Abstract
Sepsis, a leading cause of death worldwide, involves proinflammatory responses and inefficient bacterial clearance. Previously, we have shown that CD137 (4-1BB), a member of the tumor necrosis factor receptor superfamily, plays critical roles in eradicating infective Listeria monocytogenes, a gram-positive bacterium, and that stimulation of CD137 protects mice from sepsis-induced death. In this study, we unexpectedly found that CD137 activation aggravated polymicrobial sepsis due to mixed gram-positive and gram-negative bacterial infection induced by cecal ligation and puncture (CLP). CD137-deficient (CD137−/−) mice showed significantly lower mortality than CD137-sufficient (CD137+/+) mice in the CLP model. Administration of an agonistic anti-CD137 monoclonal antibody (MAb) to CD137+/+ mice decreased their survival in this infection model, while administration of a blocking anti-CD137 ligand MAb (TKS-1) to such mice increased their survival. CD137−/− mice and TKS-1-treated CD137+/+ mice had lower levels of chemokines/proinflammatory cytokines (monocyte chemoattractant protein 1, interleukin-6 [IL-6], tumor necrosis factor alpha, IL-12) and an anti-inflammatory cytokine (IL-10), exhibited improved bacterial clearance in the peritoneum, liver, and blood, and had greater numbers of infiltrated peritoneal neutrophils and macrophages in the CLP model than control mice. Our data suggest that CD137 activation aggravates polymicrobial sepsis induced by CLP.
Sepsis is a serious disorder with high rates of morbidity and mortality. The incidence of sepsis in North America has been reported to be 3.0 per 1,000 people, which means that there are 750,000 cases annually; 210,000 of these cases are fatal, and the socioeconomic burden is great (31). The sepsis syndrome develops when the initial host response to infection results in inefficient bacterial clearance and becomes excessive, producing widespread inflammation and multiorgan failure (29, 30). It is estimated that gram-positive, gram-negative, and polymicrobial sepsis accounted for 30 to 50%, 25 to 30%, and 25% of all cases, respectively, in the year 2000 (4).
The first host response against an invading pathogen involves the recruitment of leukocytes, such as neutrophils and monocytes, to infectious foci and their activation, which allows these cells to successfully localize, kill, and clear the pathogen (19). Sepsis-induced mortality is also characterized by high levels of chemokines/proinflammatory cytokines (monocyte chemoattractant protein 1 [MCP-1], interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], IL-12, gamma interferon). The cecal ligation and puncture (CLP) rodent model has been developed as a suitable animal model for polymicrobial sepsis in humans since it can produce symptoms (i.e., appendicitis and peritonitis) similar to those of patients with sepsis (10, 16, 20, 22, 23). This CLP model produces a hyperdynamic, hypermetabolic state that can lead to a hypodynamic, hypometabolic stage and eventually death. It has been used extensively to investigate the clinical manifestations of sepsis and septic shock (5, 13, 14).
CD137 is a member of the tumor necrosis factor receptor superfamily, and its role as an activating T-cell costimulatory molecule has been well defined (2). However, CD137 is also expressed on a variety of innate immune cells, and it induces responses such as tumor rejection by natural killer cells, production of cytokines by dendritic cells, proliferation, survival, cytokine production in monocytes, and abrogation of the granulocyte-macrophage colony-stimulating factor-mediated antiapoptotic action of human neutrophils (1, 3, 8, 9, 15, 21, 26). Previously, we showed that CD137 is expressed on neutrophils and that CD137-deficient (CD137−/−) mice are very susceptible to infection by Listeria monocytogenes, a gram-positive intracellular bacterium, because the antilisterial activity of their neutrophils is defective (18). Furthermore, administration of agonistic anti-CD137 monoclonal antibody (MAb) to L. monocytogenes-infected mice rescued them from death, while all control antibody-treated mice died (17). In contrast, other investigators have shown that CD137−/− and CD137 ligand-deficient (CD137L−/−) mice are resistant to septic shock induced by lipopolysaccharide (LPS), a major inflammatory component of gram-negative bacteria (40). They also showed that blocking the CD137-CD137L pathway with antibody reversed LPS-induced septic shock in CD137+/+ mice. Therefore, it seemed likely that CD137 has different roles depending on the infecting bacterial species. To further characterize the role of CD137 in bacterial infections, we investigated its role using a CLP-induced polymicrobial sepsis model, which involves various gram-negative and -positive bacteria. We found that CD137-deficient mice were resistant to CLP-induced sepsis and that blocking CD137 signaling improved the outcome of sepsis, while administration of agonistic anti-CD137 antibody aggravated sepsis.
MATERIALS AND METHODS
Mice.
Male wild-type BALB/c mice (CD137+/+) were purchased from Orient Bio-Charles River (Seoul, Republic of Korea). CD137-deficient BALB/c mice (CD137−/−) and their littermates were a kind gift from B. S. Kwon (Korean National Cancer Center) (26). Mice were used when they were 7 to 9 weeks old. All experiments were conducted according to the regulations of the Animal Committee of the University of Ulsan.
Antibodies.
