Summary
Alzheimer's disease (AD) has been associated with functional alterations in a distributed network of brain regions linked to memory function, with a recent focus on the cortical regions collectively known as the default network. Posterior components of the default network, including the precuneus and posterior cingulate, are particularly vulnerable to early deposition of amyloid β-protein, one of the hallmark pathologies of AD. In this study, we use in vivo amyloid imaging to demonstrate that high levels of amyloid deposition are associated with aberrant default network functional magnetic resonance imaging (fMRI) activity in asymptomatic and minimally impaired older individuals, similar to the pattern of dysfunction reported in AD patients. These findings suggest that amyloid pathology is linked to neural dysfunction in brain regions supporting memory function and provide support for the hypothesis that cognitively intact older individuals with evidence of amyloid pathology may be in early stages of AD.
Introduction
The deposition of fibrillar forms of amyloid-β protein is a major pathological feature of Alzheimer's disease (AD), and episodic memory impairment is the hallmark clinical symptom of early AD. Despite compelling laboratory evidence that amyloid-β exerts deleterious effects on synaptic activity associated with the formation of new memories (Walsh and Selkoe, 2004), the link between amyloid and the clinical syndrome of memory impairment in humans remains somewhat controversial. In particular, the presence of amyloid pathology found at postmortem has not correlated particularly well with antemortem clinical status, as a substantial number of older subjects without dementia have evidence of significant amyloid deposition at autopsy. It has been postulated that amyloid deposition begins many years prior to the onset of clinical symptomatology, resulting in a long prodromal phase of AD. Two recent advances in neuroimaging now allow us to explore the prodromal phase of AD: the first arises from the ability to measure amyloid deposition in living individuals and the second from the identification of sensitive markers of brain dysfunction in AD. This combination of imaging probes allows us to test the hypothesis that amyloid deposition is associated with disruption of the neural systems supporting memory function, and that amyloid-related functional alterations, similar to those reported in AD patients, can be detected in individuals even prior to significant cognitive impairment.
Fibrillar amyloid deposition can now be readily imaged in vivo with Pittsburgh Compound B (PiB) and positron emission tomography (PET) (Klunk et al., 2004). Consistent with previous autopsy reports in normal control populations (Arriagada et al., 1992; Bennett et al., 2006; Morris et al., 1996), recent PiB-PET studies have reported amyloid deposition in significant percentages of non-demented older subjects (Aizenstein et al., 2008; Buckner et al., 2005; Gomperts et al., 2008; Johnson et al., 2007; Mintun, 2006; Pike et al., 2007). It remains unknown whether these cognitively normal older individuals with evidence of amyloid deposition will ultimately develop memory impairment and clinical dementia.
It is intriguing, however, that brain regions particularly vulnerable to early amyloid deposition, appear to overlap a set of heteromodal cortices, often collectively labeled the “default network” (Raichle et al., 2001; Buckner et al., 2008). Regions within the default network show structural and functional connectivity that converges on the posterior cingulate extending into the precuneus (Buckner et al., 2008; Fransson and Marrelec, 2008; Greicius et al., 2009; Hagmann et al., 2008), which is strongly interconnected with the hippocampal formation (Greicius et al., 2004; Vincent et al., 2006). Default network regions are active during episodic and autobiographical memory retrieval and, under certain conditions, augment activity in proportion to the strength or certainty of the memory decision (Svoboda et al., 2006; Wagner et al., 2005). During tasks that demand attention to external stimuli and novel events, activity in the default network decreases (Shulman et al., 1997). During memory formation, these decreases in activity have been associated with successful encoding (Daselaar et al., 2004). When such activity decreases do not occur, new information is not properly encoded and memory falters (Daselaar et al., 2004; Miller et al., 2008a; Otten and Rugg, 2001).
Relevant to AD, functional alterations in default network activity have been reported both during cognitive tasks and in the resting state, particularly in the precuneus/posterior cingulate (PPC). Aging, even in the absence of amyloid pathology, has been associated with alterations in intrinsic connectivity, in particular connectivity between the posterior cingulate and medial prefrontal regions (Andrews-Hanna et al., 2007). These effects in aging are not specific to the default network and likely represent a general disruption of connectivity of large-scale networks. AD patients, as well as older adults with mild cognitive impairment (MCI), often a prodromal stage of AD, have shown selective disruption of default network intrinsic connectivity, most prominently in connectivity between the precuneus/posterior cingulate and medial temporal lobe regions (Bai et al., 2008; Greicius et al., 2004; Persson et al., 2008; Rombouts et al., 2005; Sorg et al., 2007). This functional alteration has also been observed during memory tasks in MCI and AD (Lustig et al., 2003; Pihlajamaki et al., 2008; Pihlajamaki et al., in press).
In a recent fMRI study comparing young and clinically normal older subjects during successful memory encoding, we observed that regions within the default network demonstrated the greatest age-related differences in fMRI activity, most prominently at the intersection of the precuneus and posterior cingulate (i.e., PPC) (Miller et al., 2008a). Older subjects who performed poorly on the associative memory task not only failed to show typical patterns of activity in the default network, but demonstrated evidence of a paradoxical reversal of the normal encoding activity in the PPC, similar to previous reports in mild AD (Lustig et al., 2003; Pihlajamaki et al., 2008) and in MCI subjects destined to develop AD (Petrella et al., 2007). We noted that the PPC region of the default network was particularly vulnerable to early amyloid deposition (Mintun et al., 2006), and speculated that older individuals with evidence of aberrant default network activity might harbor occult amyloid pathology.
