Abstract
TEL1 is important in Saccharomyces cerevisiae telomere maintenance, and its kinase activity is required. Tel1p associates with telomeres in vivo, is enriched at short telomeres, and enhances the binding of telomerase components to short telomeres. However, it is unclear how the kinase activity and telomere association contribute to Tel1p's overall function in telomere length maintenance. To investigate this question, we generated a set of single point mutants and a double point mutant (tel1KD) of Tel1p that were kinase deficient and two Xrs2p mutants that failed to bind Tel1p. Using these separation-of-function alleles in a de novo telomere elongation assay, we found, surprisingly, that the tel1KD allele and xrs2 C-terminal mutants were both partially functional. Combining the tel1KD and xrs2 C-terminal mutants had an additive effect and resembled the TEL1 null (tel1Δ) phenotype. These data indicate that Tel1p has two separate functions in telomere maintenance and that the Xrs2p-dependent recruitment of Tel1p to telomeres plays an important role even in the absence of its kinase activity.
The telomere is a highly ordered complex of proteins and DNA found at the ends of linear chromosomes that functions to protect the ends and prevents them from being recognized as double-strand DNA breaks (51). Telomeres shorten gradually due to incomplete replication (1, 20), and this shortening is counteracted by telomerase, which elongates telomeres (18, 19).
Saccharomyces cerevisiae telomeres are composed of 300 ± 50 bp of the sequence TG1-3/C1-3A. The yeast telomerase complex consists of Est2p (catalytic subunit), the RNA component TLC1, and two accessory proteins, Est1p and Est3p (50). Cells deficient for any of these telomerase components undergo progressive telomere shortening and a simultaneous decrease in growth rate, described as senescence (24, 27). Typically, a small fraction of cells, termed survivors, escape senescence and maintain telomere length by utilizing RAD52-dependent recombination (24, 26).
In addition to the telomerase complex, a number of yeast proteins are important in maintaining telomere length and integrity. These include Tel1p and Mec1p, the yeast homologues of mammalian ATM and ATR, respectively (39). While deletion of TEL1 results in short but stable telomeres, MEC1 deletion has little effect on average telomere length. However, cells lacking TEL1 that have a mutant mec1-21 allele undergo senescence, similar to telomerase null cells (36), suggesting that MEC1 plays a minor but essential role in telomere length maintenance in tel1Δ cells. It has been shown that the protein kinase activities of Tel1p and Mec1p are essential in telomere maintenance, since tel1KD cells have short telomeres and tel1Δ mec1KD cells undergo senescence (29).
In current models, Tel1p acts to maintain telomere length by regulating the access of telomerase to short telomeres. TEL1 is required for the association of Est1p and Est2p with telomeres in the late S/G2 phase of the cell cycle (16), the time when telomeres are elongated (9, 31). Additionally, in both yeast and mammalian cells, telomerase preferentially elongates the shortest telomeres (22, 30, 47). Therefore, TEL1 seems to be required mainly for the association of telomerase to short telomeres in yeast. Indeed, Tel1p preferentially binds to short telomeres (4, 21, 38) and is essential for the increased association of Est1p and Est2p to short telomeres during late S/G2 (38). However, the kinase activity of Tel1p is not required for the telomere association (21). In addition to its role in telomerase recruitment, TEL1 may also regulate telomere length by enhancing the processivity of telomerase at short telomeres (7).
The Mre11p, Rad50p, and Xrs2p (MRX) complex also plays important roles in telomere maintenance. Cells lacking any one of these components (mrxΔ) have short and stable telomeres. Since combining mrxΔ with tel1Δ has no synergistic effect on telomere shortening and mrxΔ mec1Δ cells undergo senescence, it was proposed that the MRX complex and Tel1p function in the same telomere maintenance pathway (37). In agreement with this model, the C-terminal region of Xrs2p is essential in recruiting Tel1p both to double-strand breaks (32) and to short telomeres (38). Interestingly, the mammalian functional homologue of Xrs2p, NBS1, interacts with ATM via its extreme C terminus (13), suggesting that the recruitment of Tel1p to telomeres and the recruitment of ATM to DNA damage sites are conserved.
It remains a question what exact roles the kinase activity of Tel1p and its telomere binding play in telomere maintenance. Tel1p's telomere maintenance function seems to be dependent on its kinase activity, since tel1KD cells have short telomeres (29). It has been proposed that Tel1p may regulate the recruitment of Est1p, and thus the rest of the telomerase complex (12, 23, 54), to telomeres by phosphorylating Cdc13p (3, 48). Other experiments suggest the association of Tel1p to the telomere plays a major role. The preferential binding of Tel1p to short telomeres is lost in xrs2-664 cells (38), which lack the C-terminal 190 amino acids of Xrs2p and have short telomeres, similar to xrs2Δ (41). It has been suggested that the association of Tel1p to telomeres is required for its substrate phosphorylation and, therefore, telomere length maintenance (3, 39).
To further analyze the functions of Tel1p in telomere maintenance, we generated a novel kinase-dead allele of TEL1 and new alleles of XRS2 that do not interact with Tel1p. Through these separation-of-function mutants, we show that both sets of alleles are partially active in a de novo telomere elongation assay. However, combining both the tel1KD and either of the Tel1p interaction-deficient xrs2 alleles resulted in a phenotype resembling the tel1Δ phenotype, suggesting that Tel1p has kinase-dependent and kinase-independent, but telomere binding-dependent, functions in telomere maintenance.
MATERIALS AND METHODS
Plasmids and yeast strains.
Plasmids pYM15, pYM16, and pYM17 were cloned by replacing the glutathione S-transferase (GST) module in pFA6a-kanMX6-PGAL1-GST (25) with one, two, or three copies of FLAG tags. All of the TEL1 and XRS2 alleles were generated chromosomally at the native loci of these genes. FLAG-tagged TEL1 under the GAL1 promoter was constructed by PCR-based one-step tagging (25) using pYM15, pYM16, or pYM17 as the template. The tagged TEL1 has one, two, or three FLAG tags followed by SPGIS (from the vector) and a 5× glycine linker at its N terminus. Subsequently, the GAL1 promoter was replaced by the native TEL1 promoter by a two-step gene replacement strategy using the URA3 marker as the intermediate. TEL1 mutants were generated by a two-step gene replacement strategy. XRS2-13myc and xrs2-834-13myc were constructed by PCR-based one-step tagging (25). XRS2 C-terminal point mutants were generated by inserting a mutated PCR fragment of the C terminus into xrs2-834-13myc. The regions encompassing all of the tel1 and xrs2 mutations were PCR amplified and sequenced to confirm the mutations.
