Abstract
Herein, we provide crystallographic and computational evidence that Hashimoto’s Rh2(S-PTTL)4 catalyst adopts a “chiral crown” conformation with a reactive chiral face and an unreactive achiral face. In Rh2(S-PTTL)4, all four t-butyl groups are aligned on the same face of the catalyst, and each C-(t-Bu) bond is roughly parallel to the central Rh-Rh bond. This orients the four phthalimido groups on the opposite face of the catalyst. Also described is an enantioselective and diastereoselective protocol for intermolecular Rh2(S-PTTL)4 catalyzed cyclopropanation using α-alkyl-α-diazoesters. Intermolecular cyclopropanation is selective over two competing intramolecular pathways: C–H insertion and β–hydride elimination. Based on DFT calculations and the Davies-Singleton model for cyclopropanation, a model for asymmetric induction is proposed.
Rh(II)-carboxylate catalysts derived from N-phthaloyl amino acids have emerged as exceptionally powerful tools for asymmetric synthesis. In this series, tert-leucine-derived complexes [e.g. dirhodium(II) tetrakis[N-phthaloyl-(S)-t-leucinate]—Rh2(S-PTTL)4] have emerged as highly selective catalysts in asymmetric reactions including intramolecular C–H insertion,1 tandem ylide formation/1,3-dipolarcycloaddition,2 amination3, and 2,3-sigmatropic rearrangement.4 Davies has introduced adamantylglycine analogs of these ligands, which induce even higher levels of enantioselectivity in some reactions.5
Previously, Hashimoto determined the crystal structure of the related catalyst dirhodium(II) tetrakis[N-phthaloyl-(S)-phenyl alaninate] (Rh2(S-PTPA)4), in which two phthalimido groups are oriented on each face of the dirhodium tetracarboxylate.6a Herein, we provide evidence that Rh2(S-PTTL)4 adopts a considerably different structure, which we refer to as the “chiral crown” conformation. We also demonstrate the effectiveness of Rh2(S-PTTL)4 for intermolecular enantioselective cyclopropanation with selectivity over two competing intramolecular pathways.
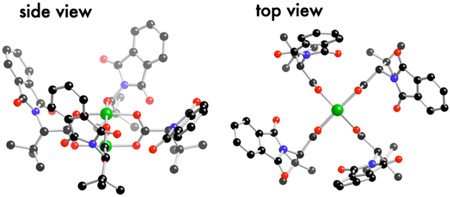
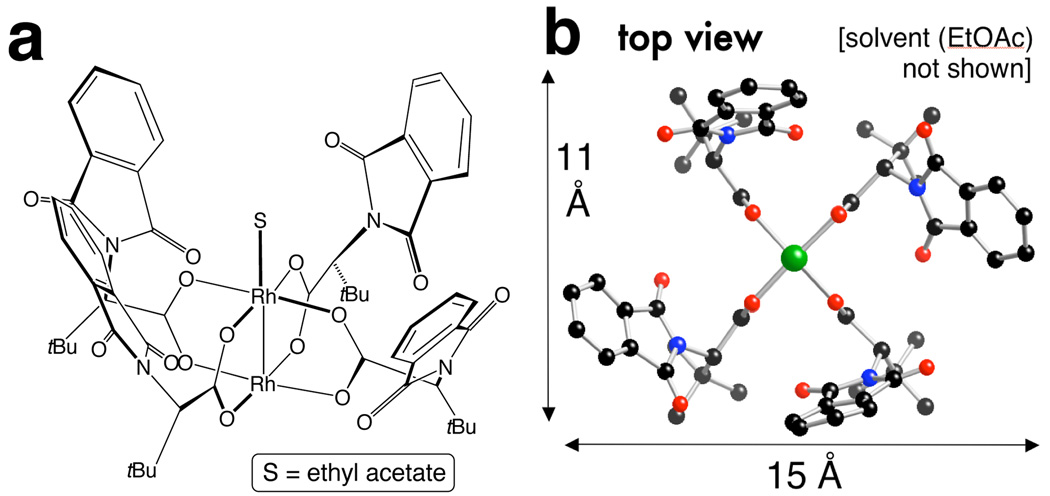
We determined the X-ray structure of Rh2(S-PTTL)4 crystals grown from ethyl acetate. As shown in Figure 1, all four t-butyl groups are oriented on the same face of the catalyst, and each C-(t-Bu) bond is roughly parallel to the central Rh-Rh bond. This orients the four phthalimido groups on the opposite face of the catalyst in a “chiral crown” structure. Ignoring solvent, the structure is C2 symmetric, and the cavity of the catalyst has a wide (∼15 Å) and narrow dimension (∼11 Å) (Fig 1). Calculations also suggest that the chiral crown structure is the lowest energy conformer of