green arrowheads) to create 16S rRNA gene clone libraries. The detection limit was 103 CFU C. difficile/g feces. (b) Temporal shifts in the intestinal bacterial community after clindamycin treatment of carrier mice as determined by 16S rRNA gene analysis. The levels of predominant bacterial groups are expressed as percentages of the clone libraries using pie charts. On day 23 1,400 clones were included (average, 280 clones/mouse), on day 25 1,202 clones were included (average, 241 clones/mouse), on day 27 1,204 clones were included (average, 241 clones/mouse), on day 29 1,081 clones were included (average, 216 clones/mouse), and on day 33 1,167 clones were included (average, 233 clones/mouse). (c) SDI for each phase of the microbiota community structure determined by 16S rRNA gene phylotypes.
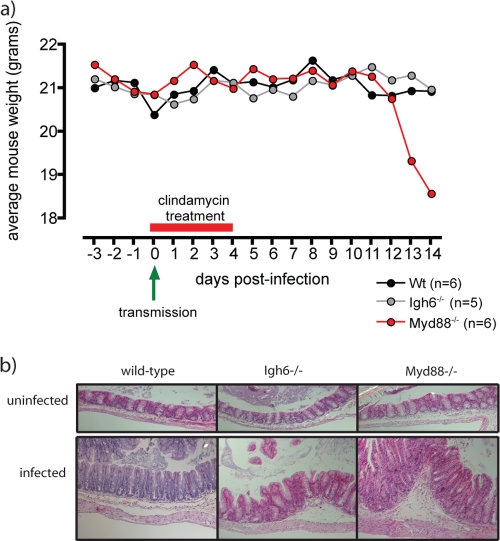
FIG. 5.
Myd88−/− mice are susceptible to severe C. difficile disease. (a) Average weights of wild-type, Igh6−/−, and Myd88−/− mice that were infected with C. difficile via transmission (green arrow) and subsequently treated with clindamycin (red bar). Wt, wild type. (b) Hemotoxylin- and eosin-stained sections of cecal mucosa from wild-type, Igh6−/−, and Myd88−/− mice that were treated with clindamycin but not infected and from mice treated with clindamycin and infected with C. difficile. Infection with C. difficile results in moderate intestinal inflammation (cecum) in wild-type and Igh6−/− mice and more severe intestinal inflammation in Myd88−/− mice. Magnification, ×20.
To perform host-to-host transmission experiments, five naïve recipient mice were housed in a sterile cage with two infected donor mice or no donor mice for 5 h. Immediately after this, the recipient mice were aseptically removed, housed individually in sterile cages, and given water that contained clindamycin (250 mg/liter) for 4 days. Mice drink 3 to 4 ml of water/day, so these mice typically ingested 0.75 to 1 mg clindamycin/day. The transmission efficiency was assessed by culturing C. difficile from feces of recipient mice after 4 days of clindamycin treatment.
To contaminate polysulfone cages (with no bedding so mice were in direct contact) for environmental spore transmission experiments, supershedders (n = 5) were placed in the cages (floor area, 800 cm2) for 1 h (1.5 to 1.8 g feces excreted/cage). Subsequently, the supershedders were removed, and the cages were left for 16 h under ambient oxygen conditions to eliminate the vegetative form of C. difficile (see Fig. 1c). Before experiments were performed, all feces were removed from the cage to ensure that transmission did not occur via coprophagy. To demonstrate spore contamination, naïve (untreated) mice were aseptically placed in a cage for 1 h and then aseptically removed, placed in a sterile cage, and given water containing clindamycin (250 mg/liter). Surface disinfection of contaminated cages was performed by adding 400 ml of a 2% Virkon solution to the cage for the periods of time indicated below. After the Virkon solution was removed, the surface was patted dry with sterile paper towels before naïve mice were placed in the cage. All animal infections were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act of 1986.
Construction and analysis of 16S rRNA gene libraries.
DNA was extracted from fresh, unfrozen fecal samples using a FastDNA Spin kit for soil and a FastPrep bead-beating instrument (both obtained from MP Biomedicals, Solon, OH). Preparation of 16S rRNA gene clone libraries and sequence analysis were performed as described previously (54), except that single reads for around 600 to 650 bp spanning the V2 to V5 variable regions were generated for each clone using primer 926r (5′-CCGTCAATTC[A/C]TTT[A/G]AGT-3′). Sequences were aligned using the NAST aligner (15) and were subjected to extensive manual correction before further analysis. Shannon diversity indices (SDI) were calculated for each sample using DOTUR (49).
Microscopy.
Cecum tissue (0.5-cm tubular sections) was carefully excised, opened, and processed for transmission electron microscopy, scanning electron microscopy, and immunogold (66) or hematoxylin and eosin staining (54) as previously described.
Nucleotide sequence accession numbers.
Sequences determined in this study have been deposited in the GenBank database under accession numbers GQ294716 to GQ300837.
RESULTS
Intestinal carriage of C. difficile in mice.
