Abstract
Objective
Interleukin-2 (IL-2) is a cytokine with multiple effects on lymphocytes including induction of CD4+ T cell proliferation. IL-2 administration has been shown to increase CD4 counts in HIV-infected people receiving antiretroviral therapy. GB virus C (GBV-C) is an apparently non-pathogenic flavivirus that replicates in CD4+ T cells and inhibits HIV replication in vitro by mechanisms including downregulation of HIV entry coreceptors (CCR5 and CXCR4) and induction of chemokines (RANTES, MIP-1α, MIP-1β, and SDF-1). GBV-C replication is significantly inhibited in vitro by activation of primary CD4+ cell cultures with IL-2 and phytohemagglutinin. We sought to determine if there is an interaction between GBV-C and IL-2 in vivo.
Methods
GBV-C viremia status was characterized in 92 HIV-infected subjects participating in a randomized trial of IL-2 and antiretroviral therapy (AIDS Clinical Trials Group Study [ACTG] 328). Changes in CD4 cell counts and HIV RNA levels in subjects assigned IL-2 were compared with those in subjects assigned antiretroviral therapy alone.
Results
Subjects lacking GBV-C viremia had a significantly greater rise in CD4 count with IL-2, compared to GBV-C viremic subjects (by 511 cells/mm3 at week 84; interaction p=0.02): GBV-C viremic subjects assigned IL-2 did not demonstrate a significant increase in CD4 count compared to subjects not assigned to receive IL-2 (95% CI for difference -255 to 397 cells/mm3).
Conclusions
GBV-C viremia was associated with a block in CD4 cell expansion following IL-2 therapy in the ACTG 328 study, and GBV-C status may be an important factor in IL-2 treatment response.
Keywords: Interleukin 2, HIV, GB virus C, GBV-C, CD4 count
INTRODUCTION
Interleukin-2 (IL-2) is a cytokine that is a key regulatory molecule in T cell biology. It is critical for proper regulation of T-cell proliferation, is a key contributor to the generation and function of CD4+CD25+ regulatory T cells, and mediates a process termed "activation-induced cell death" or AICD [1, 2]. IL-2 also exerts pleiotropic effects on other cells in the immune system including B lymphocytes, natural killer (NK) cells, monocytes, macrophages, and dendritic cells (reviewed in [3]).
The idea of enhancing immune function in HIV-infected people by administration of IL-2 is attractive, although this approach was initially controversial since IL-2 enhances HIV replication in primary CD4+ T lymphocytes [1, 4]. These concerns have been reduced due to the effectiveness of combination antiretroviral therapy (ART), and IL-2 has been administered to many HIV-infected people in an attempt to increase CD4+ T cell number, decrease HIV-induced cytokine dysregulation, enhance innate and adaptive immune responses to opportunistic infections, and to decrease the latent HIV-infected pool of cells as a result of activating resting CD4+ T cells [5, 6].
GB virus C (GBV-C, also known as hepatitis G virus) is a lymphotropic member of the family Flaviviridae that was discovered in 1995 (reviewed in [7, 8]). The virus is transmitted by sexual and blood exposure, and is quite common in humans with viremia detected in approximately 1% to 3% of healthy U.S. blood donors and approximately 10% of donors demonstrating antibodies that indicate prior GBV-C infection (reviewed in [9]). While GBV-C viremia is typically cleared within a few years in most immune competent individuals [9, 10], it persists longer in most HIV-infected people, and 80% of subjects with HIV-GBV-C co-infection maintained GBV-C viremia for more than five years in one longitudinal study [11]. Epidemiological studies have not identified an association between GBV-C infection and any known human disease. In contrast, several studies prior to the advent of effective ART found a statistically significant association between persistent GBV-C infection and prolonged survival among HIV-infected individuals (reviewed in [12–14]). Supporting a potential role for GBV-C viremia in this association, serum GBV-C RNA levels are inversely related to HIV RNA levels in vivo [15, 16]; and co-infection of human CD4+ T cells with GBV-C and HIV results in inhibition of HIV replication in vitro [17–19].