Rh2(S-PTTL)4. Starting from a completely different geometry, we carried out Monte Carlo calculations (MMFF) on Rh2(S-PTTL)4. The lowest energy conformer was similar to that found in the crystal structure, and 2.1 and 3.0 kcal/mol lower in energy than the next closest conformers. The energies of these 3 structures were then determined using DFT calculations (ADF: OLYP/TZP basis set; see Supporting Information). The chiral crown was lower in energy than the next conformer by 3.8 kcal/mol. We also minimized the energy of Rh2(S-PTTL)4 in conformations similar to that in the crystal of Rh2(S-PTPA)4—the chiral crown was more stable by 8.2 kcal/mol. We propose that Rh2(S-PTTL)4 has a reactive chiral face, and that the sterically demanding t-Bu groups block reactivity on the achiral face of the catalyst. Consistent with this hypothesis, only the Rh-atom on the chiral face is bound by solvent in the crystal structure (Fig 1a).
Figure 1.
X-ray crystal structure of Rh2(S-PTTL)4a
aRh2(S-PTTL)4 crystallized from ethyl acetate. The unit cell contained two nearly identical (but symmetry unique) molecules.
We envisaged that Rh2(S-PTTL)4 and related catalysts could prove useful for intermolecular reactions of α-alkyldiazo compounds that ordinarily give products of β-hydride elimination.7 Recent studies from our group have demonstrated that several intermolecular Rh-catalyzed processes can be favored over β-hydride elimination when sterically demanding carboxylate ligands and low reaction temperatures are employed.8 For intermolecular cyclopropanation, Rh2TPA46b is an effective catalyst.8d Recently, Hashimoto described that Rh2(S-PTTL)4 catalyzes intramolecular C–H insertion reactions in preference to β-hydride elimination, and that lowering temperature had a positive effect on selectivity over β-hydride elimination: 99:1 selectivity was observed at −78 °C vs 82:18 selectivity at 0 °C.1a
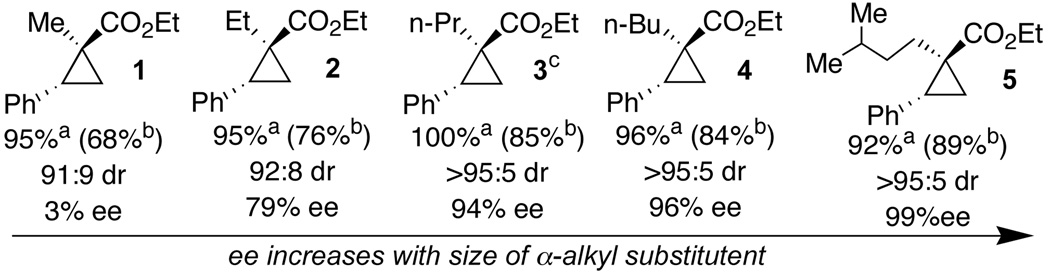
Rh2(S-PTTL)4 in hexanes at −78 °C led to cyclopropanes with high diastereoselectivity and yield. The enantioselectivity was highly sensitive to the structure of the diazoester, and larger α-alkyl substituents led to increasingly higher ee. (Scheme 1). Diasteroselectivity also increased with the size of the α-alkyl substituent. The distinction between ethyl α-diazopropionate (3% ee) and ethyl α-diazobutanoate (79% ee) was most striking. In the best case, 99% ee and >95:5 dr was observed in the reaction of styrene with ethyl 2-diazo-5-methylhexanoate. Highest yields were obtained when an excess (3 equiv) of readily available diazo esters were used. However, good yields could be obtained when the alkene and diazo compound were used in 1.1:1 stoichiometry.
