With the advent of more effective therapy for the bone marrow in acute promyelocytic leukemia (APL) central nervous system (CNS) prophylaxis has been suggested to be useful. Using data from 739 patients treated on two Spanish national trials, the authors examine the incidence of CNS relapse in APL and whether risk factors for such relapse can be identified.
Keywords: acute promyelocytic leukemia, central nervous system relapse, all-trans retinoic acid, idarubicin, prognostic factors
Abstract
Background
The prevalence of and risk factors for central nervous system recurrence in patients with acute promyelocytic leukemia are not well established and remain a controversial matter.
Design and Methods
Between 1996 and 2005, 739 patients with newly diagnosed acute promyelocytic leukemia enrolled in two consecutive trials (PETHEMA LPA96 and LPA99) received induction therapy with all-trans retinoic acid and idarubicin. Consolidation therapy comprised three courses of anthracycline monochemotherapy (LPA96), with all-trans retinoic acid and reinforced doses of idarubicin in patients with an intermediate or high risk of relapse (LPA99). Central nervous system prophylaxis was not given.
Results
Central nervous system relapse was documented in 11 patients. The 5-year cumulative incidence of central nervous system relapse was 1.7% (LPA96 3.2% and LPA99 1.2%; p=0.09). The cumulative incidence was 0%, 0.8%, and 5.5% in low-, intermediate-, and high-risk patients, respectively. Relapse risk score (p=0.0001) and the occurrence of central nervous system hemorrhage during induction (5-year cumulative incidence 18.7%, p=0.006) were independent risk factors for central nervous system relapse.
Conclusions
This study shows a low incidence of central nervous system relapse in patients with acute promyelocytic leukemia following therapy with all-trans retinoic acid and anthracycline without specific central nervous system prophylaxis. Central nervous system relapse was significantly associated with high white blood cell counts and prior central nervous system hemorrhage, which emerged as independent prognostic factors.
Introduction
The central nervous system (CNS) is the most common site of extramedullary relapse in acute promyelocytic leukemia (APL), and at least 10% of relapses involve the CNS.1 However, there is an apparent lack of documented knowledge about many aspects of this challenging clinical complication in the context of state-of-the-art treatments with all-trans retinoic acid (ATRA) and chemotherapy. The reported incidence of CNS relapses in APL ranges from 0.6% to 2%.2,3 Although CNS involvement is associated with age3 and BCR3 isoform,3,4 and possibly with the use of ATRA and the development of differentiation syndrome,5 high white blood cell (WBC) count at presentation appears to be the most consistent predictive factor.3,6 For patients without leukocytosis, who have an extremely low risk of CNS relapse, there is a general consensus that CNS prophylaxis is not indicated.7 In contrast, in patients with APL and high-risk features presenting with a high WBC count, some have argued in favor of CNS prophylaxis with intrathecal chemotherapy.6,8 Available data about disease outcome after CNS relapse are limited. The GIMEMA group reported that the survival rates of patients after CNS relapse and after isolated bone marrow relapse were similar.2 However, in a joint study by the European APL and PETHEMA groups, survival rates after CNS relapse were significantly lower than after bone marrow relapse.3 The optimal management of APL patients with CNS involvement at first relapse, whether isolated or associated with bone marrow involvement, has not been assessed critically.
We analyzed the incidence of and prognostic risk factors for CNS involvement at first relapse in a large series of newly diagnosed patients with APL who were enrolled in two consecutive Programa Español de Tratamiento en Hematología (PETHEMA) trials (LPA96 and LPA99) and treated with ATRA and anthracycline monochemotherapy without CNS prophylaxis. We also evaluated the outcome of these patients.
Design and Methods
Eligibility for inclusion in the study
The eligibility criteria for enrollment of patients with genetically diagnosed APL have been described elsewhere.9 The study protocol was approved by the Research Ethics Board of each participating hospital according to the Declaration of Helsinki.
Therapy of acute promyelocytic leukemia
Patients were included in two successive protocols (LPA96 and LPA99) that have been previously described.9 Briefly, treatment consisted of induction therapy with ATRA and idarubicin (AIDA regimen), three consolidation courses with idarubicin (two courses) and mitoxantrone (one course) with or without ATRA according to a risk-adapted strategy,9 followed by maintenance therapy with ATRA and low-dose chemotherapy with methotrexate and 6-mercaptopurine.
