Abstract
Herpes simplex virus 2 (HSV-2) strains containing mutations in the virion host shutoff (vhs) protein are attenuated for replication compared with wild-type virus in mouse embryonic fibroblasts (MEFs). However, HSV-2 vhs mutants replicate to near wild-type levels in the absence of the RNA-activated protein kinase (PKR). PKR is one of several kinases that phosphorylates the eukaryotic initiation factor 2α (eIF2α) to inhibit translation initiation, and we previously found that more of the phosphorylated form of eIF2α accumulates in MEFs infected with HSV-2 vhs mutants than with wild-type virus. Here, we show that this increase in phosphorylated eIF2α is primarily PKR dependent. Using MEFs expressing nonphosphorylatable eIF2α, we demonstrate that phosphorylated eIF2α is the primary cause of attenuated replication of HSV-2 vhs mutants and that attenuation correlates with decreased accumulation of viral proteins. Normally, HSV antagonizes eIF2α phosphorylation through the action of ICP34.5, which redirects protein phosphatase 1α (PP1α) to dephosphorylate eIF2α during infection. We show that ICP34.5 does not accumulate efficiently in MEFs infected with HSV-2 vhs mutant viruses, suggesting that the accumulation of phosphorylated eIF2α and the attenuated phenotype of HSV-2 vhs mutants in MEFs result from a deficiency in ICP34.5.
vhs is a tegument protein conserved among the neurotropic alphaherpesviruses (18, 47, 50). vhs is an endoribonuclease (15, 53, 59) that degrades host transcripts to inhibit host protein synthesis (24, 28, 29, 40) and viral transcripts, possibly to aid progression through the temporal cascade of viral gene expression (28, 39, 40). In primary MEFs at low multiplicities of infection (MOI), replication of herpes simplex virus 2 (HSV-2) vhs mutants is reduced 10- to 100-fold by 24 h postinfection (p.i.) compared with that of wild-type virus (13, 27). HSV-2 vhs mutants replicate to wild-type levels in mouse embryonic fibroblasts (MEFs) lacking the alpha/beta interferon (IFN-α/β) receptor and in RNA-activated protein kinase (PKR)-deficient MEFs (13, 27), suggesting that HSV-2 vhs has a role in counteracting the PKR-mediated arm of the IFN-α/β response in MEFs. One target of PKR is the translation initiation factor eukaryotic initiation factor 2α (eIF2α), and indeed, phosphorylated eIF2α accumulates in MEFs infected with HSV vhs mutants (43).
eIF2, composed of α, β, and γ subunits, is a key factor in cap-dependent translation initiation (44). eIF2α is phosphorylated by a group of kinases including PKR, PKR-like ER kinase (PERK), and the general control nonderepressible-2 kinase (GCN2). These kinases are activated by double-stranded RNA (dsRNA), ER stress, and amino acid deprivation, respectively, each of which often occurs during virus infection (3, 21). Phosphorylation of the eIF2α subunit prevents formation of the 80S ribosomal complex by inhibiting guanine nucleotide exchange (20, 33, 44), resulting in the formation of stalled initiation complexes and translation arrest. Viruses that trigger but cannot counteract eIF2α phosphorylation may be compromised for viral protein synthesis and replication due to the translation arrest imposed. In addition, stalled initiation complexes induce the formation of stress granules, which are dynamic structures that provide a means for the transient inhibition of protein synthesis (25). Fibroblasts infected with HSV-1 vhs mutants have more stress granules than do cells infected with wild-type virus (16), a finding that correlates well with the increase in phosphorylated eIF2α identified in MEFs infected with vhs mutants (43). Stress granule formation could have a detrimental effect on virus replication by sequestering and preventing translation of viral messages or host mRNAs encoding proteins critical for virus replication (2, 30).
HSV-1 encodes several antagonists of PKR and PERK, including ICP34.5, US11, and gB (7-10, 12, 36, 37). Of particular interest is the PKR antagonist ICP34.5, which counteracts eIF2α phosphorylation by binding to protein phosphatase 1α (PP1α) and redirecting it to dephosphorylate eIF2α (22, 23). Infection of fibroblasts and neurons with HSV-1 lacking ICP34.5 leads to greatly increased levels of phosphorylated eIF2α, translation arrest, and strongly attenuated virus replication compared to those of cells infected with wild-type virus (5, 12). These observations highlight the functional importance of PKR antagonism by ICP34.5.
While wild-type HSV prevents accumulation of phosphorylated eIF2α in primary MEFs, MEFs infected with vhs mutants exhibit increased levels of phosphorylated eIF2α. Levels in cells infected with vhs mutants are increased by 6 h p.i., and the difference from wild-type virus-infected cells becomes magnified as the infection progresses (43). The accumulation of phosphorylated eIF2α is more pronounced in MEFs infected with HSV-2 vhs mutants than in those infected with HSV-1 vhs mutants (43). The mechanism by which phosphorylated eIF2α is increased in MEFs infected with vhs mutants has not been determined. Phosphorylated eIF2α could accumulate because of a defect in the regulation of phosphatase or kinase activities, or both. HSV-2 vhs mutants (13) but not HSV-1 vhs mutants (43) are attenuated primarily through the action of PKR, distinguishing between the roles of HSV-1 and HSV-2 vhs in promoting virus replication. We reasoned that the increase in phosphorylated eIF2α in MEFs infected with HSV-2 vhs mutants may be PKR mediated, and this increase may be responsible for attenuating the replication of HSV-2 vhs mutant viruses due to translation arrest and/or formation of stress granules. In this study, we examined these possibilities and identified the probable cause of the increase in phosphorylated eIF2α in MEFs infected with HSV-2 vhs mutants.
MATERIALS AND METHODS
Mice.
129Sv/Ev (wild-type) mice and PKR−/− mice (129Sv/Ev background) (58) were housed and bred at the Department of Comparative Medicine, Saint Louis University School of Medicine, in accordance with institutional and Public Health Service guidelines.
Cells and viruses.
Vero cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 3% newborn and 3% fetal calf serum with 1% penicillin-streptomycin. B6 (wild-type) and eIF2α S51A MEFs (B6 background) (referred to hereafter as S51A MEFs) (48) were provided by Donalyn Scheuner and Randy Kaufman and were used through passage 11. B6 (wild-type), PERK−/− (B6 background), and GCN2−/− (B6 background) MEFs (60, 61) were obtained from Doug Cavener and used through passage 5. 129Sv/Ev (wild-type) and PKR−/− (129Sv/Ev background) MEFs were prepared from embryos at 15 to 18 days gestation and used through passage 5. TIA-1−/− and TIAR−/− MEF cell lines (30) were provided by Cathy Miller with permission from Paul Anderson. MEFs were cultured in DMEM supplemented with 10% bovine growth serum and 1% penicillin-streptomycin. MEFs were seeded into 12-well plates and allowed to divide for 24 to 48 h until contact inhibited prior to infection.
All HSV-2 strains were in the wild-type 333 background (14). The vhs-deficient mutant 333d41 and its marker rescue virus, 333d41R (52), were provided by David Leib. 333d41 has a 939-bp deletion between two XcmI sites and lacks vhs RNase activity. Vhs RNase-deficient (333D215N) and rescue (333D215NR) HSV-2 strains were constructed as previously described (27). 333D215N has a single amino acid substitution that abrogates RNase activity while retaining the capacity for vhs to form complexes with VP16 and VP22 and to serve structural roles in the virion. A thymidine kinase-deficient (ΔTK−) virus (32) was provided by Jim Smiley. The ΔTK− virus has a 180-bp deletion in the TK gene that eliminates TK activity. All HSV-1 strains were in the wild-type strain 17 background. Strain 17syn+ (6) was obtained from David Leib. 17termA, which contains a truncated ICP34.5 gene, and its marker rescue virus, 17termAR (4), were provided by David Leib with permission from Rick Thompson. Viruses were propagated on Vero cells, and titers were determined on Vero cell monolayers by standard plaque assay (26).