Healthy immunocompetent C57BL/6 mice were orally challenged with the human virulent strain C. difficile M68, and subsequently fecal shedding was monitored. Intriguingly, challenged mice reproducibly became carriers, shedding low levels of C. difficile (<500 CFU/g [fresh weight] feces) for many months (Fig. 1a). Further, low levels of C. difficile spores (∼102 spores/g) were consistently detected in dried fecal pellets collected from the bedding of cages harboring carrier mice, which were aseptically moved to sterile cages once a week. During the initial experiments mice were pretreated with neomycin; however, subsequent experiments demonstrated that prior antibiotic treatment is not required for infection of mice with C. difficile (data not shown). Importantly, the same strain of C. difficile (strain M68) that was used to infect mice was routinely isolated from the feces of carriers, based on comparative genomic hybridization results (see Fig. S1 in the supplemental material). The carrier mice appeared to be generally healthy and did not develop any obvious clinical symptoms or pathological lesions in their intestinal tissues (data not shown). Therefore, C. difficile M68 can asymptomatically and persistently colonize the intestine of mice at low levels in a manner reminiscent of the colonization of human carriers (46).
Antibiotic induction of a supershedder state.
In the clinical setting, treatment with antibiotics is the major risk factor for C. difficile disease (59). To determine the effect of antibiotic treatment on the dynamics of C. difficile shedding, we treated carrier mice with clindamycin, to which C. difficile M68 is resistant. Between 2 and 3 days after treatment was initiated, we observed a dramatic 106-fold increase in the levels of C. difficile shedding for all of the treated mice, establishing what we refer to as a supershedding state (Fig. 1a and b). Significantly, the supershedding state was maintained as long as mice were being treated with clindamycin (see Fig. S2 in the supplemental material). Typically, 12 to 16 days after a 3-day course of clindamycin the levels of C. difficile shedding dropped abruptly to the previous carrier shedding levels (Fig. 1b). After long-term exposure (17 days) to clindamycin, many mice were found to be in the carrier state 12 to 16 days after cessation of treatment (45 of 105 mice). However, a significant number of mice were persistent supershedders for 3 to 5 weeks (55 of 105 mice) or occasionally for months (5 of 105 mice) (see Fig. S2 in the supplemental material). Thus, after longer-term antibiotic treatment mice were more prone to remain in the supershedder state for extended periods. It is also noteworthy that colonized mice were susceptible to multiple cycles of clindamycin-induced supershedding (Fig. 1b), indicating that mice do not clear the infection or become resistant to the supershedding state.
Total C. difficile cells (vegetative cells and spores) or only spores (ethanol resistant) in fecal samples can easily be enumerated using CCEY selective medium in combination with the ethanol shock method (47). We found that immediately after excretion 10 to 20% of the total C. difficile vegetative cells and spores shed in the feces of supershedders were spores (Fig. 1c), representing a >105-fold increase (P < 0.005) compared to the level excreted by carrier mice (Fig. 1a and c). After excretion from supershedders, feces were maintained in the ambient atmosphere for up to 30 days and periodically sampled to monitor the viability of environmental C. difficile total cells and spores. After 7 h spores accounted for all of the culturable C. difficile (Fig. 1c). Thus, while the vegetative form of C. difficile in excreted feces was short lived (<7 h), spores survived at a constant level over a 30-day period (Fig. 1c). Since sporulation requires vegetative growth (43, 55), these observations suggest that C. difficile sporulates in the intestinal tract prior to excretion. Together, these results demonstrate that antibiotic treatment per se of carrier mice induces a supershedding state, resulting in the release of high levels of C. difficile spores into the environment, which can persist even after withdrawal of antibiotics. Importantly, the C. difficile supershedding state exists in the absence of obvious clinical disease (lethargy, ruffled fur, hunched position, etc.).
Antibiotic disruption of C. difficile carrier microbiota.
Since we established that C. difficile M68 can establish both a carrier state and a supershedder state in mice, we decided to investigate the changes in the intestinal microbiota associated with these states. Hence, we performed a detailed 16S rRNA gene sequence analysis of a total of 6,122 clones amplified from the feces of C. difficile-infected mice (n = 5) at five time points before and after clindamycin treatment (Fig. 2a and b; see Table S1 in the supplemental material). This analysis confirmed that in carrier mice C. difficile was a minor component of the microbiota, as C. difficile 16S rRNA gene sequences were not detected even though >1,200 clones were sequenced (Fig. 2a and b). Given the low level of C. difficile shedding from carrier mice, we did not expect to detect any 16S rRNA clones representing this species. Indeed, the carrier mice maintained a diverse microbiota community structure (SDI, 3.6) (Fig. 2c) dominated by members of the Firmicutes and Bacteroidetes phyla (Fig. 2a), a typical profile for a stable mammalian intestinal microbiota (20, 35).