Prophylaxis and treatment of central nervous system relapse
Patients did not receive CNS prophylaxis. When CNS relapse occurred, treatment comprised weekly intrathecal triple therapy (ITT) with methotrexate, hydrocortisone, and cytarabine until complete clearance of blasts in the cerebrospinal fluid, followed by less frequent ITT treatments as consolidation. Some patients received further craniospinal irradiation at the physician’s discretion. Systemic treatment with ATRA plus chemotherapy or arsenic trioxide was also given as induction or consolidation or both, even for patients with isolated CNS involvement. Autologous or allogeneic stem cell transplant was given, in some cases, as intensification therapy.
Study definitions and end-points
The remission-induction response was assessed according to the criteria revised recently by Cheson et al.10 Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML–RARA-specific band visualized at diagnosis, using a previously described nested reverse-transcriptase polymerase chain reaction (RT-PCR) assay for PML–RARA amplification11 with a sensitivity level of 10−4. Persistent molecular disease and molecular relapse were defined as PCR positivity in two consecutive bone marrow samples collected at the end of consolidation therapy and at any time after consolidation therapy, respectively. CNS relapse was confirmed by lumbar puncture and cytological examination of cerebrospinal fluid, which was performed only in patients with clinically suspected CNS relapse. Morphological and molecular status was assessed by examining bone marrow aspirates from all patients at the time of CNS relapse.
Prognostic factors
Twenty-seven characteristics of the patients and disease were analyzed to establish their relationship to CNS relapse. In addition to the characteristics listed in Table 1, the following variables were also considered: serum lactate dehydrogenase (LDH), peripheral blast count, fibrinogen level, relapse-risk score, cytogenetics, FLT3-internal tandem duplication (FLT3-ITD) mutation, and a number of surface antigen markers (CD2, CD7, CD11b, CD34, CD13, HLA-DR, and CD56). Occurrence of clinically and radiologically evident CNS hemorrhage at diagnosis or during induction, and development of differentiation syndrome were also included. Differentiation syndrome was diagnosed according to the presence of two or more of the symptoms and signs described by Frankel et al.12
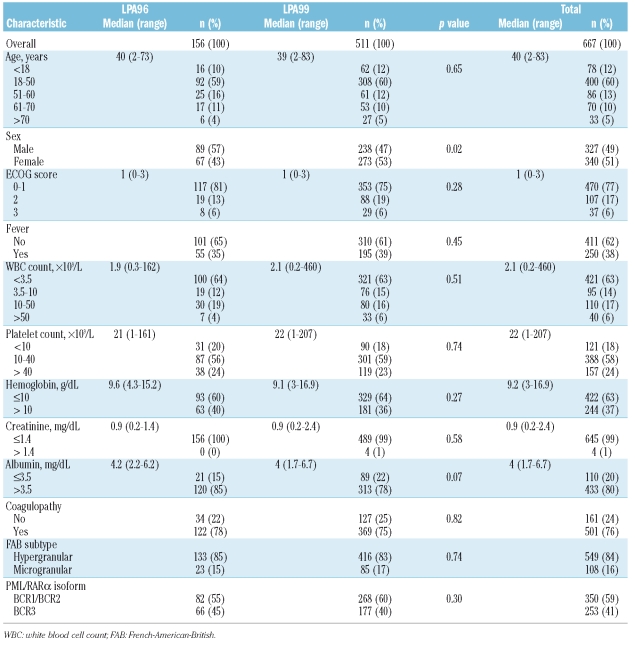
Table 1.
Main demographic and baseline characteristics of 667 acute promyelocytic leukemia patients who achieved complete remission with the AIDA regimen (study population).
Statistical methods
The χ2 test, with Yates’ correction if necessary, was used to analyze differences in the distribution of variables between subsets of patients. The probability of CNS involvement at first relapse was estimated by the cumulative incidence method, and univariate comparison between the cumulative incidence curves was performed using the Gray test.14 Isolated bone marrow relapse (molecular or morphological), death in complete remission, and development of secondary acute leukemia or myelodysplastic syndrome was each considered as a competing risk event. Multivariate analysis was performed using a logistic regression model.14 Overall survival after relapse was estimated by the Kaplan–Meier method, and comparisons were made by the log-rank test.15 The patients’ follow-up was updated in June 2008, and the median follow-up was 83 months (range, 32–136 months).
