Abstract
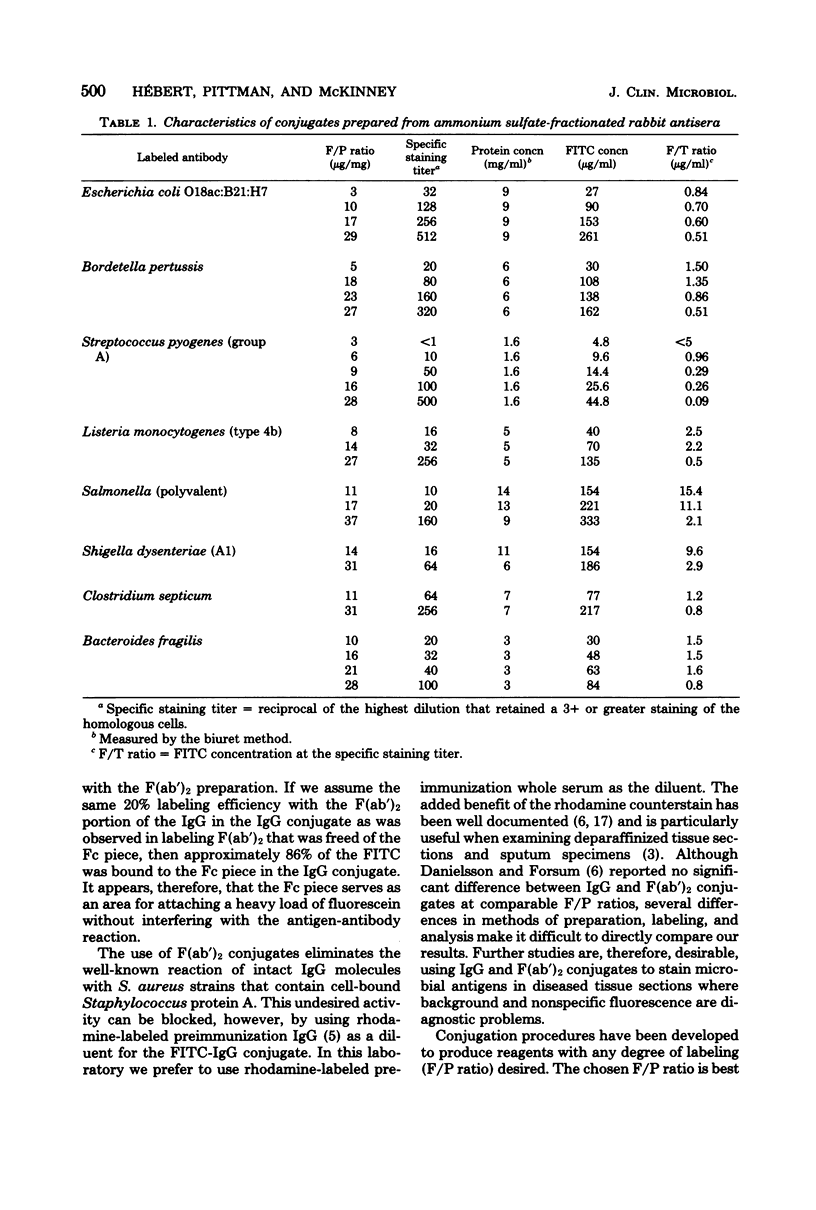
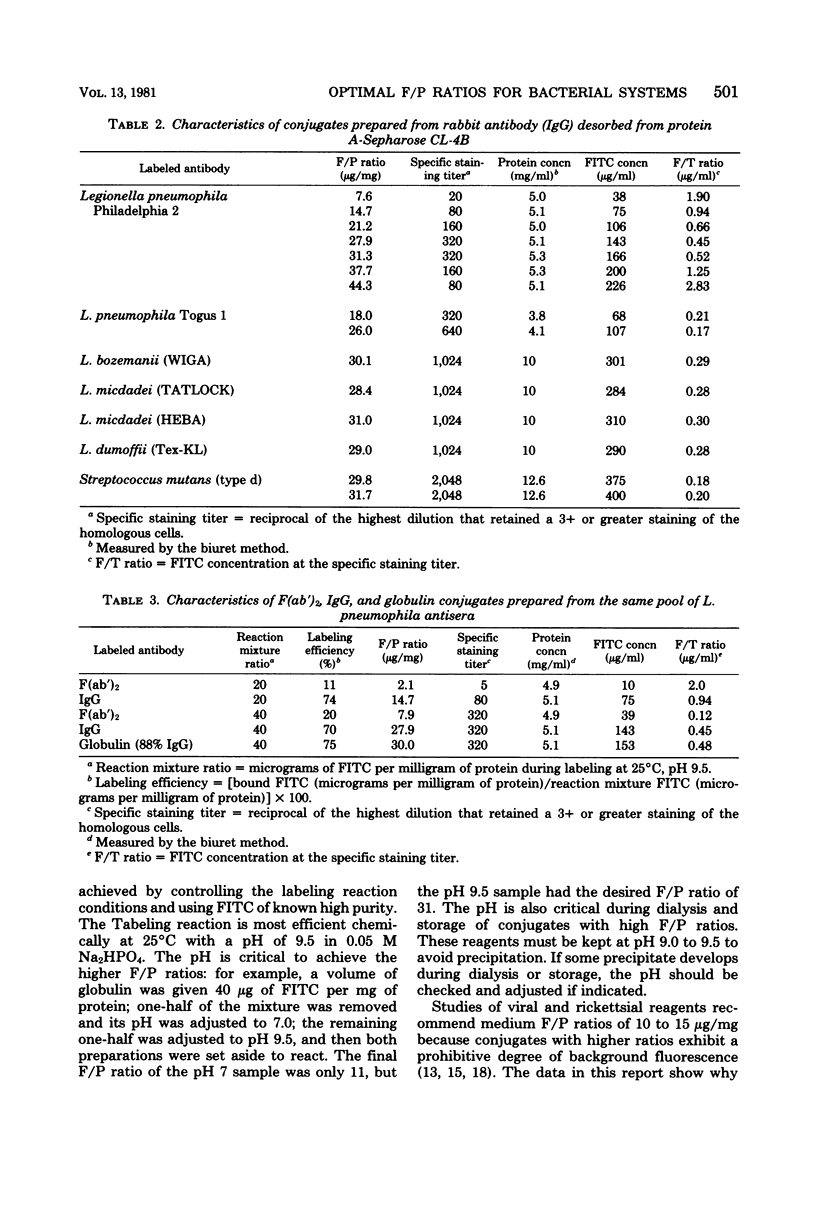
A number of bacterial systems were studied with specific direct fluorescent-antibody reagents prepared from rabbit antiserum fractions and having a wide range of fluorescein-to-protein ratios. These systems included Bacteroides, Bordetella, Clostridium, Escherichia, Legionella, Listeria, Salmonella, Shigella, and Streptococcus. For all systems studied, a fluorescein-to-protein ratio of 30 was optimal for conjugates prepared from ammonium sulfate fractions (greater than 75% gamma globulin) and pure immunoglobulin G desorbed from the Sepharose-bound protein A of Staphylococcus aureus. A pepsin digestion procedure is described that yielded the F(ab')2 piece of pure immunoglobulin G; this was labeled and studied at two fluorescein-to-protein ratios.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., Steigerwalt A. G., McDade J. E. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann Intern Med. 1979 Apr;90(4):656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson D., Forsum U. Diagnosis of Neisseria infections by defined immunofluorescence. Methodologic aspects and applications. Ann N Y Acad Sci. 1975 Jun 30;254:334–349. doi: 10.1111/j.1749-6632.1975.tb29185.x. [DOI] [PubMed] [Google Scholar]
- Danielsson D. The demonstration of N. gonorrhoeae with the aid of fluorescent antibodies. 5. A comparison of different techniques--absorption, one-step inhibition, and counterstaining--for elimination of cross reactions. Acta Derm Venereol. 1965;45(1):61–73. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hebert G. A., Pelham P. L., Pittman B. Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol. 1973 Jan;25(1):26–36. doi: 10.1128/am.25.1.26-36.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert G. A., Pittman B., Cherry W. B. Factors affecting the degree of nonspecific staining given by fluorescein isothiocyanate labelled globulins. J Immunol. 1967 Jun;98(6):1204–1212. [PubMed] [Google Scholar]
- Hébert G. A., Tzianabos T., Gamble W. C., Chappell W. A. Development and characterization of high-titered, group-specific fluorescent-antibody reagents for direct identification of rickettsiae in clinical specimens. J Clin Microbiol. 1980 May;11(5):503–507. doi: 10.1128/jcm.11.5.503-507.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerla H. C., Hierholzer J. C. Physicochemical and serological characteristics of respiratory virus fluorescein-isothiocyanate conjugates for fluorescent-antibody diagnosis. J Clin Microbiol. 1975 May;1(5):451–461. doi: 10.1128/jcm.1.5.451-461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A. ENZYMATIC DIGESTION OF RABBIT GAMMA GLOBULIN AND ANTIBODY AND CHROMATOGRAPHY OF DIGESTION PRODUCTS. Methods Med Res. 1964;10:134–141. [PubMed] [Google Scholar]
- SMITH C. W., MARSHALL J. D., Jr, EVELAND W. C. Use of contrasting fluorescent dye as counterstain in fixed tissue preparations. Proc Soc Exp Biol Med. 1959 Oct;102:179–181. doi: 10.3181/00379727-102-25182. [DOI] [PubMed] [Google Scholar]
- Tzianabos T., Hebert G. A., White L. A. Preparation of rabies fluorescein isothiocyanate-labeled immune globulin from mouse hyperimmune ascitic fluids. J Clin Microbiol. 1976 Jun;3(6):609–612. doi: 10.1128/jcm.3.6.609-612.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