In vitro infection and assessment of virus replication.
Cells were infected at low (0.01) or high (10) MOI. Mock infection was carried out by the addition of uninfected Vero cell lysate. At 1 h p.i., the virus was removed, cells were gently washed twice with phosphate-buffered saline at room temperature, and DMEM containing 10% bovine growth serum was added for the remainder of the incubation period. Cultures were collected 24 h p.i. and were frozen at −80°C. Virus titers were determined by standard plaque assay on Vero cell monolayers (26). Statistical significance of differences in viral titers was determined by t test.
Western blot analysis.
At 3, 6, or 9 h p.i., as specified in the figure legends, the medium was completely removed from the cell monolayers. Cells were lysed on ice in 200 μl buffer containing 50 mM Tris-Cl (pH 8), 150 mM NaCl, and 1% NP-40 supplemented with complete mini EDTA-free protease inhibitor cocktail tablets and PhosStop phosphatase inhibitor cocktail tablets (Roche). An equal volume of 2× reducing sample buffer was added to the samples, and they were stored at −80°C. For analysis of PKR levels in cell lysates, samples were combined with an equal volume of 2× nonreducing sample buffer in order to distinguish PKR from a cross-reactive band with the same mobility under reducing conditions. Samples were boiled for 4 min and proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to Immobilon-P membranes (Millipore), which were incubated overnight with rabbit polyclonal antibodies against eIF2α (Santa Cruz), phosphorylated eIF2α (Biosource), HSV-1 or HSV-2 (Dako), PKR (Santa Cruz), S6 ribosomal protein (Cell Signaling), or HSV-2 ICP34.5 (55) (provided by Phil Krause) diluted in Tris-buffered saline-Tween 20 with 1% nonfat milk. The membranes were then incubated for 1 h with an alkaline phosphatase-conjugated anti-rabbit secondary antibody (Promega). Colorimetric signal was detected using the BCIP/NBT substrate (Promega). Western blots were scanned and bands were quantified using Image J software (http://rsb.info.nih.gov/ij/).
Assessment of PKR activation.
129Sv/Ev (wild-type) MEFs in six-well plates were left untreated, mock infected by the addition of Vero cell lysate, or infected at an MOI of 10. At 3 h p.i., medium was removed and replaced with phosphate-free DMEM supplemented with 10% bovine growth serum for 1 h. From 4 to 6 h p.i., cells were incubated in the presence of 50 μCi [32P]ATP (Perkin Elmer). Cells were lysed at 6 h p.i. as described above for Western blot analysis. PKR was immunoprecipitated from lysates by using an anti-PKR antibody conjugated to protein A/G agarose beads (Santa Cruz) for 3.5 h on a rocker at 4°C in the presence of PhosStop phosphatase inhibitor cocktail (Roche). Beads were washed three times with ice-cold lysis buffer. Immunoprecipitated proteins were analyzed by Western blot analysis for PKR and autoradiography, using Kodak Biomax light film (Sigma).
An in vitro PKR kinase assay was designed to assess the capacity of lysates from 333- and 333d41-infected MEFs to activate PKR, presumably by the presence of dsRNA. PKR was immunoprecipitated from uninfected wild-type 129Sv/Ev MEFs as described above. Anti-PKR antibody also was used in an immunoprecipitation with PKR−/− MEF lysate as a negative control. As a stimulus for PKR activation, lysates were prepared in parallel as follows. PKR−/− MEFs were untreated, mock infected with Vero cell lysate, or infected with 333 or 333d41 virus at an MOI of 10. At 6 h p.i., the MEFs were lysed in lysis buffer supplemented with RNasin (Promega) and centrifuged at 400 × g in a Sorvall MC12V microcentrifuge for 10 min to pellet the nuclear fraction. The postnuclear fraction was mixed with the immunoprecipitated PKR in the presence of kinase assay reaction buffer consisting of 25 mM Tris-HCl (pH 7.5), 20 μM EGTA, magnesium-ATP cocktail (Upstate), and 10 μCi [γ-32P]ATP (3,000 Ci/mmol; Perkin Elmer). Samples were incubated at 30°C for 15 min to incorporate radioactive phosphate. As a positive control for kinase activity, 2.5 μg poly(I:C) (Sigma) was added to immunoprecipitated PKR in the kinase assay. After incubation, 2× reducing sample buffer was added, and samples were analyzed by Western blot analysis as described above. 32P signal was detected by autoradiography.
Real-time reverse transcriptase PCR.
129Sv/Ev MEFs were infected at an MOI of 10 as described above. At 5 h p.i., medium was removed, and cells were collected in TRI reagent (Molecular Research Center, Inc.). Samples were frozen at −80°C until further processing. RNA was isolated according to the TRI reagent manufacturer's protocol, treated with Turbo DNase (Ambion), and quantified using a Nanodrop ND-1000 (Thermo Scientific). cDNA was synthesized from 500 ng RNA by using the Transcriptor first-strand cDNA synthesis kit (Roche) primed with random hexamers according to the manufacturer's specifications. ICP34.5 was amplified (forward primer, 5′-AGACTCCCAAATGGTCCCTGCGTA-3′; reverse primer, 5′-TGTTCGCCCACTCTGCGT-3′) and normalized to the signal from amplification of 18S rRNA (forward primer, 5′-GTAACCCGTTGAACCCCATT-3′; reverse primer, 5′-CCATCCAATCGGTAGTAGCG-3′) using FastStart SYBR green master mix (Roche) on an ABI 7500 system. Cycling conditions were as follows: 50°C for 2 min; 95°C for 10 min; 40 cycles at 95°C for 15 s; 60°C for 1 min. Products were dissociated at 95°C for 15 s. The threshold cycle (CT) value was calculated from the average of duplicate wells, and the ICP34.5 signal for each sample was normalized to the 18S rRNA signal content by determination of ΔCT. The change in ICP34.5 signal for the 333d41, 333d41R, or ΔTK virus relative to that of wild-type virus was determined using the 2ΔΔCT method (31, 45).
RESULTS
Accumulation of phosphorylated eIF2α in MEFs infected with HSV-2 lacking RNase activity correlates with decreased accumulation of viral proteins.
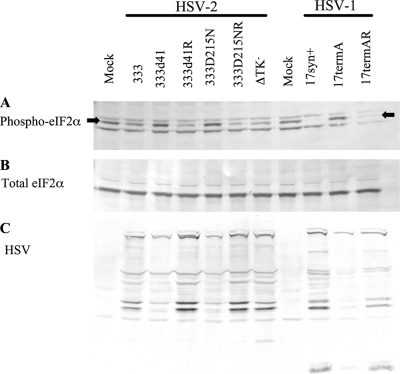
HSV-2 vhs is a multifunctional protein with RNase activity and a structural role within the virus tegument. MEFs infected with an HSV-2 vhs deletion mutant contain more phosphorylated eIF2α than do MEFs infected with wild-type virus (43). To determine whether the increase in phosphorylated eIF2α depends on vhs RNase activity, we compared the vhs null mutant 333d41 with 333D215N (Table 1), in which a single amino acid substitution ablates RNase activity but leaves the protein complex formation and stability of vhs intact (27). Wild-type 129Sv/Ev MEFs were infected with HSV-2 333, 33341, 333d41R, 333D215N, 333D215NR, and the ΔTK− virus (Table 1) at an MOI of 10, and samples were collected for Western blot analysis. Samples were collected at 6 h p.i., the time at which a difference in the levels of phosphorylated eIF2α in MEFs infected with wild-type and vhs mutant viruses is unambiguously detectable (43). Cells infected with HSV-1 17termA, which lacks ICP34.5, were included as a positive control. Indeed, 17termA-infected MEFs showed the expected increase in phosphorylated eIF2α compared to that in cells infected with its wild-type and marker rescue viruses (Fig. 1) (12, 57). Levels of phosphorylated eIF2α (Fig. 1A) relative to total eIF2α (Fig. 1B) were decreased in cells infected with 333 and rescue viruses 333d41R and 333D215NR compared with those in mock-infected cells, as would be expected due to the activity of ICP34.5. The levels of phosphorylated eIF2α did not differ between cells infected with 333 and those infected with the marker rescue viruses, confirming that the marker rescue viruses are phenotypically wild type. MEFs infected with 333d41 and 333D215N contained more phosphorylated eIF2α compared with those infected with the wild-type and marker rescue viruses, demonstrating that the increase in phosphorylated eIF2α results from the loss of vhs RNase activity. In addition, MEFs infected with the ΔTK− virus did not show an increase in phosphorylated eIF2α compared to those infected with wild-type 333. Therefore, increased levels of phosphorylated eIF2α specifically occur in the absence of vhs RNase activity.