Results
Accrual and patients’ characteristics
Between November 1996 and June 2005, 793 consecutive patients with a genetic diagnosis of APL were registered from 82 institutions in Spain, The Netherlands, Belgium, Argentina, Uruguay, and the Czech Republic (see Appendix). Details about the non-eligible, non-evaluable, and evaluable patients have been reported elsewhere.16,17
The main demographic and baseline characteristics of the 667 patients who achieved complete remission are shown in Table 1. Patients enrolled in the LPA96 and LPA99 trials were similar for all baseline characteristics except for sex (more females were included in the LPA99 trial, p=0.02).
Incidence and characteristics of central nervous system relapse
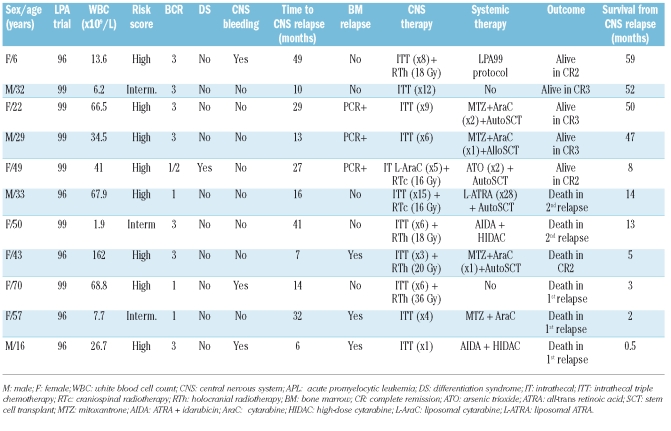
CNS involvement at first relapse was documented in 11 patients: in eight of these it was isolated (although three had positive PML–RARA in the bone marrow by RT-PCR) whereas in three it was simultaneous with overt involvement of the bone marrow. The median time to CNS relapse was 16 months (range, 6–49 months) compared with 16 months (range, 5–74 months) for isolated bone marrow relapse. The characteristics of the patients who experienced CNS relapse are summarized in Table 2. Seven patients were female, and four were male. At the time of the primary APL diagnosis, the median age was 33 years (range, 6–70 years) and WBC count was 34.5×109/L (range, 1.9–162×109/L). Eight of the 11 CNS relapses occurred in the 149 patients classified at diagnosis as being at high-risk (cumulative incidence at 5 years, 5.45%), and the other three were in the 381 patients classified as being at intermediate-risk (cumulative incidence at 5 years, 0.8%). No CNS relapses were observed in low-risk patients. Three patients experienced clinical CNS hemorrhage during induction therapy. One additional patient (9%) had differentiation syndrome compared with 22 of 73 relapsed patients (30%) without CNS involvement. The overall 5-year cumulative incidence of CNS relapse was 1.7%, with a trend toward a higher incidence in the LPA96 trial (3.2%) than in the LPA99 trial (1.2%) (p=0.09). The cumulative incidence of isolated bone marrow relapse was 14.7% in the LPA96 trial and 10.6% in the LPA99 trial (p=0.08). The relative frequency of CNS involvement among relapsed patients overall was 13%: five of 28 relapses (18%) in the LPA96 trial and six of 56 relapses (11%) in the LPA99 trial. No extramedullary sites of relapse other than CNS were observed in either PETHEMA trial.
Table 2.
Pretreatment characteristics of patients who had a central nervous system relapse and time of occurrence, treatment, and outcome the relapse.
Treatment of central nervous system relapse
Treatment of CNS relapse included ITT for all patients except one, who was treated with intrathecal liposomal cytarabine. Six patients received further CNS irradiation (4 holocranial and 2 craniospinal). Except for two patients, both with CNS relapse without morphological bone marrow involvement, all remaining patients received systemic treatment (Table 2).
Outcome of patients with central nervous system relapse
Of the eight patients with CNS involvement without morphological relapse in the bone marrow (3 PCR positive) one died during CNS treatment. The remaining seven patients achieved a complete clearance of blasts from the cerebrospinal fluid and relief of symptoms. The subsequent outcome of these patients was as follows: (i) two patients experienced a second relapse and died despite receiving consolidation therapy with liposomal ATRA or intensive chemotherapy; (ii) three patients are alive in complete remission after salvage therapy for a second CNS or bone marrow relapse; and (iii) two patients did not relapse after receiving ATRA plus intensive chemotherapy or arsenic trioxide followed by autologous stem cell transplantation.
The three patients with simultaneous CNS and bone marrow involvement died during systemic therapy with ATRA plus intensive chemotherapy (2 patients) or during aplasia following autologous stem cell transplantation.
The median survival was 13 months in patients who had a CNS relapse and 20 months in those who had isolated bone marrow relapse (p=0.61).
