Abstract
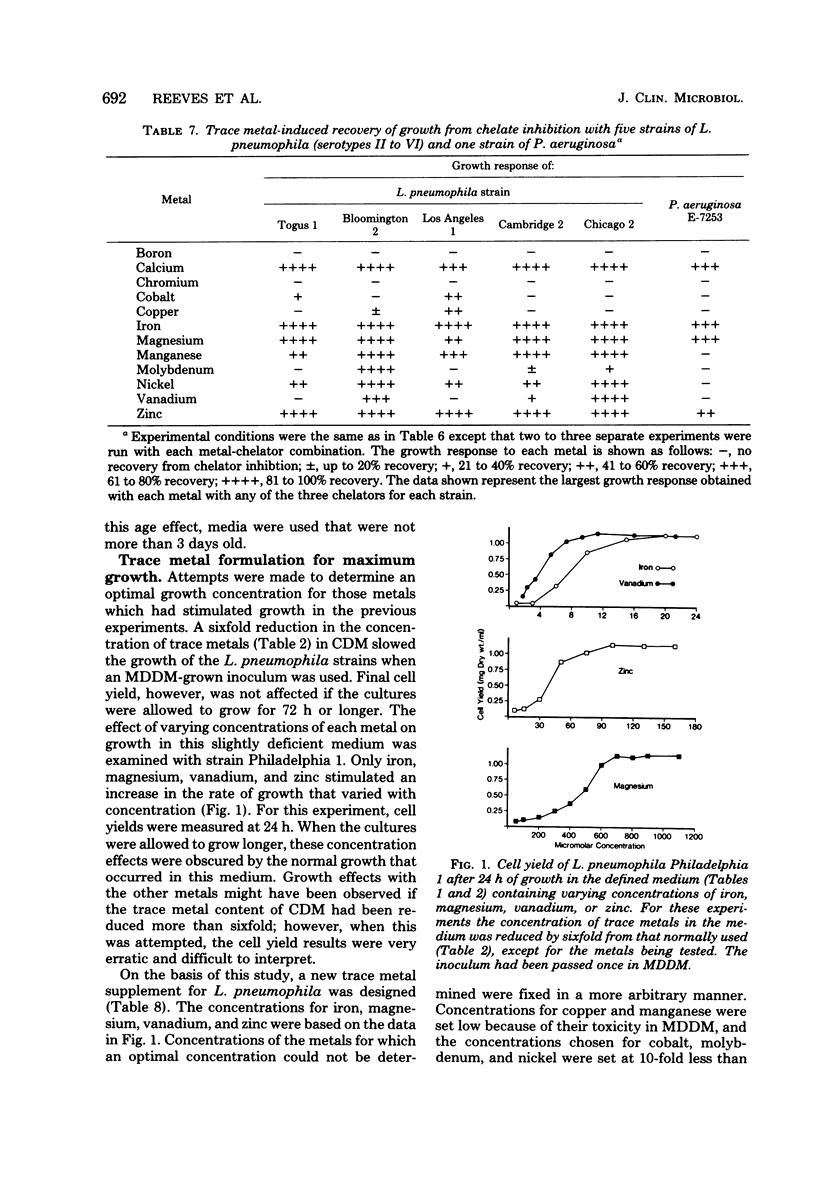
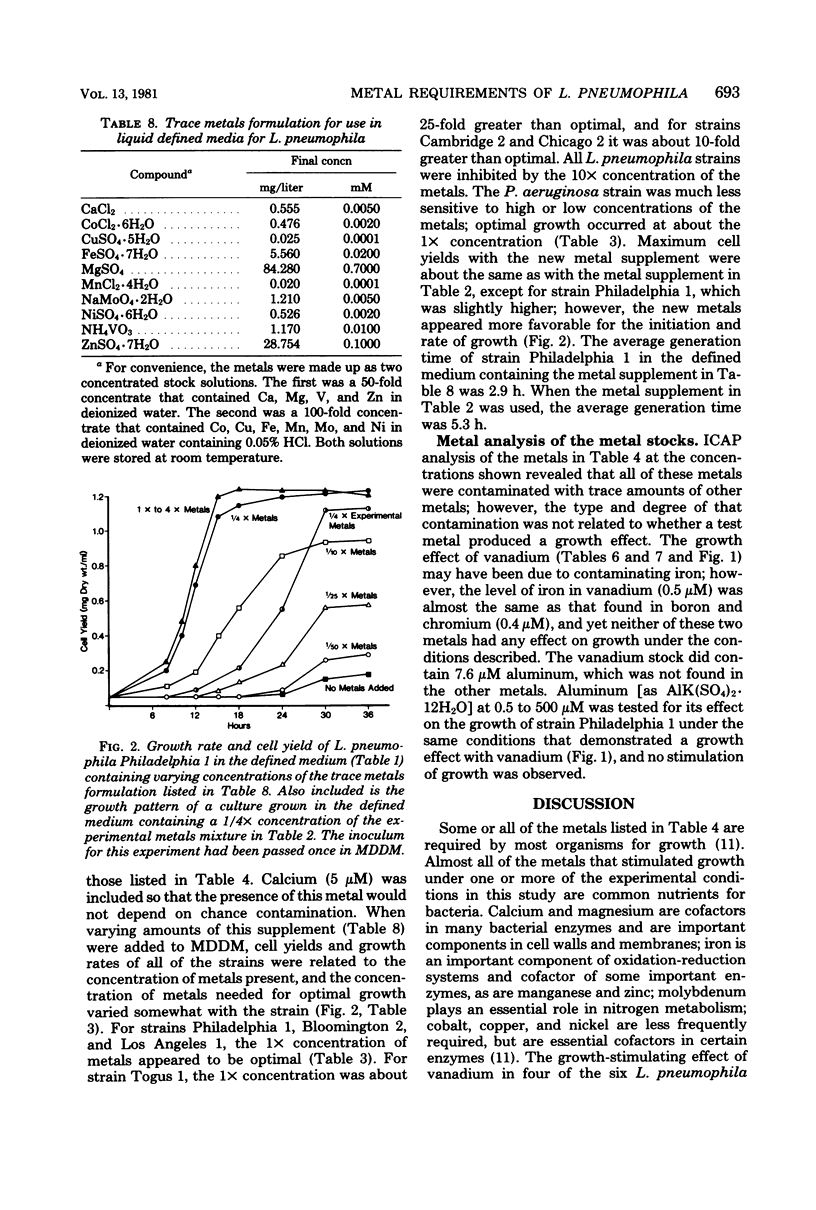
Serial passage of six strains of Legionella pneumophila and one strain of Pseudomonas aeruginosa in a liquid chemically defined medium deficient in trace metals resulted in the death of five L. pneumophila strains and very limited growth in the remaining strain and the P. aeruginosa strain. Addition of either iron or magnesium restored growth to almost normal levels in all of the strains when early-passage inocula were used. A low concentration of magnesium stimulated growth with cobalt, copper, iron, manganese, molybdenum, vanadium, or zinc. When a complete defined medium containing trace metals was used, growth was inhibited by adding the chelators ethylenediaminetetraacetic acid, citrate, or 2,2'-bipyridyl. Chelator inhibition was partly or fully relieved with either calcium, cobalt, copper, iron, magnesium, molybdenum, nickel, vanadium, or zinc. P. aeruginosa differed from L. pneumophila in that it required higher concentrations of each chelator to inhibit growth and that its growth was stimulated by only four metals: calcium, iron, magnesium, and zinc. A trace-metal supplement for L. pneumophila was designed which included all metals stimulating growth in these experiments and which proved to be sufficient for optimal growth of all the strains.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., McKenna C. E., Lie R. F., Traylor T. G., Kamen M. D. The vanadium effect in nitrogen fixation by azotobacter. Biochim Biophys Acta. 1972 Mar 30;264(1):25–38. doi: 10.1016/0304-4165(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Burns R. C., Fuchsman W. H., Hardy R. W. Nitrogenase from vanadium-grown Azotobacter: isolation, characteristics, and mechanistic implications. Biochem Biophys Res Commun. 1971 Feb 5;42(3):353–358. doi: 10.1016/0006-291x(71)90377-9. [DOI] [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England A. C., 3rd, McKinney R. M., Skaliy P., Gorman G. W. A fifth serogroup of Legionella pneumophila. Ann Intern Med. 1980 Jul;93(1):58–59. doi: 10.7326/0003-4819-93-1-58. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. J., Smith D. K., Schnute S. F., Werber S. L., Weinberg E. D. Pseudomonas culture longevity: control by phosphate. Microbios. 1971 Dec;4(15):205–215. [PubMed] [Google Scholar]
- George J. R., Pine L., Reeves M. W., Harrell W. K. Amino acid requirements of Legionella pneumophila. J Clin Microbiol. 1980 Mar;11(3):286–291. doi: 10.1128/jcm.11.3.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutner S. H. Inorganic nutrition. Annu Rev Microbiol. 1972;26:313–346. doi: 10.1146/annurev.mi.26.100172.001525. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Thacker L., Harris P. P., Lewallen K. R., Hebert G. A., Edelstein P. H., Thomason B. M. Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann Intern Med. 1979 Apr;90(4):621–624. doi: 10.7326/0003-4819-90-4-621. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Wilkinson H. W., Sommers H. M., Fikes B. J., Sasseville K. R., Yungbluth M. M., Wolf J. S. Legionella pneumophila serogroup six: isolation from cases of legionellosis, identification by immunofluorescence staining, and immunological response to infection. J Clin Microbiol. 1980 Sep;12(3):395–401. doi: 10.1128/jcm.12.3.395-401.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. P. Cooling towers and evaporative condensers. Ann Intern Med. 1979 Apr;90(4):667–670. doi: 10.7326/0003-4819-90-4-667. [DOI] [PubMed] [Google Scholar]
- Morris G. K., Patton C. M., Feeley J. C., Johnson S. E., Gorman G., Martin W. T., Skaliy P., Mallison G. F., Politi B. D., Mackel D. C. Isolation of the Legionnaires' disease bacterium from environmental samples. Ann Intern Med. 1979 Apr;90(4):664–666. doi: 10.7326/0003-4819-90-4-664. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L., HAAS V., BARKER H. A. Metabolism of glucose by Butyribacterium rettgeri. J Bacteriol. 1954 Aug;68(2):227–230. doi: 10.1128/jb.68.2.227-230.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