In contrast, a single exposure to clindamycin resulted in dramatic alteration of the carrier microbiota (Fig. 2a and b; see the supplemental material). Initially, the microbiota disruption was characterized by a tremendous loss of species richness (SDI, 1.5) (Fig. 2a and c), particularly in the Bacteroidetes and obligate anaerobic Firmicutes species, and a dramatic expansion of the facultative anaerobes Escherichia coli and Enterococcus casseliflavis (Fig. 2b). Following a highly reproducible 3-day lag period after clindamycin exposure, the C. difficile supershedding state was established (Fig. 2a) and C. difficile 16S rRNA genes accounted for ∼5% of the libraries (Fig. 2b). This time point coincided with partial recovery of the diversity of the microbiota (SDI, 2.5) (Fig. 2c), particularly for the Firmicutes phylum (Fig. 2b; see Table S1 in the supplemental material). The subsequent decrease in the level of C. difficile colonization was associated with a significant rebound in species diversity (SDI, 3.1) (Fig. 2c) typified by large increases in the range of Firmicutes species and a slower recovery of the richness of the Bacteriodetes phylum (Fig. 2a). Our analysis was deep enough to monitor changes at the species level. However, we did not identify any specific genus or group of bacteria that were reproducibly associated with the reduction in C. difficile shedding (see Table S1 in the supplemental material). Finally, within 10 days after exposure to clindamycin both the composition and the richness of the microbiota had recovered to levels similar, but not identical, to the levels before clindamycin treatment (SDI, 3.3) (Fig. 2a and c).
C. difficile supershedders promote spore-mediated transmission.
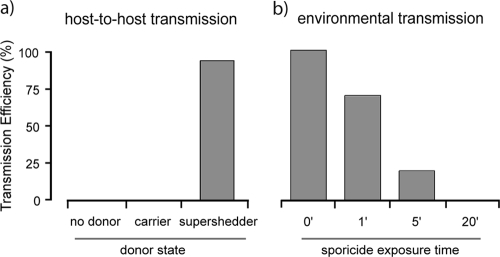
Supershedders are the most contagious individuals in natural populations (36, 64). Consequently, we tested whether C. difficile could be transmitted from colonized mice to naïve mice (i.e., mice that had never been colonized by C. difficile) and whether transmissibility was related to shedding levels. After naïve mice were housed with supershedding mice in the same cage for 5 h, the majority (19 of 20) (Fig. 3a) of naïve recipient mice were colonized with C. difficile. In contrast, none (Fig. 3a) of the 15 naïve recipient mice housed with C. difficile carriers were colonized even though we demonstrated that low numbers of spores were present in the feces of the former mice. Thus, the high level of shedding of C. difficile from supershedder mice promotes efficient host-to-host transmission, whereas the carrier state does not.
FIG. 3.
Host transmission of C. difficile via supershedding mice and environmental spore contamination. (a) Transmission efficiency, indicating the percentage of naïve mice that acquired C. difficile infection from donor mice. The number of naïve mice housed with no donor was 10, the number of naïve mice housed with carrier mice was 15, and the number of naïve mice housed with supershedding mice was 20. (b) Percentage of naïve mice that acquired C. difficile infection from a spore-contaminated environment. The number of naïve mice housed in a contaminated environment with no sporicide was 15, the number of naïve mice housed in a contaminated environment treated for 1 min with sporicide was 10, the number of naïve mice housed in a contaminated environment treated for 5 min with sporicide was 10, and the number of naïve mice housed in a contaminated environment treated for 20 min with sporicide was 10. Groups of 5 or 10 naïve mice were housed in cages previously contaminated by supershedders. The transmission efficiency was determined as described in Materials and Methods.
Next we investigated if direct contact was essential for C. difficile transmission by housing naïve mice in cages previously occupied by supershedders. These investigations showed that environmental spores are a very effective agent for C. difficile transmission, as all 15 naïve mice were colonized when they were housed in cages previously contaminated by supershedders (Fig. 3b). During these experiments the feces were removed from the cages to ensure that transmission was not due to coprophagy. Indeed, we found that even short-term housing of supershedding mice in contaminated cages was sufficient to trigger transmission to naïve mice subsequently placed in the same cages, suggesting that brief exposure to environmental contamination can result in infection (data not shown).
Since we could readily trigger transmission of C. difficile between mice, we were in a position to assess different disinfection regimens for their potential to block transmission. Treatment of C. difficile spore-contaminated cages with alcohol-based disinfectants did not prevent transmission (data not shown), reflecting the findings for clinical settings (59). Instead, we found that a rigorous 20-min surface disinfection regimen using the strong sporicidal agent Virkon was necessary to reduce environmental spore contamination enough to eliminate transmission (0 of 10 mice) (Fig. 3b). Importantly, 1- and 5-min Virkon-based surface disinfection regimens resulted in only 30% and 80% reductions in the transmission efficiency, respectively (Fig. 3b). Indeed, use of stringent precautions in our animal facility, such as proper disinfection and aseptic handing of mice, is required to prevent inadvertent C. difficile transmission.