Prognostic factors for central nervous system relapse
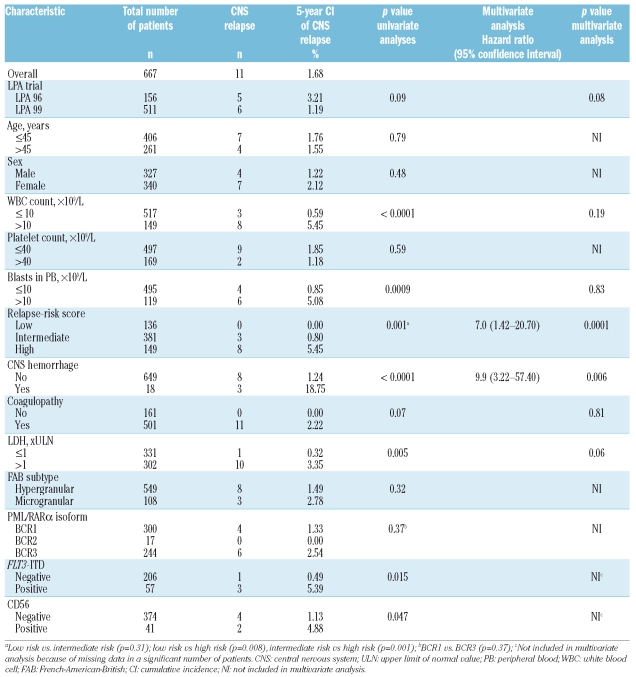
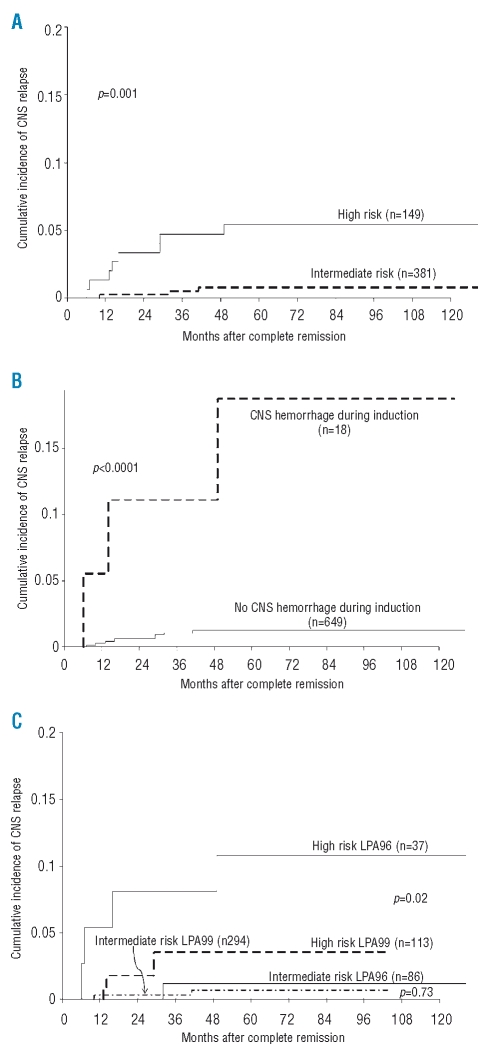
The univariate analysis identified the following characteristics as being associated with CNS relapse: high WBC count in the peripheral blood at presentation (p<0.0001), high blast count in the peripheral blood (p=0.0009), both with a count of 10×109/L as the most significant cut-off; relapse-risk score (p=0.001); clinical CNS hemorrhage during induction chemotherapy (p<0.0001); elevated serum LDH concentration (p=0.005); FLT3-ITD mutations (p=0.015); and CD56 positivity (p=0.047) (Table 3). Multivariate analysis was also carried out: insufficient data were available for FLT3-ITD mutation status and CD56 expression. Multivariate analysis identified the following independent prognostic factors for CNS relapse: relapse-risk score (p=0.0001) (Figure 1A) and clinical CNS hemorrhage during induction chemotherapy (p=0.006) (Figure 1B). Because univariate analysis showed a trend for a lower cumulative incidence of CNS relapse among patients treated in the LPA99 trial (p=0.09), the LPA96 and LPA99 trials were compared according to the relapse-risk group. For high-risk patients, the 5-year cumulative incidences of CNS relapse were 10.8% in the LPA96 trial and 3.6% in the LPA99 trial (p=0.02; Figure 1C). For intermediate-risk patients, the 5-year cumulative incidences were 1.2% and 0.7% in the LPA96 and LPA99 trials, respectively (p=0.73).