Strains for the de novo telomere elongation assay were derived from UCC5913 (9). RAD52 was deleted from UCC5913, and the resulting strain was crossed to yPH500 (42) to generate JHUY877. 2FLAG-TEL1 alleles were crossed into JHUY877 and XRS2-13myc alleles were generated in JHUY877 by PCR-based gene replacement. See Table S1 in the supplemental material for a list of strains.
Southern blotting for native telomere length analysis.
Strains for native telomere length analysis were grown on yeast extract-peptone-dextrose (YPD) plates or yeast extract-galactose plates (for strains with TEL1 controlled by the GAL1 promoter) for ∼4 days (two restreaks) after sporulation. Genomic DNA was then prepared from each strain, digested by XhoI, and fractionated by 1% agarose electrophoresis (36). The blots were probed with a subtelomeric Y′ fragment probe or a poly(dA-dC)·(dG-dT) probe (GE Healthcare, Piscataway, NJ).
Tel1p purification and kinase analysis.
Spores of yYM167, -147, -149, -151, and -153 (2FLAG-TEL1 mec1Δ sml1Δ or 2FLAG-tel1 mec1Δ sml1Δ) were grown in yeast extract-peptone-galactose to mid-log phase, and ∼100 ml of each strain was harvested. Cells were washed twice with H2O and lysed in lysis buffer (20 mM HEPES [pH 7.5], 0.1 mM EDTA, 0.5 mM EGTA, 2 mM MgCl2, 20% glycerol, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 μg/ml pepstatin A, and 2× protease inhibitors [Roche, Indianapolis, IN]) with 150 mM KCl by bead beating. Whole-cell extracts were precleared with protein G-Sepharose (GE Healthcare, Piscataway, NJ) and incubated with anti-FLAG antibody M2 (Sigma, St. Louis, MO) and protein G-Sepharose. The resin was washed with lysis buffer with 300 mM KCl, and 2FLAG-Te1p was eluted with 2 mg/ml of 3× FLAG peptide (Sigma, St. Louis, MO).
For the kinase assay, 40 fmol of 2FLAG-Telp1 was incubated with 1 pmol of PHAS-I (Stratagene, La Jolla, CA) and 5 μCi of [γ-32P]ATP in 20 mM HEPES (pH 7.5), 50 mM KCl, 10 mM MnCl2, and 1 mM dithiothreitol for 30 min at 30°C. The reaction mixtures were subsequently fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the gel was exposed to a PhosphorImager screen (Fujifilm Medical Systems, Stamford, CT).
De novo telomere elongation assay.
The de novo telomere elongation assays were performed essentially as described previously (9). Data analysis was done with the ImageQuant software (version 5.2; GE Healthcare, Piscataway, NJ). The elongation efficiency (percent extended) was calculated as follows within each lane: (extended telomere seed)/(extended telomere seed + unextended telomere seed), where the unextended telomere seed refers to the intensity of the HO endonuclease-generated band and the extended telomere seed refers to the intensity of the smear above the unextended telomere seed.
Pulldown assay and immunoprecipitation.
In the pulldown assay, ∼50 ml of mid-log-phase yYM188 (tel1Δ::2FLAG-TEL1) cells was used for each reaction mixture. Cells were washed with H2O and lysed in lysis buffer with 100 mM KCl by bead beating as described above. The whole-cell extract was incubated with no peptide, 0.2 μg of biotinylated pepYM1 (wild-type Xrs2p C-terminal 20 amino acids, bSGSGDGDDDDDDGPKFTFKRRKG; b indicates biotinylation), pepYM2 (equivalent to the xrs2-D842A/843A mutant; bSGSGDGDDDDaaGPKFTFKRRKG; aa indicates mutations) or pepYM3 (equivalent to the xrs2-K846A/F847A mutant; bSGSGDGDDDDDDGPaaTFKRRKG), and streptavidin-agarose (Pierce, Rockford, IL). The resin was washed in the same buffer and analyzed by Western blotting.
For the immunoprecipitation experiments, ∼100 ml of mid-log-phase cells was harvested and lysed as described above. Whole-cell extracts were immunoprecipitated with anti-FLAG M2 antibody or anti-myc 9E10 antibody (National Cell Culture Center, Minneapolis, MN) and protein G-Sepharose. The resin was washed in the same buffer and analyzed by Western blotting.
RESULTS
Kinase-deficient Tel1p proteins lack kinase activity and are defective in telomere maintenance at native telomeres.
To dissect the roles of Tel1p kinase activity in specific steps of telomere maintenance, we generated four alleles with point mutations in the kinase domain. The TEL1 allele used in this study was tagged at the N terminus with two copies of the FLAG tag at the endogenous TEL1 locus. The tag does not interfere with telomere length maintenance of TEL1 (see Fig. S1 in the supplemental material).
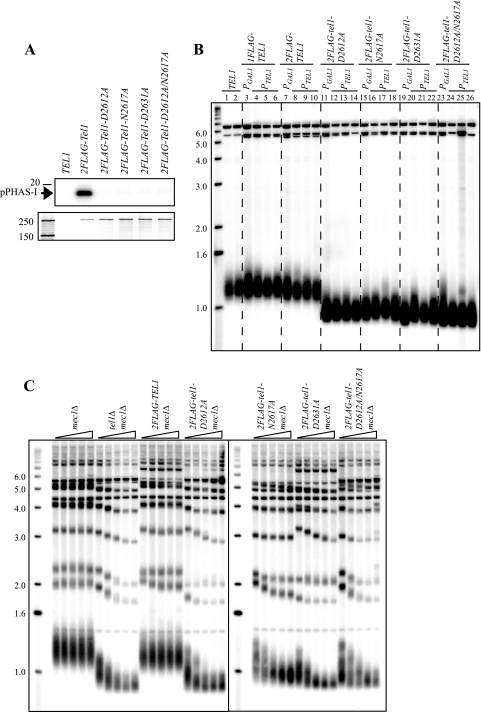
It has been reported that the kinase activity of Tel1p is required for telomere maintenance (17, 29), and the kinase-deficient Tel1p generated in these studies contained four point mutations in one allele. To minimize the effect of mutations on protein conformation, we individually mutated three out of the four residues in the active site to generate the D2612A, N2617A, and D2631A mutants of Tel1p. We also generated the D2612A/N2617A double point mutant, which is analogous to the kinase-dead mutant of mammalian ATM (5). We refer to this double point mutant as tel1KD. We assayed these mutant 2FLAG-Tel1p proteins in an in vitro kinase assay and found that all four mutants lacked kinase activity (Fig. 1A). tel1 mutant cells had short telomeres compared to the wild-type cells when either overexpressed behind the GAL1 promoter or expressed from its endogenous promoter (Fig. 1B), similar to other kinase-deficient alleles (29). The GAL1-regulated constructs were overexpressed by at least 40-fold (data not shown), and telomere elongation was not seen in these strains (Fig. 1B), indicating that these alleles are indeed null and not hypomorphic.
