Abstract
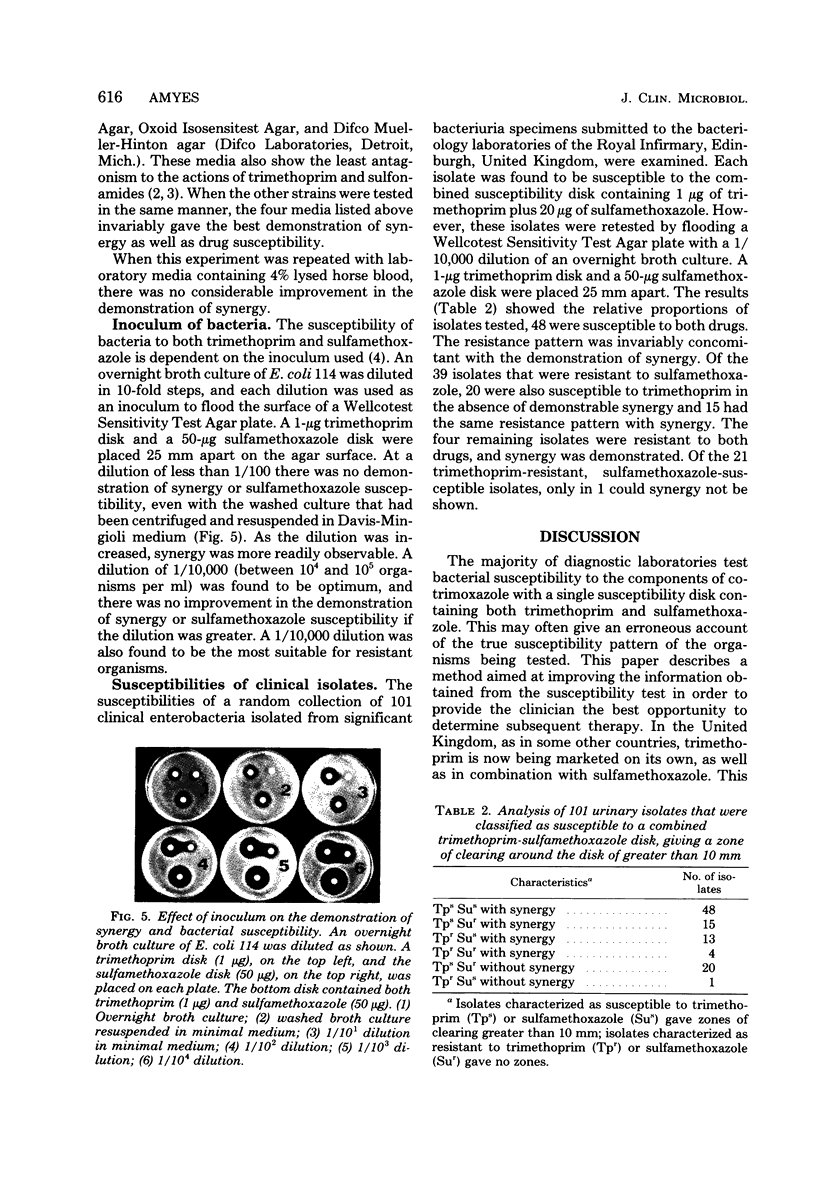
It is impossible to test accurately bacterial susceptibility to the trimethoprim-sulfamethoxazole combination co-trimoxazole with a single combined susceptibility disk. However, a variety of factors still affect the result even when separate trimethoprim and sulfamethoxazole disks are used. Experiments with separate disks showed that the optimum conditions for testing the susceptibilities of enterobacteria to these drugs were to flood-seed an agar plate with an inoculum of 10(4) to 10(5) organisms per ml, take off the excess liquid, and place a disk of 1 microgram of trimethoprim and another of 50 micrograms of sulfamethoxazole on the surface of the agar with their centers exactly 25 mm apart. This method not only allowed the determination of resistance but also distinguished synergy.