C. difficile supershedders transmit disease.
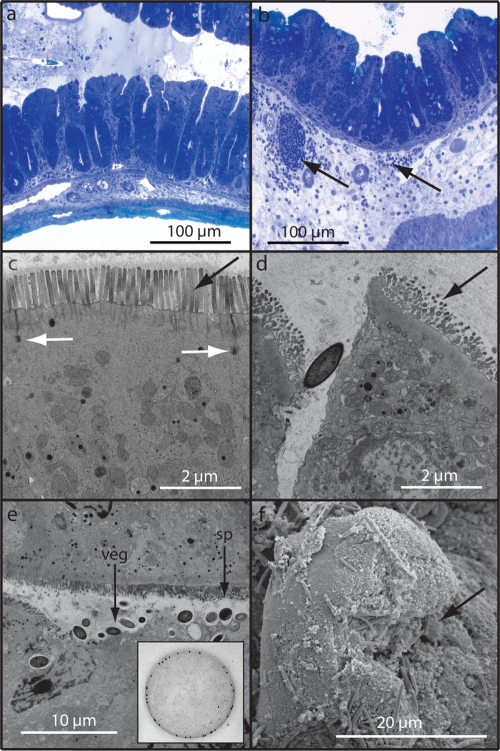
The fact that C. difficile transmission was so efficient allowed us to investigate C. difficile colonization and host interactions following natural infection of mice rather than artificial challenge. When naturally infected mice were treated with clindamycin to induce the supershedder state, they did not develop debilitating disease (see Fig. S3a in the supplemental material). However, an inflammatory exudate was routinely found in the feces of these supershedders. We thus examined the gastrointestinal tracts of supershedder mice to identify any associated pathology. Clindamycin-treated, uninfected control mice showed no sign of pathological lesions (Fig. 4a and c). In contrast, during formation of the supershedder state (days 4 to 7 postinfection), clear signs of mucosal damage were evident in the large intestines (cecum and colon) of the majority of supershedding mice, including epithelial cell death, microvillus effacement, and disrupted epithelial tight junctions (Fig. 4d and e). Both the vegetative and spore forms of C. difficile were readily observed in the intestinal lumen, and sometimes large numbers were observed (Fig. 4e). Individual cells or clumps of C. difficile could be seen in close association with and sometimes attached to or within damaged tissue (Fig. 4f). Importantly, immunogold labeling with a C. difficile antibody clearly demonstrated that the bacteria observed were in fact C. difficile (Fig. 4e, inset).
FIG. 4.
Supershedders efficiently transmit C. difficile disease to naïve recipients. (a to f) Representative images that illustrate epithelial damage and the inflammatory response in ceca of recipient mice (100 mice) after clindamycin treatment (see Fig. S3 in the supplemental material). (a) Toluidine blue staining of the cecum from a clindamycin-treated control mouse. (b) Toluidine blue staining demonstrating edema and immune cell infiltrate (arrows) within the cecal submucosa of a C. difficile-infected mouse. (c) Transmission electron micrograph of the intact epithelial brush border (black arrow) and tight junctions (white arrows) of the cecum from a clindamycin-treated control mouse. (d) Disrupted tight junction and microvillus effacement within the cecal submucosa of a C. difficile-infected mouse (arrow) (compare with panel c). (e) C. difficile vegetative cells and spores associated with epithelial effacement and cellular invasion and immunogold labeling of C. difficile within the lumen (inset). (f) Matt of C. difficile cells (arrows) overlaying damaged and necrotic microvilli.
On days 10 to 13 postinfection, as C. difficile shedding levels were declining (see Fig. S3a in the supplemental material), supershedders continued to display obvious signs of intestinal inflammation. Pathological lesions consisting of hyperplasia, inflammatory cell infiltration, and submucosa edema were commonly observed (Fig. 4b). Regions of severe inflammation were associated with large aggregates of C. difficile cells that appeared to form exaggerated mats covering the epithelial surface (see Fig. S3b in the supplemental material), which was marked by destroyed or necrotic microvilli (Fig. 4f). The type of inflammatory lesions was variable, ranging from mild to severe. Importantly, despite this clear intestinal damage, wild-type mice did not lose weight or succumb to C. difficile infection. Indeed, these mice quickly recovered as no signs of intestinal damage or pathology were evident following termination of clindamycin treatment and the subsequent return of supershedding mice to the carrier state (data not shown).
Myd88 protects against severe C. difficile disease.
C. difficile disease is normally associated with immunocompromised or elderly individuals, and although normal supershedder mice displayed evidence of intestinal damage, they did not develop overt disease. Therefore, we exposed groups of knockout mice with mutations in genes associated with immune defense to microbial pathogens. Igh6−/− mice, defective in B-cell responses (29), that were infected with C. difficile maintained their weight and remained asymptomatic during the experiment (Fig. 5a). Further, these mice did not exhibit severe mucosal damage compared to similarly infected wild-type mice (Fig. 5b). In contrast, we found that on days 10 to 12 postinfection Myd88−/− mice (1), deficient in innate signaling at the mucosa, became moribund and began to rapidly lose weight (Fig. 5a). Analysis of the large intestine of infected mice revealed more pronounced intestinal disease in Myd88−/− mice than in wild-type and Igh6−/− mice (Fig. 5b), including increased hyperplasia, cellular infiltrate, and edema. Interestingly, 48% (28 of 60) of the Myd88−/− infected mice succumbed to infection, whereas clindamycin-treated, uninfected Myd88−/− mice (n = 15) remained healthy. Postmortem examination revealed a variety of pathological lesions at systemic sites in moribund Myd88−/− mice that were not observed in wild-type infected mice, particularly in the kidneys and lungs (see Fig. S4 in the supplemental material). Therefore, mice deficient in Myd88 are susceptible to severe C. difficile intestinal disease and are prone to succumbing to an infection that is associated with systemic pathologies.