Here we studied another group of older individuals with a combination of PET amyloid imaging and an associative memory fMRI paradigm. Given the convergence of evidence that suggests the default network is important to memory function and the observation that this network is functionally disrupted in MCI and AD, we explored whether amyloid burden might be directly associated with altered default network activity in asymptomatic and minimally impaired older individuals. If found, such an association would support the hypotheses that amyloid deposition is related to dysfunction in brain networks supporting memory formation and that asymptomatic individuals with significant amyloid deposition possess functional alterations similar to those observed in clinically-diagnosed AD.
Results
Fifty-three subjects participated in this study; eighteen healthy young adults, age 18-30, and thirty-five older adults, age 60-90, who were recruited from studies of aging and cognition ongoing at the Brigham and Women's Hospital and Massachusetts General Hospital. Older subjects were deemed eligible for this study if they were living independently in the community, had a Mini-Mental State Examination (MMSE) score of 27-30, and performed within 1.5 S.D. of normal on screening neuropsychological measures. The Clinical Dementia Rating (CDR), based on a detailed interview with the subject and a study partner who has daily contact with the subject, revealed that 22 of the older subjects had a CDR of 0. Thirteen of the subjects were classified as CDR 0.5, in the range of questionable dementia, based on their reports of subjective memory complaints, which were corroborated by their study partner. The CDR 0.5 subjects selected for this study, however, did not demonstrate objective memory impairment on neuropsychological testing, and showed no significant differences from the CDR 0 subjects on any other measures. These CDR 0.5 subjects did not meet Petersen criteria for MCI (Petersen et al., 2001), and thus were classified as “not normal, not MCI” per the Alzheimer's Disease Research Center Uniform Dataset criteria (Morris et al., 2006), but might be considered to be “pre-MCI” on the basis of their subjective memory complaints. Demographic information for all subjects is presented in Table 1.
Table 1.
Demographic variables and memory performance
| Subjects | Young | All Older | CDR 0 | CDR 0.5 |
| n = 53 | n=18 | n=35 | n=22 | n=13 |
| Age | 23.7 ± 2.9 | 76.6 ± 6.1 | 75.9 ± 6.7 | 77.8 ± 4.8 |
| Gender | 4 M / 14 F | 12 M / 23 F | 5 M / 17 F | 7 M / 6 F |
| Education | 16.5 ± 1.3 | 16.1 ± 2.5 | 16 ± 2 | 17 ± 3 |
| MMSE | N/A | 28.9 ± .98 | 28.9 ± .92 | 28.9 ± 1.1 |
| CDR-SB | N/A | .63 ± 1.1 | .02 ± .11 | 1.7 ± 1.4* |
| Buschke 30 Minute Delayed Recall | N/A | 7.56 ± 3.41 | 7.81 ± 3.53 | 7.09 ± 3.27 |
| Face-name Task Accuracy | 72.9% ± 9.4% correct 53.8% ± 10.1% high confidence hits |
69.0% ± 10.2% correct 43.6% ± 16.8% high confidence hits |
70.7% ± 10.0% correct 47.3% ± 16.0% high confidence hits |
66.1% ± 10.1% correct 37.2% ± 16.7% high confidence hits |
Values are listed as mean ± SD.
p>0.05 difference between CDR 0.5 and CDR 0.
MMSE = Mini-Mental Status Examination.
CDR-SB= Clinical Dementia Rating Sum of Boxes
Approximately one third of the non-demented older adults have amyloid deposition
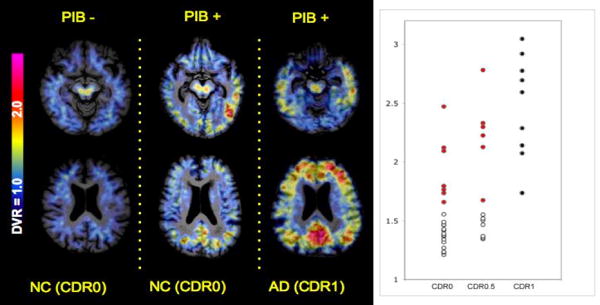
The non-demented older subjects in this study demonstrated a wide range of amyloid deposition on PiB-PET imaging. Figure 1 demonstrates the PiB distribution volume ratio (DVR) maps, using the cerebellum as reference tissue, in a PiB negative (PiB-) older control (left), showing only non-specific PiB retention in the white matter; compared to a PiB positive (PiB+) older control (center) with significant cortical PiB retention in the PPC, lateral temporo-parietal, and prefrontal regions, in a similar pattern to that typically observed in mild AD patients (right).
Figure 1.
A. Distribution volume ratio (DVR) maps of 11-C PiB activity in representative subjects from the study. Color scale from blue = low (1.0 DVR) to red = very high (3.0 DVR) levels of PiB retention. Shown on the Left is a 76 year old cognitively normal woman (CDR rating of 0) showing no evidence of specific cortical PiB retention (PiB-); Middle is a 75 year old woman, also cognitively normal (CDR 0) demonstrating elevated PiB retention in several regions of heteromodal cortices, especially precuneus/posterior cingulate (PPC) regions; Right is a 77 year old man, diagnosed with mild AD (CDR 1) demonstrating high levels of PiB retention in multiple cortical areas. B. Scatterplot of the anatomically defined PPC DVR values for the CDR 0 (n=22) and CDR 0.5 (n=13) groups compared to a group of AD patients (all CDR 1.0) imaged in a previous study (Johnson et al., 2007). Dots shown in red represent the CDR 0 and CDR 0.5 subjects who were classified as PiB+, based on a cut-off of PPC DVR >1.6, the lower end of PPC DVR values in the AD patients.