GBV-C replicates in primary human T and B lymphocytes in vitro [20–22], and incubation of infected lymphocyte cultures with IL-2 and phytohaemogglutinin resulted in decreased GBV-C replication in vitro [23]. To examine the possibility that GBV-C might influence the IL-2 response of HIV-infected individuals, the GBV-C viremia status and change in CD4 following assignment to IL-2 therapy or no IL-2 therapy in subjects in the AIDS Clinical Trials Group 328 study [5] were characterized.
METHODS
The ACTG 328 study, an open label, randomized trial of intermittent recombinant human interleukin-2 (IL-2) by intravenous (IV) or subcutaneous (SC) administration in subjects with HIV infection receiving combination antiretroviral therapy (ART) compared to ART alone, has been described previously [5]. Briefly, participants in the ACTG 328 study were HIV-infected individuals with baseline CD4 cell counts between 50 and 350 cells/mm3 on one occasion within 30 days prior to study entry, and who had never received protease-inhibitor or IL-2 therapy. Subjects did not have active HIV-related complications at the time of enrollment and all subjects initiated one of three ART regimens (Indinavir combined with either ZDV+3TC, ZDV+ddI, or d4T+ddI, of which at least one nucleoside reverse transcriptase inhibitor was new for the subject). One hundred fifty nine subjects whose HIV RNA concentration decreased to ≤ 5,000 copies/mm3 after 11 weeks of therapy were randomly assigned at 12 weeks to receive no IL-2 (n=52), 9 million international units (MIU) IV IL-2 daily for 5 days every 8 weeks for 72 weeks (n=53), or 7.5 MIU IL-2 SC twice daily for 5 days every 8 weeks for 72 weeks (n=54). Per protocol, thirty subjects in the IV IL-2 group switched to the SC IL-2 regimen after 3 or 6 cycles if their CD4 counts increased by 25% or ≥ 100 cells/mm3 compared to their baseline values [5]. Subjects were monitored and the increase in CD4 count at weeks 60 (primary endpoint) and 84 (secondary endpoint) of over 50% of week 12 CD4 count were assessed as well as change in HIV RNA at weeks 60 and 84.
Available samples from ACTG 328 at the time of randomization (week 12) were analyzed for GBV-C RNA by real time RT-PCR (limit of detection 105 copies/ml) [24] by laboratory personnel who were not aware of any clinical data. GBV-C antibody testing was not done, as the commercial assay is no longer available (Georg Hess, Roche Diagnostics, personal communication). Change in CD4 cell count and HIV RNA following IL-2 therapy (week 12 to week 60, and week 12 to week 84), was analyzed by treatment group and 12 week GBV-C classification. The proportion of subjects in each group with an increase in CD4 count at weeks 60 and 84 of over 50% of week 12 count was also analyzed. In addition, the proportion of subjects at weeks 60 and 84 with an HIV RNA increase of greater than or equal to 0.7 log10 compared to week 12 was analyzed by logistic regression (a secondary endpoint). To address whether the subset of subjects with samples for GBV-C testing responded to IL-2 therapy differently than the entire study population, the proportion of subjects with a CD4 response of 50% or more at week 60 was compared, by treatment assignment, between those with samples tested for GBV-C and those without. The same analysis was performed for the week 84 responses. All analyses were performed in the statistical environment R [25].
RESULTS
Administration of IL-2 (IV and/or SC) in combination with ART was shown to significantly increase CD4 cell counts in the ACTG 328 study population when compared to subjects who received ART without IL-2, and there was no change in plasma HIV RNA [5]. Fifty eight percent of the subjects in ACTG study 328 (92 of 159) had samples available from the week 12 visit for GBV-C RNA testing. These 92 subjects did not have significant differences in baseline characteristics or changes in CD4 and HIV RNA responses following randomization observed when compared to the total study cohort (data not shown). GBV-C viremia was detected in 41% of subjects (38/92), similar to that observed in other studies of HIV-infected people [11].