TABLE 1.
Viruses used in this work
| Virus | Description | Reference |
|---|---|---|
| 333 | HSV-2 wild type | 14 |
| 333d41 | vhs mutant in the 333 background | 52 |
| 333d41R | Repaired 333d41 virus | 52 |
| 333D215N | Point mutation in 333 vhs ablates RNase activity | 27 |
| 333D215NR | Repaired point mutant virus | 27 |
| ΔTK− virus | Thymidine kinase-deficient virus in the 333 background | 32 |
| 17syn+ | HSV-1 wild type | 6 |
| 17termA | ICP34.5−/− in the 17syn+ background | 4 |
| 17termAR | Repaired 17termA virus | 4 |
FIG. 1.
Accumulation of phosphorylated eIF2α (Phospho-eIF2α) is associated with loss of HSV-2 vhs nuclease activity. Wild-type 129Sv/Ev MEFs were infected at an MOI of 10 with the indicated viruses. Lysates were collected at 6 h p.i. and analyzed by Western blot analysis for phosphorylated eIF2α (middle band indicated by arrows) (A), total eIF2α (B), or HSV-1 and HSV-2 proteins (C).
Phosphorylation of eIF2α occurs in response to virus infection to shut down protein synthesis and prevent virus replication. To assess whether the increase in eIF2α phosphorylation in MEFs infected with HSV-2 vhs mutants correlates with decreased accumulation of viral proteins, a replicate Western blot was prepared and probed with a mixture of polyclonal antisera against HSV-1 and HSV-2 (Fig. 1C). Loading was equilibrated to the total eIF2α signal (Fig. 1B). Small amounts of viral proteins accumulated in MEFs infected with the HSV-2 vhs mutants 333d41 and 333D215N compared to amounts in MEFs infected with wild-type and marker rescue viruses, indicating that vhs is necessary for accumulation of viral proteins to wild-type levels and that its RNase activity is critical in the process. Viral proteins accumulated to similar levels in MEFs infected with wild-type 333 and the marker rescue viruses 333d41R and 333D215NR, confirming that the rescue viruses reflect the characteristics of the wild-type virus and, thus, decreases observed with the mutant viruses result from loss of vhs RNase activity. Viral proteins accumulated to wild-type levels in MEFs infected with the ΔTK− virus at 6 h p.i., distinguishing the phenotypes of the TK and vhs mutants. As expected, levels of HSV-1 proteins were greatly reduced in MEFs infected with 17termA compared to in those infected with 17 and 17termAR (1, 11).
PKR mediates most of the eIF2α phosphorylation in MEFs infected with vhs mutants.
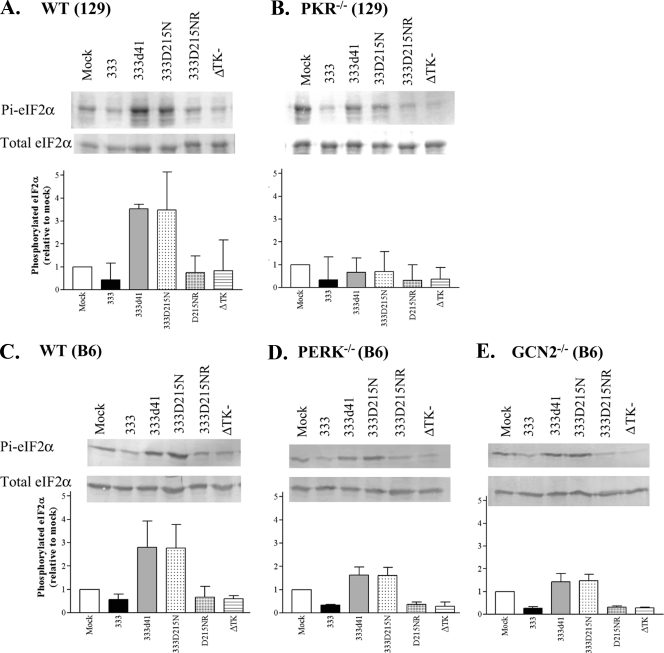
HSV-2 vhs mutants replicate inefficiently in the presence of PKR (13). PKR is one of the kinases that modifies eIF2α, so we determined whether PKR was responsible for phosphorylating eIF2α in MEFs during infection with vhs mutant viruses. 129Sv/Ev (wild-type) MEFs and PKR−/− MEFs (129Sv/Ev background) were infected with 333, 333d41, 333D215N, 333D215NR, and the ΔTK− virus at an MOI of 10, and samples were collected at 6 h p.i. for Western blot analysis. For each sample, the phosphorylated eIF2α signal was normalized to the total eIF2α signal, and then comparisons were made between samples. In 129Sv/Ev MEFs, wild-type and rescue viruses suppressed the level of phosphorylated eIF2α to below that found in mock-infected cells (Fig. 2A). 333d41 and 333D215N infection resulted in the accumulation of phosphorylated eIF2α to levels 3.5-fold higher than mock treatment and eightfold higher than 333 and D215NR infection (Fig. 2A), as previously observed (43). In PKR−/− MEFs, however, the level of phosphorylated eIF2α in cells infected with vhs mutants was slightly lower than the amount found in mock-infected cells and was only twofold higher than in cells infected with wild-type and rescue viruses (Fig. 2B). Infection with the ΔTK− virus produced the same suppression of phosphorylated eIF2α as that produced by wild-type and rescue viruses (Fig. 2A), indicating that the phosphorylation increases are specific to cells infected with vhs mutants. These data indicate that PKR modulates the level of phosphorylated eIF2α during infection with vhs mutants. Because other kinases also contribute to the accumulation of phosphorylated eIF2α in the absence of PKR, we examined the effects of the eIF2α kinases PERK and GCN2. In B6 (wild-type), PERK−/−, and GCN2−/− MEFs, 333 and 333D215NR effectively suppressed the accumulation of phosphorylated eIF2α (Fig. 2C, D, and E). In B6 MEFs infected with 333d41 or 333D215N, phosphorylated eIF2α accumulated to levels threefold higher than those in mock-infected MEFs (Fig. 2C). In contrast to our observations in PKR−/− MEFs, phosphorylated eIF2α in PERK−/− (Fig. 2D) and GCN2−/− (Fig. 2E) MEFs infected with vhs mutants was not suppressed below the levels in mock-infected MEFs, although it was reduced to levels only approximately 1.5-fold over those in mock-treated cells. This suggests that PERK and GCN2 contribute to the phosphorylation of eIF2α at 6 h p.i., but to a lesser extent than does PKR. In B6 (wild-type), PERK−/−, and GCN2−/− MEFs infected with 333d41 or 333D215N, phosphorylated eIF2α accumulated to levels about fivefold higher than those found in MEFs infected with wild-type virus, confirming that PKR is the kinase primarily responsible for the increase in phosphorylated eIF2α in cells infected with vhs mutants compared to those infected with wild-type virus. In summary, the levels of phosphorylated eIF2α in MEFs infected with vhs mutants fall below the level in mock-infected cells only in PKR−/− MEFs and are closest to the levels found after infection with wild-type virus (within twofold) in PKR−/− MEFs. These results indicate that PKR mediates most of the phosphorylation of eIF2α in the absence of vhs RNase activity, whereas PERK and/or GCN2 make comparatively minor contributions.