Table 3.
Main comparisons of cumulative incidence of central nervous system relapse according to the characteristics of the patients and disease.
Figure 1.
Cumulative incidence of central nervous system relapse according to (A) relapse-risk group: intermediate (white blood cell count ≤10×109/L and a platelet count ≤40×109/L) versus high (white blood cell count >10×109/L). No relapse occurred among low-risk patients (WBC count ≤10×109/L and a platelet count >40×109/L); (B) occurrence of central nervous system hemorrhage during induction; and (C) LPA trial and relapse-risk group: LPA96 versus LPA99.
Discussion
This study shows a relatively low incidence of CNS involvement at first relapse in APL patients receiving front-line therapy with ATRA and anthracycline monochemotherapy without specific CNS prophylaxis. A comparison of this incidence in two sequential PETHEMA trials showed that risk-adapted consolidation with ATRA and reinforced anthracycline for intermediate- and high-risk patients (LPA99 trial) significantly reduced the incidence of CNS relapse in the latter setting. The relapse-risk score, which is a composite of WBC and platelet counts,18 and the occurrence of clinical CNS hemorrhage during induction were the main risk factors for CNS relapse. Although a sizable proportion of patients experiencing CNS involvement at first relapse will develop subsequent bone marrow relapse, some of these patients will still respond to treatment and may survive over the long term.
Despite the sequential nature of the two trials, there was an apparent unbiased improvement in the 5-year cumulative incidence of CNS involvement from 3.2% in the LPA96 trial to 1.2% in the LPA99 trial. The difference was not significant for this crude comparison, which included all patients, but became significant when high-risk patients were analyzed separately. One explanation of the lower CNS relapse rate in the LPA99 study could be that the more dose-intensive chemotherapy in this trial reduced the systemic disease burden, decreasing the risk of seeding of APL cells in the CNS and, thereby, the incidence of CNS relapse. Another explanation is that the higher-dose intensity of anthracyclines of the LPA99 trial may offer a more effective treatment of the CNS sanctuary. Notably, in contrast to other anthracyclines, the active metabolite of idarubicin, idarubicinol, can penetrate the blood–brain barrier.19–21
The variable incidence of CNS involvement at first relapse observed in different series should be interpreted with caution. Variables with potential impact on the incidence of this complication may have been unbalanced and may bias the direct comparison between different series. Different proportions of patients with leukocytosis at presentation, a well-recognized risk factor for CNS relapse,3 is the most obvious potential cause of bias, but other more subtle factors should also be considered. In this respect, the sample size and the length of follow-up are particularly important and usually considered, but some other factors related to competing events, particularly bone marrow relapse, should also be considered. Theoretically, a high incidence of bone marrow relapse can lead to an apparent low incidence of CNS involvement at first relapse. This may explain, at least in part, the different incidence rates of CNS relapse reported for series with similar sample size and follow-up.2,3 The apparently lower incidence of CNS relapse reported by the European APL Group3 (4 cases among 582 patients) than in the GIMEMA Group2 (16 cases among 740 patients) was observed in the context of a higher incidence of hematologic relapses in the former (150/582 vs. 131/740). Interestingly, the European APL Group reported a 2-year cumulative incidence of CNS relapse of 0% in 340 patients treated with ATRA combined with chemotherapy, including high-dose cytarabine and intrathecal prophylaxis in patients with WBC counts greater than 10×109/L.8 However, full evaluation of these results requires both independent confirmation and a longer follow-up because a considerable proportion of CNS relapses may occur more than 2 years after the time of attainment of the first complete remission (e.g., 5 of 11 cases in our present study).3
As reported previously, our study confirms that APL patients with high WBC count at diagnosis (>10×109/L) have an increased risk of CNS relapse.3 However, the relapse-risk score18 remained as an independent risk factor in multivariate analysis after removing WBC count from the regression equation. An additional and novel finding is the association between the occurrence of clinical CNS hemorrhage before or during remission induction and subsequent CNS relapse. This has not been reported before and could have potential therapeutic implications. Regarding other potential risk factors, some authors have suggested previously that FLT3-ITD mutations, which correlate with leukocytosis,22 and an increased expression of adhesion molecules, such as CD56, can promote leukemic infiltration in the CNS.23–25 In this study, we were unable to assess CD56 expression and FLT3-ITD mutation as predictive factors for CNS relapse in multivariate analysis because of an insufficient number of patients with adequate data. Other factors previously related to extramedullary relapse, such as age (< 45 years),3 BCR3 isoform,3,4 and development of differentiation syndrome5 were not significant determinants of CNS relapse in our study.