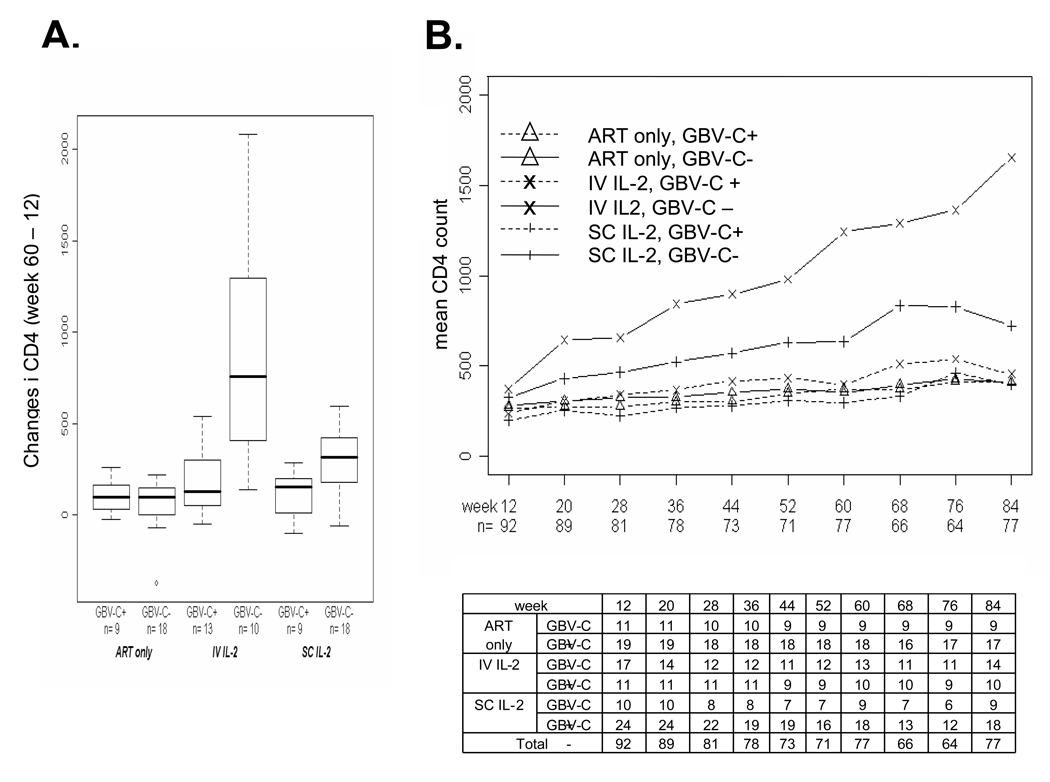
Subjects without GBV-C viremia who were assigned to receive IL-2 had greater increases in CD4 counts than those with GBV-C viremia at week 60 (Figure 1A), by 679 and 193 cells/mm3 for those assigned to receive IV and SC IL2 respectively. These differences in change in CD4 based on GBV-C viremia status persisted throughout the 84 weeks of the study (Fig. 1B). The interaction was significant for subjects receiving IL-2 at weeks 60 and 84 (p=0.01, 95% confidence interval (CI) for difference 98 to 692 cells/mm3 [Table 1]; week 84 interaction p=0.02, 95% CI for difference 97 to 926; Table 1). Subjects who did not have GBV-C viremia demonstrated a significant increase in CD4 counts with assignment of IL-2 (Table 1, 95% CI 257 to 617, p<105) while subjects with GBV-C viremia did not demonstrate a significant increase in CD4 counts with assignment to IL-2 (Table 1, 95% CI −194 to 278).
Figure 1.