FIG. 2.
eIF2α phosphorylation in MEFs infected with HSV-2 vhs mutant viruses is mediated primarily by PKR. Wild-type 129Sv/Ev (A), PKR−/− (B), wild-type B6 (C), PERK−/− (D), and GCN2−/− (E) MEFs were infected at an MOI of 10 with the indicated viruses. Lysates were collected 6 h p.i. and analyzed by Western blot analysis. Band intensities were quantified with ImageJ. The ratio of phosphorylated eIF2α (Pi-eIF2α) compared to total eIF2α was determined and expressed as a proportion of the mock-infected MEF sample (set to 1). Data represent the means ± standard deviation (SD) of the results for three to four experiments. A representative Western blot from each data set is shown.
Phosphorylated eIF2α is the primary cause for attenuated replication and reduced accumulation of viral proteins in MEFs infected with vhs mutant viruses.
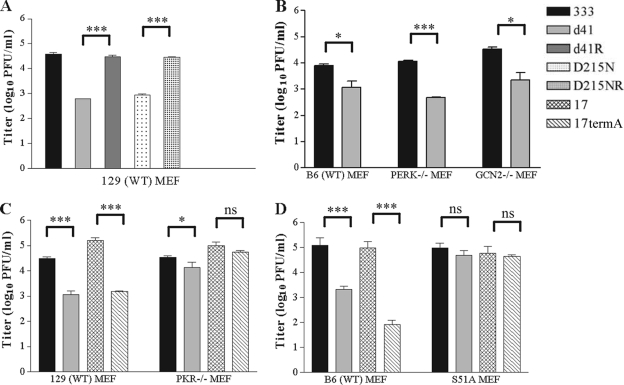
Replication of HSV-2 vhs mutant viruses is attenuated 10- to 100-fold compared with replication of wild-type and marker rescue viruses when MEFs are infected at a low MOI (Fig. 3A) (13, 27). In the absence of PKR, replication of 333d41 recovers to near wild-type levels (Fig. 3C) (13). To establish the effects of the eIF2α kinases PERK and/or GCN2 on replication of 333d41, we infected B6 (wild-type), PERK−/−, and GCN2−/− (B6 background) MEFs with 333 and 333d41 at a low MOI (0.01). Replication of 333d41 was equally attenuated (approximately 10-fold) in PERK−/− and GCN2−/− MEFs as well as B6 MEFs at 24 h p.i. (Fig. 3B), suggesting that neither kinase contributes greatly to the attenuation of vhs mutant virus replication in MEFs, consistent with their relatively minor contributions to eIF2α phosphorylation in MEFs infected with vhs mutants (Fig. 2).
FIG. 3.
Replication of an HSV-2 vhs mutant virus is restored to wild-type levels in PKR−/− and S51A MEFs. (A) Wild-type 129Sv/Ev MEFs were infected at a low MOI (0.01) with the indicated viruses. Samples were collected 24 h p.i. Data represent virus titers from duplicate samples from one experiment and are consistent with published data. Wild-type B6, PERK−/−, and GCN2−/− MEFs (B); 129Sv/Ev and PKR−/− MEFs (C); or B6 and S51A MEFs (D) were infected at a low MOI (0.01). Viral titers from samples collected 24 h p.i. are represented as the means ± standard error of the mean of the results for two experiments (B) or three experiments (C and D). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05.
Given that PKR is critical to attenuation of 333d41 replication and PKR mediates the majority of the eIF2α phosphorylation in MEFs infected with vhs-deficient virus (Fig. 2), we predicted that 333d41 should replicate like wild-type virus in the absence of eIF2α phosphorylation. To test this prediction, we infected B6 (wild-type) MEFs and S51A MEFs (B6 background), which contain a nonphosphorylatable eIF2α with a serine-to-alanine mutation at the phosphoacceptor site (48), with 333 and 333d41 at a low MOI (0.01) as described above. If our prediction was correct, replication of 333d41 in S51A MEFs should resemble that of HSV-1 lacking ICP34.5, an antagonist of PKR-mediated eIF2α phosphorylation (57). Therefore, infections with HSV-1 17syn+ (wild type) and 17termA (ICP34.5−/−) (Table 1) were also included. As expected, replication of 333d41 and 17termA was attenuated relative to that of 333 and 17syn+, respectively, in 129Sv/Ev and B6 MEFs 24 h after low-MOI (0.01) infection (Fig. 3C and D). 17termA replicated to wild-type levels in PKR−/− and S51A MEFs (Fig. 3C and D), demonstrating the role ICP34.5 plays in counteracting PKR-mediated eIF2α phosphorylation in MEFs (57). In PKR−/− MEFs, the 333d41 titer recovered to nearly the level of the wild-type virus (Fig. 3C), as previously observed (13). Significantly, 333d41 replicated to wild-type virus level in S51A MEFs (Fig. 3D), demonstrating that eIF2α phosphorylation is the primary reason for attenuation of vhs mutant virus replication in MEFs.
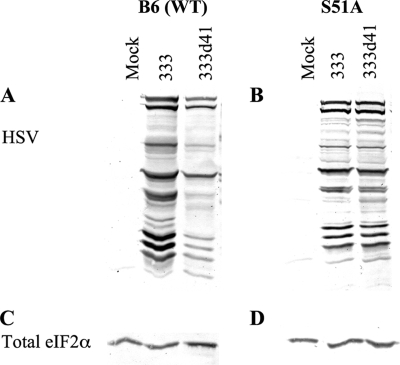
In MEFs and other cell types infected with HSV-1 ICP34.5 null mutants, phosphorylated eIF2α accumulates and leads to arrest of viral protein translation (1, 11). In MEFs infected with vhs mutants, smaller amounts of viral proteins accumulate by 6 h p.i. (Fig. 1C) than in MEFs infected with wild-type virus, so we sought to determine the reason for the reduced accumulation of viral proteins. If eIF2α phosphorylation inhibits translation of viral transcripts in MEFs infected with vhs mutants, the accumulation of viral proteins should recover to wild-type levels in cells containing nonphosphorylatable eIF2α. If, however, the abundant host transcripts maintained in the absence of vhs compete with viral messages for translation machinery and thus attenuate viral protein synthesis independently of eIF2α phosphorylation, then the decrease in accumulation of viral proteins should occur even in the absence of phosphorylated eIF2α. To evaluate the attenuation of viral protein synthesis in the absence of vhs, B6 (wild-type) and S51A MEFs were mock infected or infected with 333 and 333d41 at an MOI of 10. At 6 h p.i., cells were lysed and analyzed by Western blot analysis. Loadings were approximately equivalent based on the amount of total eIF2α (Fig. 4C and D). Western blot analysis of the membranes, using a polyclonal antibody against HSV proteins, revealed that in 333d41-infected wild-type MEFs, viral protein bands detected at 6 h p.i. were less intense than those in 333-infected cells (Fig. 4A). Thus, accumulation of viral proteins was reduced in MEFs infected with vhs mutant virus when more phosphorylated eIF2α was present. In S51A MEFs, the intensities of viral proteins detected by Western blot analysis 6 h after infection with 333 or 333d41 were indistinguishable (Fig. 4B), indicating that eIF2α phosphorylation is the primary cause of the reduced accumulation of viral proteins in MEFs infected with HSV-2 vhs mutants.