Full text
PDF
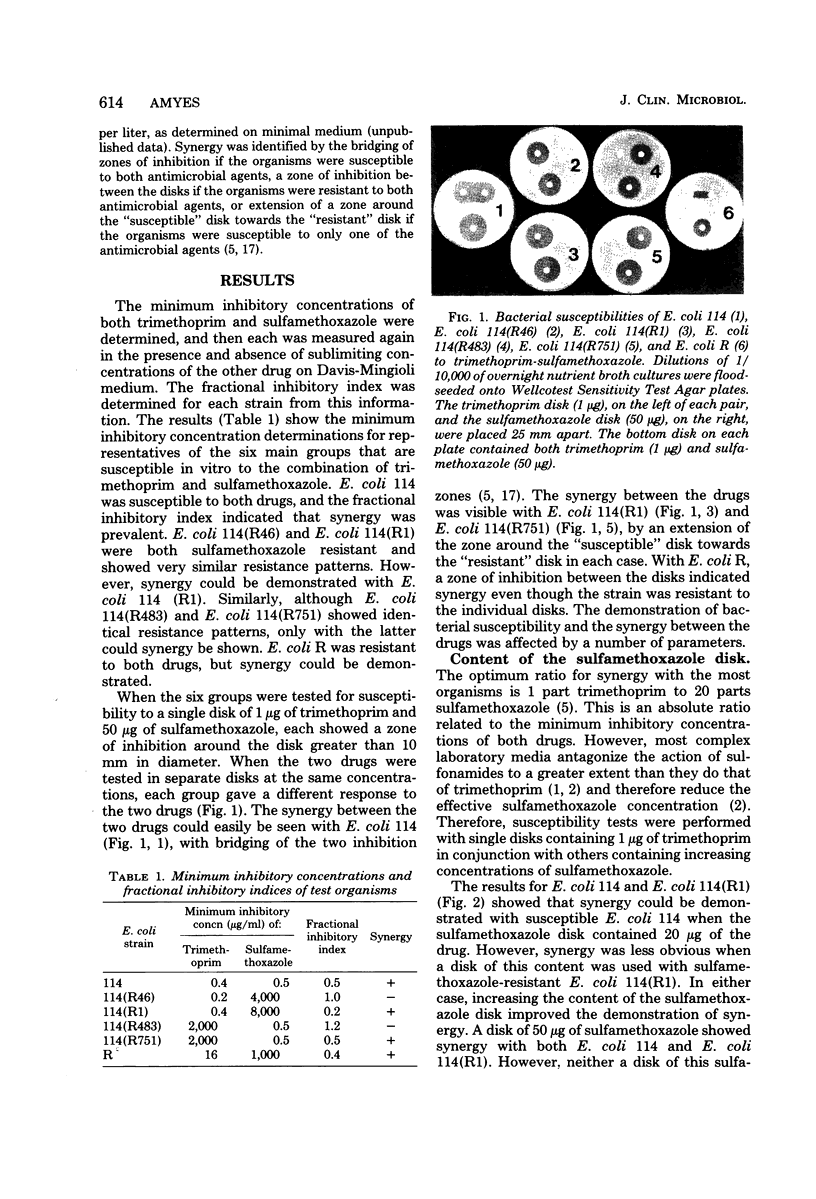


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Smith J. T. Trimethoprim antagonists: effect of uridine in laboratory media. J Antimicrob Chemother. 1978 Sep;4(5):421–429. doi: 10.1093/jac/4.5.421. [DOI] [PubMed] [Google Scholar]
- Amyes S. G., Smith J. T. Trimethoprim-sensitivity testing and thymineless mutants. J Med Microbiol. 1974 May;7(2):143–153. doi: 10.1099/00222615-7-2-143. [DOI] [PubMed] [Google Scholar]
- Bushby S. R. Combined antibacterial action in vitro of trimethoprim and sulphonamides. The in vitro nature of synergy. Postgrad Med J. 1969 Nov;45(Suppl):10–18. [PubMed] [Google Scholar]
- Bushby S. R., Hitchings G. H. Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother. 1968 May;33(1):72–90. doi: 10.1111/j.1476-5381.1968.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrell J. H., Garrod L. P., Waterworth P. M. Trimethoprim: laboratory and clinical studies. J Clin Pathol. 1968 Mar;21(2):202–209. doi: 10.1136/jcp.21.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Fleming M. P. R factors conferring resistance to trimethoprim but not sulphonamides. J Gen Microbiol. 1972 Dec;73(3):573–575. doi: 10.1099/00221287-73-3-573. [DOI] [PubMed] [Google Scholar]
- Jobanputra R. S., Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974 May;7(2):169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- Kerry D. W., Hamilton-Miller J. M., Brumfitt W. Trimethoprim and rifampicin: in vitro activities separately and in combination. J Antimicrob Chemother. 1975 Dec;1(4):417–427. doi: 10.1093/jac/1.4.417. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Burchall J. J. Reversal of the antimicrobial activity of trimethoprim by thymidine in commercially prepared media. Appl Microbiol. 1971 Nov;22(5):812–817. doi: 10.1128/am.22.5.812-817.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Lord V. L., Gunasekera H. K., Leiberman P. J., Luxton D. E. Comparison of trimethoprim alone with trimethoprim sulphamethoxazole in the treatment of respiratory and urinary infections with particular reference to selection of trimethoprim resistance. Lancet. 1980 Jun 14;1(8181):1270–1273. doi: 10.1016/s0140-6736(80)91732-8. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Waterworth P. M. Practical aspects of testing sensitivity to trimethoprim and sulphonamide. Postgrad Med J. 1969 Nov;45(Suppl):21–29. [PubMed] [Google Scholar]