The low incidence of CNS relapse does not argue in favor of systematic CNS prophylaxis even in patients with high-risk APL. The burden for these patients and the potential risks of additional medical complications (e.g., hemorrhage or neurological toxicity)26 following lumbar puncture would be unlikely to outweigh the possible benefits. Because there is no clear benefit of systematic CNS prophylaxis, its indication in APL is still a matter of debate. Based on our results, however, prophylaxis could be considered for those patients with clinical or radiological signs of CNS hemorrhage during induction.
In conclusion, we found a low incidence of CNS involvement at first relapse in newly diagnosed patients with APL following therapy with ATRA and anthracycline monochemotherapy without specific CNS prophylaxis. CNS relapse was significantly associated with WBC counts greater than 10×109/L and CNS hemorrhage during induction treatment, which emerged as independent prognostic factors.
Acknowledgments
the authors thank Miguel Priego for data collection and management.
Appendix
The following institutions and clinicians participated in the study: Argentina (Grupo Argentino de Tratamiento de la Leucemia Aguda)—Complejo Médico Policia Federal, La Plata; Fundaleu, Buenos Aires: S. Pavlovsky, G. Milone; Hospital Clemente Álvarez, Rosario: S. Ciarlo; Hospital de Clínicas, Buenos Aires; Hospital General San Martín, La Plata: M. Gelemur; Hospital Rossi, La Plata: S. Saba; Hospital San Martín de Paraná, Entre Ríos: P. Negri; Instituto Privado de Hematología, Paraná; Instituto de Trasplante de Médula Ósea, La Plata: V. Prates; Czech Republic—Faculty Hospital, Brno: M. Protivankova; Spain (Programa Español de Tratamiento de las Hemopatías Malignas)—Basurtuko Ospitalea, Bilbao: J. M. Beltrán de Heredia; Complejo Hospitalario de Segovia: J. M. Hernández; Complexo Hospitalario Xeral-Calde, Lugo; J. Arias; Complejo Hospitalario, León: F. Ramos; Fundación Jiménez Díaz, Madrid: A. Román; Hospital 12 de Octubre, Madrid: J. de la Serna; Hospital Carlos Haya, Málaga: S. Negri; Hospital Central de Asturias, Oviedo: C. Rayón; Hospital Clinic, Barcelona: J. Esteve; Hospital Clínico de Valladolid: F.J. Fernández-Calvo; Hospital Clínico San Carlos, Madrid: J. Díaz Mediavilla; Hospital Clínico San Carlos (H. Infantil), Madrid: C. Gil; Hospital Clínico Universitario, Santiago de Compostela: M. Pérez; Hospital Clínico Universitario, Valencia: M. Tormo; Hospital Clínico Universitario Lozano Blesa, Zaragoza: M. Olave; Hospital de Cruces, Baracaldo: E. Amutio; Hospital del Mar, Barcelona: C. Pedro; Hospital de Navarra, Pamplona: A. Gorosquieta; Hospital Dr Negrín, Las Palmas: T. Molero; Hospital Dr Peset, Valencia: M. J. Sayas; Hospital Dr Trueta, Girona: R. Guardia; Hospital General de Albacete: J. R. Romero; Hospital General de Alicante: C. Rivas; Hospital General de Alicante (Oncología Pediátrica): C. Esquembre; Hospital General de Castellón: R. García; Hospital General de Especialidades Ciudad de Jaén: A. Alcalá; Hospital General de Jerez de la Frontera: A. León; Hospital General de Murcia: M.L. Amigo; Hospital General de Valencia: M. Linares; Hospital Germans Trias i Pujol, Badalona: J. M. Ribera; Hospital Insular de Las Palmas: J. D. González San Miguel; Hospital Juan Canalejo, A Coruña: G. Debén; Hospital Joan XXIII, Tarragona: L. Escoda; Hospital La