Hybridomas producing a neutralizing MAb against CD137L (TKS-1) (17) and an agonistic MAb against CD137 (3E1) (15) were kind gifts from H. Yagita (Juntendo University, Tokyo, Japan) and R. Mittler (Emory University, Atlanta, GA), respectively. The MAbs were produced from ascites of nude mice and were purified with a protein G column (Sigma-Aldrich, St. Louis, MO). Control rat immunoglobulin G (IgG) was purchased from Sigma. The following antibodies were obtained from BD PharMingen (San Diego, CA): phycoerythrin (PE)-conjugated anti-Ly6G, PE-conjugated anti-F4/80, fluorescein isothiocyanate (FITC)-conjugated anti-CD11b, FITC-conjugated anti-Fas, and FITC-conjugated anti-annexin V antibodies. To obtain heat-inactivated 3E1 (HI-3E1) and heat-inactivated TKS-1 (HI-TKS-1), 3E1 and TKS-1 were incubated for 20 min at 80°C.
Induction of sepsis by CLP.
Sepsis was induced by CLP as previously described (7, 24, 25, 32, 39). Briefly, mice were anesthetized by intraperitoneal injection of a mixture of Zoletil 50 (50 mg/kg) and Rompun (10 mg/kg). Under sterile conditions, a 1- to 2-cm incision was made in the lower left abdomen, and the cecum was exposed. The distal portion of the cecum was ligated with a 4-0 silk suture, punctured twice with a 21- or 26-gauge needle, and replaced in the peritoneal cavity, and the peritoneal wall and skin incisions were closed. Sham-operated mice were subjected to similar laparotomy without CLP. Immediately after surgery, all animals received a subcutaneous injection of 1 ml of sterile saline (0.9% NaCl). Survival was recorded every 12 h for 7 days.
Analysis of blood and leukocytes in the peritoneal cavity.
At 4, 8, 16, and 24 h after surgery using a 21-gauge needle for CLP, mice (≥6 mice/group) were killed, and blood was collected and saved for analysis of bacteria and cytokines. The peritoneal cavities were washed with 3 ml of sterile phosphate-buffered saline (PBS), and the lavage fluids were collected. After a 10-μl aliquot of the lavage fluid was removed for assessment of the bacterial CFU, the remaining fluid was centrifuged at 400 × g for 5 min at 4°C, and the supernatants were collected and stored at −80°C to determine cytokine concentrations. The cell pellets were treated with red blood cell lysing buffer, washed twice, and resuspended in PBS containing 3 mM EDTA. For flow cytometry, cells were incubated with 1 μg/ml of anti-mouse Fcγ MAb (clone 2.4G2) for 15 min to block nonspecific binding of MAbs and then stained with 1 μg/ml of FITC- or PE-conjugated anti-CD11b, -Ly6G, -F4/80, -Fas, or -annexin V MAb.
Bacterial counts.
Serial dilutions of homogenized liver samples (200- to 500-mg samples in 2 ml sterile saline), whole blood, and peritoneal lavage fluid were plated on tryptic soy and brain heart infusion agar plates. The tryptic soy agar plates were incubated aerobically at 37°C for 24 h, and the brain heart infusion agar plates were incubated anaerobically for 48 h. Colonies were counted, and the results are expressed below in CFU/ml, CFU/g, and CFU/mouse for blood, liver, and peritoneal lavage fluid samples, respectively, as previously described (34).
Cytokine analysis with CBA.
The cytokines in the peritoneal exudates and sera were quantified using a cytometric bead array (CBA) kit (BD Biosciences) with a FACSCaliber cytometer equipped with CellQuestPro and CBA software. According to the manufacturers, the theoretical lower limits of detection of IL-6, IL-10, MCP-1, TNF-α, and IL-12 are 5.0, 17.5, 52.7, 7.3, and 10.7 pg/ml, respectively.
Statistical analysis.
All data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA). Survival curves were analyzed by using a log rank test, and paired data were analyzed using a t test. Means and standard errors of the means were calculated in experiments with multiple data points. A P value of <0.05 was considered statistically significant.
RESULTS
CD137-deficient mice are resistant to CLP-induced sepsis.
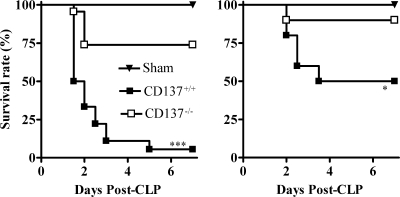
First, we compared the survival rates of CD137−/− mice and their wild-type littermates (CD137+/+) in the CLP sepsis model. In the severe sepsis experiment (using 21-gauge needles and two punctures), only 33% of the CD137+/+ mice (6 of 18 mice) were alive on day 2 post-CLP, compared to 74% of the CD137−/− mice (17 of 23 mice) (Fig. 1, left panel). By day 7 post-CLP, nearly all of the CD137+/+ mice were dead (17 of 18 mice; 5% survival), whereas no more CD137−/− mice had died. In the moderate CLP experiment (using 26-gauge needles and two punctures), the CD137−/− mice were also more resistant to sepsis than the CD137+/+ mice (Fig. 1, right panel). On day 7 post-CLP, 90% of the CD137−/− mice were still alive (9 of 10 mice), compared to 50% of the CD137+/+ mice (5 of 10 mice). Sham surgery did not cause any mortality, and the survival of CD137−/− mice having a C57BL/6 background with CLP-induced sepsis was also greater than that of wild-type C57BL/6 mice with CLP-induced sepsis, indicating that the CD137 effects were not mouse strain specific (data not shown).