Effect of GB virus C (GBV-C) on change in CD4 count among study participants. Subjects received twelve weeks of combination antiretroviral therapy (ART) prior to randomization to receive no interleukin 2 (IL-2) (ART only) or intravenous (IV) IL-2 or subcutaneous (SC) IL-2. The change in CD4 following assignment into treatment groups at 48 weeks after IL-2 randomization (week 60 – week 12) are shown in panel A. The average CD4 count over time for all 92 subjects for whom a sample was available for GBV-C viremia testing is shown (panel B). The table indicates the number of subjects from this substudy for whom CD4 data were available at each time point.
Table 1.
Interaction between GBV-C viremia and interleukin 2 (IL-2) assignment for CD4 change
| Treatment Group | IL-2 vs no IL-2 ‡ | IV IL-2 vs SC IL-2 | |||
|---|---|---|---|---|---|
| Week 60 – week 12 | IV IL-2 (n) | SC IL-2 (n) | No IL-2 (n) | ||
| GBV-C neg | *859 (10) | *299 (18) | *62 (18) | †437 (p<10−5, 257 to 617) | †560 (p<10−6, 362 to 758) |
| GBV-C pos | *180 (13) | *106 (9) | *108 (9) | †42 (p=0.73, −194 to 278) | †74 (p=0.50, −144 to 291) |
| GBV-C Neg vs Pos | p<10−7 | p=0.06 | P=0.71 | §†395 (p=0.01, 98 to 692) | §†486 (p=0.002, 193 to 781) |
| Week 84-week 12 | IV IL-2 (n) | SC IL-2 (n) | No IL-2 (n) | ||
| GBV-C neg | *1273 (10) | *395 (18) | *126 (17) | †582 (p<10−4, 327 to 837) | †878 (p<10−7,619 to 1137) |
| GBV-C pos | *226 (14) | *207 (9) | *148 (9) | †71 (p=0.67, −255 to 397) | †19 (p=0.89, −262 to 299) |
| GBV-C Neg vs Pos | p<10−9 | p=0.17 | P=0.90 | §†511 (p=0.02, 97 to 926) | §†860 (p<10−4, 478 to 1241) |
Change in CD4 count. IV = intravenous IL-2 = interleukin 2, SC = subcutaneous. GBV-C positive (negative) = GBV-C RNA detected (not detected) in plasma as described in the methods.
CD4 change from week 12 analyzed as a linear model.
the two IL-2 treatment groups (IV and SC) are pooled. For the IV vs SC comparison, three treatment groups were used and the contrast between IV and SQ estimated.
Interaction tests are based on likelihood ratio tests and are the interaction of the difference between treatment groups with GBV-C viremia.
Among those without GBV-C viremia, CD4 responses were significantly greater among those assigned to receive IV IL-2 when compared to those assigned to receive SC IL-2 at both week 60 and week 84 (Table 1, p<10−6 and p<10−7 respectively). In contrast, subjects with GBV-C viremia assigned to IV IL-2 did not demonstrate a significant increase over those assigned SC (p=0.50 at week 60, p=0.89 at week 84 respectively). The comparison of CD4 response in the IV versus SC comparison for those GBV-C non-viremic to those non-viremic was significant (interaction p=0.002 at week 60 and p<10−4 at week 84).
There were no differences in HIV RNA levels between GBV-C viremic and non viremic patients, irrespective of IL-2 treatment group, and GBV-C viral load did not correlate with change in CD4 counts (data not shown). IL-2 administration was not associated with significant differences in GBV-C viral load at any time point, nor did CD4 count changes correlate with GBV-C viral load (data not shown).
DISCUSSION
In this substudy of ACTG 328, the GBV-C viremia status was characterized at the time subjects were randomly assigned to IL-2 or no IL-2 in combination with ART. Among individuals with GBV-C viremia, there was no significant difference in CD4 count increases in subjects assigned to receive IL-2 compared to subjects who were not assigned IL-2. Thus, IL-2 failed to stimulate CD4 cell expansion in people with GBV-C viremia. In contrast, CD4 counts increased significantly among those without GBV-C viremia assigned to IL-2 (437 cells/mm3 greater than those not assigned IL-2 at week 60). Treatment interaction was significant based on GBV-C viremia status at week 60 (p=0.01) and week 84 (p=0.02), providing strong statistical credibility to the interaction of GBV-C viremia with IL-2 [26, 27].