DISCUSSION
We demonstrate here for the first time that human virulent C. difficile can establish an asymptomatic and persistent carrier state in healthy mice, mimicking the situation with human carriers (46) (Fig. 6). Further, we show that carrier mice are not significantly contagious, even though they excrete low levels of C. difficile spores. However, using these carrier mice, we obtained the first experimental evidence that antibiotic treatment triggers a supershedding state involving high levels of spore excretion, leading to C. difficile transmission in the absence of obvious clinical disease. Symptomatic hospital patients with overt C. difficile clinical disease are currently the primary target of infection control measures in hospitals (24, 59). However, our results suggest that antibiotic treatment of C. difficile carriers per se triggers excretion of high levels of spores. Thus, in hospitals it may be prudent to monitor patients undergoing treatment with antibiotics for evidence of C. difficile spore shedding.
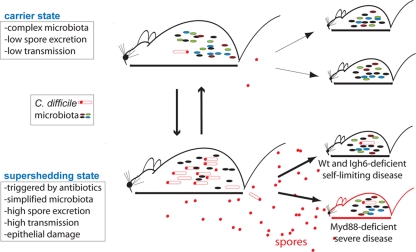
FIG. 6.
Relationship between C. difficile carrier state and supershedding state in response to antibiotic treatment and role of the host immune system in severe disease. C. difficile carrier mice possess a stable and diverse intestinal microbiota community (small rods) which includes low levels of C. difficile (red rods with spores). As a result, carrier mice excrete low levels of spores and are poor donors for C. difficile infection. Antibiotic treatment of carrier mice reduces the diversity of the intestinal microbiota community, allowing C. difficile to proliferate and sporulate (red circles). As a result, a supershedding state occurs with high levels of spore excretion and efficient host-to-host transmission. Withdrawing the antibiotic allows the intestinal microbiota to rediversify and suppresses C. difficile levels, thereby reestablishing the carrier state and reducing the contagiousness of the host. Transmission of C. difficile to Myd88-deficient mice, but not transmission of C. difficile to Igh6−/− mice, results in severe disease, implicating Toll-like receptor signaling in protection against virulent C. difficile overgrowth. Wt, wild type.
A stable intestinal microbiota community protects the host from both indigenous and exogenous pathogens through a phenomenon termed “colonization resistance” (53, 54, 58). Antibiotic-mediated disruption of colonization resistance can promote intestinal overgrowth of pathogens such as Enterococcus (10, 18) and Salmonella enterica (4, 8, 14, 34, 50). Here we used 16S rRNA gene analysis to monitor the temporal shifts in the bacterial community of carriers during the loss and subsequent recovery of colonization resistance to C. difficile. We show that reduced bacterial diversity is one of the major changes in the intestinal microbiota that allows C. difficile to proliferate and sporulate within the intestinal tract. In addition to C. difficile proliferation after antibiotic treatment, we noted that Enterococcus and E. coli also thrived. Although we did not monitor the transmission of Enterococcus and E. coli in our experiments, it has previously been documented that transmission of Enterococcus (18) and S. enterica (34) increases significantly after treatment of infected hosts with antibiotics. Thus, bacterial groups associated with antibiotic-associated diarrhea may exploit common antibiotic-induced perturbations in the intestinal microbiota to cause contagious diarrhea and disseminate.
Disrupting the carrier microbiota with a short course of clindamycin appears to result in ecological succession (37) in which the disturbance of the initial community is followed by orderly changes in the composition and richness until a stable climax community is reestablished. Recent analyses of murine (2) and human (16, 28) intestinal bacterial communities in response to longer-term antibiotic therapy revealed similar patterns of biodiversity dynamics, although the stable, postantibiotic communities differed significantly from the preantibiotic communities. Ecological succession is a fundamental concept in ecology that is used to understand and predict the impact of a disturbance on ecosystems, and the classic example is the recovery of a forest after clear-cutting. Ecological successions are marked by distinct stages in which opportunistic organisms exploit the reduced species diversity to proliferate but are replaced by organisms that compete more efficiently as the community diversity increases during recovery (37). In terms of antibiotic disruption of the mammalian intestinal microbiota ecosystem, C. difficile, E. coli, and enterococci are considered opportunistic organisms that thrive in the intestinal microbiota community during periods when the biodiversity is low. This idea is consistent with a recent analysis of the intestinal microbiota of patients that are prone to relapsing with C. difficile overgrowth and disease (12).