FIG. 1.
tel1 mutant alleles are similar to tel1Δ in native telomere length maintenance. (A) Kinase activity assay of mutant Tel1p proteins. Tel1p purified from the indicated strains was fractionated by 6% SDS-PAGE and stained by silver staining (bottom panel). The same amounts of Tel1p were tested in a kinase assay using PHAS-I as the substrate (top panel). The position of phosphorylated PHAS-I (pPHAS-I) is indicated by an arrow. Sizes of protein markers (in kDa) are shown on the left. (B) Telomere lengths of strains with chromosomal TEL1 or tel1 alleles expressed from the GAL1 promoter PGAL1 (lanes 3, 7, 11, 15, 19, and 23, corresponding to strains yYM23, -27, -94, -97, -100, and -103) or from the native TEL1 promoter PTEL1 (the rest of the samples are strains in which the GAL1 promoter was replaced by the TEL1 promoter, e.g., strains for lanes 4 to 6 were derivatives of yYM23 [lane 3], strains for lanes 8 to 10 were derivatives of yYM27 [lane 7], etc.). Only strains with PGAL1-TEL1 alleles were grown in galactose-containing medium, and other strains were grown in YPD. (C) Telomere lengths of strains with TEL1 and tel1 alleles combined with mec1Δ and sml1Δ (sml1Δ is not labeled in the figure; parental strains were JHUY817 and yYM242, -244, -246, -248, and -250). Cells were serially streaked on YPD plates, starting from spore colonies every 48 h. Genomic DNA was prepared from streaks 1, 4, 7, 10, and 13 (symbolized by gradient triangles) and analyzed by Southern blotting. Genomic DNA for panel B was prepared from cells after the second restreaking. DNA in panel B was probed with a subtelomeric Y′ probe, and for panel C it was probed with a poly(dA-dC)·(dG-dT) probe. Sizes of DNA markers (in kb) are indicated on the left (B and C).
tel1Δ cells have short but stable telomeres. When combined with mec1-21, yeast cells show progressive telomere shortening and eventually senesce upon successive restreaking (36). To examine the tel1 mutant alleles in more detail, we generated tel1 mec1Δ mutants (also with sml1Δ to suppress the mec1Δ lethality [52]). Similar to tel1Δ, cells with each of the four 2FLAG-tel1 mutant alleles alone had short and stable telomeres over serial restreaking (see Fig. S2 in the supplemental material) and when combined with mec1Δ resulted in further telomere shortening (Fig. 1C). Interestingly, it has been reported that tel1Δ mec1-21 cells senesce around restreak four, and this senescence is followed by Y′ subtelomeric element amplification and the emergence of survivors (36). We did not observe survivors by serial streaking at up to restreak 35 (data not shown; see Fig. S3A in the supplemental material). Moreover, we found no evidence of subtelomeric or telomeric DNA amplification typical of type I or type II survivors in tel1Δ mec1Δ or 2FLAG-tel1 mec1Δ cells at restreak 21 or 13, respectively (Fig. 1C; see also Fig. S3B in the supplemental material). Consistent with the apparent absence of survivors on plates, the slow growth of tel1Δ mec1Δ cells is not dependent on RAD52 (see Fig. S3 in the supplemental material). The differences in our results and the tel1Δ mec1-21 results may be due to strain differences or the fact that mec1-21 may have some residual kinase activity. From our results with tel1Δ mec1Δ we speculate that the kinase activity of either Tel1p or Mec1p is required for the generation of survivors (see Discussion).
The tel1KD allele has partial activity in the de novo telomere elongation assay.
Although each of the 2FLAG-tel1 alleles including the 2FLAG-tel1KD kinase-dead allele has short telomeres, as does tel1Δ null, we found subtle differences in telomere length. We observed gradual telomere shortening in tel1Δ and tel1Δ mec1Δ cells at earlier restreaks but not in 2FLAG-tel1 and 2FLAG-tel1 mec1Δ cells (Fig. 1C; see also Fig. S2 in the supplemental material). Furthermore, the telomere length of tel1Δ cells at later restreaks was slightly shorter than that of 2FLAG-tel1 cells (see Fig. S2 in the supplemental material).
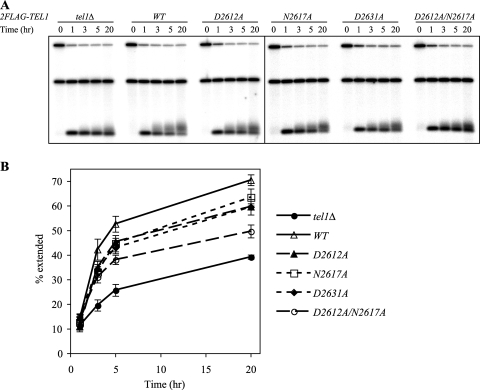
To further examine the differences seen in native telomere length in tel1 and tel1Δ cells, we utilized the more sensitive de novo telomere elongation assay (9), which follows telomere extension within the first few hours after the creation of a short telomere. As previously reported, tel1Δ was defective in de novo telomere elongation (15) (Fig. 2A). In contrast, 2FLAG-tel1 cells showed elongation at intermediate levels, while the 2FLAG-tel1KD strain had the least telomere extension (Fig. 2, compare tel1 strains to the wild type). The levels of telomere elongation were quantified by calculating the fraction of the nascent telomere seed that was elongated (Fig. 2B) (see Materials and Methods). These data strongly suggest that the 2FLAG-tel1KD allele has kinase-independent functions for telomere maintenance.