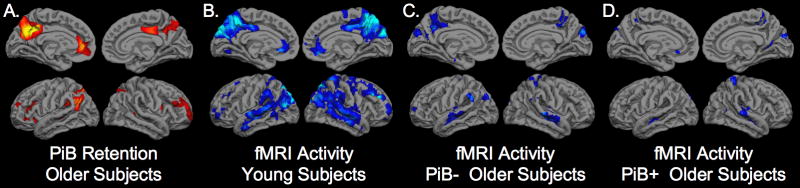
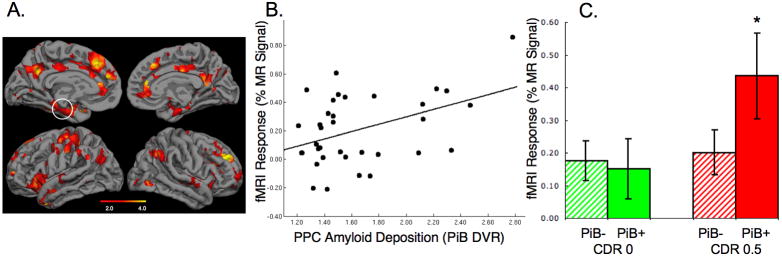
The partial volume corrected DVR values for the PPC demonstrate that approximately 30% of the older subjects in both the CDR 0 and CDR 0.5 groups fell within the DVR range of AD patients (PPC DVR>1.6; see Figure 1). These individuals were classified as PiB+ (n=13). The average volume of the right and left combined PPC region from FreeSurfer was 15317 +/-2423 mm3 and the PPC volume did not differ between the PiB+ and PiB- subjects (p=.82). In a whole brain map level analysis, the PiB+ older subjects demonstrated higher PiB retention in the PPC, lateral parietal, and medial prefrontal regions, compared to PiB- subjects (Figure 2A).
Figure 2.
A. Anatomic distribution of PiB retention for the group-wise comparison of PiB+ (n=13) > PiB- (n=22) older subjects thresholded at p<0.001 FWE corrected for multiple comparisons. B-D. SPM2 one-sample t-test of young subjects (B), PiB- older subjects (C), and PiB+ older subjects (D) demonstrating significant task-related decreases in fMRI activity (deactivation shown in blue) during successful encoding of face-name pairs (Fixation > high confidence correct “hit” (HCH) responses based on subsequent memory testing).
fMRI default network activity during successful encoding in young and older adults
As a comparison group for the older subjects, we analyzed fMRI data from a group of young subjects to identify regions functionally engaged during successful memory formation, in particular, default network regions, which showed task-induced decreases in fMRI activity (deactivation). Results are shown in Figure 2B. Significant task-induced deactivation (shown in blue) was found in the PPC, lateral temporo-parietal, and medial prefrontal regions (p<0.001). As noted in a previous report (Buckner et al., 2005), the pattern of deactivation within the default network overlaps the distribution of amyloid deposition, particularly evident in the PPC region bilaterally. Similar to previous studies, we noted that older subjects demonstrated decreased default network activity, even among the PiB- older individuals (Figure 2C). The decrease in default network activity was particularly evident in the older subjects with high amyloid burden (PiB+), who demonstrated minimal deactivation in the PPC or other default network regions, even at a more liberal threshold of p<0.05 (see Figure 2D). This qualitative observation is consistent with an association of amyloid deposition with impaired default network activity. This possibility was tested explicitly in the analyses below.
Amyloid deposition is associated with aberrant default network activity in older subjects
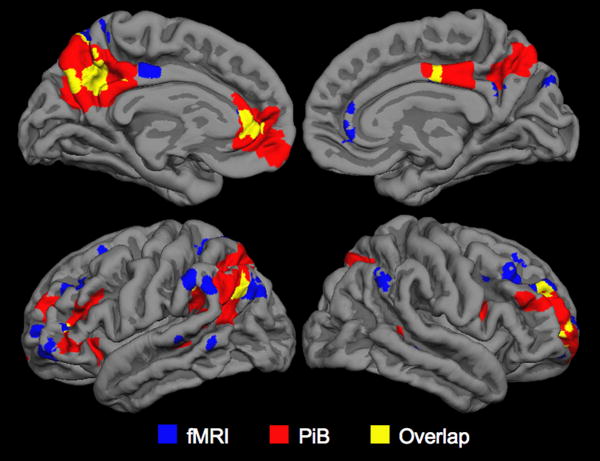
We then compared the pattern of fMRI default network activity between the PiB- and PiB+ older subjects. Significant differences in default network activity (p<0.001) were found in the left PPC (MNI coordinates -6, -57, 36), left medial prefrontal regions (-3, 42, 27) and lateral parietal regions (-60, -54, 36). We then investigated the anatomic overlap between the regions showing greatest difference in PiB retention (Figure 2A) and the regions showing the greatest differences in fMRI default activity (difference between Figure 2C and 2D) by creating an intersection of the two maps (Figure 3). Figure 3 demonstrates the regions showing overlap between high amyloid burden and fMRI activity differences in the older subjects, particularly notable in the left PPC. We also noted that regions of greatest difference in PiB+ and PiB- overlapped the regions showing the most significant default network activity in the young subjects (Figure 2B).
Figure 3.
Whole brain map demonstrating the intersection between the PiB+ > PiB- maps for PiB DVR (shown in red); the PiB+ > PiB- fMRI default activity (shown in blue) and overlap (shown in yellow) with peak region in the PPC (MNI coordinates -6, -57, 36).