Other workers have successfully used hamsters (38, 45) and mice (13, 41, 57) to investigate aspects of acute C. difficile disease and protective immunity. Previous murine infection models have relied on mice devoid of an intestinal microbiota (germfree or antibiotic depletion prior to infection), so these models were not exploited to study the complete infection cycle, including intestinal carriage and host-to-host transmission. Further, previous work on other enteric infections has suggested that bacteria grown in laboratory medium and administered by oral gavage may have a distinct pattern of colonization compared to the pattern seen following host-to-host transmission (39, 60, 61). Here, using direct environmental transmission of C. difficile spores, we demonstrate that naturally infected immunocompetent mice develop intestinal damage and inflammation that resolve after the antibiotic is removed and the intestinal microbiota recovers. These results mimic the expected outcome of C. difficile infection in healthy, immunocompetent adult humans (5, 32). Our ability to trigger C. difficile transmission with relevant antibiotic treatment of carriers places in context the entire C. difficile infection and transmission cycle and potentially has great significance.
Severe C. difficile disease likely occurs in immunocompromised or elderly individuals because of aberrant interactions between the host immune system, the intestinal microbiota, and C. difficile. Presumably, specific elements of the immune system are compromised in such individuals, making them susceptible to severe disease, although this hypothesis has not been formally addressed. We demonstrate here that the innate immune system of mice protects against C. difficile severe disease, specifically signaling through the Myd88 pathway (Fig. 6). Defective innate immune signaling has not previously been implicated in C. difficile disease susceptibility, but this pathway has been shown to play a role in a variety of intestinal disorders, such as Crohn's disease and ulcerative colitis, due to disrupted signaling between the luminal bacteria and the intestinal mucosa (65). The identification of Myd88−/− mice as mice that are susceptible to severe C. difficile disease generates a number of testable hypotheses regarding the precise mechanism(s) that the host uses to resist the potentially harmful effects of virulent C. difficile overgrowth, including the roles of Myd88-mediated cytokine cascades (22), antimicrobial peptides (56), and the epithelial barrier function (44). Severe C. difficile intestinal disease in humans can culminate in multiple-organ dysfunction syndrome (17) that can be associated with renal failure (3, 7) and acute respiratory distress syndrome (27). We observed these syndromes in many Myd88-deficient mice, demonstrating that transgenic knockout mice can be used to study host susceptibility to severe C. difficile disease.
Understanding the interactions between C. difficile, the intestinal microbiota, and the host immune system in response to different antibiotic treatments and host genotypes is required to fully appreciate the mechanisms of C. difficile carriage, disease, and transmission. The C. difficile strain used in our studies represents only one clinically relevant ribotype, so it is important to test other strains (i.e., ribotype 027) in the mouse model. Further, the ability to make targeted mutations in C. difficile (26, 40), as well as the availability of several C. difficile genomes (http://www.sanger.ac.uk/Projects/C_difficile), should allow studies of toxin production and sporulation during C. difficile disease and transmission. Thus, the availability of a murine model for investigating the complete infection and transmission cycle of C. difficile could be extremely valuable for assessing the potential of novel therapeutics, immunization, probiotics, and transmission prevention measures to control C. difficile.
Supplementary Material
Acknowledgments
This work was funded by the Wellcome Trust. T.D.L. was funded by the Royal Society of London.
We are grateful to Stanley Falkow and Bronwyn MacInnis for invaluable input and thoughtful discussions, to Cordy Brandt and Nicola Goodwin for technical assistance with the animal infections, to the Sanger Sequencing Team for 16S rRNA clone sequencing, and to Denise Drudy for supplying C. difficile strain M68.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 June 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Antonopoulos, D. A., S. M. Huse, H. G. Morrison, T. M. Schmidt, M. L. Sogin, and V. B. Young. 2009. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 772367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrich, J., G. H. Sodeck, G. Sengolge, C. Konnaris, M. Mullner, A. N. Laggner, and H. Domanovits. 2005. Clostridium difficile causing acute renal failure: case presentation and review. World J. Gastroenterol. 111245-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346334-339. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett, J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145758-764. [DOI] [PubMed] [Google Scholar]
- 7.Bishara, J., N. Peled, S. Pitlik, and Z. Samra. 2008. Mortality of patients with antibiotic-associated diarrhoea: the impact of Clostridium difficile. J. Hosp. Infect. 68308-314. [DOI] [PubMed] [Google Scholar]