FIG. 1.
The level of survival of CD137−/− mice with CLP-induced sepsis is higher than that of CD137+/+ mice. BALB/c CD137−/− mice and CD137+/+ littermates were subjected to CLP using 21-gauge (left panel) or 26-gauge (right panel) needles and two punctures. Sham-treated mice were subjected to laparotomy without CLP, and mouse survival was monitored every 12 h for 7 days. Each group contained 10 to 23 mice, and the results of two or three different experiments were pooled. *, P < 0.05 for a comparison with CD137+/+ mice, as determined by a log rank test; ***, P < 0.001 for a comparison with CD137+/+ mice, as determined by a log rank test.
Blocking CD137 signaling increases the survival of mice with CLP-induced sepsis, whereas stimulation of CD137 decreases it.
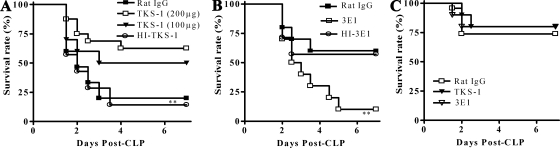
Our finding that CD137-deficient mice were more resistant to CLP-induced sepsis prompted us to investigate whether blocking or stimulating CD137 signaling affected the severity of sepsis. TKS-1 and 3E1 are MAbs which bind to CD137L and CD137, respectively. It has been shown that TKS-1 blocks CD137 signaling by binding to CD137L and inhibiting CD137-CD137L interactions (37). 3E1 has been used as an agonistic antibody that stimulates CD137 signaling in a variety of immune cells, including T cells, dendritic cells, natural killer cells, and neutrophils (1, 3, 9, 17, 18, 21). Wild-type mice were inoculated intraperitoneally with control antibody, TKS-1, or 3E1 and the CLP procedure was performed 2 h later. Figure 2A shows that treatment with TKS-1 increased the survival of the mice in a dose-dependent manner. On day 7 post-CLP, the survival rate for mice treated with 200 μg TKS-1 was 62.5% (10 of 16 mice), while that for control mice was only 20% (3 of 15 mice). Conversely, 3E1 treatment significantly reduced the survival of mice with CLP-induced sepsis (Fig. 2B). The survival rate for control antibody-treated mice on day 5 was 60% (12 of 20 mice) in the moderate CLP model, and that for the 3E1-treated mice was only 10% (2 of 20 mice). Since neither TKS-1 pretreatment nor 3E1 pretreatment affected the survival of CD137−/− mice in the CLP model, the effects described above were clearly CD137 dependent (Fig. 2C). In addition, another clone of an agonistic antibody, 3H3, had the same aggravating effect on CLP as 3E1 (data not shown), and HI-3E1 and HI-TKS-1 had little effect on the survival of CLP-treated mice, indicating that the effects of the antibodies were due to specific inhibition or triggering of CD137 (Fig. 2A and B).
FIG. 2.
Blocking CD137 signaling in CLP increases survival, while stimulation of CD137 decreases survival. (A) CD137+/+ BALB/c mice were inoculated intraperitoneally with rat IgG (200 μg), 3E1 (100 or 200 μg), or HI-TKS-1 (200 μg). After 2 h, they were subjected to CLP using 21-gauge needles and two punctures. (B) CD137+/+ mice were inoculated intraperitoneally with 200 μg of rat IgG, 3E1, or HI-3E1. After 2 h, they were subjected to CLP using 26-gauge needles. (C) CD137−/− mice were treated intraperitoneally with 200 μg of TKS-1, 3E1, or rat IgG 2 h before CLP using 21-gauge needles. Mouse survival was determined for 7 days. Each group contained 10 to 20 mice, and the results of two or three different experiments were pooled. **, P < 0.01 for a comparison with rat IgG-treated mice, as determined by a log rank test.
The enhanced survival of mice with CLP-induced sepsis in the absence of CD137 signaling is correlated with enhanced clearance of bacteria.
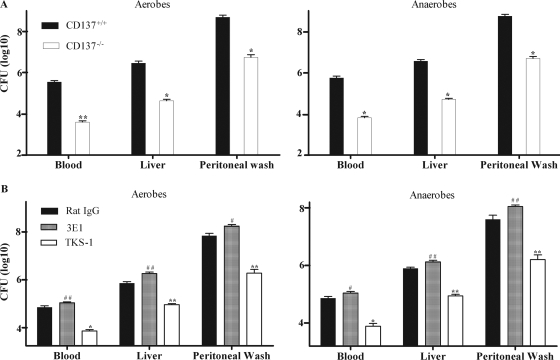
We investigated whether the enhanced survival of mice with CLP-induced sepsis resulting from CD137 deficiency or CD137 blockade was related to enhanced bacterial clearance. As shown in Fig. 3A, the bacterial clearance in the organs of CD137−/− mice in the CLP model was greater to that in the organs of CD137+/+ mice. At 24 h post-CLP, the numbers of CFU of aerobes and anaerobes in the blood, peritoneum, and liver of CD137−/− mice were 70 to 90 times lower than those in the CD137+/+ mice (Fig. 3A). The number of CFU at 8 h post-CLP exhibited the same pattern (data not shown).