FIG. 4.
eIF2α phosphorylation attenuates accumulation of viral protein in MEFs infected with vhs mutants. B6 (A and C) and S51A (B and D) MEFs were mock infected or infected at an MOI of 10, and samples were collected at 6 h p.i. Proteins were separated by SDS-PAGE and analyzed by Western blot analysis with antibodies against viral proteins (A and B). Loading was equilibrated to total eIF2α levels detected by Western blot analysis (C and D). WT, wild type.
Stress granule formation is not a major cause of attenuated replication of vhs mutant viruses.
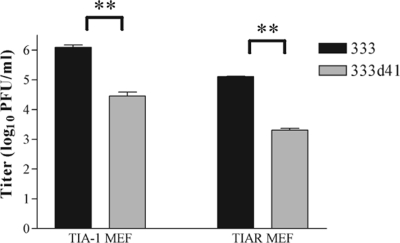
Phosphorylated eIF2α triggers a variety of cellular stress responses, including the formation of stress granules (25). Thus, the increase in phosphorylated eIF2α in MEFs infected with vhs mutants compared with levels in MEFs infected with wild-type virus may increase stress granule formation during infection. Indeed, more stress granules have been observed in fibroblasts infected with HSV-1 vhs mutants (16). The self-aggregating RNA-binding proteins TIA-1 and TIAR mediate, in part, the shuttling of transcripts into stress granules. The process of shuttling transcripts could impact virus replication, particularly if viral transcripts or transcripts encoding required host proteins are sequestered in stress granules (2). If stress granule formation attenuates replication of HSV-2 vhs mutants, 333d41 should replicate to near wild-type levels in TIA-1−/− and TIAR−/− MEFs, which are unable to form stress granules. However, 333d41 did not replicate as well as 333 in either TIA-1−/− or TIAR−/− MEFs when assessed 24 h after infection at a low MOI (0.01) (Fig. 5), indicating that stress granule formation is not the predominant reason for attenuated replication of HSV-2 vhs mutant virus in MEFs. This result supports the likelihood that translational arrest mediated by eIF2α causes the attenuated replication of HSV-2 vhs mutant viruses.
FIG. 5.
Replication of 333d41 remains attenuated in the absence of stress granule formation. TIA-1−/− and TIAR−/− MEFs were infected at a low MOI (0.01) in duplicate, and samples were collected at 24 h p.i. Viral titers represent the means ± standard error of mean of the results for two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05.
PKR levels and activity are not increased in MEFs infected with vhs mutants compared with those in MEFs infected with wild-type virus.
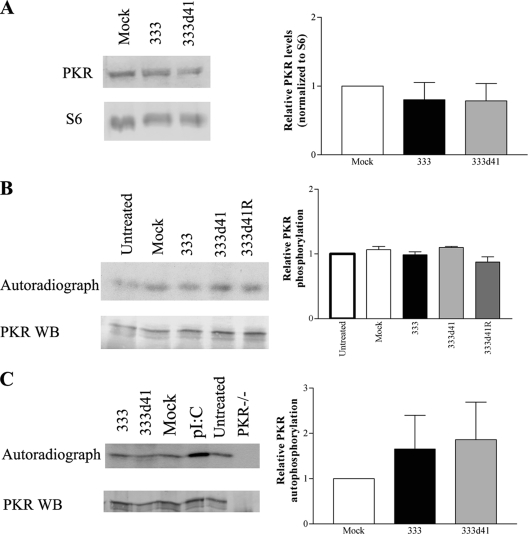
Next, we sought to determine the mechanism by which phosphorylated eIF2α accumulates in MEFs infected with HSV-2 vhs mutants. Because the majority of the increase in phosphorylated eIF2α is PKR mediated, we examined PKR levels and activity. PKR levels may be higher in the absence of vhs if, for example, vhs normally degrades PKR transcripts. To evaluate PKR protein levels, 129Sv/Ev (wild-type) MEFs were mock infected or infected with 333 or 333d41, and cells were lysed at 6 h p.i. and analyzed by Western blot analysis. PKR levels were normalized to S6 ribosomal protein. MEFs infected with 333 and 333d41 contained equivalent amounts of PKR (Fig. 6A), demonstrating that vhs is not critical for regulating PKR levels early after infection and, thus, an increase in PKR cannot explain the increase in phosphorylated eIF2α in MEFs infected with vhs mutants compared to those infected with wild-type virus.
FIG. 6.
PKR levels and activity are equivalent during infection with 333 and 333d41. (A) 129Sv/Ev MEFs were infected at an MOI of 10. Samples were collected 6 h p.i. Proteins were separated by SDS-PAGE, and PKR levels were analyzed by Western blot analysis. Loading was normalized to levels of S6 ribosomal protein. Band intensities were quantified using ImageJ software, and relative PKR levels are expressed in reference to the mock-infected sample (set to 1). The graph represents the means ± SD of the results for four experiments. (B) 129Sv/Ev MEFs were infected at an MOI of 10. Cells were labeled with [32P]ATP from 4 to 6 h p.i. and lysed 6 h p.i. PKR was immunoprecipitated, and proteins were separated by SDS-PAGE and analyzed by Western blotting and autoradiography. ImageJ software was used to quantify bands, and the 32P signal was normalized to the PKR signal on the Western blot. Autophosphorylation of PKR is expressed relative to the untreated sample (set to 1). The graph represents the means ± SD of the results for two experiments. (C) PKR−/− MEFs were infected at an MOI of 10 and lysed at 6 h p.i., and postnuclear supernatants were used to stimulate exogenous PKR in an in vitro kinase assay. The lysates or poly(I:C) were incubated with PKR immunoprecipitated from 129Sv/Ev MEFs in the presence of [γ32P]ATP. Proteins were separated by SDS-PAGE and analyzed as described for panel B. Quantified data represents the means ± SD of the results for six experiments. Representative Western blots (WB) and/or autoradiographs are shown for each experiment.
Alternatively, PKR could be more active in MEFs infected with vhs mutants because the loss of RNase activity could lead to an increase in dsRNA resulting from undegraded complementary transcripts. Another possibility is that US11, which binds and inhibits PKR activation (8, 46), could be synthesized to lower levels, resulting in increased PKR activity. However, a lack of US11 is likely not the cause of the initial increase in phosphorylated eIF2α, because US11 is synthesized and inhibits PKR late in infection (35), whereas we observe the increase in eIF2α phosphorylation by 6 h p.i. To assess PKR activation, we immunoprecipitated PKR from 32P-labeled MEFs that had been untreated, mock infected with Vero cell lysate, or infected at an MOI of 10 with 333, 333d41, 333d41R, 333D215N, 333D215NR, the ΔTK− virus, 17syn+, or 17termA. Relatively equal amounts of phosphorylated PKR were found under all conditions (Fig. 6B and data not shown), indicating that PKR is active at or near its basal level in MEFs 6 h after infection with the HSV-1 and HSV-2 viruses tested. Importantly, the presence or absence of vhs RNase activity did not affect the activation of PKR (Fig. 6B and data not shown), suggesting that increased PKR activation is not the cause of the increased levels of phosphorylated eIF2α observed during infection with vhs mutants compared with levels observed with wild-type and marker rescue viruses.