FIG. 2.
Kinase-deficient tel1 alleles partially inhibit de novo telomere elongation. (A) Southern blotting analysis for the de novo telomere elongation assay in strains with TEL1 and tel1 alleles. Strains used were yYM159 (tel1Δ) and spores derived from yYM278-282 (2FLAG-TEL1 and 2FLAG-tel1s). The time, in hours, shows the duration for which the telomere seed was allowed to be extended. Plasmid pRS416 in the strains was used as a control to another experiment and did not affect this assay (data not shown). (B) Quantitative analysis of results shown in panel A. The fraction of the extended telomere seed is plotted against the time of telomere seed elongation. Data from three independent experiments are presented as means ± standard errors of the means. Solid lines, wild-type (2FLAG-TEL1) and tel1Δ strains; dashed lines, strains with point mutations.
Xrs2p C-terminal mutants can abolish the interaction with Tel1p.
Tel1p associates with telomeres and is recruited to short telomeres in vivo (4, 21, 38). To examine the role of telomere association of Tel1p in telomere elongation, we sought to generate mutations in Xrs2p that abolish the recruitment of Tel1p. Previous studies implicated the C terminus of Xrs2p in telomere maintenance and showed that the extent of telomere shortening was proportional to the length of C-terminal truncation (49). Based on the observation that the C-terminal 21 amino acids of NBS1 are required in mediating the NBS1-ATM interaction (13), we hypothesized that the corresponding region in Xrs2p is important in mediating its interaction with Tel1p. Accordingly, we generated a small C-terminal truncation mutant of Xrs2p that lacks the last 20 amino acids, the region homologous to the ATM-recruiting region of NBS1 (13). We also generated targeted double point mutants within the C-terminal 20 amino acids, D838A/D839A, D842A/D843A, and K846A/F847A in residues corresponding to NBS1 residues that are critical for the NBS1-ATM interaction.
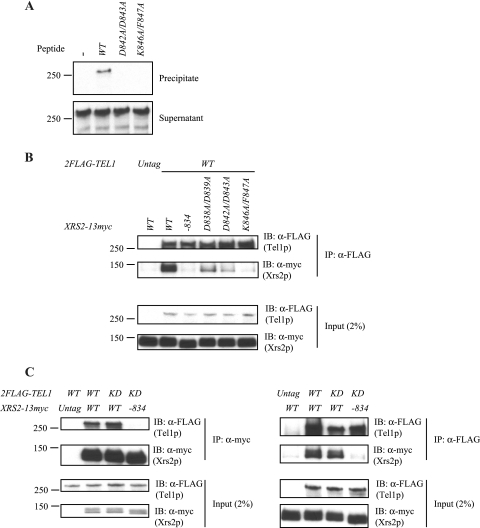
In agreement with our prediction of the importance of the Xrs2p C terminus in mediating the interaction with Tel1p, a biotinylated peptide corresponding to the C-terminal 20 amino acids of wild-type Xrs2p was able to precipitate Tel1p, while peptides with the D842A/D843A or the K846A/F847A mutations did not precipitate Tel1p (Fig. 3A).
FIG. 3.
Tel1p interacts with Xrs2p via the extreme C terminus of Xrs2p. (A) Tel1p interacts with the C-terminal peptide of Xrs2p. 2FLAG-Tel1p was precipitated with a biotinylated wild-type or mutant C-terminal peptide of Xrs2p. The precipitate (top panel) and the supernatant (bottom panel) were blotted for Tel1p with anti-FLAG antibody. (B) Tel1p interacts with Xrs2p via the C-terminal 20 amino acids of Xrs2p in vivo, and residues K846 and F847 of Xrs2p are required. Strains expressing 2FLAG-Tel1p and the indicated wild-type or mutant Xrs2p-13myc (derived from yYM260, -269, -271, -273, and -274) were immunoprecipitated (IP) with an anti-FLAG antibody. Two percent of the input material and the immunoprecipitate were examined by Western blotting (IB) for Tel1p (anti-FLAG; top panels) and Xrs2p (anti-myc; bottom panels). (C) Kinase-deficient Tel1p retains the ability to interact with Xrs2p. Xrs2p-13myc (anti-myc IP; left) or 2FLAG-Tel1p (anti-FLAG IP; right) was immunoprecipitated from the indicated strains (derived from yYM260, -309, and -312). The input and immunoprecipitate were examined as for panel B. KD refers to the 2FLAG-tel1-D2612A/N2617A allele. “Untag” indicates that the allele was not tagged. Sizes of protein markers (in kDa) are shown on the left.
To examine this interaction on full-length proteins in vivo, wild-type Xrs2p and the C-terminal mutants were tagged at the C terminus with a 13myc epitope at its endogenous locus. We confirmed that this tag did not significantly affect native telomere length compared to the XRS2 allele (see Fig. S4 in the supplemental material). The xrs2-13myc C-terminal mutants generated in this study caused only slight telomere shortening compared to the significant shortening seen in xrs2Δ cells (see Fig. S4 in the supplemental material), suggesting that these alleles are only minimally dysfunctional in native telomere length maintenance.
To directly test whether these mutations affect the association of Xrs2p with Tel1p, we examined their interaction by coimmunoprecipitation. Compared to the wild-type protein, double mutations D838A/D839A or D842A/D843A of Xrs2p compromised the interaction of Xrs2p-13myc with 2FLAG-Tel1p, while the C-terminal deletion and K846A/F847A mutations nearly abolished detectable interaction (Fig. 3B; see also Fig. S5 in the supplemental material). In agreement with the observation that the recruitment of Tel1p to short telomeres is independent of its kinase activity (21), kinase-dead Tel1p still interacted with Xrs2p (Fig. 3C). We conclude that the C-terminal 20 amino acids of Xrs2p are required for the interaction of Xrs2p with Tel1p and residues K846 and F847 in this region of Xrs2p are important for the interaction.
Xrs2p C-terminal mutants are partially functional in de novo telomere elongation.