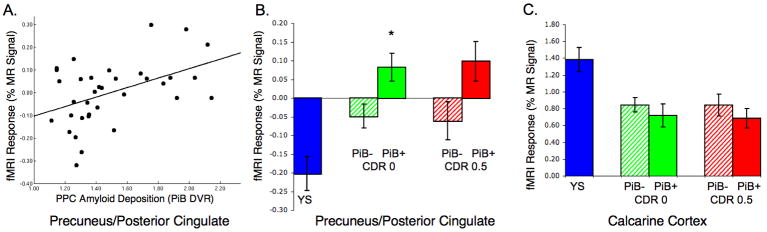
To examine the relationship between PiB retention and fMRI activity among the older subjects using unbiased regional analyses, we extracted both the PiB DVR and the fMRI signal response from a functionally defined ROI based on the intersection between the highest amyloid burden (PiB+ > PiB- DVR map; Figure 2A) and the region with most significant default network activity deactivation in the young subjects (Figure 2B), which was found in the PPC (MNI -9, -60, 39). Relating the PiB DVR values and fMRI activity within this ROI, we found a significant positive correlation among all older subjects, that is increasing amyloid was associated with increased fMRI signal in PPC (R= .46; p=0.006; see Figure 4A). Furthermore, we found that while young subjects demonstrated significant deactivation and PiB- older subjects demonstrated diminished deactivation, the PiB+ subjects demonstrated evidence of a positive MR signal response in this region. That is, the normal memory task-induced decrease in fMRI activity observed in young adults, demonstrated paradoxical increases in fMRI signal in the PiB+ older adults, similar to previous reports in older adults with MCI and clinically diagnosed AD (Lustig et al., 2003; Petrella et al., 2007; Pihlajamaki et al., 2008). We next investigated whether there was a relationship between PiB status, clinical status, and fMRI response.
Figure 4.
A. Regression plot demonstrating significant correlation between PiB DVR values and fMRI activity extracted from the precuneus/posterior cingulate ROI (R= .46; p=0.006). B. Bar graph showing the fMRI response in ROI from divided by CDR and PiB status, demonstrating a main effect of PiB but not of CDR status. PiB+ CDR 0 subjects demonstrated significantly higher fMRI activity than PiB- CDR 0 subjects (p=0.003). C. Bar graph of fMRI response extracted from control region in the calcarine cortex did not reveal any relationship with PPC amyloid levels.
Dichotomous grouping by PiB and CDR revealed that the reversal of PPC deactivation was evident in both CDR 0 and CDR 0.5 PiB+ subjects (Figure 4B). Interestingly we observed a main effect of PiB level on PPC fMRI activity (ANOVA; main effect of PiB p=0.019), but no main effect of CDR. Even among CDR 0 group alone, PiB+ subjects showed significantly higher fMRI signal compared to CDR 0 PiB- subjects in the PPC (p=0.003). We also performed a similar ROI analysis across the groups on a control region, taken from the calcarine cortices. Although all older subject groups demonstrated less fMRI activation than young subjects, we did not find any difference in MR signal between the PiB+ and PiB- older groups in the calcarine ROI (Figure 4C).
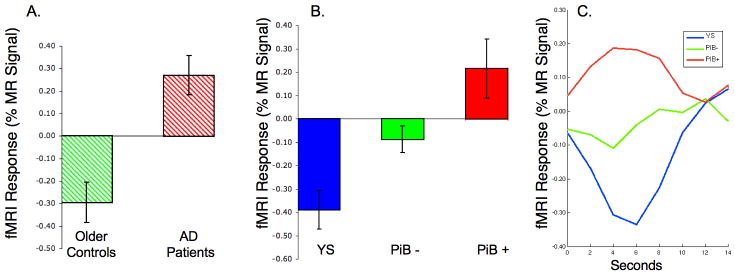
In order to directly test the hypothesis that cognitively intact older subjects with amyloid deposition show aberrant default network activity similar to that observed in AD, we investigated the fMRI signal response in an a priori PPC region of interest (ROI) based on the previously published fMRI studies comparing mild AD patients to normal older controls (Celone et al., 2006; Pihlajamaki et al., 2008). We identified an ROI based on the region within the PPC where AD patients demonstrated greater fMRI activity than older controls (Figure 5A), and utilized this unbiased “out of sample” ROI to further interrogate the relationship between amyloid and fMRI activity in the current sample of non-demented older individuals. We again found that PiB+ non-demented older individuals showed a complete loss of normal default activity, with paradoxical increase in fMRI activity, very similar to our findings in clinical AD patients (Figure 5B). Figure 5C illustrates time course of the MR signal extracted from this ROI with a “reversal” of the normal default activity in the PiB+ individuals (Figure 5C).
Figure 5.
A. Bar graph of the NC vs. AD fMRI response in precuneus/posterior cingulate region (MNI coordinates 0, -75, 33). (Note that these are subjects from previously published studies: Celone 2006; Pihlajamaki 2008) B. fMRI activity extracted from same ROI comparing Young subjects (blue), PiB- Older Subjects (green) and PiB+ Older Subjects (red) from the current study, with PiB+ subjects demonstrating similar pattern of aberrant default activity to that seen in AD patients. C. MR Signal timecourse in PPC ROI demonstrating deactivation in young subjects, minimal deactivation in PiB- older subjects, and reversal of default activity (paradoxical activation) in PiB+ older subjects.
Exploratory analyses reveal amyloid associated activity increases in multiple regions engaged in memory encoding
To further explore whether amyloid deposition in the PPC was associated with altered functional activity in other brain regions, we regressed the PiB DVR value from the anatomically defined PPC ROI into the whole brain functional maps for successful encoding, with age and task performance included as co-variates (Figure 6A). We again found significant positive correlations between PiB retention and fMRI activity in multiple regions of the default network, including portions of the medial prefrontal cortex (MNI coordinates -3, 45, 16; R=.78, p<0.00001), posterior cingulate (0, -48,15; R= .58, p<0.0002) and the precuneus (-3, -57,39; R=.59, p<0.0003); such that higher levels of PiB retention were associated with increasingly positive (aberrant) MR signal response.
Figure 6.
A. Whole brain exploratory analysis of PPC PiB DVR and fMRI activity across all 35 older subjects demonstrating significant positive correlations in multiple regions in the default network, as well as regions which activated during memory encoding. B. Regression of PiB and fMRI response from hippocampal ROI (circled region in 6A; R=.44; p<0.01) C. ANOVA of PiB and CDR groups demonstrated a significant effect on PiB on hippocampal activity only among the CDR 0.5 group (p<0.05).