Scheme 1.
Effects of α-alkyl substitution on stereoselectivity
In general, reactions were carried out in hexane at −78 °C with 0.5 mol% Rh2(S-PTTL)4 a Isolated yield with 3 equiv. diazo compound and limiting alkene. bIsolated yield with 1.1 equiv of alkene and limiting diazo compound. cThe yield and ee of 3 dropped in reactions carried out with 3.0 equiv diazoester at 0 °C (54%, 88% ee) and 25 °C (43%, 85% ee).
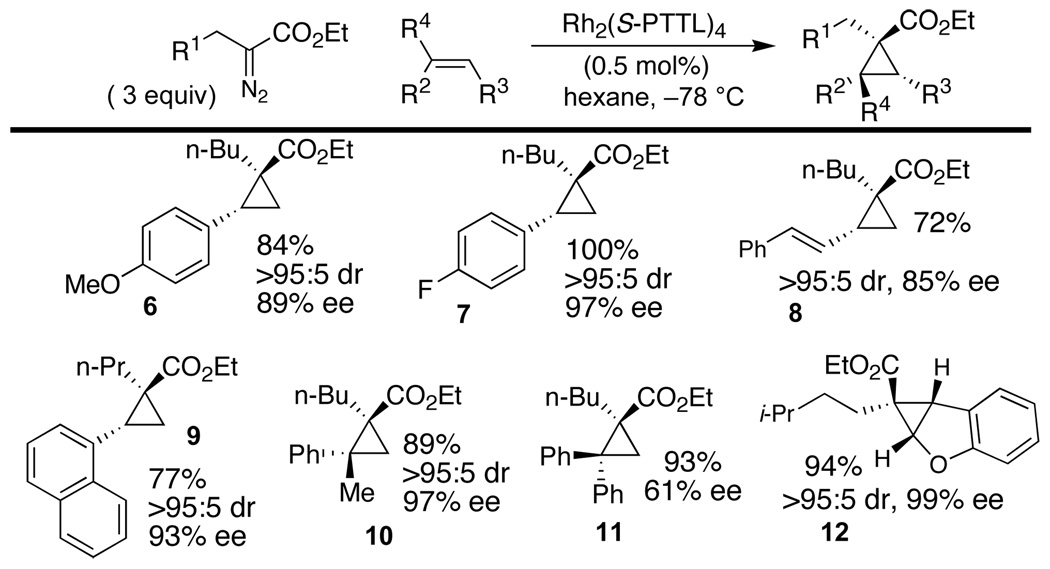
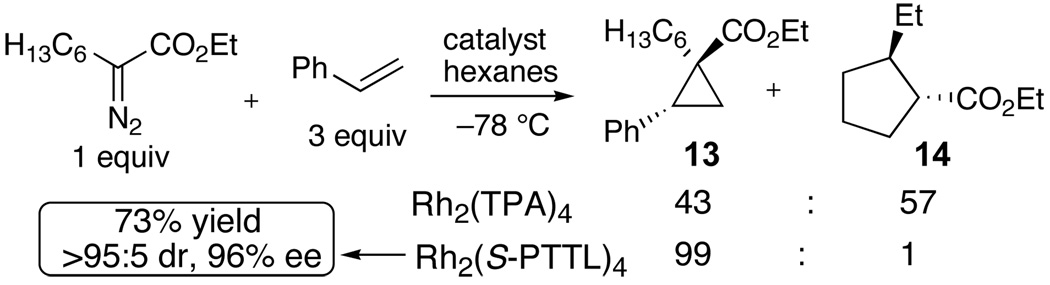
The scope of the cyclopropanation reaction was studied (Scheme 2). High diastereoselectivity and enantioselectivity was achieved in cyclopropanation reactions with styrenes, α-methylstyrene, 1-vinylnapthalene, benzofuran and (E)-1-phenylbutadiene. Moderate enantioselectivity (61% ee) was observed in the reaction between 1,1-diphenylethylene and ethyl α-diazohexanoate. Enantioselective cyclopropanation of styrene with ethyl α-diazooctanoate was selective over intramolecular C-H insertion1a,5,7b (Scheme 3). Thus, the Rh2(S-PTTL)4 catalyzed reaction gave cyclopropane 13 (99:1 dr, 96% ee) with 99:1 selectivity over C–H insertion product 14. The analogous Rh2TPA4 catalyzed reaction was poorly selective, and gave 14:13 with 57:43 selectivity.