To corroborate these results, an in vitro kinase assay was utilized. Lysates from PKR−/− MEFs infected with 333 and 333d41 were tested for their capacity to activate exogenous PKR immunoprecipitated from wild-type MEFs. Immunoprecipitated PKR not exposed to lysate (untreated) did not differ from PKR exposed to mock-infected cell lysate, indicating that PKR is not activated by the addition of PKR−/− MEF cell lysate (Fig. 6C). PKR activation was equivalently low upon exposure to postnuclear lysates from 333- and 333d41-infected PKR−/− MEFs (Fig. 6C). This result suggests that the levels of dsRNA did not differ in the cytoplasm of the infected cells from which the lysates were derived at 6 h p.i., a time point at which we can clearly detect increased levels of phosphorylated eIF2α in the 333d41-infected MEFs. PKR was enzymatically active, as confirmed by addition of poly(I:C). In a second in vitro kinase assay, PKR was immunoprecipitated from MEFs 6 h after infection with 333 and 333d41 and subjected to an in vitro kinase assay to measure autophosphorylation (data not shown). Results corroborated those shown in Fig. 6B and C. Together, these results indicate that the increase in phosphorylated eIF2α in MEFs infected with vhs mutants is not due to a detectable increase in PKR activation.
Less ICP34.5 accumulates in MEFs infected with vhs mutant viruses.
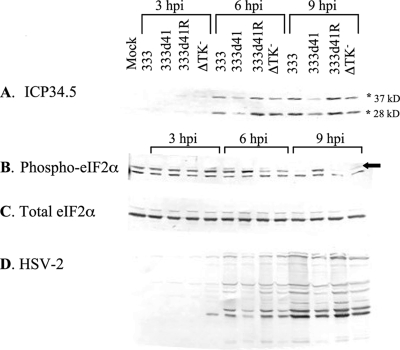
Experiments thus far have demonstrated that changes in PKR level or activity do not explain the accumulation of phosphorylated eIF2α in MEFs infected with vhs mutants. An alternative hypothesis is that the PKR antagonist ICP34.5, which is critical for redirecting PP1α to dephosphorylate eIF2α during infection (22), is not synthesized efficiently in MEFs infected with vhs mutants compared to in those infected with wild-type virus. To examine levels of ICP34.5 protein at various times throughout infection, wild-type 129Sv/Ev MEFs were mock infected or infected with 333, 333d41, 333d41R, or the ΔTK− virus and collected at 3, 6, and 9 h p.i. Samples were assessed by Western blot analysis with antibodies against ICP34.5, phosphorylated eIF2α, eIF2α, and HSV-2. Loading was normalized to total eIF2α (Fig. 7C). The ICP34.5 antibody recognizes two bands of approximately 28 and 37 kDa (Fig. 7A), both of which are diminished in the presence of small interfering RNA against HSV-2 ICP34.5 (55), suggesting that this antibody reacts with two different isoforms of ICP34.5. The nature of these isoforms has not been determined. Markedly less ICP34.5 accumulated in MEFs infected with 333d41 than in MEFs infected with 333 and 333d41R at 6 and 9 h p.i. (Fig. 7A), suggesting that the increase in phosphorylated eIF2α in MEFs infected with vhs mutants could be due to reduced ICP34.5/PP1α-mediated dephosphorylation of eIF2α. Indeed, the lower levels of ICP34.5 correlated with increased phosphorylated eIF2α (Fig. 7B, top band) and decreased accumulation of viral proteins in this time course (Fig. 7D). Like vhs mutants, TK mutants replicate less efficiently than does wild-type virus in contact-inhibited MEFs (57). Consistent with this observation, the ΔTK− virus accumulated reduced amounts of many viral proteins compared to 333 by 9 h p.i. (Fig. 7D); however, ICP34.5 levels remained similar to wild-type levels at both 6 and 9 h p.i. (Fig. 7A). This result indicates that the reduced amount of ICP34.5 seen in 333d41-infected MEFs is specific to vhs mutants.
FIG. 7.
ICP34.5 accumulation is reduced in MEFs infected with 333d41 compared to in those infected with 333 and 333d41R. 129Sv/Ev MEFs were mock infected or infected at an MOI of 10 with the indicated viruses. Samples were collected at 3, 6, and 9 h p.i. Proteins were separated by SDS-PAGE and analyzed by Western blot analysis for ICP34.5 (A), phosphorylated eIF2α (Phospho-eIF2α) (B), total eIF2α (C), or HSV-2 proteins (D). Asterisks indicate the two isoforms of ICP34.5. The arrow indicates the band corresponding to phosphorylated eIF2α. The data are representative of the results for two independent experiments.
ICP34.5 transcript accumulates to the same levels in MEFs infected with 333 and 333d41.
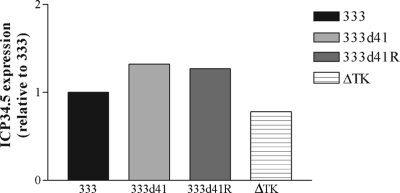
The lower levels of ICP34.5 protein accumulation in 333d41-infected MEFs could be explained by the reduced accumulation of ICP34.5 transcript. We used real-time reverse transcriptase PCR to evaluate ICP34.5 transcript levels in wild-type 129Sv/Ev MEFs infected with 333, 333d41, 333d41R, and the ΔTK− virus. Mock-infected cells were included as a negative control. At 5 h p.i., equal levels of ICP34.5 mRNA were detected in all infected cell types (Fig. 8), indicating that decreased accumulation of ICP34.5 protein at 6 h p.i. in MEFs infected with 333d41 (Fig. 7) does not result from reduced transcript levels.
FIG. 8.
ICP34.5 mRNA levels in MEFs infected with 333d41 are equal to those in MEFs infected with 333 and 333d41R. 129Sv/Ev MEFs were infected at an MOI of 10. Samples were collected 5 h p.i. RNA was isolated and reverse transcribed before analysis of the ICP34.5 transcript levels by real-time PCR. Data are representative of the results for two independent experiments.
ICP34.5 accumulates to wild-type levels in S51A MEFs.
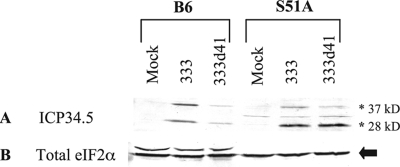
Many viral proteins accumulate to lower levels in MEFs infected with 333d41 than in those infected with wild-type and marker rescue viruses (Fig. 1C and 4A), but viral proteins accumulate to wild-type levels in MEFs that have a nonphosphorylatable eIF2α (Fig. 4B). At 5 h p.i., the amount of ICP34.5 transcript available for translation is not a limiting factor in ICP34.5 protein accumulation (Fig. 8). This raises the possibility that translation of ICP34.5 may not initiate efficiently in MEFs infected with 333d41. Therefore, we compared ICP34.5 levels in wild-type and S51A MEFs to determine whether eIF2α phosphorylation contributes to the decreased accumulation of ICP34.5 in MEFs infected with 333d41. B6 (wild-type) and S51A (B6 background) MEFs were mock infected or infected with 333 or 333d41 and collected at 6 h p.i. for analysis of ICP34.5 protein levels by Western blotting. In B6 MEFs, the 28- and 37-kDa isoforms of ICP34.5 were present in approximately equal intensities, and the levels of both isoforms were reduced in MEFs infected with 333d41 compared with those in MEFs infected with 333 (Fig. 9A and B). In S51A MEFs, however, the 28-kDa isoform predominated, and levels of ICP34.5 in 333d41- and 333-infected cells were nearly identical (Fig. 9C and D). These results suggest that eIF2α phosphorylation occurring in the absence of vhs attenuates the translation of ICP34.5, which likely exacerbates the increase in phosphorylated eIF2α.
FIG. 9.
ICP34.5 accumulation is hindered in the presence of phosphorylated eIF2α. B6 and S51A MEFs were infected at an MOI of 10. Six hours p.i., samples were collected, and proteins were analyzed by Western blot analysis with antibodies against ICP34.5 (A) or eIF2α loading control (B). ICP34.5 isoforms are indicated with asterisks, and eIF2α is indicated by an arrow. Data are representative of the results for two independent experiments.
