Abstract
Inhibition of soluble epoxide hydrolase (sEH) has been shown to be renal protective in rat models of salt-sensitive hypertension. Here, we hypothesize that targeted disruption of the sEH gene (Ephx2) prevents both renal inflammation and injury in deoxycorticosterone acetate plus high salt (DOCA-salt) hypertensive mice. Mean arterial blood pressure (MAP) increased significantly in the DOCA-salt groups, and MAP was lower in Ephx2−/− DOCA-salt (129 ± 3 mmHg) compared with wild-type (WT) DOCA-salt (145 ± 2 mmHg) mice. Following 21 days of treatment, WT DOCA-salt urinary MCP-1 excretion increased from control and was attenuated in the Ephx2−/− DOCA-salt group. Macrophage infiltration was reduced in Ephx2−/− DOCA-salt compared with WT DOCA-salt mice. Albuminuria increased in WT DOCA-salt (278 ± 55 μg/day) compared with control (17 ± 1 μg/day) and was blunted in the Ephx2−/− DOCA-salt mice (97 ± 23 μg/day). Glomerular nephrin expression demonstrated an inverse relationship with albuminuria. Nephrin immunofluorescence was greater in the Ephx2−/− DOCA-salt group (3.4 ± 0.3 RFU) compared with WT DOCA-salt group (1.1 ± 0.07 RFU). Reduction in renal inflammation and injury was also seen in WT DOCA-salt mice treated with a sEH inhibitor {trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid; tAUCB}, demonstrating that the C-terminal hydrolase domain of the sEH enzyme is responsible for renal protection with DOCA-salt hypertension. These data demonstrate that Ephx2 gene deletion decreases blood pressure, attenuates renal inflammation, and ameliorates glomerular injury in DOCA-salt hypertension.
Keywords: deoxycorticosterone acetate, blood pressure, Ephx2, high salt, albuminuria, NF-κB, glomerular injury
epoxyeicosatrienoic acids (EETs), the products of cytochrome P-450 epoxygenase metabolism of arachidonic acid, have been shown to cause vasodilation, possess anti-inflammatory properties, prevent migration in vascular smooth muscle cells as well as prevent platelet aggregation (2, 11, 16, 39, 42). EETs cause vasodilation by activating large-conductance Ca2+-activated K+ channels in smooth muscle that leads to hyperpolarization and subsequent vasodilation (3, 10, 18). In addition, epoxygenase metabolites prevent the activation of NF-κB that in turn leads to the activation of downstream inflammatory cytokines (9, 38). EETs, however, are quickly degraded by soluble epoxide hydrolase (sEH) to their less active diols, resulting in dimished EET cardioprotective actions (16, 48, 50).
Past studies have demonstrated that pharmacological inhibition of sEH prevents EET degradation and enhances the renal and cardioprotective effects of these metabolites (11, 16, 17). Antihypertensive and renal-protective therapeutic actions for sEH inhibitors have been repeatedly demonstrated in angiotensin-dependent hypertension (19, 20, 21, 52). For instance, Zhao et al. (52) determined that hypertensive rats treated with an sEH inhibitor were protected against renal injury as seen by reduced collagen deposition and albuminuria. In addition to reduced renal injury with sEH inhibition, Cyp2c23, the key enzyme in the generation of EETs, has been shown to be upregulated when inflammatory pathways were inhibited (7, 8). For example, another study demonstrated that chemokine receptor 2b (CCR2b) inhibition enhanced renal Cyp2c23 expression in angiotensin-induced salt-sensitive hypertension (7). sEH inhibition has also been examined in non-angiotensin II models of hypertension and has been shown to lower blood pressure (29, 50). For instance, Loch et al. (29) demonstrated that sEH inhibition lowered blood pressure in rats treated with deoxycorticosterone acetate plus high salt (DOCA-salt).
Mice that are sEH null (Ephx2−/−) have been generated, and studies have began to examine cardiovascular function and blood pressure regulation (30, 35, 45, 46, 51). Specifically, Ephx2−/− mice have been shown to have lower blood pressure as well as are protected against postischemic injury and ventricular dysfunction (35, 45, 46). The Ephx2 gene contains two domains: a hydrolase and a phosphatase domain (37). There are currently no known selective inhibitors of the N-terminal phosphatase domain that are active in vivo, and the sEH inhibitors inhibit the epoxide hydrolase activity of the C-terminal domain without affecting the phosphatase activity of the N-terminal domain (22, 33, 34). Definitive evidence that the phosphatase domain does not contribute to blood pressure regulation and renal damage in the Ephx2−/− mice is lacking. Accordingly, the hypothesis of the current study was that Ephx2 gene deletion reduces renal injury and inflammation with salt-sensitive hypertension and that this protection resides in the deficiency in the C-terminal hydrolase domain.