- 8.Bohnhoff, M., B. L. Drake, and C. P. Miller. 1955. The effect of an antibiotic on the susceptibility of the mouse's intestinal tract to Salmonella infection. Antibiot. Annu. 3453-455. [PubMed] [Google Scholar]
- 9.Borriello, S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C)13-19. [DOI] [PubMed] [Google Scholar]
- 10.Brandl, K., G. Plitas, C. N. Mihu, C. Ubeda, T. Jia, M. Fleisher, B. Schnabl, R. P. DeMatteo, and E. G. Pamer. 2008. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455804-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazier, J. S. 2008. Clostridium difficile: from obscurity to superbug. Br J. Biomed Sci. 6539-44. [DOI] [PubMed] [Google Scholar]
- 12.Chang, J. Y., D. A. Antonopoulos, A. Kalra, A. Tonelli, W. T. Khalife, T. M. Schmidt, and V. B. Young. 2008. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 197435-438. [DOI] [PubMed] [Google Scholar]
- 13.Chen, X., K. Katchar, J. D. Goldsmith, N. Nanthakumar, A. Cheknis, D. N. Gerding, and C. P. Kelly. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 1351984-1992. [DOI] [PubMed] [Google Scholar]
- 14.Croswell, A., E. Amir, P. Teggatz, M. Barman, and N. H. Salzman. 2009. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 772741-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 725069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dethlefsen, L., S. Huse, M. L. Sogin, and D. A. Relman. 2008. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson, G., C. Hickey, and J. Trinder. 2003. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 291030. [DOI] [PubMed] [Google Scholar]
- 18.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, C. K. Hoyen, J. A. Hanrahan, A. M. Hujer, R. A. Hutton-Thomas, C. C. Whalen, R. A. Bonomo, and L. B. Rice. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 3431925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drudy, D., N. Harnedy, S. Fanning, R. O'Mahony, and L. Kyne. 2007. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin. Microbiol. Infect. 13298-304. [DOI] [PubMed] [Google Scholar]
- 20.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 3081635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fawley, W. N., S. Underwood, J. Freeman, S. D. Baines, K. Saxton, K. Stephenson, R. C. Owens, Jr., and M. H. Wilcox. 2007. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect. Control Hosp. Epidemiol. 28920-925. [DOI] [PubMed] [Google Scholar]
- 22.Fukata, M., K. Breglio, A. Chen, A. S. Vamadevan, T. Goo, D. Hsu, D. Conduah, R. Xu, and M. T. Abreu. 2008. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J. Immunol. 1801886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerding, D. N. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin. Infect. Dis. 38646-648. [DOI] [PubMed] [Google Scholar]
- 24.Gerding, D. N., C. A. Muto, and R. C. Owens, Jr. 2008. Measures to control and prevent Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1)S43-S49. [DOI] [PubMed] [Google Scholar]
- 25.Goorhuis, A., D. Bakker, J. Corver, S. B. Debast, C. Harmanus, D. W. Notermans, A. A. Bergwerff, F. W. Dekker, and E. J. Kuijper. 2008. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin. Infect. Dis. 471162-1170. [DOI] [PubMed] [Google Scholar]
- 26.Heap, J. T., O. J. Pennington, S. T. Cartman, G. P. Carter, and N. P. Minton. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70452-464. [DOI] [PubMed] [Google Scholar]
- 27.Jacob, S. S., J. C. Sebastian, D. Hiorns, S. Jacob, and P. K. Mukerjee. 2004. Clostridium difficile and acute respiratory distress syndrome. Heart Lung 33265-268. [DOI] [PubMed] [Google Scholar]
- 28.Jernberg, C., S. Lofmark, C. Edlund, and J. K. Jansson. 2007. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 156-66. [DOI] [PubMed] [Google Scholar]
- 29.Kaisho, T., F. Schwenk, and K. Rajewsky. 1997. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science 276412-415. [DOI] [PubMed] [Google Scholar]
- 30.Kawano, A., M. Ikeda, R. Iritani, A. Kinoshita, K. Watanabe, T. Hayao, T. Kokubo, and S. Matsushita. 2007. Colitis associated with Clostridium difficile in specific-pathogen-free C3H-scid mice. J. Vet. Med. Sci. 69973-975. [DOI] [PubMed] [Google Scholar]
- 31.Kelly, C. P., and J. T. LaMont. 2008. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 3591932-1940. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers, E. J., and C. M. Surawicz. 2008. Clostridium difficile infection. Lancet 3711486-1488. [DOI] [PubMed] [Google Scholar]
- 33.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342390-397. [DOI] [PubMed] [Google Scholar]
- 34.Lawley, T. D., D. M. Bouley, Y. E. Hoy, C. Gerke, D. A. Relman, and D. M. Monack. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 76403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ley, R. E., M. Hamady, C. Lozupone, P. J. Turnbaugh, R. R. Ramey, J. S. Bircher, M. L. Schlegel, T. A. Tucker, M. D. Schrenzel, R. Knight, and J. I. Gordon. 2008. Evolution of mammals and their gut microbes. Science 3201647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd-Smith, J. O., S. J. Schreiber, P. E. Kopp, and W. M. Getz. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockwood, J. L., M. F. Hoopes, and M. P. Marchetti. 2007. Invasion ecology, vol. 1. Blackwell Publishing, Oxford, United Kingdom.