DISCUSSION
Loss of HSV-2 vhs renders HSV-2 hypersensitive to the PKR-mediated arm of the IFN-α/β response in MEFs (13). When we examined the substrates of PKR to determine the mechanism by which vhs counteracts the effects of PKR, we found that the PKR substrate eIF2α is phosphorylated more heavily in MEFs infected with HSV-2 vhs mutants than in those infected with wild-type virus (43). In this study, we show that the accumulation of phosphorylated eIF2α is primarily PKR dependent and is associated with the RNase function of HSV-2 vhs. Phosphorylation of eIF2α causes the attenuated replication of HSV-2 vhs mutants in MEFs and correlates with decreased accumulation of viral proteins. The formation of stress granules does not affect replication of HSV-2 vhs mutant viruses, suggesting that translational shutoff plays the major role in attenuating replication of HSV-2 vhs mutants in MEFs. Although PKR is responsible for most of the accumulation of phosphorylated eIF2α in MEFs infected with HSV-2 vhs mutants, vhs does not directly regulate PKR activity, because neither the level of PKR protein nor its activity is detectably increased in MEFs infected with vhs mutants compared to in those infected with wild-type virus, though differences in kinase activity below our level of detection could potentially render measurable effects on the substrate. We did, however, observe a marked decrease in the steady-state levels of ICP34.5 in MEFs infected with vhs mutant virus compared to in MEFs infected with wild-type virus, which correlates with the attenuated replication of the vhs mutant. While many HSV proteins accumulate to lower levels in MEFs infected with vhs mutants compared to in those infected with wild-type virus, our data are consistent with the hypothesis that a defect in ICP34.5 accumulation results in the attenuated replication of HSV-2 vhs mutants in MEFs. During infection with wild-type or vhs mutant viruses, ICP34.5 transcript levels are equal in wild-type MEFs. In addition, ICP34.5 protein accumulates to equivalent levels in S51A MEFs infected with vhs mutant or wild-type virus, suggesting phosphorylated eIF2α reduces the efficiency of ICP34.5 translation, which in turn exacerbates the increase in phosphorylated eIF2α.
Our data support an important role for vhs in establishing an environment conducive to the rapid production of viral proteins early in infection. It has been proposed that synthesis of HSV proteins in cells infected with vhs mutants could be reduced due to competition between cellular and viral transcripts for translation machinery (51). Our data suggest this is not strictly the case because during infection of MEFs containing nonphosphorylatable eIF2α, the accumulation of viral proteins from wild-type virus and that from vhs mutant viruses are indistinguishable, despite the fact that the vhs mutant is unable to degrade host transcripts. Thus, virus-induced phosphorylation of eIF2α, which results in a limiting number of initiation-competent translation complexes, may create the need for vhs during infection. Our data are consistent with a model in which vhs reduces competition between viral and cellular transcripts for initiation-competent translation factors in the presence of basal levels of phosphorylated eIF2α very early in infection, when the levels of viral transcripts are relatively low. During this early phase, we hypothesize that HSV-2 vhs activity facilitates translation of immediate early transcripts, transcripts that are imported with the virion, and/or transcripts from other gene classes that are nonetheless transcribed early to promote viral protein synthesis. ICP34.5, for example, is transcribed beginning 1 to 3 h after infection with HSV-1 (42). Consequently, ICP34.5 made at early times p.i. mediates the dephosphorylation of eIF2α to a level below the basal level found in untreated cells, which may be advantageous to the translation of viral proteins. In the scenario of a wild-type virus infection, ICP34.5 continues to accumulate and carries out its critical role in counteracting eIF2α phosphorylation throughout the replication cycle, thereby promoting viral protein synthesis and replication. In the absence of vhs, we propose that ICP34.5 synthesis is delayed at very early times p.i. because of increased competition for initiation-competent translation factors. As a result, ICP34.5 protein fails to accumulate to levels sufficient to reduce the basal amount of phosphorylated eIF2α and to counteract the accumulation of phosphorylated eIF2α during infection. The increase in phosphorylated eIF2α then further attenuates the translation of a number of viral proteins, including ICP34.5. An insufficient quantity of ICP34.5 exacerbates the increase in phosphorylated eIF2α, resulting in further attenuation of viral protein synthesis. This model is particularly appealing in light of the correlation between the stronger and faster RNase activity of HSV-2 vhs, compared with that of HSV-1 vhs (17, 19), and the enhanced accumulation of phosphorylated eIF2α in MEFs infected with HSV-2 vhs mutants compared to in those infected with HSV-1 vhs mutants. Future studies will address the early effects of vhs activity on viral transcript and protein levels in the presence and absence of phosphorylated eIF2α.
The early defect in the replication of HSV-2 vhs mutant viruses manifests as delayed and/or attenuated accumulation of viral proteins. This defect could be in virion composition, reducing the capacity of the virus to efficiently invade the target cells. However, the RNase activity-deficient mutant, 333D215N, contains a single amino acid substitution in vhs and is incorporated into the virion at normal levels (27). It also associates normally with VP16 and VP22 (27). These observations indicate that 333D215N virions are relatively intact, yet this virus is attenuated for viral protein synthesis and replication in vitro to the same extent as the vhs deletion mutant 333d41 (Fig. 1 and 3) (27). The replication and eIF2α phosphorylation phenotypes of vhs mutants in MEFs are, therefore, most likely due to loss of RNase activity and not loss of virion integrity.
Phosphorylation of eIF2α is a major antiviral response in MEFs that needs to be overcome for efficient virus replication, and in this study, we identify a role for HSV-2 vhs in facilitating the accumulation of ICP34.5 to antagonize eIF2α phosphorylation through its modulation of PP1α phosphatase activity. ICP34.5 is a multifunctional protein with roles beyond antagonizing eIF2α (9, 41, 56). Therefore, we speculate that vhs mutants may also exhibit other ICP34.5-associated deficiencies, such as an inability to counteract PKR-mediated autophagy (41, 54), which could contribute to the attenuated phenotype of vhs mutants in vivo. In addition, ICP34.5 binds TBK-1 to inhibit IRF3 translocation and induction of IFN and IFN-stimulated genes (56). Thus, the reduced levels of ICP34.5 accumulated during infection with 333d41 could contribute to the observed IFN sensitivity of HSV-2 vhs mutants in vitro and in vivo (13, 27, 38).
While our study highlights the role for HSV-2 vhs RNase function in efficient ICP34.5 accumulation in MEFs, in vivo, the role of vhs is more complicated. In MEFs and in mice, HSV-2 vhs plays a critical role in counteracting the IFN-α/β response (27, 38), but unlike MEFs (13), the PKR-mediated arm of the type I IFN response is not the primary cause for attenuation of HSV-2 vhs mutants in vivo (38). These observations suggest that HSV-2 vhs mutants fail to counteract additional effectors of the type I IFN response in vivo, resulting in attenuated virus replication and pathogenicity. Because infection of mice requires the virus to enter and replicate in a variety of cell types that differ in their capacities to induce and respond to IFN, the RNase function of vhs may be utilized in a variety of ways as the virus replicates in vivo. Thus, it will be interesting to determine whether cultures of vaginal epithelial cells or neurons recapitulate the in vivo phenotype of the vhs mutant virus. It will also be important to determine whether HSV-2 vhs facilitates the accumulation of other viral proteins with roles in counteracting the IFN-α/β response, such as ICP0 or UL13 (34, 49), which may contribute to in vivo phenotypes of HSV-2 vhs mutants.