Princesa, Madrid: R. de la Cámara; Hospital Materno-Infantil de Las Palmas: A. Molines; Hospital do Meixoeiro, Vigo: C. Loureiro; Hospital Montecelo, Pontevedra: M. J. Allegue; Hospital Mutua de Terrasa: J. M. Martí; Hospital Niño Jesús, Madrid: L. Madero; Hospital Ntra. Sra. de Sonsoles, Ávila: M. Cabezudo; Hospital Ramón y Cajal, Madrid: J. García-Laraña; Hospital Reina Sofía, Córdoba: R. Rojas; Hospital Río Carrión, Palencia: F. Ortega; Hospital Río Hortega, Valladolid: M. J. Peñarrubia; Hospital San Jorge, Huesca: F. Puente; Hospital San Rafael, Madrid: B. López-Ibor; Hospital Sant Pau, Barcelona: S. Brunet; Hospital San Pedro de Alcántara, Cáceres: J. M. Bergua; Hospital Santa María del Rosell, Cartagena: J. Ibáñez; Hospital Severo Ochoa, Leganés: P. Sánchez; Hospital Son Dureta, Palma de Mallorca: A. Novo; Hospital de Tortosa: L. L. Font; Hospital Txagorritxu, Vitoria: J. M. Guinea; Hospital Universitario del Aire, Madrid: A. Montero; Hospital Universitario de Salamanca: M. González; Hospital Universitario La Fe, Valencia: M. A. Sanz, G. Martín, J. Martínez; Hospital Universitario La Fe (Hospital Infantil), Valencia: A. Verdeguer; Hospital Universitario La Paz (Hospital Infantil), Madrid: P. García; Hospital Universitario Marqués de Valdecilla, Santander: E. Conde García; Hospital Universitario Príncipe de Asturias, Alcalá de Henares: J. García; Hospital Universitario Puerta del Mar, Cádiz: F. J. Capote; Hospital Universitario Puerta de Hierro, Madrid: I. Krsnik; Hospital Universitario Vall D’Hebron, Barcelona: J. Bueno; Hospital Universitario Materno-Infantil Vall D’Hebron, Barcelona: P. Bastida; Hospital Universitario Virgen de la Arrixaca, Murcia: P. Rosique; Hospital Universitario Virgen de la Arrixaca (Pediatría), Murcia: J. L. Fuster; Hospital Universitario Virgen del Rocío, Sevilla: R. Parody; Hospital Universitario Virgen de la Victoria, Málaga: I. Pérez; Hospital Virgen del Camino, Pamplona: J. Molina; Hospital Xeral Cíes, Vigo; C. Poderós; Institut Catala d’Oncologia, Hospitalet de Llobregat; R. Duarte; The Netherlands (The Dutch–Belgian Hemato-Oncology Cooperative Group, HOVON)—VU Medical Center Amsterdam: G. J. Ossenkoppele; Academic Medical Center, University of Amsterdam: J. van der Lelie; Erasmus University Medical Center, Rotterdam: B. Lowenberg, P. Sonneveld, M. Zijlmans; University Medical Center, Groningen: E. Vellenga; Gasthuisberg Hospital, Leuven: J. Maertens; OLVG Hospital, Amsterdam: B. de Valk; Den Haag Hospital, Leyenburg: P.W. Wijermans; Medical Spectrum Twente Hospital, Enschede: M.R. de Groot; Academic Hospital Maastricht: H.C. Schouten; St. Antonius Hospital, Nieuwegein: D.H. Biesma; Sophia Hospital, Zwolle: M. van Marwijk Kooy; Uruguay, Hospital Maciel, Montevideo: E. de Lisa.
Footnotes
Authorship and Disclosures
PM and MAS, conceived the study, and analyzed and interpreted the data; PM, BL and MAS wrote the paper; PM performed the statistical analyses; JD-M, GD, VP, MT, VR, IP, IF, MV, CRi, JG, JdlS, JE, JMB, CRa, MG, JDG, SN and SB included data on patients treated in their institutions, reviewed the manuscript and contributed to the final draft.
The authors reported no potential conflicts of interest.
Funding: this study was supported in part by the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja (grant 2006/0137), Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0031).