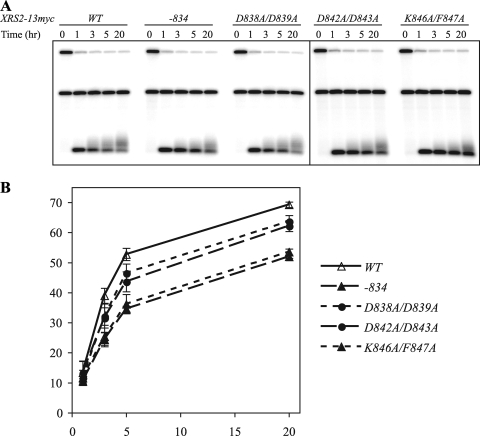
To examine the role of Tel1p recruitment in a more sensitive assay, we tested the xrs2 C-terminal mutants in the de novo telomere elongation assay. xrs2Δ completely blocks telomere seed extension in this assay (8). In contrast, we found that the XRS2 C-terminal mutants were partially active in the de novo telomere elongation assay (Fig. 4, compare xrs2 mutant strains to the wild-type strain). The two XRS2 mutants, xrs2-834-13myc and xrs2-K846A/F847A-13myc, which did not bind to Tel1p (Fig. 3B; see also Fig. S5 in the supplemental material), were the least active in de novo telomere elongation (Fig. 4). These data indicate that xrs2 alleles that do not recruit Tel1p still have partial functions in telomere elongation.
FIG. 4.
XRS2 C-terminal mutants are partially defective in the de novo telomere elongation assay. (A) Southern blotting analysis for the de novo telomere elongation assay in strains with 13myc-tagged XRS2 and xrs2 alleles (derived from yYM286, -288, -289, -292, and -293). The time, in hours, shows the duration for which the telomere seed was allowed to be extended. (B) Quantitative data analysis of the results shown in panel A. The fraction of the extended telomere seed is plotted against the time of telomere seed elongation. Data from two independent experiments are presented as means ± standard errors of the means. Solid line, the wild-type strain (XRS2-13myc); lines with short or long dashes, the mutant strains.
tel1KD combined with XRS2 C-terminal mutants resembles tel1Δ.
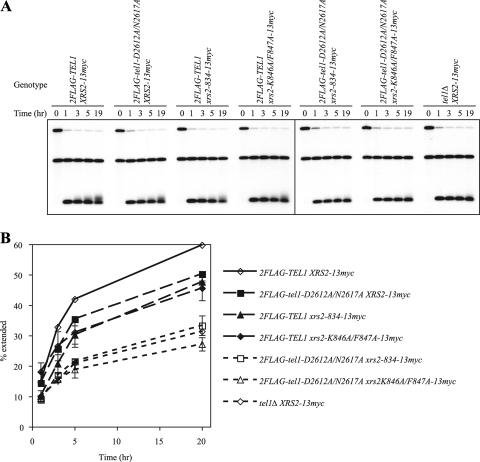
Having shown that both Tel1p kinase activity and its telomere association contribute to telomere elongation, we were interested in whether these activities act in the same or parallel pathways. To this end, we combined the tel1KD allele (2FLAG-tel1-D2612A/N2617A) independently with each of the two xrs2 alleles that were deficient in interaction with Tel1p, xrs2-834-13myc and xrs2-K846A/F847A-13myc, in the de novo telomere elongation assay. As observed previously, single mutants of tel1 and xrs2 were partially active in de novo telomere elongation (Fig. 5). However, double mutants that combined the tel1KD allele and one of the XRS2 C-terminal mutants were as defective as tel1Δ (Fig. 5).
FIG. 5.
tel1KD combined with xrs2 C-terminal mutants synergistically inhibits de novo telomere elongation. (A) Southern blotting analysis for de novo telomere elongation in strains with different combinations of 2FLAG-TEL1 and XRS2-13myc alleles (derived from yYM319, -321, -300, -301, -308, -305, and -324). The time, in hours, shows the duration for which the telomere seed was allowed to be extended. (B) Quantitative data analysis of the results shown in panel A. The fraction of the extended telomere seed is plotted against the time of telomere seed elongation. Data from two independent experiments are presented as means ± standard errors of the means (data for strains 2FLAG-TEL1 XRS2-13myc and 2FLAG-tel1-D2612A/N2617A XRS2-13myc were from one experiment, but similar results have been obtained in other experiments). Solid line, the wild-type strain; lines with long dashes, strains with single mutations; lines with short dashes, the tel1Δ strain and strains with double mutations.
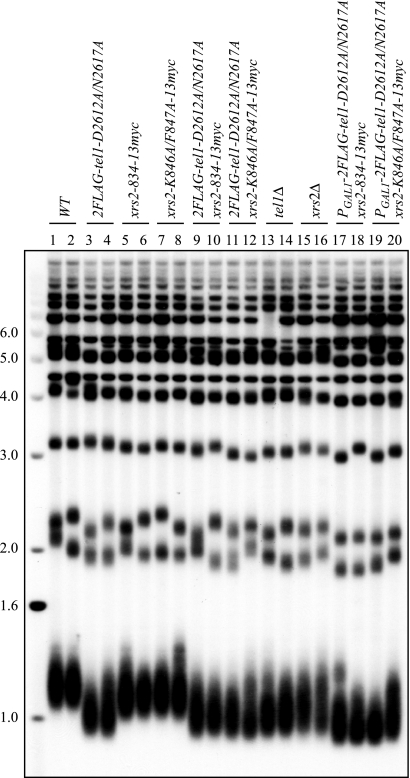
We also found additive effects of these two mutations on telomere length at native telomeres. Cells with the tel1KD allele or the xrs2 C-terminal mutations showed some telomere shortening. When combined, the double mutants have telomeres slightly shorter than tel1KD cells but much shorter than the xrs2 mutants. Importantly, these double mutants have telomeres as short as tel1Δ cells (Fig. 6). This indicates that the effects of Tel1p kinase-dead mutations and Xrs2p C-terminal mutations are additive.
FIG. 6.