Scheme 2.
Enantioselective cyclopropanation with α-alkyl diazoesters
Scheme 3.
Catalyst dependent selectivity over C-H insertion
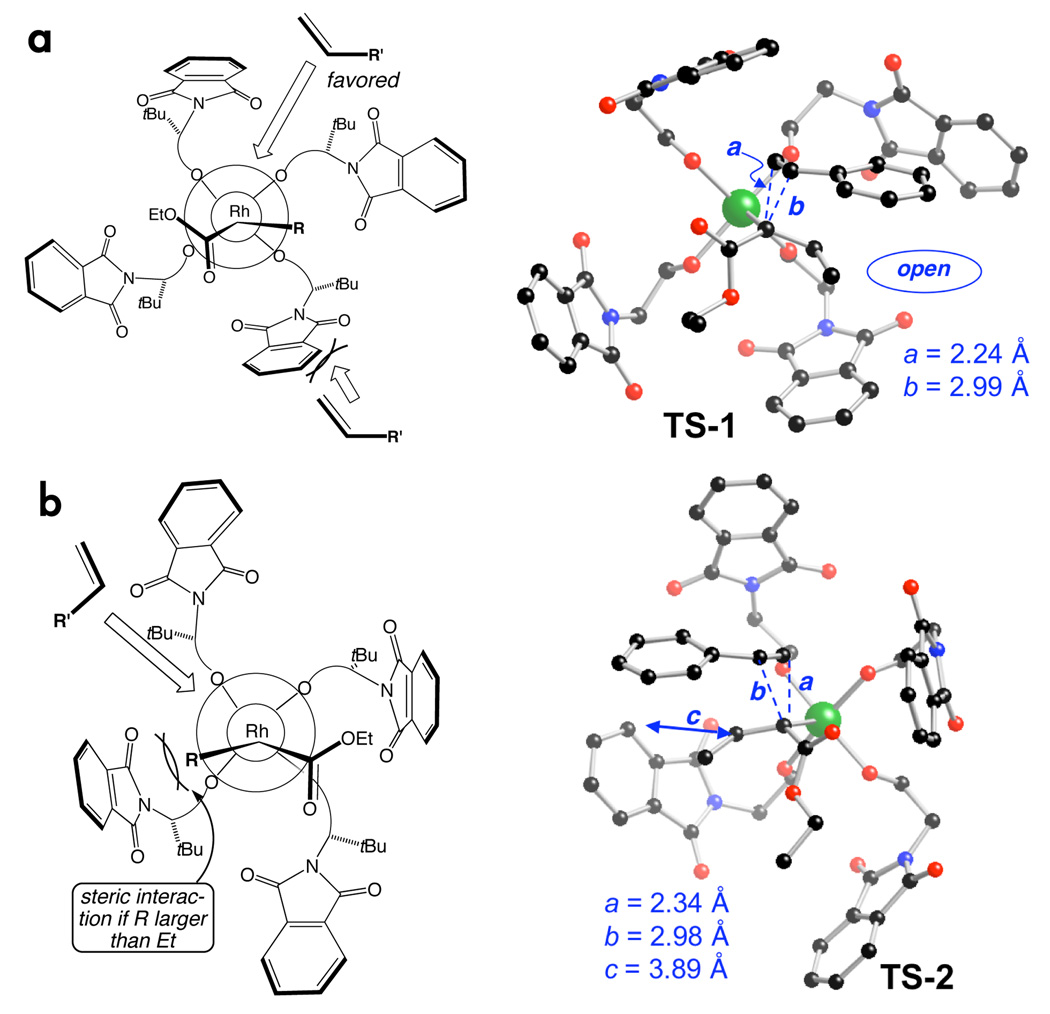
To explain the enantioselectivity, we propose a model (Fig 2a) in which the carbene is aligned with the wide dimension of the C2 symmetric chiral cavity (see Fig 1). This leaves the Si–face of the carbene accessible for reaction with the alkene via end-on9 approach. When the α-alkyl substitutent is small (Me or Et), we propose that there is competing reactivity on the Re-face via a confor-mation in which the carbene is aligned with the narrow dimension of the chiral cavity (Fig 2b). DFT calculations were used to support this hypothesis.