METHODS
Experimental hypertension groups.
All animal studies were performed in accordance with the Medical College of Georgia Animal Care and Use Committee. Wild-type (WT) C57BL/6J mice (Jackson Laboratory, Sacramento, CA) and homozygous Ephx2 gene-deleted mice (Ephx2−/−) from Jackson Laboratories that were backcrossed with C57BL/6J mice for 10 generations were utilized. Adult WT and Ephx2−/− male mice weighing ∼25 g were randomly assigned into four groups; control, high salt (1% NaCl drinking water), DOCA alone, and DOCA plus high salt (DOCA-salt). Mice were anesthetized with 2% isoflurane with a continuous flow of 95% O2-5% CO2. DOCA pellets (50 mg) were subcutaneously implanted, and mice were assigned to the DOCA alone and DOCA-salt groups. High salt was administered via drinking water containing 1% NaCl. Following 21 days of DOCA and/or high salt, mice were placed into metabolic cages, and a 24-h urine sample was collected. Animals were then anesthetized with pentobarbital sodium, and tissues were collected. All tissue samples were immediately frozen in liquid nitrogen and then stored at −80°C. Blood pressure and heart rate were measured using radiotelemetry. Mice were implanted with telemetry catheters as described previously (27).
Renal injury.
An index of renal injury measured in the current study was albuminura and was determined with an ELISA kit purchased from Exocell (Philadelphia, PA). Renal injury was further assessed histologically. Kidney sections embedded and frozen in Optimal Cutting Temperature (Tissue-Tek, Hatfield, PA) medium were sliced into 4-μm sections and stained with Masson's trichrome for collagen III deposition according to the manufacturer's recommended protocols (Richard Allan Scientific, Kalamazoo, MI). Ten images were taken per mouse, and values were averaged. To quantify the Masson's trichrome staining, 10 random images each from all groups were assigned random numbers and scored by a blinded observer on a scale of 0 to 10 for collagen deposition. A score between 0 and 2 corresponded to low Masson's trichome staining intensity, 3–6 corresponded to intermediate staining, and 7–10 corresponded to high to very high staining intensity. Nephrin, a protein involved in the maintenance of the glomerular slit diaphragm, was also examined via immunofluorescence. Five-micrometer frozen kidney sections were incubated overnight at room temperature with goat anti-human nephrin primary antibody 1:50 (sc-19000, Santa Cruz Biotechnology) followed by rabbit anti-goat Cy-3 fluorescent-tagged secondary antibody 1:400 for 1 h (Zymed). Slides were mounted using Prolong Gold anti-fade (Invitrogen). Desmin immunofluorescence was carried out using a 1:50 dilution of mouse anti-human desmin primary antibody (Dako, Carpinteria, CA) followed by a 1:800 dilution of FITC-tagged goat anti-mouse secondary antibody (Zymed). Photographs were taken at ×400.
Renal inflammation.
We determined whether mRNA expression of proinflammatory cytokines is altered in the DOCA-salt groups. Total RNA was extracted from 20 mg of kidney cortex using an RNeasy Plus Mini-kit (Qiagen) according to the manufacturer's protocol. RNA concentrations were determined using absorbance at 260 nm. Reverse transcription was performed on 2 μg of RNA from each sample using an RT2 PCR Array First Strand Kit (SuperArray Bioscience). Each cDNA synthesis reaction was diluted before being added to an RT2 Real-Time SYBR Green PCR Mastermix (SuperArray Bioscience), which was aliquoted onto a 96-well PCR Array plate, one sample per plate; each well contained a primer pair for a different gene or control. Thermal cycling and real-time detection were done with a Bio-Rad iCycler (Bio-Rad Laboratories, Hercules, CA): 1) 95°C for 10 min and 2) 95°C for 15 s followed by 60°C for 60 s (repeated 40 times). Melt-curve analysis was completed after each PCR reaction. Threshold cycle (Ct) values were normalized to a set of housekeeping genes to get a ΔCt value, and fold-changes were calculated using the equation (2−ΔCt test)·(2−ΔCt control)−1. Student's t-test was used for statistical analysis, and changes greater than ±2 and P < 0.05 were considered significant. Superarray results were confirmed by real-time PCR on three genes present on the arrays, picked at random.