At a less stringent statistical threshold of p<0.005 at the whole brain level, we also observed significant positive correlations between PPC PiB DVR and MR signal response in some additional brain regions. Many of these regions, including inferior prefrontal cortices and hippocampus, typically demonstrate increased MR signal during successful encoding in young and older subjects (Daselaar et al., 2003; Duverne et al., 2008; Miller et al., 2008a). ROI analyses within the hippocampus (centered on -21, -30, -15) also demonstrated a positive correlation between PiB and fMRI activity (R=.44; p=<0.01; Figure 6B), but ANOVA revealed that this relationship was driven primarily by hippocampal hyperactivation among the PiB+ CDR 0.5 subjects (p<0.05; Figure 6C). Finally, we performed an exploratory, vertex to vertex, surface based analysis to investigate the relationship between PiB retention and fMRI activity at the voxel level on the cortical surface. Although this analytic approach has some technical and statistical power limitations, we did observe small regions of significant local correlations at a less stringent threshold of p<0.01, most prominently in medial prefrontal regions of the default network (see supplemental data online at www.cell.com/neuron/).
We did not observe a significant relationship overall between PiB retention and behavioral performance on the associative memory task, likely because this study included only older individuals who did not have evidence of significant memory impairment. It is also possible that the increased fMRI activity in some memory-related regions, such as the hippocampus, might serve as compensatory mechanism to maintain memory performance in the setting of amyloid pathology and disrupted default network activity.
Discussion
This study investigated the relationship between in vivo amyloid deposition and neural activity during memory formation in older persons without dementia or objective cognitive impairment. Similar to the pattern observed in AD patients, a substantial subset of non-demented older individuals demonstrated evidence of amyloid deposition in the default network, most prominently in key nodes of the network thought to be involved in successful memory function. Here for the first time in humans, we demonstrate that amyloid deposition in asymptomatic and minimally impaired older individuals is associated with aberrant neural activity in the default network, consistent with findings reported in AD patients. Our results support the hypothesis that amyloid pathology is related to disrupted synaptic activity in the networks supporting memory function, even prior to cognitive impairment.
Aberrant default network activity during both task fMRI and resting state, has been a consistent finding in patients with clinical AD and in subjects at high risk for AD. Multiple groups have confirmed impaired intrinsic functional connectivity in the default network during the resting state in MCI and AD (Bai et al., 2008; Greicius et al., 2004; Rombouts et al., 2005; Sorg et al., 2007), which appears to be over and above more general age-related disruption of large-scale networks (Andrews-Hanna et al., 2007). Lustig et al. reported a similar observation of disrupted default network activity during memory tasks in AD subjects, showing a reversal of MR signal response that was strikingly similar to the present observation in PiB+ subjects (Lustig et al., 2003). We have also previously seen this aberrant default network activity, that is, failure of task-induced deactivation, in late MCI and in mild AD patients using face-name block design fMRI paradigms (Celone et al., 2006; Pihlajamaki et al., 2008). Petrella and colleagues, using a similar face-name encoding paradigm, found that impaired deactivation in MCI subjects was predictive of subsequent cognitive decline into clinical AD (Petrella et al., 2007). Two recent papers have also reported diminished deactivation in older subjects with genetic risk for AD (Persson et al., 2008; Pihlajamaki et al., in press). Our finding that cognitively intact and minimally impaired older individuals with high amyloid burden demonstrate disrupted default network activity, similar to the pattern found in AD, suggests that amyloid deposition may be responsible for dysfunction of brain networks supporting memory function, and suggests the possibility that these subjects may be indeed be in early (preclinical) stages of AD.
The mechanistic underpinnings of the aberrant default network activity remain to be elucidated. The PPC region typically demonstrates hypometabolism on FDG-PET and decreased perfusion in SPECT and arterial spin labeling (ASL) MRI in AD (Johnson et al., 1998; Johnson et al., 2005; Minoshima et al., 1997). This region has also demonstrated abnormal metabolism and perfusion in subjects with MCI who go on to develop clinical AD, and even in normal older subjects with genetic risk factors for AD or who subsequently develop dementia (Jagust et al., 2006; Johnson and Albert, 2000; Reiman et al., 2001). Thus, it is possible that abnormal PPC activity during memory encoding in these subjects might reflect the inability to modulate activity below an already decreased level of activity during the resting state. There have, however, also been reports of hyperperfusion in the PPC in very mildly impaired subjects, and it is possible that the paradoxical increase in MR signal reflects an abnormally increased level of activity at baseline (Sojkova et al., 2008). Studies relating amyloid to FDG-PET and ASL MR perfusion, as well as intrinsic functional connectivity during the resting state in these older subjects with elevated PiB retention are ongoing, and we will eventually be able to relate fMRI activity to baseline perfusion, metabolism, and activity in these subjects.
Recent molecular and electrophysiological studies in amyloid precursor protein (APP) transgenic mouse models may be relevant to our findings of abnormally increased activity in regions with high amyloid burden. A recent mouse study suggests that the presence of amyloid plaques may result in abnormally increased neuronal activity in surrounding neurons (Busche et al., 2008). Another study reported evidence of aberrant neuronal excitation that reached the level of non-convulsive seizure activity in mice overexpressing human APP (Palop et al., 2007). Thus, our observation of paradoxically increased fMRI signal during memory encoding might reflect a local excitatory response to amyloid pathology, interfering with the normal inhibition of default network neurons during memory encoding. AD pathology has also been associated with loss of inhibitory neuronal function (Baig et al., 2005; Hyman et al., 1992), and it is possible that our findings are due to an imbalance of excitatory and inhibitory inputs related to amyloid pathology.