IL-2 upregulates CCR5 and CXCR4 expression on T cells [28, 29] while GBV-C interactions with CD4 cells results in decreased CCR5 surface density [18, 19, 30, 31], suggesting that GBV-C dampens T cell activation. Furthermore, addition of IL-2 and phytohemagglutinin (PHA) to peripheral blood mononuclear cells (PBMCs) obtained from HIV-GBV-C co-infected individuals resulted in significant reduction in GBV-C replication [23]. Taken together with the results observed in the ACTG cohort, these data suggest a specific interaction between GBV-C and IL-2-mediated proliferation and potentially activation, both of which would have a beneficial effect on HIV disease (32).
Limitations of this analysis include the fact that samples were not available for GBV-C testing in 42% of the randomized subjects in ACTG 328, and that this study was designed retrospectively. Despite these limitations, significant differences in CD4 cell count responses were observed between GBV-C positive and GBV-C negative subjects following both 60 and 84 weeks of therapy. In addition, subjects with samples studied for GBV-C were not significantly different from the entire ACTG 328 cohort either at baseline or in CD4 and HIV change following IL-2 within the treatment groups (data not shown). Thus, further investigations of GBV-C status in randomized studies of IL-2 administration in HIV-infected people are warranted. If the results observed in this study are validated, the measurement of GBV-C viremia status would be important prior to administration of IL-2 therapy, particularly if the goal is to increase CD4 cell number.
In this study GBV-C viremia was associated with a block in IL-2-related T cell proliferation. This, in combination with the in vitro evidence that IL-2 decreases GBV-C replication suggests that there is an interaction between GBV-C and IL-2 signaling pathways. Further study of mechanisms by which GBV-C influences IL-2 response are warranted.
ACKNOWLEDGMENTS
This work was supported in part by Merit Review Grants from the Veterans Administration (JTS and JX), and by an NIH RO1 grant (AI-58740, JTS), ACTU grants, (AI-69424, AI-27660, RM and JF). We are grateful to Rebecca Gelman PhD and Deborah Wang Cheng MS for providing the ACTG data and to Suhong Zhang PhD for an analysis of an earlier data set, and Jennifer Nowack for assistance with specimens. Supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Nat Rev Immunol. 2003;4:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 5.Mitsuyasu R, Gelman R, Cherng DW, Landay A, Fahey J, Reichman R, et al. The Virologic, Immunologic, and Clinical Effects of Interleukin 2 With Potent Antiretroviral Therapy in Patients With Moderately Advanced Human Immunodeficiency Virus Infection: A Randomized Controlled Clinical Trial—AIDS Clinical Trials Group 328. Arch Intern Med. 2007;167:597–605. doi: 10.1001/archinte.167.6.597. [DOI] [PubMed] [Google Scholar]
- 6.Davey RT, Pertel PE, Benson C, Cassell DJ, Gazzard BG, Holodniy M, et al. Safety, tolerability, pharmacokinetics, and efficacy of an interleukin-2 agonist among HIV-infected patients receiving highly active antiretroviral therapy. J Interferon Cytokine Res. 2008;28:89–100. doi: 10.1089/jir.2007.0064. [DOI] [PubMed] [Google Scholar]
- 7.Linnen J, Wages J, Zhang-Keck Z-Y, Fry KE, Krawczynski KZ, Alter H, et al. Molecular cloning and disease association of hepatitis G virus: A transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 8.Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, et al. Isolation of novel virus-like sequences associated with human hepatitis. Nature Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23:137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 10.Alter HJ. G-pers creepers, where'd you get those papers? A reassessment of the literature on the hepatitis G virus. Transfusion. 