The combination of tel1KD and an xrs2 C-terminal mutant resembles tel1Δ at native telomeres, and overexpressing tel1KD does not rescue the short telomeres. Southern blotting analysis results show the native telomere lengths of strains expressing combinations of the TEL1 and XRS2 alleles. The wild-type (WT) strains were derived from yYM256. Strains for lanes 3 to 20 were derived from yYM309, -310, -268, -269, -274, -275, -311, -312, -325, -326, -333 to -336, and -327 to -330. Both TEL1 and XRS2 alleles are tagged (except for tel1Δ and xrs2Δ). The blot was probed with a poly(dA-dC)·(dG-dT) probe. Sizes of DNA markers (in kb) are indicated on the left.
One explanation for this additive effect is that the Tel1p kinase-dead mutant might have been hypomorphic. As shown in Fig. 1, overexpression of tel1KD from a GAL1 promoter did not rescue short telomeres (Fig. 1B, compare lane 23 to lanes 8 to 10). Western analysis showed that the tel1KD allele was overexpressed by ∼40-fold (not shown). To further examine this, we overexpressed the tel1KD allele in combination with two different xrs2 C-terminal mutants and examined telomere length (Fig. 6). We found that overexpression of the kinase-dead tel1KD in the xrs2 C-terminal mutant background did not restore telomere length to the length observed in xrs2 C-terminal mutants alone. Thus, the kinase-dead allele is not simply hypomorphic. We conclude that Tel1p has a role in telomere length maintenance that is independent of its kinase activity.
DISCUSSION
Te1p kinase activity and binding to telomeres have separate functions.
To examine the role of TEL1 in telomere maintenance, we generated mutations in the kinase domain of Tel1p. These mutant alleles showed loss of Tel1p kinase activity, short and stable telomeres, and senescence when combined with mec1Δ, similar to tel1Δ (Fig. 1; see also Fig. S2 and S3 in the supplemental material). Using the de novo telomere elongation assay we found that the mutant tel1 alleles supported some telomere elongation; they elongated the telomere seed more efficiently than tel1Δ but less efficiently than TEL1, indicating that the kinase-dead Tel1p protein plays a role in telomere lengthening.
To examine the function of Tel1p recruitment to telomeres, we generated an Xrs2p mutant truncated for the C-terminal 20 amino acids and mutants with point mutations in this C-terminal region. Unlike other C-terminal XRS2 mutants, these XRS2 mutations only minimally affected the native telomere length (see Fig. S4 in the supplemental material). Each Xrs2p mutant showed a different degree of loss of association with Tel1p (Fig. 3B), and this correlated with the degree of reduction of de novo telomere elongation. The correlation of protein interaction and telomere elongation defects suggests that the deficiencies of these xrs2 mutants in de novo telomere elongation are due to their defects in recruiting Tel1p. Interestingly, the two Xrs2p mutants (xrs2-834 and xrs2-K846/F847A) that most completely eliminated the interaction with Tel1p were still partially active in the de novo telomere elongation assay (Fig. 4). These data indicate that kinase-proficient Tel1p is partially functional in telomere elongation even when not bound to telomeres.
In contrast to the mild shortening of native telomeres we observed in our xrs2 C-terminal mutants, published reports on the C-terminal-truncated xrs2-664 allele, which was used to establish the binding of Tel1p to short telomeres (38), showed significant telomere shortening similar to xrs2Δ mutants (41). This led to the conclusion that telomere binding by Tel1p is a prerequisite for its target protein phosphorylation (39). The discrepancy between the results with the xrs2-664 allele and the alleles analyzed in this study could be explained if the region between amino acids 665 and 834 of Xrs2p has Tel1p-independent telomere functions. Consistent with this hypothesis, it has been shown that telomere length is sensitive to the extent of truncation at the Xrs2p C terminus (49).
The kinase activity of Tel1p is required for telomere maintenance.
It was reported previously that the kinase activity of Tel1p is required for telomere maintenance (17, 29). Our data support this conclusion and extend it. As described above, Tel1p kinase, which has a greatly reduced association with Xrs2, showed some elongation activity in the de novo assay (Fig. 3 and 4). Thus, the kinase activity of Tel1p plays a role in telomere length maintenance even when it is not associated with the telomere.
Although it has been known for some time that Tel1p kinase activity is required for telomere maintenance, it is not clear what protein target(s) Tel1p regulates in telomere maintenance. In vitro, Tel1p phosphorylates a number of proteins. However, when the Tel1p preferred phosphorylation sites in some of these proteins were mutated, no telomere effect was observed (28). Tel1p and Mec1p phosphorylate Cdc13p, and it is proposed this regulates the Cdc13p-Est1p interaction, which is critical for telomerase recruitment to telomeres (11, 34, 35). However, it is not known whether the Cdc13p-Est1p interaction is eliminated by the S-to-A mutations. In addition to Cdc13p phosphorylation by Tel1p, there are likely other substrates of Tel1p and Mec1p kinases that are important for telomere maintenance. In wild-type cells, the association of Est2p with telomeres peaks at both G1 and late S/G2 (14, 46). The binding of Est2p to telomeres diminished in tel1Δ cells. Interestingly, not only the late S/G2 (dependent on the Cdc13p-Est1p interaction) but also the G1 Est2p (dependent mostly on the Ku-TLC1 interaction [14, 43]) binding was also reduced (16). Therefore, it is likely that Tel1p may phosphorylate another substrate(s) to facilitate the recruitment of Est2p during G1.
Tel1p has kinase-independent functions in telomere maintenance.
One explanation of why Tel1p associates with telomeres is that this association is necessary for the phosphorylation of its telomeric protein target(s). While this may be the case, since the kinase-dead Tel1p, which can still associate with telomeres via the interaction with Xrs2p (Fig. 3C), retains some function in de novo telomere elongation (Fig. 2), our data strongly support the conclusion that the association of Tel1p to telomeres has additional functions.
The xrs2 mutants xrs2-834 and xrs2-K846A/F847A failed to interact with Tel1p (Fig. 3B; see also Fig. S5 in the supplemental material), and cells with either of these alleles had slightly shorter native telomeres (see Fig. S4 in the supplemental material) and reduced de novo telomere elongation (Fig. 4) compared to wild-type cells. This indicates that “free” (not telomere-bound) kinase-proficient Tel1p is not sufficient for the wild-type level of telomere maintenance. However, we cannot rule out the possibility that Tel1p has some residual interaction with xrs2-834 or xrs2-K846A/F847A in vivo. These interactions are not significant, because expressing tel1-D2612A/N2617A on the xrs2 mutant background resembles tel1Δ in de novo telomere elongation (Fig. 5).