The PPC is of particular interest because of the convergence of this region's vulnerability to early amyloid deposition, and its critical role in memory function, both during encoding and retrieval. The PPC and lateral parietal cortices typically deactivate during successful memory encoding of novel information (Daselaar et al., 2004; Duverne et al., 2008) and then typically activate during successful memory retrieval (Wagner et al., 2005), metamemory processes (Chua et al. 2006) and during autobiographical recall (Svoboda et al., 2006). As daily life involves a constant alternation between the processes of encoding of new information, retrieval of previously encountered information, and assessment as to whether information is novel or familiar, the PPC may be nearly continuously “toggling” between an activated and deactivated state, which may be particularly metabolically demanding. We have also recently noted that the PPC is a prominent “hub” in intrinsic cortical connectivity, and that the topography of hubs with high connectivity in young subjects overlapped the pattern of amyloid deposition in AD patients (Buckner et al., 2009). Laboratory evidence suggests that neuronal activity may directly increase production of amyloid-beta peptides (Cirrito et al., 2005). Thus, the critical role of the PPC in memory function and its heightened fluctuations in activity might serve to increase the production of amyloid β-protein in this region.
Our findings may also help to elucidate a long-standing conundrum regarding the role of amyloid in memory impairment in AD patients. Memory impairment in AD was often attributed primarily to pathology in the medial temporal lobe, where tangle formation and neuronal loss predominate (Hyman et al., 1984). Although transgenic mice show very high levels of amyloid deposition in the hippocampus, human autopsy series and PiB imaging studies have consistently found relatively low levels of amyloid plaques in the hippocampus and related medial temporal lobe regions, compared to the very high levels of amyloid pathology observed in heteromodal association cortices (Klunk et al., 2004). Our findings suggest that amyloid deposition in the default network is associated with altered neural activity in multiple nodes of the distributed network supporting memory function.
Interestingly, our exploratory analyses also revealed a relationship between PiB in the PPC and fMRI activity in the hippocampus, which was primarily due to increased activation among the PiB + CDR 0.5 subjects. This finding is consonant with our previous studies and those of other groups reporting hippocampal hyperactivation in very mild MCI subjects (Celone et al., 2006; Dickerson et al., 2005; Kircher et al., 2007; Miller et al., 2007) and in subjects at genetic risk for AD (Bondi et al., 2005; Bookheimer et al., 2000). Given the reciprocal anatomic and functional connectivity between the PPC and the hippocampus through the entorhinal cortices, it is conceivable that amyloid deposition and abnormal activity in the default network contributes to synaptic dysfunction and eventual neuronal loss in the hippocampus and surrounding medial temporal cortices. Recent PiB and volumetric MRI studies suggest that cognitively normal older adults with high levels of amyloid deposition have reduced whole-brain (Fotenos et al., 2008) and hippocampal volume (Mormino et al., 2008), as well as selective cortical thinning (Dickerson et al., 2009), relative to their amyloid-free peers. Taken together with our current findings, these studies suggest that amyloid deposition in the default network may have both local and remote effects on critical nodes in the distributed network supporting memory function.
The relationship of amyloid-related disruption to memory performance remains to be elucidated. The subjects included in this study did not have significant memory impairment, as we wanted to examine the relationship of amyloid pathology to memory network function prior to dementia, and thus it may not be surprising that we did not find a significant relationship between amyloid levels and task performance. It remains unclear whether the increased activity, particularly in the hippocampus, might be compensatory enabling these individuals to maintain memory performance. It is also possible that the increased hippocampal activation may also be indicative of impending memory failure, as suggested by our previous fMRI studies in MCI (Dickerson et al., 2004; Miller et al., 2008b). We are following these subjects longitudinally to determine if the combination of amyloid imaging and fMRI might be a sensitive predictor of subsequent cognitive decline.
There are several limitations to the current study. We have a relatively small sample of older subjects who fell in the PiB+ range of that seen in AD patients. Many of our analyses combined the CDR 0 and CDR 0.5 subjects to have adequate power to observe significant relationships on the whole brain map level, however, a significant difference in fMRI activity is observed among the CDR 0 group alone based on PiB status in the ROI analyses. We chose to focus on the PPC in this study because of its intriguing role in memory function, and the prominent early amyloid deposition in this region. While we found evidence that PPC amyloid deposition was associated with dysfunction in the PPC and multiple other regions comprising the default network, it is likely that amyloid deposition in other brain regions contribute to the altered functional activity observed in the default network. The issue of regional specificity may be difficult to fully elucidate given the high degree of colinearity among regional PiB levels. Furthermore, PiB binds selectively to fibrillar forms of amyloid β-protein, and recent evidence suggests that oligomeric forms may be the principal species responsible for impaired neural activity (Shankar et al., 2008). Although it is likely that brain regions with high fibrillar amyloid deposition also harbor high levels of oligomeric A-β, perhaps in “toxic halos” surrounding plaques (Meyer-Luehmann et al., 2008), we cannot yet image soluble forms of amyloid β-protein in living humans. It is also possible that the observed functional alterations are related to other pathophysiological alterations that may co-occur with amyloid deposition, such as tangle pathology and or neuronal loss. Most importantly, we do not yet have clinical follow-up on these subjects to determine if the presence of amyloid deposition and impairment of memory related fMRI activity are indeed predictors of subsequent cognitive decline and eventual progression to clinical AD.
In summary, our findings suggest that amyloid pathology in older humans is associated with aberrant neural responses during memory formation. Alterations in default network activity, similar to those observed in patients with clinical AD, were found even in asymptomatic and minimally impaired older individuals with high levels of amyloid deposition. Aberrant default network activity may be a sensitive indicator of amyloid-related synaptic toxicity that will eventually result in memory impairment. Longitudinal studies are clearly needed, but our findings are consistent with the premise that cognitively intact older individuals with amyloid pathology may already be in the prodromal stages of AD. This combination of molecular and functional imaging techniques may prove useful in monitoring disease progression prior to significant clinical symptomatology, as well as the response to amyloid-modifying agents in clinical trials of subjects at-risk for developing AD.