1997;37(6):569–572. doi: 10.1046/j.1537-2995.1997.37697335149.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton JT, Chaloner K. GB Virus C and survival in HIV-positive people. AIDS. 2004;18:2343–2344. doi: 10.1097/00002030-200411190-00021. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GBV-C viremia on survival of HIV infected individuals: A meta-analysis. HIV Med. 2006;7:173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Polgreen PM, Xiang J, Chang Q, Stapleton JT. GB Virus type C/Hepatitis G virus: A nonpathogenic flavivirus associated with prolonged survival in HIV-infected individuals. Microbes and Infect. 2003;5:1255–1261. doi: 10.1016/j.micinf.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 16.Bjorkman P, Flamholc L, Molnegren V, Marshall A, Guner N, Widell A. Enhanced and resumed GB virus C replication in HIV-1-infected individuals receiving HAART. AIDS. 2007;21:1641–1643. doi: 10.1097/QAD.0b013e32823bc9b7. [DOI] [PubMed] [Google Scholar]
- 17.Xiang J, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, et al. Effect of coinfection with GB Virus C (Hepatitis G virus) on survival among patients with HIV infection. N Engl J Med. 2001;345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 18.Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1 α, MIP-1 β, and SDF-1. Lancet. 2004;363:2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Knauer O, Donhauser N, Eichenmuller M, Helm M, Fleckenstein B, et al. Inhibition of HIV strains by GB Virus C in cell culture can be mediated by CD4 and CD8 T-lymphocyte derived soluble factors. AIDS. 2005;19 doi: 10.1097/01.aids.0000180097.50393.df. 12567-1272. [DOI] [PubMed] [Google Scholar]
- 20.Fogeda M, Navas S, Martin J, Casqueiro M, Rodriguez E, Arocena C, et al. In vitro infection of human peripheral blood mononuclear cells by GB virus C/Hepatitis G virus. J Virol. 1999;73:4052–4061. doi: 10.1128/jvi.73.5.4052-4061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang J, Wunschmann S, Schmidt WN, Shao J, Stapleton JT. Full-length GB Virus C (Hepatitis G Virus) RNA transcripts are infectious in primary CD4-Positive T cells. J Virol. 2000;74:9125–9133. doi: 10.1128/jvi.74.19.9125-9133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 23.George SL, Xiang J, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology. 2003;316:191–201. doi: 10.1016/s0042-6822(03)00585-3. [DOI] [PubMed] [Google Scholar]
- 24.Souza IE, Allen JB, Xiang J, Klinzman D, Diaz R, Zhang S, et al. Optimal testing for GB Virus C viremia: Effect of primer selection on estimates of GBV-C prevalence and response to antiretroviral therapy. J Clin Microbiol. 2006;44:3105–3113. doi: 10.1128/JCM.02663-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- 26.Peto R. Statistical aspects of cancer trials. In: Price P, Sikoa K, editors. Treatment of Cancer. London: Chapman and Hall; 1995. pp. 1039–1043. [Google Scholar]
- 27.Follman D. Subgroups and interactions. In: Geller N, editor. Advances in Clinical Trial Biostatistics. New York: Marcel Dekker, Inc.; 2004. pp. 121–139. [Google Scholar]
- 28.Yang Y-F, Tomura M, Iwasaki M, Mukai T, Gao P, Ono S, et al. IL-12 as well as IL-2 upregulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. 2001:116–125. doi: 10.1023/a:1011059906777. [DOI] [PubMed] [Google Scholar]
- 29.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, et al. Regulation of CC chemokine receptor 5 in Hepatitis G virus infection. AIDS. 2003;17:1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- 31.Maidana Giret MT, Silva TM, Levi JE, Bassichetto KC, Ana N, Sabino E, et al. GBV-C infection is associated with less T cell activation in recently HIV-infected subjects and is independent of HIV-1 viral load. 4th IAS Conf HIV Pathog Treat 2007; 2007. Abstract No. MOAA105. [Google Scholar]
- 32.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–177. [PubMed] [Google Scholar]