Figure 2.
DFT-optimized geometries of transition structures for cyclopropanation by attack of the (a) Si- and (b) Re-face.
Previously, Gaussian DFT calculations on cyclopropanation reactions with carbenoids of Rh2(O2CH)4 have been carried out using the hybrid B3LYP functional with a LANL2DZ basis set and effective core potential on Rh atoms and a 6–311+G(d) on the other atoms.9a However, such calculations are intractably slow for large carbenoids from Rh2(S-PTTL)4. Accordingly, we turned to ADF calculations using the OLYP/TZP (small frozen core) level of theory. The cyclopropanation of styrene by a model carbenoid [(CO2Et)(Et)C=Rh2(O2CH)4] was used for calibration: transition structures with similar geometries were found in OLYP/TZP calculations (using ADF) and in B3LYP/Lanl2dz/6-31G(d) Gaussian 03 calculations. For the OLYP-optimized geometries, single point B3LYP/Lanl2dz/6-31G(d) energy calculations were carried out to better estimate the barriers of the transition structures.10a
For the Rh2(S-PTTL)4 catalyzed reaction to form 2, two10b simi lar energy11 transition structures (TS-1 and TS-2) were located with a barrier of 11 kcal/mol (Fig 2). The C2 symmetry of the chiral cavity of the catalyst is retained in TS-1 and TS-2. In TS-2, where the carbene is aligned along the narrow dimension of the chiral cavity, the α-ethyl group is only 3.89 Å away from a phthaloyl moiety. Inspection of the model indicates that simple replacement of the α-ethyl group by a larger n-alkyl group would result in a significant steric interaction. In contrast, the space arou nd the α-alkyl group of TS-1 is open, and able to support larger alkyl groups. We believe that reaction via TS-2 competes when R is Me or Et, but TS-1 is dominant when R is larger than Et.
In summary, a chemoselective, enantioselective and diastereoselective protocol for intermolecular Rh2(S-PTTL)4 catalyzed cyclopropanation is described for reactions of alkenes with α-alkyl-α-diazoesters. Calculations and X-ray crystallography support a chiral crown conformation for Rh2(S-PTTL)4 in which the catalyst has a reactive chiral face and an unreactive achiral face.
Supplementary Material
Full experimental and computational details, 1H and 13C NMR spectra, stereochemical assignments, and crystallographic data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NIH (GM068640) for support.