Experimental Procedures
Subjects
All subjects provided written informed consent prior to any experimental procedures. This study was approved by and conducted under the auspices of the Partners Human Research Committee at Brigham and Women's Hospital and Massachusetts General Hospital. Young subjects were recruited from advertisements posted in the hospital and the Internet. Older subjects were recruited from ongoing longitudinal studies of cognition and aging at BWH and MGH. Three of the 35 older subjects in this study also participated in our previous study of age-related differences in memory encoding (Miller et al., 2008a). All older subjects underwent a detailed screening and clinical and neuropsychological evaluation. Subjects were excluded from this study if they 1) were undergoing evaluation or treatment for memory complaint; 2) met Petersen criteria for mild cognitive impairment (MCI) (Petersen, 2004) with significant memory impairment (1.5 S.D. below age and education norms on standard clinical tests), as these subjects are at a high likelihood of progression to clinical dementia over a short time frame; 3) met NINDS criteria for clinical dementia (McKhann et al., 1984); or 4) had an MMSE score of 26 or below.
MR Image Acquisition
Both young and older subjects were scanned using a Siemens Trio 3.0 T scanner (Siemens Medical Systems, Erlangen Germany). High-resolution T1-weighted structural images were acquired using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MP-RAGE) sequence: repetition time (TR) = 2530 msec, echo time (TE) = 3.45 msec, inversion time (TI) = 1100 msec, flip angle (FA) = 7°, field of view (FOV) = 256 mm, matrix 192 × 256, slice thickness = 1.33 mm, 128 sagittal slices. Blood-oxygen-level-dependent (BOLD) fMRI data were acquired using a T2*-weighted gradient-echo echo-planar imaging (EPI) sequence: TR = 2000 msec, TE = 30 msec, FA = 90°, FOV = 200 mm, matrix 64 × 64 (in-plane resolution 3.125 × 3.125 mm2). Thirty oblique coronal slices with a thickness of 5.0 mm and an interslice gap of 1.0 mm were acquired, oriented perpendicular to the anterior-posterior commissural line. Six functional runs were acquired consisting of 102 whole-brain acquisitions with 5 TR discarded for T1 stabilization. The total functional scanning time was 25 min 30 sec.
PiB PET imaging
11C- PiB was prepared as described by Mathis et al (Mathis et al., 2002) and PiB data were acquired at MGH, as previously described (Gomperts et al., 2008; Johnson et al., 2007). Following a transmission scan, 10-15 mCi 11C-PiB was injected as a bolus and followed immediately by a 60-minute dynamic acquisition. PiB PET data were reconstructed with ordered set expectation maximization, corrected for attenuation, and each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method with cerebellar cortex as the reference tissue input function, was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) (Price et al., 2005).
We utilized an individually anatomically defined regional DVR calculated for the PPC to classify subjects as PiB+ vs. PiB-, as this region typically demonstrates the most consistent elevation of PiB retention in non-demented older subjects (Gomperts et al., 2008; Mintun et al., 2006). The anatomically defined PPC region was determined on each subject's high-resolution MP-RAGE image with FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) using a semi-automated parcellation method based on a probabilistic atlas (Fischl et al., 2004).
fMRI associative memory paradigm
The fMRI paradigm was a mixed block and event-related design adapted from a previously published fMRI block design paradigm (Celone et al., 2006) and a subsequent memory event-related design (Miller et al., 2008a). Briefly, the mixed design task was scanned during six encoding runs, each consisting of two 40 sec blocks of Novel and two Repeated face-name pair blocks (also 40 sec long) alternating with 25 sec periods of visual Fixation on a white cross-hair. Each face-name pair was shown for 4.75 sec. Each Novel block consisted of 7 face name pairs, resulting in a total of 84 novel face-name pairs shown over the course of the experiment. Subjects were instructed to try to remember the name associated with each face, and to indicate with a button press whether or not they thought the name was a good “fit” for the face (a purely subjective decision to aid deep associative encoding (Sperling et al., 2003). Responses were collected using a fiber-optic response box held in the right hand. Visual stimuli were presented using MacStim 2.5 software (WhiteAnt Occasional Publishing, West Melbourne, Australia). Images were projected through a collimating lens onto a screen attached to the head coil.
After the scanning session, all subjects underwent a forced-choice associative recognition memory test in which the 84 Novel faces seen during scanning were presented on a computer screen. Each face was shown with two names printed underneath: the correct name that was paired with the face during scanning and one incorrect name that was previously paired with a different face. The subjects were instructed to indicate the correct name with a button press, and then to indicate whether they had high or low confidence in their name choice (to minimize effects of correct guessing). Successful memory encoding was operationalized as a “high confidence hit” (HCH), for those face-name stimuli for which the subject chose the name that was correctly associated with that face during the scan, and indicated they had high confidence in their decision during the post-scan test.
fMRI data analysis
Functional MRI data were preprocessed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) for Matlab (The Mathworks, Inc, Natick, Massachusetts, USA). Functional data were realigned using INRIAlign, a motion correction algorithm unbiased by local signal changes (Freire and Mangin, 2001). The data were then normalized to the standard SPM2 EPI template and resampled into 3 mm isotropic resolution in MNI space. Data were then smoothed using an 8 mm Gaussian kernel. A high pass filter of 260 sec was used to remove low frequency signal (e.g. drifts across entire fMRI run). The data were modeled by convolving a canonical haemodynamic response function with the onsets from successfully encoded face-name pairs.