FIG. 3.
Enhanced bacterial clearance in the absence of CD137 signaling after CLP. (A) CD137+/+ and CD137−/− mice were subjected to CLP using 21-gauge needles. After 24 h, they were killed to obtain blood, peritoneal wash fluid, and liver homogenates. Aerobic and anaerobic bacterial colony counts were obtained as described in Materials and Methods. The results are expressed in CFU/ml of blood, CFU/g of liver, and CFU/ml of peritoneal lavage fluid. The data are data from three pooled experiments, and each group contained six to eight mice. (B) BALB/c CD137+/+ mice were inoculated intraperitoneally with 200 μg of 3E1, TKS-1, or rat IgG. After 2 h, they were subjected to CLP using 21-gauge needles. The numbers of bacteria in the blood, liver, and peritoneum at 16 h post-CLP were determined as described above for panel A. The data are means and standard errors of the means obtained for individual mice and are representative of at least three independent experiments in which each group contained six to eight mice. # and *, P < 0.05 for a comparison with rat IgG-treated mice or CD137+/+ mice; ## and **, P < 0.01 for a comparison with rat IgG-treated mice or CD137+/+ mice.
Modulation of CD137 signaling using antibodies also affected the bacterial clearance rates in mice with CLP-induced sepsis. Administration of TKS-1 decreased the number of bacteria in the organs of mice with CLP-induced sepsis, while administration of 3E1 increased the number of bacteria (Fig. 3B). The numbers of CFU for 3E1-treated mice were twofold higher than those for control antibody-treated mice, while the numbers of CFU for TKS-1-treated mice were about 80 times lower. These results indicate that stimulation of CD137 signaling reduces bacterial clearance and leads to decreased survival of mice with induced sepsis.
CD137 blockade increases leukocyte infiltration into the peritoneal cavity during CLP-induced sepsis.
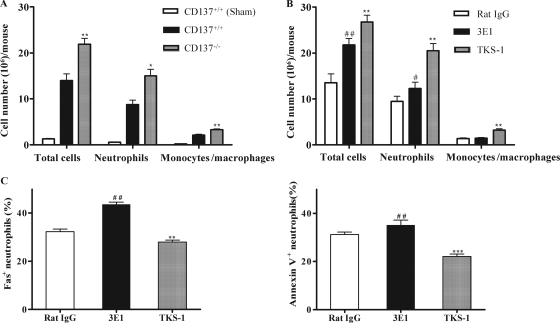
In the CLP-induced sepsis model, neutrophils and monocytes are first recruited into the peritoneum and play an important role in the host defense (19, 24, 25). A possible explanation for the increased bacterial clearance and survival rates of CD137-deficient mice was the potential difference in the leukocyte populations recruited to the peritoneum after CLP. As shown in Fig. 4A, at 16 h post-CLP, CD137−/− mice always had a higher number of infiltrated peritoneal cells, including neutrophils and monocytes/macrophages, than CD137+/+ mice. Most of peritoneal cells were neutrophils, and the percentage of neutrophils and monocytes/macrophages among the peritoneal cells in CD137−/− mice was also higher than the percentage in CD137+/+ mice at 4, 8, 16, and 24 h post-CLP (data not shown). These results suggested that the enhanced bacterial clearance in the CD137−/− mice might be due to the greater neutrophil and monocyte/macrophage recruitment into the peritoneal cavity after CLP.
FIG. 4.
Blocking CD137 signaling increases the number of peritoneal cells in mice subjected to CLP. (A) CD137+/+ and CD137−/− mice were subjected to CLP. At 16 h post-CLP, the peritoneal cavities were washed with 3 ml of sterile PBS. The cells were harvested by centrifugation, counted, stained with PE-conjugated anti-Ly6G or -F4/80 or FITC-conjugated anti-CD11b antibody and analyzed by fluorescence-activated cell sorting. CD11b+ Ly6G+ cells were considered neutrophils, and CD11b+ F4/80+ cells were considered monocytes/macrophages. (B) BALB/c CD137+/+ mice were inoculated intraperitoneally with 200 μg of 3E1, TKS-1, or rat IgG. After 2 h, they were subjected to CLP using 21-gauge needles. At 16 h post-CLP, the numbers of total cells, neutrophils, and monocytes/macrophages were determined as described above for panel A. (C) 3E1 treatment increases the percentage of Fas+ and annexin V+ neutrophils in CLP mice. The peritoneal cells described above for panel B were stained with FITC-conjugated anti-Fas or -annexin V or PE-conjugated anti-Ly6G antibody. They were first gated on PE-conjugated Ly6G, and the percentage of Fas+ or annexin V+ neutrophils was analyzed by fluorescence-activated cell sorting. The data are means and standard errors of the means obtained for individual mice and are representative of at least three independent experiments in which each group contained six to eight mice. # and *, P < 0.05 for a comparison with rat IgG-treated mice or CD137+/+ mice; ## and **, P < 0.01 for a comparison with rat IgG-treated mice or CD137+/+ mice; ***, P < 0.001 for a comparison with rat IgG-treated mice or CD137+/+ mice.