- 38.Lyras, D., J. R. O'Connor, P. M. Howarth, S. P. Sambol, G. P. Carter, T. Phumoonna, R. Poon, V. Adams, G. Vedantam, S. Johnson, D. N. Gerding, and J. I. Rood. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 4581176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Schoolnik, and A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connor, J. R., D. Lyras, K. A. Farrow, V. Adams, D. R. Powell, J. Hinds, J. K. Cheung, and J. I. Rood. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 611335-1351. [DOI] [PubMed] [Google Scholar]
- 41.Onderdonk, A. B., R. L. Cisneros, and J. G. Bartlett. 1980. Clostridium difficile in gnotobiotic mice. Infect. Immun. 28277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozaki, E., H. Kato, H. Kita, T. Karasawa, T. Maegawa, Y. Koino, K. Matsumoto, T. Takada, K. Nomoto, R. Tanaka, and S. Nakamura. 2004. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J. Med. Microbiol. 53167-172. [DOI] [PubMed] [Google Scholar]
- 43.Paredes, C. J., K. V. Alsaker, and E. T. Papoutsakis. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 3969-978. [DOI] [PubMed] [Google Scholar]
- 44.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118229-241. [DOI] [PubMed] [Google Scholar]
- 45.Razaq, N., S. Sambol, K. Nagaro, W. Zukowski, A. Cheknis, S. Johnson, and D. N. Gerding. 2007. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J. Infect. Dis. 1961813-1819. [DOI] [PubMed] [Google Scholar]
- 46.Riggs, M. M., A. K. Sethi, T. F. Zabarsky, E. C. Eckstein, R. L. Jump, and C. J. Donskey. 2007. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 45992-998. [DOI] [PubMed] [Google Scholar]
- 47.Riley, T. V., J. S. Brazier, H. Hassan, K. Williams, and K. D. Phillips. 1987. Comparison of alcohol shock enrichment and selective enrichment for the isolation of Clostridium difficile. Epidemiol. Infect. 99355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupnik, M. 2007. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin. Microbiol. Infect. 13457-459. [DOI] [PubMed] [Google Scholar]
- 49.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 711501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekirov, I., N. M. Tam, M. Jogova, M. L. Robertson, Y. Li, C. Lupp, and B. B. Finlay. 2008. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 764726-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Songer, J. G., and M. A. Anderson. 2006. Clostridium difficile: an important pathogen of food animals. Anaerobe 121-4. [DOI] [PubMed] [Google Scholar]
- 52.Stabler, R. A., D. N. Gerding, J. G. Songer, D. Drudy, J. S. Brazier, H. T. Trinh, A. A. Witney, J. Hinds, and B. W. Wren. 2006. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 1887297-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stecher, B., and W. D. Hardt. 2008. The role of microbiota in infectious disease. Trends Microbiol. 16107-114. [DOI] [PubMed] [Google Scholar]
- 54.Stecher, B., R. Robbiani, A. W. Walker, A. M. Westendorf, M. Barthel, M. Kremer, S. Chaffron, A. J. Macpherson, J. Buer, J. Parkhill, G. Dougan, C. von Mering, and W. D. Hardt. 2007. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 52177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30297-341. [DOI] [PubMed] [Google Scholar]
- 56.Vaishnava, S., C. L. Behrendt, A. S. Ismail, L. Eckmann, and L. V. Hooper. 2008. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 10520858-20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vernet, A., G. Corthier, F. Dubos-Ramare, and A. L. Parodi. 1989. Relationship between levels of Clostridium difficile toxin A and toxin B and cecal lesions in gnotobiotic mice. Infect. Immun. 572123-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vollaard, E. J., and H. A. Clasener. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vonberg, R. P., E. J. Kuijper, M. H. Wilcox, F. Barbut, P. Tull, P. Gastmeier, P. J. van den Broek, A. Colville, B. Coignard, T. Daha, S. Debast, B. I. Duerden, S. van den Hof, T. van der Kooi, H. J. Maarleveld, E. Nagy, D. W. Notermans, J. O'Driscoll, B. Patel, S. Stone, and C. Wiuff. 2008. Infection control measures to limit the spread of Clostridium difficile. Clin. Microbiol. Infect. 14(Suppl. 5)2-20. [DOI] [PubMed] [Google Scholar]
- 60.Wiles, S., W. P. Hanage, G. Frankel, and B. Robertson. 2006. Modelling infectious disease—time to think outside the box? Nat. Rev. Microbiol. 4307-312. [DOI] [PubMed] [Google Scholar]
- 61.Wiles, S., K. M. Pickard, K. Peng, T. T. MacDonald, and G. Frankel. 2006. In vivo bioluminescence imaging of the murine pathogen Citrobacter rodentium. Infect. Immun. 745391-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson, K. H. 1983. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J. Clin. Microbiol. 181017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson, K. H., M. J. Kennedy, and F. R. Fekety. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woolhouse, M. E., C. Dye, J. F. Etard, T. Smith, J. D. Charlwood, G. P. Garnett, P. Hagan, J. L. Hii, P. D. Ndhlovu, R. J. Quinnell, C. H. Watts, S. K. Chandiwana, and R. M. Anderson. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. USA 94338-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xavier, R. J., and D. K. Podolsky. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448427-434. [DOI] [PubMed] [Google Scholar]
- 66.Yu, J., R. Rossi, C. Hale, D. Goulding, and G. Dougan. 2009. Interaction of enteric bacterial pathogens with murine embryonic stem cells. Infect. Immun. 77585-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.