Cross-modality data analyses
The anatomic overlap between the regions showing the greatest PiB retention and the regions showing the greatest differences in fMRI activity between the PiB+ and PiB- subjects was determined using a surface-based conjunction analysis in template space. In order to create an unbiased (Vul et al., in press), functionally defined PPC ROI to sample both PiB (DVR values) and fMRI (% MR signal change) in the older subjects, a 6mm spherical ROI (centered on MNI -9, -60, 39) was created based on the overlap between Figure 2A (PiB map peak) and Figure 2B (YS peak deactivation). The PiB DVR value extracted from the functionally defined PPC ROI was highly correlated with the anatomically defined PPC DVR values (R=.94). Regression analyses and ANOVA were performed on the PiB and the fMRI values extracted from the functionally defined PPC ROI.
To further explore this relationship using an independently determined ROI and to determine if PiB + non-demented subjects demonstrated alterations similar to those we have observed in AD patients, we utilized an a priori region of interest (ROI) determined from a separate group analysis of 15 elderly controls (EC) and 15 mild AD (CDR 1) previously reported in other papers (Celone et al., 2006; Pihlajamaki et al., 2008). A spherical ROI was defined around the peak location within the PPC coordinates (MNI coordinates 0, -75, 33) for which AD subjects demonstrated significantly greater fMRI activity compared to normal older controls.
We also investigated the data set more extensively using an exploratory approach across the whole brain. To identify brain regions showing task-induced deactivation during the encoding task, SPM2 whole brain maps of Fixation > High-Confidence Hits (HCH) were generated for each subject, using a one-sample T-test thresholded at p<0.001. Two additional regressors were modeled to account for age and performance related variance in fMRI activity. The resulting maps were regressed against the mean PPC PiB DVR values with a statistical threshold at p<0.005 to allow exploratory analyses of additional brain regions that demonstrated a sub-threshold relationship between amyloid burden and fMRI activity. Finally, we performed an exploratory vertex to vertex surface-based analysis to investigate the relationship of local amyloid deposition to fMRI activity within subject. As this approach has limited statistical power compared to an ROI approach, due to the interpolation across differing acquisition matrices and potential surface misregistration, and is limited to the grey matter voxels within the cortical ribbon, we utilized a more liberal threshold of p<0.01 (see Supplemental material online at www.cell.com/neuron/).
Supplementary Material
Acknowledgments
We thank Mary Foley, Larry White, and the Athinoula A. Martinos Center MRI Core for assistance with MRI imaging. The Massachusetts General Hospital Molecular Imaging PET Core provided assistance with PiB PET amyloid imaging. Bill Klunk and Chet Mathis from the University of Pittsburgh generously provided expertise in PiB imaging and analyses. We thank the Massaschusetts Alzheimer's Disease Research Center (ADRC) and Sarah Rastegar for assistance with subject recruitment. We thank Meghan Frey, Elisha Eng, and Lauren Olson for assistance with neuropsychological testing. We thank Bill Klunk, Brad Dickerson, Akram Bakkour, Deborah Blacker, Ali Atri, and Patrizia Vannini for assistance with analyses and insightful comments. This work was supported by the National Institute on Aging R01AG027435 (RAS and KAJ), the Massachusetts Alzheimer's Disease Research Center P50AG005134 (RAS, KAJ, BTH, DJS), an Anonymous Medical Foundation (RAS), the Howard Hughes Medical Institute (RLB), and the Alzheimer's Association (RAS).
Footnotes
Disclosures: Dr. Keith Johnson has consulted for GE Healthcare, who holds the commercial licensing and distribution rights for PiB PET imaging.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer's disease. Acta Neuropathol. 2005;110:393–401. doi: 10.1007/s00401-005-1060-2. [DOI] [PubMed] [Google Scholar]
- Bennett D, Schneider J, Arvanitakis Z, Kelly J, Aggarwal N, Shah R, Wilson R. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen F, Liu H, Hedden T, Andrews-Hanna J, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. Journal of Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat D, Greve D, Chua E, Rand-Giovannetti E, Rentz D, Bertram L, Mullin K, Tanzi R, Blacker D, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The Relationship between Aging, Performance, and the Neural Correlates of Successful Memory Encoding. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Marzloff K, Wenniger JJ, Dawson TM, Bredt DS, Snyder SH. Relative sparing of nitric oxide synthase-containing neurons in the hippocampal formation in Alzheimer's disease. Ann Neurol. 1992;32:818–820. doi: 10.1002/ana.410320618. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer's disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Albert MS. Perfusion abnormalities in prodromal AD. Neurobiol Aging. 2000;21:289–292. doi: 10.1016/s0197-4580(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang Y, Huang GF, Debnath ML, Klunk WE. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008a;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008b;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated {beta}-amyloid deposition in elderly subjects. Brain. 2008 doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46:1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS ONE. 2007;2:e1104. doi: 10.1371/journal.pone.0001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Depeau KM, Blacker D, Sperling RA. Impaired Medial Temporal Repetition Suppression is Related to Failure of Parietal Deactivation in Alzheimer Disease. Am J Geriatr Psychiatry. 2008;16:283–292. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, O'Keefe K, Bertram L, Tanzi R, Dickerson B, Blacker D, Albert M, Sperling R. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord. doi: 10.1097/WAD.0b013e3181a785c9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E varepsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks:Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sojkova J, Beason-Held L, Zhou Y, An Y, Kraut MA, Ye W, Ferrucci L, Mathis CA, Klunk WE, Wong DF, Resnick SM. Longitudinal cerebral blood flow and amyloid deposition: an emerging pattern? J Nucl Med. 2008;49:1465–1471. doi: 10.2967/jnumed.108.051946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. doi: 10.1111/j.1745-6924.2009.01125.x. in press. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.