Blocking CD137 signaling by treatment with TKS-1 also stimulated infiltration of neutrophils and macrophages into the peritoneum of CLP-induced mice (Fig. 4B). Interestingly, injection of 3E1 also increased the number of neutrophils, but the neutrophils had higher levels of Fas and annexin V, which are indicators of early apoptotic cells (Fig. 4C).
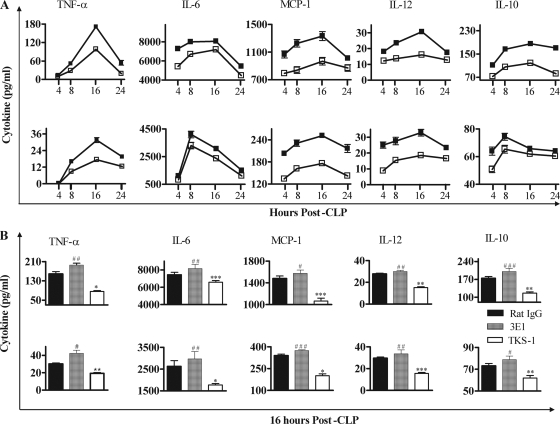
CD137−/− mice and TKS-1-treated mice have lower levels of chemokines/proinflammatory cytokines after CLP.
Since the severity of sepsis is correlated with the levels of proinflammatory cytokines (22, 25) and the anti-inflammatory cytokine IL-10 suppresses infiltration of neutrophils and macrophages in murine peritonitis (6, 37), we compared the levels of cytokines in CD137−/− and CD137+/+ mice in the CLP model. As shown in Fig. 5A, the levels of chemokines/proinflammatory cytokines (MCP-1, IL-6, TNF-α, IL-12) and IL-10 in the peritoneum and serum of CD137−/− mice were significantly lower than the levels in the peritoneum and serum of CD137+/+ mice up to 24 h post-CLP. In the peritoneum (Fig. 5A, top panel), all cytokine levels peaked at 16 h post-CLP and decreased thereafter. It is noteworthy that the times of peak cytokine levels were the same as those of leukocyte recruitment. The kinetics of cytokine levels in the serum were the same as the kinetics in peritoneum in both CD137−/− and CD137+/+ mice (Fig. 5A, bottom panel).
FIG. 5.
Blocking CD137 signaling reduces the levels of inflammatory cytokines and chemokines in the peritoneum and blood of CLP-treated mice. (A) CD137+/+ (▪) and CD137−/− (□) mice were killed and bled at 4, 8, 16, or 24 h post-CLP, and their peritonea were washed with 3 ml of PBS. After centrifugation at 400 × g for 5 min, cytokine concentrations in the peritoneum (top panels) and blood (bottom panels) were determined with a CBA kit. The data are expressed as pg cytokine/ml of peritoneal wash fluid or serum. (B) BALB/c CD137+/+ mice were inoculated intraperitoneally with 200 μg of 3E1, TKS-1, or rat IgG. After 2 h, they were subjected to CLP using 21-gauge needles. At 16 h post-CLP, cytokine concentrations in the peritoneum (top panel) and blood (bottom panel) were determined as described above for panel A. The data are means and standard errors of the means. The results of three independent experiments were similar, and therefore the data for six to eight mice/group were pooled. # and *, P < 0.05 for a comparison with rat IgG-treated mice; ## and **, P < 0.01 for a comparison with rat IgG-treated mice; ### and ***, P < 0.001 for a comparison with rat IgG-treated mice.
In addition, administration of 3E1 increased the levels of cytokines in the mice with CLP-induced sepsis, while administration of TKS-1 decreased these levels (Fig. 5B). It is clear, therefore, that CD137 signaling enhanced the levels of chemokines/proinflammatory cytokines, while blocking CD137 signaling reduced their levels, and this effect may well have influenced the levels of survival of mice with CLP-induced sepsis.
DISCUSSION
In this study, we show that CD137 is detrimental to mice with polymicrobial CLP-induced sepsis because of reduced antibacterial activities caused by CD137 signaling. First, the survival rates of CD137-deficient mice are higher, and the numbers of bacteria in their tissues are lower. Second, inhibition of the CD137 interaction with CD137L by administration of anti-CD137L antibody increases the survival of mice with CLP-induced sepsis. Last, activation of CD137 by injection of agonistic anti-CD137 antibody decreases the survival of mice with sepsis.