We speculate that the kinase-independent functions of TEL1 include recruiting other factors. TEL1 has been shown to be required for the recruitment of Est1p and Est2p to short telomeres (38). Although Tel1p may phosphorylate Cdc13p to enhance its association with Est1p, it remains possible that Tel1p itself interacts and recruits Est1p and/or Est2p. Alternatively, Tel1p may recruit another telomere protein to contribute to telomere elongation, for example, Tel2p (2).
The two functions of Tel1p are additive.
When tel1-D2612A/N2617A was combined with xrs2-834 or xrs2-K846A/F847A, de novo elongation was as deficient as for tel1Δ (Fig. 5). This indicates that Tel1p has two functions in telomere maintenance: acting as a kinase and binding to telomeres (via interacting with Xrs2p), and the kinase-independent role of Tel1p requires telomere association.
TEL1 and telomeric recombination.
In this study, we were surprised to not observe survivors or telomeric recombination in tel1Δ mec1Δ and tel1KD mec1Δ cells (Fig. 1C and data not shown; see also Fig. S3 in the supplemental material). To confirm this lack of survivor generation, we examined tel1Δ mec1Δ rad52Δ cells, and they grew in a similar manner to tel1Δ mec1Δ cells. We did not observe the further reduction in growth rate that would be expected if telomere maintenance required recombination. Our results are different than those reported previously for tel1Δ mec1-21 strains, in which survivors and amplification of the subtelomeric Y′ fragment were observed (36). One possible explanation for this discrepancy is that since mec1-21 contains a single point mutation (G882S) located outside of the kinase domain (29), the residual kinase activity of mec1-21 might have allowed for the generation of survivors. Therefore, we hypothesize that the kinase activity of Tel1p or Mec1p may be required for signaling telomeric recombination and the generation of survivors.
In addition to regulating telomere length, TEL1 is also involved in telomere end capping. The absence of TEL1 increases gross chromosomal rearrangements (on the tlc1Δ or est2Δ background) (33) and fusion of telomeres to an induced double-strand break (6, 10). In addition, TEL1 also inhibits chromosomal circularization in the absence of telomerase in Schizosaccharomyces pombe (45). While there is evidence that the Tel1p kinase activity is required for telomere end protection (6), it will be interesting to test whether telomere binding also contributes to this capping function of Tel1p.
Implications of this study for the DNA damage response.
In addition to its roles in telomere maintenance, Tel1p is known to function in DNA damage signaling along with Mec1p. In mammalian cells, the Tel1p homologue ATM is the central regulator of the DNA damage response, although in yeast the major DNA damage sensor is Mec1p (53). ATM is recruited to sites of DNA damage by the NBS1 component of the MRN complex, similar to the recruitment of Tel1p to telomeres by Xrs2p. Interestingly, a mouse model that expresses a truncated NBS1 lacking the C-terminal ATM interaction region (Nbs1ΔC/ΔC) manifests only a subset of Atm−/− phenotypes (44). While there may be residual ATM recruitment by the mutant MRNΔC/ΔC (13), another possibility is that ATM, like Tel1p, has roles in DNA damage response that are independent of binding to the damage sites via the MRN complex.
Conversely, while it has been established that ATM signals DNA damage by phosphorylating many protein targets (40), it has not been investigated whether ATM has any kinase-independent functions. In light of our data showing Tel1p's kinase-independent functions in telomere maintenance, it will be interesting to test an AtmKD knock-in animal model to examine whether ATM has kinase-independent roles in DNA damage response.
Supplementary Material
Acknowledgments
We are grateful for Carla Connelly's technical assistance and thank Brendan Cormack, Mary Armanios, and the Greider lab members for their helpful comments on the manuscript. We also thank Dan Gottschling for providing the initial de novo telomere elongation strain.
This work was supported by National Institutes of Health grant RO1GM043080 to C.W.G. Y.M. is a Leukemia and Lymphoma Society fellow.
Footnotes
Published ahead of print on 13 July 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allsopp, R. C., H. Vaziri, C. Patterson, S. Goldstein, E. V. Younglai, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 8910114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, C. M., D. Korkin, D. L. Smith, S. Makovets, J. J. Seidel, A. Sali, and E. H. Blackburn. 2008. Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break. Genes Dev. 22854-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi, A., and D. Shore. 2008. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol. Cell 31153-165. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi, A., and D. Shore. 2007. Increased association of telomerase with short telomeres in yeast. Genes Dev. 211726-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 2811677-1679. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. W., and E. H. Blackburn. 2003. Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol. Cell 111379-1387. [DOI] [PubMed] [Google Scholar]
- 7.Chang, M., M. Arneric, and J. Lingner. 2007. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 212485-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diede, S. J., and D. E. Gottschling. 2001. Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr. Biol. 111336-1340. [DOI] [PubMed] [Google Scholar]
- 9.Diede, S. J., and D. E. Gottschling. 1999. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases alpha and delta. Cell 99723-733. [DOI] [PubMed] [Google Scholar]
- 10.DuBois, M. L., Z. W. Haimberger, M. W. McIntosh, and D. E. Gottschling. 2002. A quantitative assay for telomere protection in Saccharomyces cerevisiae. Genetics 161995-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, S. K., and V. Lundblad. 1999. Est1 and Cdc13 as comediators of telomerase access. Science 286117-120. [DOI] [PubMed] [Google Scholar]
- 12.Evans, S. K., and V. Lundblad. 2002. The Est1 subunit of Saccharomyces cerevisiae telomerase makes multiple contributions to telomere length maintenance. Genetics 1621101-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falck, J., J. Coates, and S. P. Jackson. 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 434605-611. [DOI] [PubMed] [Google Scholar]
- 14.Fisher, T. S., A. K. Taggart, and V. A. Zakian. 2004. Cell cycle-dependent regulation of yeast telomerase by Ku. Nat. Struct. Mol. Biol. 111198-1205. [DOI] [PubMed] [Google Scholar]