HSV-2 vhs mutants are strikingly different from HSV-1 vhs mutants. HSV-1 vhs mutants are mildly attenuated for replication, compared with wild-type virus, in MEFs after low-MOI infection, and their replication does not recover to wild-type levels in the absence of PKR (43). HSV-2 vhs mutants are attenuated 10- to 100-fold relative to wild-type virus in MEFs after low-MOI infection (13, 27), but they replicate to near wild-type levels in MEFs lacking PKR (13). Moreover, at 6 h p.i., twofold more phosphorylated eIF2α accumulates in MEFs infected with HSV-1 vhs mutants than in those infected with wild-type virus, while MEFs infected with HSV-2 vhs mutants show an eightfold increase over those infected with wild-type virus (43). These data suggest that HSV-1 and HSV-2 vhs proteins have different roles during infection because each fills a specific niche based on coevolution with other viral proteins critical for evading the host immune response or adaptation to the repertoire of host proteins in the unique cell types they encounter in vivo. Both of these possibilities encourage the study of other immune evasion factors in HSV-2 for comparison with HSV-1. It is likely that the kinetics, strength of activity, or even function differs between homologous proteins in HSV-1 and HSV-2, and the interplay between these proteins may be important in understanding pathogenicity.
Acknowledgments
This work was supported by award R01-AI057573 from the Public Health Service to L.A.M. and a Saint Louis University Presidential Fellowship to K.M.W.
We thank Gina Cano-Monreal, Maria Korom, Hong Wang, John Tavis, as well as the Blight, Diamond, Klein, Leib, Stuart, Wang, and Yu labs and Paul Olivo for support and helpful discussions regarding this work. Thanks go to Paul Anderson and Cathy Miller, Randy Kaufman and Donalyn Scheuner, and Doug Cavener for MEFs; Phil Krause for antibody; and David Leib, Jim Smiley, and Rick Thompson for viruses.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Alexander, D. E., S. L. Ward, N. Mizushima, B. Levine, and D. A. Leib. 2007. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J. Virol. 81:12128-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckham, C. J., and R. Parker. 2008. P bodies, stress granules, and viral life cycles. Cell Host Microbe 3:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlanga, J. J., I. Ventoso, H. P. Harding, J. Deng, D. Ron, N. Soneberg, L. Carrasco, and C. deHaro. 2006. Antiviral effect of the mammalian translation initiation factor 2 alpha kinase GCN2 against RNA viruses. EMBO J. 25:1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 68:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, S. M., J. Harland, A. R. MacLean, J. Podlech, and J. B. Clements. 1994. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J. Gen. Virol. 75:2367-2377. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. M., D. A. Ritchie, and J. H. Subak-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. [DOI] [PubMed] [Google Scholar]
- 7.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, G., K. Yang, and B. He. 2003. Dephosphorylation of eIF-2α mediated by the γ134.5 protein of herpes simplex virus type 1 is required for viral response to interferon but is not sufficient for efficient viral replication. J. Virol. 77:10154-10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, G., Z. Feng, and B. He. 2005. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2α dephosphorylation by the γ134.5 protein. J. Virol. 79:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou, J., and B. Roizman. 1992. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duerst, R. J., and L. A. Morrison. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322:158-167. [DOI] [PubMed] [Google Scholar]
- 14.Duff, R., and F. Rapp. 1971. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat. New Biol. 233:48-50. [DOI] [PubMed] [Google Scholar]
- 15.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 73:7153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esclatine, A., B. Taddeo, and B. Roizman. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 78:8582-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everly, D. N., Jr., and G. S. Read. 1997. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J. Virol. 71:7157-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenwick, M. L., and J. Clark. 1982. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J. Gen. Virol. 61:121-125. [DOI] [PubMed] [Google Scholar]
- 19.Fenwick, M. L., and S. A. Owen. 1988. On the control of immediate early (alpha) mRNA survival in cells infected with herpes simplex virus. J. Gen. Virol. 69:2869-2877. [DOI] [PubMed] [Google Scholar]
- 20.Gale, M., Jr., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He, B. 2006. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13:393-403. [DOI] [PubMed] [Google Scholar]
- 22.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, B., M. Gross, and B. Roizman. 1998. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 24.Hill, T. M., R. R. Sinden, and J. R. Sadler. 1983. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis by different mechanisms in Friend erythroleukemia cells. J. Virol. 45:241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korom, M., K. M. Wylie, and L. A. Morrison. 2008. Selective ablation of virion host shutoff protein RNase activity attenuates herpes simplex virus 2 in mice. J. Virol. 82:3642-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, W., Y. Li, N. Kedersha, P. Anderson, M. Emara, K. M. Swiderek, G. T. Moreno, and M. A. Brinton. 2002. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 76:11989-12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 32.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrick, W. C. 1992. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 56:291-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulvey, M., J. Poppers, D. Sternberg, and I. Mohr. 2003. Regulation of eIF2α phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J. Virol. 77:10917-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulvey, M., C. Arias, and I. Mohr. 2006. Resistance of mRNA translation to acute endoplasmic reticulum stress-inducing agents in herpes simplex virus type 1-infected cells requires multiple virus-encoded functions. J. Virol. 80:7354-7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulvey, M., C. Arias, and I. Mohr. 2007. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J. Virol. 81:3377-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61:604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orvedahl, A., D. Alexander, Z. Talloczy, Q. Sun, Y. Wei, W. Zhang, D. Burns, D. A. Leib, and B. Levine. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23-35. [DOI] [PubMed] [Google Scholar]
- 42.Pasieka, T. J., T. Baas, V. S. Carter, S. C. Proll, M. G. Katze, and D. A. Leib. 2006. Functional genomic analysis of herpes simplex virus type 1 counteraction of the host innate response. J. Virol. 80:7600-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasieka, T. J., B. Lu, S. D. Crosby, K. M. Wylie, L. A. Morrison, D. E. Alexander, V. D. Menachery, and D. A. Leib. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pestova, T. V., V. G. Kolupaeva, I. B. Lomakin, E. V. Pilipenko, I. N. Shatsky, V. I. Agol, and C. U. Hellen. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA 98:7029-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 49.Shibaki, T., T. Suzutani, I. Yoshida, M. Ogasawara, and M. Azuma. 2001. Participation of type I interferon in the decreased virulence of the UL13 gene-deleted mutant of herpes simplex virus type 1. J. Interferon Cytokine Res. 21:279-285. [DOI] [PubMed] [Google Scholar]
- 50.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase. J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smiley, J. R., M. M. Elgadi, and H. A. Saffran. 2001. Herpes simplex virus vhs protein. Methods Enzymol. 342:440-451. [DOI] [PubMed] [Google Scholar]
- 52.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taddeo, B., W. Zhang, and B. Roizman. 2006. The U(L)41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 103:2827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talloczy, Z., W. Jiang, H. W. Virgin IV, D. A. Leib, D. Scheuner, R. J. Kaufman, E. L. Eskelinen, and B. Levine. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. USA 99:190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang, S., A. S. Bertke, A. Patel, K. Wang, J. I. Cohen, and P. R. Krause. 2008. An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc. Natl. Acad. Sci. USA 105:10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the gamma134.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward, S. L., D. Scheuner, J. Poppers, R. J. Kaufman, I. Mohr, and D. A. Leib. 2003. In vivo replication of an ICP34.5 second-site suppressor mutant following corneal infection correlates with in vitro regulation of eIF2α phosphorylation. J. Virol. 77:4626-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelus, B. D., R. S. Stewart, and J. Ross. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 70:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, P., B. McGrath, S. Li, A. Frank, F. Zambito, J. Reinert, M. Gannon, K. Ma, K. McNaughton, and D. R. Cavener. 2002. The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22:3864-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, P., B. C. McGrath, J. Reinert, D. S. Olsen, L. Lei, S. Gill, S. A. Wek, K. M. Vattem, R. C. Wek, S. R. Kimball, L. S. Jefferson, and D. R. Cavener. 2002. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 22:6681-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]