CD137 is expressed on a variety of immune cells, including activated T cells, dendritic cells, neutrophils, and natural killer cells, while CD137L is expressed exclusively on antigen-presenting cells, such as dendritic cells. This expression pattern raises the possibility that CD137-CD137L interactions are involved in multiple steps in innate and adaptive immune responses (35). However, although the role of CD137 as a costimulatory molecule in T-cell activation and differentiation has been well established, its roles in bacterial infections are poorly understood, except for our previous report that CD137-deficient mice are more susceptible to sepsis induced by L. monocytogenes (a gram-positive intracellular bacterium) (18). Recently, we demonstrated that injection of agonistic anti-CD137 antibody into CD137-sufficient mice significantly enhanced the survival of Listeria-infected mice (17). However, our previous observations of the effect of CD137 in Listeria infections are opposite the results of the present study with the CLP model. We hypothesize that CD137 plays opposite roles in gram-negative and gram-positive bacterial infections in combination with signaling via Toll-like receptors (TLRs). TLRs are pattern recognition receptors that play critical roles in the recognition of pathogen molecules. TLR4 recognizes predominantly the LPS of gram-negative bacteria, while TLR2 binds primarily gram-positive bacterial cell wall components (12). In a previous study we showed that combined activation of neutrophils with anti-CD137 antibody and heat-killed L. monocytogenes had a strong synergistic effect on the production of IL-6 and TNF-α (17). Although we did not examine combined TLR4 and CD137 signaling, it is possible that the signals aggravate sepsis in gram-negative bacterial infections. Other investigators have also shown that CD137−/− mice are more resistant to LPS-induced septic shock than wild-type mice (40), which is consistent with our present finding that CD137−/− mice with polymicrobial sepsis have higher survival rates. Thus, CD137 signaling in combination with TLR stimulation by a gram-positive bacterium may convert neutrophils into efficient bacterium-killing cells, while CD137 signaling in combination with TLR stimulation by a gram-negative bacterium may aggravate sepsis. Further studies are needed to address these possibilities.
In the CLP model, mouse survival is closely correlated with the numbers of bacterial CFU in tissues, infiltration of neutrophils and monocytes/macrophages, and levels of cytokines and chemokines. Bacteria spreading from infection sites and entering the bloodstream are rapidly trapped in the liver, bound to the surface of Kupffer cells and macrophages in the liver, and subsequently killed by infiltrated neutrophils (11, 27, 38). The liver is impaired in mice with lethal sepsis induced by CLP and also in humans with sepsis, and this impairment is associated with ineffective bacterial clearance, leading to bacterial dissemination and high mortality rates (4, 22, 25). Cytokines and chemokines play a critical role in recruitment of leukocytes to inflamed tissues, but they are often described as double-edged swords. An appropriate concentration of cytokines is necessary for the recruitment and activation of immune cells in response to foreign antigens, but when excess cytokines are produced, they damage the host. A successful host defense response during sepsis requires a fine balance of anti- and proinflammatory cytokines (25). Several reports have demonstrated that inflammatory cytokines can serve as both markers and mediators of the severity of sepsis and that elevated levels of these cytokines predict mortality following CLP (22, 33, 36). In the present study we found that the absence of CD137 or blockade of the CD137-CD137L interaction following CLP led to a marked increase in survival accompanied by an almost twofold-lower number of bacteria in the liver and reduced levels of chemokines/proinflammatory cytokines (MCP-1, IL-6, TNF-α, IL-12). In contrast, CD137 activation by injection of agonistic antibody 3E1 decreased survival, and there were concurrent increases in the levels of cytokines and chemokines and in the numbers of bacteria in the liver. We also found that CD137 interfered with infiltration of cells into infection sites. The use of CD137−/− mice or blocking the CD137-CD137L interaction by administering anti-CD137L MAb following CLP resulted in significant increases in the numbers of neutrophils and macrophages (Fig. 4). Although we do not know the mechanism by which CD137 suppresses cell infiltration in CLP-induced sepsis, this mechanism may well involve IL-10. It has been shown that IL-10 strongly suppresses infiltration of neutrophils and macrophages into the peritoneum in a murine peritonitis model (6, 37), and our results also show that serum and peritoneal IL-10 levels in CLP-treated mice are inversely correlated with the numbers of infiltrated neutrophils and macrophages (Fig. 5).
In conclusion, our results indicate that blockade of CD137 signaling improves and CD137 activation aggravates polymicrobial sepsis induced by CLP. This suggests that a regimen that controls CD137-CD137L interactions may aid in the treatment of clinical sepsis.
Acknowledgments
We thank Byoung S. Kwon (National Cancer Center, Korea) and R. Mittler (Emory University, Atlanta, GA) for their kind gifts of antibodies and for helpful comments and discussions.
Seong-A Ju and Sang-Chul Lee were supported by the Basic Research Promotion Fund (grant KRF-2007-412-J00302), and Quang-Tam Nguyen and Sang-Min Park were supported by the 2nd Project of BK21 funded by the Korean Ministry of Education, Science and Technology.
We have no financial conflicts of interest.