- 15.Frank, C. J., M. Hyde, and C. W. Greider. 2006. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol. Cell 24423-432. [DOI] [PubMed] [Google Scholar]
- 16.Goudsouzian, L. K., C. T. Tuzon, and V. A. Zakian. 2006. S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol. Cell 24603-610. [DOI] [PubMed] [Google Scholar]
- 17.Greenwell, P. W., S. L. Kronmal, S. E. Porter, J. Gassenhuber, B. Obermaier, and T. D. Petes. 1995. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82823-829. [DOI] [PubMed] [Google Scholar]
- 18.Greider, C. W., and E. H. Blackburn. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43405-413. [DOI] [PubMed] [Google Scholar]
- 19.Greider, C. W., and E. H. Blackburn. 1987. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51887-898. [DOI] [PubMed] [Google Scholar]
- 20.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345458-460. [DOI] [PubMed] [Google Scholar]
- 21.Hector, R. E., R. L. Shtofman, A. Ray, B. R. Chen, T. Nyun, K. L. Berkner, and K. W. Runge. 2007. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol. Cell 27851-858. [DOI] [PubMed] [Google Scholar]
- 22.Hemann, M. T., M. A. Strong, L. Y. Hao, and C. W. Greider. 2001. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 10767-77. [DOI] [PubMed] [Google Scholar]
- 23.Hughes, T. R., S. K. Evans, R. G. Weilbaecher, and V. Lundblad. 2000. The Est3 protein is a subunit of yeast telomerase. Curr. Biol. 10809-812. [DOI] [PubMed] [Google Scholar]
- 24.Lendvay, T. S., D. K. Morris, J. Sah, B. Balasubramanian, and V. Lundblad. 1996. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 1441399-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 26.Lundblad, V., and E. H. Blackburn. 1993. An alternative pathway for yeast telomere maintenance rescues est1− senescence. Cell 73347-360. [DOI] [PubMed] [Google Scholar]
- 27.Lundblad, V., and J. W. Szostak. 1989. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57633-643. [DOI] [PubMed] [Google Scholar]
- 28.Mallory, J. C., V. I. Bashkirov, K. M. Trujillo, J. A. Solinger, M. Dominska, P. Sung, W. D. Heyer, and T. D. Petes. 2003. Amino acid changes in Xrs2p, Dun1p, and Rfa2p that remove the preferred targets of the ATM family of protein kinases do not affect DNA repair or telomere length in Saccharomyces cerevisiae. DNA Repair (Amsterdam) 21041-1064. [DOI] [PubMed] [Google Scholar]
- 29.Mallory, J. C., and T. D. Petes. 2000. Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase. Proc. Natl. Acad. Sci. USA 9713749-137754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcand, S., V. Brevet, and E. Gilson. 1999. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 183509-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcand, S., V. Brevet, C. Mann, and E. Gilson. 2000. Cell cycle restriction of telomere elongation. Curr. Biol. 10487-490. [DOI] [PubMed] [Google Scholar]
- 32.Nakada, D., K. Matsumoto, and K. Sugimoto. 2003. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 171957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennaneach, V., and R. D. Kolodner. 2004. Recombination and the Tel1 and Mec1 checkpoints differentially effect genome rearrangements driven by telomere dysfunction in yeast. Nat. Genet. 36612-617. [DOI] [PubMed] [Google Scholar]
- 34.Pennock, E., K. Buckley, and V. Lundblad. 2001. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104387-396. [DOI] [PubMed] [Google Scholar]
- 35.Qi, H., and V. A. Zakian. 2000. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev. 141777-1788. [PMC free article] [PubMed] [Google Scholar]
- 36.Ritchie, K. B., J. C. Mallory, and T. D. Petes. 1999. Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 196065-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritchie, K. B., and T. D. Petes. 2000. The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics 155475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabourin, M., C. T. Tuzon, and V. A. Zakian. 2007. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol. Cell 27550-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabourin, M., and V. A. Zakian. 2008. ATM-like kinases and regulation of telomerase: lessons from yeast and mammals. Trends Cell Biol. 18337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3155-168. [DOI] [PubMed] [Google Scholar]
- 41.Shima, H., M. Suzuki, and M. Shinohara. 2005. Isolation and characterization of novel xrs2 mutations in Saccharomyces cerevisiae. Genetics 17071-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 12219-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stellwagen, A. E., Z. W. Haimberger, J. R. Veatch, and D. E. Gottschling. 2003. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 172384-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stracker, T. H., M. Morales, S. S. Couto, H. Hussein, and J. H. Petrini. 2007. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature 447218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian, L., B. A. Moser, and T. M. Nakamura. 2008. Recombination-based telomere maintenance is dependent on Tel1-MRN and Rap1 and inhibited by telomerase, Taz1, and Ku in fission yeast. Mol. Cell. Biol. 281443-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taggart, A. K., S. C. Teng, and V. A. Zakian. 2002. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 2971023-1026. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira, M. T., M. Arneric, P. Sperisen, and J. Lingner. 2004. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117323-335. [DOI] [PubMed] [Google Scholar]
- 48.Tseng, S. F., J. J. Lin, and S. C. Teng. 2006. The telomerase-recruitment domain of the telomere binding protein Cdc13 is regulated by Mec1p/Tel1p-dependent phosphorylation. Nucleic Acids Res. 346327-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsukamoto, Y., C. Mitsuoka, M. Terasawa, H. Ogawa, and T. Ogawa. 2005. Xrs2p regulates Mre11p translocation to the nucleus and plays a role in telomere elongation and meiotic recombination. Mol. Biol. Cell 16597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega, L. R., M. K. Mateyak, and V. A. Zakian. 2003. Getting to the end: telomerase access in yeast and humans. Nat. Rev. Mol. Cell Biol. 4948-959. [DOI] [PubMed] [Google Scholar]
- 51.Verdun, R. E., and J. Karlseder. 2007. Replication and protection of telomeres. Nature 447924-931. [DOI] [PubMed] [Google Scholar]
- 52.Zhao, X., E. G. Muller, and R. Rothstein. 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2329-340. [DOI] [PubMed] [Google Scholar]
- 53.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408433-439. [DOI] [PubMed] [Google Scholar]
- 54.Zhou, J., K. Hidaka, and B. Futcher. 2000. The Est1 subunit of yeast telomerase binds the Tlc1 telomerase RNA. Mol. Cell. Biol. 201947-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.