References
- 1.Evans GD, Grimwade DJ. Extramedullary disease in acute promyelocytic leukemia. Leuk Lymphoma. 1997;33:219–29. doi: 10.3109/10428199909058422. [DOI] [PubMed] [Google Scholar]
- 2.Specchia G, Lo Coco F, Vignetti M, Avvisati G, Fazi P, Albano F, et al. Extramedullary involvement at relapse in acute promyelocytic leukemia patients treated or not with all-trans retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche dell’Adulto. J Clin Oncol. 2001;19:4023–8. doi: 10.1200/JCO.2001.19.20.4023. [DOI] [PubMed] [Google Scholar]
- 3.de Botton S, Sanz M, Chevret S, Dombret H, Martin G, Thomas X, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. European APL Group; PETHE-MA Group. Leukemia. 2006;20:35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 4.Liso V, Specchia G, Pogliani EM, Palumbo G, Mininni D, Rossi V, et al. Extramedullary involvement in patients with acute promyelocytic leukemia: a report of seven cases. Cancer. 1998;83:1522–8. [PubMed] [Google Scholar]
- 5.Ko BS, Tang JL, Chen YC, Yao M, Wang CH, Shen MC, et al. Extramedullary relapse after all-trans retinoic acid treatment in acute promyelocytic leukemia. The occurrence of retinoic acid syndrome is a risk factor. Leukemia. 1999;13:1406–8. doi: 10.1038/sj.leu.2401495. [DOI] [PubMed] [Google Scholar]
- 6.Breccia M, Carmosino I, Diverio D, De Santis S, De Propris MS, Romano A, et al. Early detection of meningeal localization in acute promyelocytic leukaemia patients with high presenting leucocyte count. Br J Haematol. 2003;120:266–70. doi: 10.1046/j.1365-2141.2003.04056.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Guidelines on the management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 8.Adès L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24:5703–10. doi: 10.1200/JCO.2006.08.1596. [DOI] [PubMed] [Google Scholar]
- 9.Sanz MA, Martín G, González M, León A, Rayón C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA Group. Programa de Estudio y Traitmiento de las Hemopatías Malignas. Blood. 2004;103:1237–43. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Bolufer P, Barragán E, Sánz MA, Martín G, Bornstein R, Colomer D, et al. Preliminary experience in external quality control of RT-PCR PML-RAR α detection in promyelocytic leukemia. Leukemia. 1998;12:2024–8. doi: 10.1038/sj.leu.2401225. [DOI] [PubMed] [Google Scholar]
- 12.Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP., Jr The ‘retinoic acid syndrome’ in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–6. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 14.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16.Sanz MA, Montesinos P, Vellenga E, Rayón C, de la Serna J, Parody R, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA group. Blood. 2008;112:3130–4. doi: 10.1182/blood-2008-05-159632. [DOI] [PubMed] [Google Scholar]
- 17.de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 18.Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–53. [PubMed] [Google Scholar]
- 19.Borchmann P, Hübel K, Schnell R, Engert A. Idarubicin: a brief overview on pharmacology and clinical use. Int J Clin Pharmacol Ther. 1997;35:80–3. [PubMed] [Google Scholar]
- 20.Dreyer ZE, Kadota RP, Stewart CF, Friedman HS, Mahoney DH, Kun LEM, et al. Phase 2 study of idarubicin in pediatric brain tumors: Pediatric Oncology Group study POG 9237. Neuro Oncol. 2003;5:261–7. doi: 10.1215/S115285170200056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid JM, Pendergrass TW, Krailo MD, Hammond GD, Ames MM. Plasma pharmacokinetics and cerebrospinal fluid concentrations of idarubicin and idarubicinol in pediatric leukemia patients: a Childrens Cancer Study Group report. Cancer Res. 1990;50:6525–8. [PubMed] [Google Scholar]
- 22.Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. NCRI Adult Leukaemia Working Party. Blood. 2005;106:3768–76. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti M, Falanga A, Giovanelli S, Oldani E, Barbui T. All-trans-retinoic acid increases adhesion to endothelium of the human promyelocytic leukaemia cell line NB4. Br J Haematol. 1996;93:360–6. doi: 10.1046/j.1365-2141.1996.4911029.x. [DOI] [PubMed] [Google Scholar]
- 24.Di Noto R, Lo Pardo C, Schiavone EM, Ferrara F, Manzo C, Vacca C, et al. All-trans retinoic acid (ATRA) and the regulation of adhesion molecules in acute myeloid leukemia. Leuk Lymphoma. 1996;21:201–9. doi: 10.3109/10428199209067601. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara F, Morabito F, Martino B, Specchia G, Liso V, Nobile F, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000;18:1295–300. doi: 10.1200/JCO.2000.18.6.1295. [DOI] [PubMed] [Google Scholar]
- 26.Atra A, Pinkerton CR, Bouffet E, Norton A, Hobson R, Imeson JD, et al. Acute neurotoxicity in children with advanced stage B-non-Hodgkin’s lymphoma and B-acute lymphoblastic leukaemia treated with the United Kingdom Childrens Cancer Study group 9002/9003 protocols. Eur J Cancer. 2004;40:1346–50. doi: 10.1016/j.ejca.2004.02.011. [DOI] [PubMed] [Google Scholar]