Editor: F. C. Fang
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Asai, T., B. K. Choi, P. M. Kwon, W. Y. Kim, J. D. Kim, D. S. Vinay, B. M. Gebhardt, and B. S. Kwon. 2007. Blockade of the 4-1BB (CD137)/4-1BBL and/or CD28/CD80/CD86 costimulatory pathways promotes corneal allograft survival in mice. Immunology 121349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho, R. H., B. Kwon, H. Yagita, S. La, E. A. Lee, J.-E. Kim, H. Akiba, J. Kim, J. H. Suh, D. S. Vinay, S. A. Ju, B. S. Kim, R. S. Mittler, K. Okumura, and B. S. Kwon. 2004. Blockade of 4-1BB (CD137)/4-1BB ligand interactions increases allograft survival. Transpl. Int. 17351-361. [DOI] [PubMed] [Google Scholar]
- 3.Choi, B. K., J. S. Bae, E. M. Choi, W. J. Kang, S. Sakaguchi, D. S. Vinay, and B. S. Kwon. 2004. 4-1BB-dependent inhibition of immunosuppression by activated CD4+ CD25+ T cells. J. Leukoc. Biol. 75785-791. [DOI] [PubMed] [Google Scholar]
- 4.Chung, S. W., S. R. Hall, and M. A. Perrelle. 2009. Role of haem oxygenase-1 in microbial host defence. Cell. Microbiol. 11199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebong, S., D. Call, J. Nemzek, G. Bolgos, D. Newcomb, and D. Remick. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect. Immun. 676603-6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feterowski, C., M. Mack, H. Weighardt, B. Bartsch, S. K. Moore, and B. Holzmann. 2004. CC chemokine receptor 2 regulates leukocyte recruitment and IL-10 production during acute polymicrobial sepsis. Eur. J. Immunol. 343664-3673. [DOI] [PubMed] [Google Scholar]
- 7.Flierl, M. A., D. Rittirsch, H. Gao, L. M. Hoesel, B. A. Nadeau, D. E. Day, F. S. Zetoune, J. V. Sarma, M. S. H. Lang, J. L. M. Ferrara, and P. A. Ward. 2008. Adverse functions of IL-17A in experimental sepsis. FASEB J. 222198-2205. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima, A., T. Yamaguchi, W. Ishida, K. Fukata, R. S. Mittler, H. Yagita, and H. Ueno. 2005. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J. Immunol. 1754897-4903. [DOI] [PubMed] [Google Scholar]
- 9.Futagawa, T., H. Akiba, T. Kodama, K. Takeda, Y. Hosoda, H. Yagita, and K. Okumura. 2002. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14275-286. [DOI] [PubMed] [Google Scholar]
- 10.Godshall, C. J., A. B. Lentsch, J. C. Peyton, M. J. Scott, and W. G. Cheadle. 2001. STAT4 is required for antibacterial defense but enhances mortality during polymicrobial sepsis. Clin. Diagn. Lab. Immunol. 81044-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory, S. H., L. K Barczynski, and E. J. Wing. 1992. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J. Leukoc. Biol. 51421-424. [DOI] [PubMed] [Google Scholar]
- 12.Hansson, U. H., J. Allen, M. Osman, G. Squires, N. Klein, and R. E. Callard. 2004. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. J. Immunol. 111173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbard, W. J., M. Choudhry, M. G. Schwacha, J. D. Kerby, L. W. Rue III, K. I. Bland, and I. H. Chaudry. 2005. Cecal ligation and puncture. Shock 2452-57. [DOI] [PubMed] [Google Scholar]
- 14.Jacob, A., M. Zhou, R. Wu, V. J. Halpern, T. S. Ravikumar, and P. Wang. 2007. Pro-inflammatory cytokines from kupffer cells downregulate hepatocyte expression of adrenomedullin binding protein-1. Biochim. Biophys. Acta 1772766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, Y. J., S. O. Kim, S. Shimada, M. Otsuka, A. S. Nebi, B. S. Kwon, T. H. Watts, and J. Han. 2007. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat. Immunol. 8601-609. [DOI] [PubMed] [Google Scholar]
- 16.Kato, T., A. Murata, H. Ishida, H. Toda, N. Tanaka, H. Hayashida, M. Monden, and N. Matsuura. 1995. Interleukin 10 reduces mortality from severe peritonitis in mice. Antimicrob. Agents Chemother. 391336-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, S. C., S. A. Ju, B. H. Sung, S. K. Heo, H. R. Cho, E. A. Lee, J. D. Kim, I. H. Lee, S. M. Park, Q. T. Nguyen, J. H. Suh, and B. S. Kim. 2009. Stimulation of the molecule 4-1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect. Immun. 772168-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, S. C., S. A. Ju, H. N. Pack, S. K. Heo, J. H. Suh, S. M. Park, B. K. Choi, B. S. Kwon, and B. S. Kim. 2005. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect. Immun. 735144-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsukawa, A. 2007. STAT proteins in innate immunity during sepsis: lessons from gene knockout mice. Acta Med. Okayama 61239-245. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa, A., C. M. Hogaboam, N. W. Lukacs, P. M. Lincoln, R. M. Strieter, and S. L. Kunkel. 1999. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between mcp-1 and leukotriene B4. J. Immunol. 1636148-6154. [PubMed] [Google Scholar]