References
- 1.a) Minami K, Saito H, Tsutsui H, Nambu H, Anada M, Hashimoto S. Adv. Synth. Catal. 2005;347:1483. [Google Scholar]; b) Tsutsui H, Yamaguchi Y, Kitagaki S, Nakamura S, Anada M, Hashimoto S. Tetrahedron: Asymmetry. 2003;14:817. [Google Scholar]; c) Saito H, Oishi H, Kitagaki S, Nakamura S, Anada M, Hashimoto S. Org. Lett. 2002;4:3887. doi: 10.1021/ol0267127. [DOI] [PubMed] [Google Scholar]
- 2.a) Shimada N, Anada M, Nakamura S, Nambu H, Tsutsui H, Hashimoto S. Org. Lett. 2008;10:3603. doi: 10.1021/ol8013733. [DOI] [PubMed] [Google Scholar]; b) Tsutsui H, Shimada N, Abe T, Anada M, Nakajima M, Nakamura S, Nambu H, Hashimoto S. Adv. Synth. Catal. 2007;349:521. [Google Scholar]
- 3.a) Anada M, Tanaka M, Washio T, Yamawaki M, Abe T, Hashimoto S. Org. Lett. 2007;9:4559. doi: 10.1021/ol702019b. [DOI] [PubMed] [Google Scholar]; b) Yamawaki M, Tsutsui H, Kitagaki S, Anada M, Hashimoto S. Tetrahedron Lett. 2002;43:9561. [Google Scholar]
- 4.Kitagaki S, Yanamoto Y, Tsutsui H, Anada M, Nakajima M, Hashimoto S. Tetrahedron Lett. 2001;42:6361. [Google Scholar]
- 5.a) Denton JR, Cheng K, Davies HML. Chem. Commun. 2008:1238. doi: 10.1039/b719175h. [DOI] [PubMed] [Google Scholar]; b) Reddy RP, Davies HML. J. Am. Chem. Soc. 2007;129:10312. doi: 10.1021/ja072936e. [DOI] [PubMed] [Google Scholar]; c) Reddy RP, Davies HML. Org. Lett. 2006;8:5013. doi: 10.1021/ol061742l. [DOI] [PubMed] [Google Scholar]; d) Reddy RP, Lee GH, Davies HML. Org. Lett. 2006;8:3437. doi: 10.1021/ol060893l. [DOI] [PubMed] [Google Scholar]
- 6.a) Hashimoto S, Watanabe N, Sato T, Shiro MS, Ikegami S. Tetrahedron Lett. 1993;33:5109. [Google Scholar]; b) Hashimoto S, Watanabe N, Ikegami S. Tetrahedron Lett. 1992;33:2709. [Google Scholar]
- 7.a) Taber DF, Herr RJ, Pack SK, Geremia JM. J. Org. Chem. 1996;61:2908. doi: 10.1021/jo952098j. [DOI] [PubMed] [Google Scholar]; b) Taber DF, Joshi PV. J. Org. Chem. 2004;69:4276. doi: 10.1021/jo0303766. [DOI] [PubMed] [Google Scholar]
- 8.a) Panne P, Fox JM. J. Am. Chem. Soc. 2007;129:22. doi: 10.1021/ja0660195. [DOI] [PubMed] [Google Scholar]; b) DeAngelis A, Panne P, Yap GPA, Fox JM. J. Org. Chem. 2008;73:1435. doi: 10.1021/jo702308f. [DOI] [PubMed] [Google Scholar]; c) DeAngelis A, Taylor MT, Fox JM. J. Am. Chem. Soc. 2009;131:1101. doi: 10.1021/ja807184r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Panne P, DeAngelis A, Fox JM. Org. Lett. 2008;10:2987. doi: 10.1021/ol800983y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Nowlan DT, Gregg TM, Davies HML, Singleton DA. J. Am. Chem. Soc. 2003;125:15902. doi: 10.1021/ja036025q. [DOI] [PubMed] [Google Scholar]; (b) Doyle MP. Acc. Chem. Res. 1986;19:348. [Google Scholar]
- 10.(a) OLYP/TZP [ADF program] calculations overestimate the barrier relative to B3LYP/Lanl2dz/6-311+G(d) [Gaussian 03]. However, the geometries of the transition structures were similar.(b) A third higher energy TS-3, shown in the supporting information, was also located. This TS corresponds to attack on the ‘southern’ face of the carbenoid as shown in Fig 2a.
- 11.TS-2 was lower in energy than TS-1 by 1.4 kcal/mol at the OLYP/TZP level of theory; their energies reversed (TS-1 lower by 0.3 kcal/mol) after a single point calculation [B3LYP/Lanl2dz&6-31G(d) method]. We consider these energetic differences to be within the margin of error for the level of theory.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental and computational details, 1H and 13C NMR spectra, stereochemical assignments, and crystallographic data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.