- 21.Melero, I., J. V. Johnston, W. W. Shufford, R. S. Mittler, and L. Chen. 1998. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell. Immunol. 190167-172. [DOI] [PubMed] [Google Scholar]
- 22.Moreno, S. E., J. C. Alves-Filho, F. Rios-Santos, J. S. Silva, S. H. Ferreira, F. Q. Cunha, and M. M. Teixeira. 2006. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J. Immunol. 1771264-1271. [DOI] [PubMed] [Google Scholar]
- 23.Murphey, E. D., D. N. Herndon, and E. R. Sherwood. 2004. Gamma interferon does not enhance clearance of Pseudomonas aeruginosa but does amplify a proinflammatory response in a murine model of postseptic immunosuppression. Infect. Immun. 726892-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ness, T. L., C. M. Hogaboam, R. M. Strieter, and S. L. Kunkel. 2003. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J. Immunol. 1713775-3784. [DOI] [PubMed] [Google Scholar]
- 25.Ness, T. L., K. J. Carpenter, J. L. Ewing, C. J. Gerard, C. M. Hogaboam, and S. L. Kunkel. 2004. CCR1 and CC chemokine ligand 5 interactions exacerbate innate immune responses during sepsis. J. Immunol. 1736938-6948. [DOI] [PubMed] [Google Scholar]
- 26.Nishimoto, H., S. W. Lee, H. Hong, K. G. Potter, M. Maeda-Yamamoto, T. Kinoshita, Y. Kawakami, R. S. Mittler, B. S. Kwon, C. F. Ware, M. Croft, and T. Kawakami. 2005. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood 1064241-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen, K. A., F. J. Pixley, K. S. Thomas, M. V. Manzanares, B. J. Ray, A. F. Horwitz, J. T. Parsons, H. E. Beggs, E. R. Stanley, and A. H. Bouton. 2007. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 1791275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Pinheiro da Silva, F., M. Aloulou, D. Skurnik, M. Benhamou, A. Andremont, I. T. Velasco, M. Chiamolera, J. S. Verbeek, P. Launay, and R. C. Monteiro. 2007. CD16 promotes Escherichia coli sepsis through an FcRγ inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat. Med. 131368-1374. [DOI] [PubMed] [Google Scholar]
- 30.Plitas, G., B. M. Burt, H. M. Nguyen, Z. M. Bamboat, and R. P. DeMatteo. 2008. Toll-like receptor 9 inhibition reduced mortality in polymicrobial sepsis. J. Exp. Med. 2051277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramnath, R. D., S. W. Ng, A. Guglielmotti, and M. Bhatia. 2008. Role of MCP-1 in endotoxemia and sepsis. Int. Immunopharmacol. 8810-818. [DOI] [PubMed] [Google Scholar]
- 32.Reddy, R. C., G. H. Chen, K. Tateda, W. C. Tsai, S. M. Phare, P. Mancuso, M. P. Golden, and T. J. Standiford. 2001. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 281L537-L543. [DOI] [PubMed] [Google Scholar]
- 33.Remick, D. G., G. Bolgos, S. Copeland, and J. Siddiqui. 2005. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect. Immun. 732751-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott, M. J., W. G. Cheadle, J. J. Hoth, J. C. Peyton, K. Subbarao, W. H. Shao, and B. Haribabu. 2004. Leukotriene B4 receptor (BLT-1) modulates neutrophil influx into the peritoneum but not the lung and liver during surgically induced bacterial peritonitis in mice. Clin. Diagn. Lab. Immunol. 11936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo, S. K., H. Y. Park, J. H. Choi, W. Y. Kim, Y. H. Kim, H. W. Jung, B. Kwon, H. W. Lee, and B. S. Kwon. 2003. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J. Immunol. 171576-583. [DOI] [PubMed] [Google Scholar]
- 36.Sherwood, E. R., V. T. Enoh, E. D. Murphey, and C. Y. Lin. 2004. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab. Investig. 841655-1665. [DOI] [PubMed] [Google Scholar]
- 37.Shin, H. H., J. E. Lee, and H. S. Choi. 2007. Absence of 4-1BB increases cell influx into the peritoneal cavity in response to LPS stimulation by decreasing macrophage IL-10 levels. FEBS Lett. 5814355-4360. [DOI] [PubMed] [Google Scholar]
- 38.Steel, D. M., and A. S. Whitehead. 1994. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 1581-88. [DOI] [PubMed] [Google Scholar]
- 39.Tschop, J., A. Martignoni, H. S. Goetzman, L. G. Choi, Q. Wang, J. G. Noel, C. K. Ogle, T. A. Pritts, J. A. Johannigman, A. B. Lentsch, and C. C. Caldwell. 2008. γδ T cells mitigate the organ injury and mortality of sepsis. J. Leukoc. Biol. 83581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinay, D. S., B. K. Choi, J. S. Bae, W. Y. Kim, B. M. Gebhardt, and B. S. Kwon. 2004. 4-1BB-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 1734218-4229. [DOI] [PubMed] [Google Scholar]