Next, we examined macrophage infiltration into the kidney utilizing ED-1 immunohistochemistry. Five-micrometer frozen kidney sections were cut and incubated overnight at room temperature with mouse anti-rat CD-68 primary antibody (1:100, Serotec, Raleigh, NC) followed by the secondary antibody goat anti-mouse IgG HRP (1:50, Serotec) for 1 h at room temperature. Slides were incubated with AEC substrate chromogen (Dako) for 20 min, rinsed, and counterstained with Mayers hematoxylin for 30 s. Photographs were taken at ×400 magnification, and CD-68 positive cells were counted in a blinded fashion. The number of positive cells was calculated per square millimeter. To further assess cytokines and activation of inflammatory pathways, we measured MCP-1 and NF-κB activity. Urinary monocyte chemmoattractant protein-1 (MCP-1) excretion was measured using an ELISA purchased from BD Bioscience (MCP-1 ELISA kit, Minneapolis, MN). To assess activation of proinflammatory cytokines, an NF-κB activity assay was performed on kidney samples. Renal NF-κB activity was measured using a TransAM NFκBp65 activation assay purchased from Active Motif (Carlsbad, CA) and performed according to the manufacturer's protocol.
Inhibitor studies.
To determine that renal protection resides within the hydrolase domain of the Ephx2 gene, sEH inhibitor studies were performed in WT and Ephx2−/− DOCA-salt mice. These groups of mice were given the sEH inhibitor tAUCB at a dose of 10 mg/day in their food for 21 days. Blood pressure, albuminuria, and urinary MCP-1 excretion were examined following the 21-day treatment period using the methods explained previously.
Statistical analysis.
All data are presented as means ± SE. For analysis of mean arterial pressure (MAP), statistical significance was determined using a two-way ANOVA followed by a Bonferroni post hoc test to identify individual differences between specific groups and treatment time. A P value of <0.05 was considered as statistically significant.
RESULTS
Blood pressure.
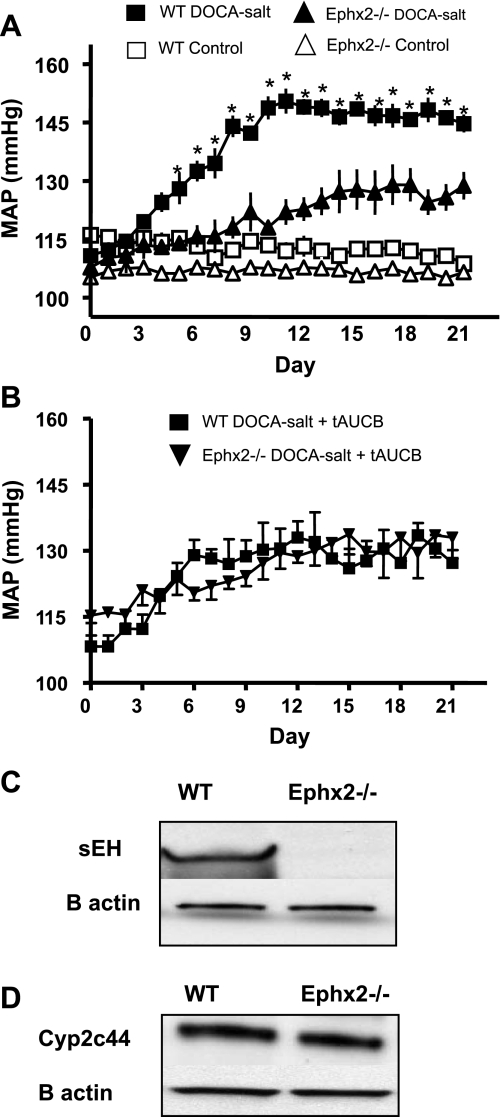
MAP increased significantly from baseline in the WT DOCA-salt group at day 6 and remained increased for the remainder of the treatment period (Fig. 1A, *P < 0.05 vs. WT control). MAP also increased significantly in the Ephx2−/− DOCA-salt group compared with control at day 8 and remained elevated during the treatment period (+P < 0.05 vs. Ephx2−/− control). A separate set of WT and Ephx2−/− DOCA-salt mice were treated with tAUCB, a sEH inhibitor that selectively inhibits the hydrolase domain of the enzyme. MAP in the WT DOCA-salt group with tAUCB was significantly lower than WT DOCA-salt, and no statistical change was seen compared with Ephx2−/− DOCA-salt or Ephx2−/− DOCA-salt plus tAUCB (Fig. 1B). Heart rate decreased significantly in the WT DOCA-salt compared with WT control (470 ± 12 vs. 576 ± 9 beats/min, P < 0.05) and also decreased in the Ephx2−/− DOCA-salt group compared with control (507 ± 15 vs. 572 ± 8 beats/min, P < 0.05). However, heart rate was significantly higher in the Ephx2−/− DOCA-salt group compared with WT DOCA-salt (P < 0.05).
Fig. 1.
Mean arterial pressure (MAP) increased significantly in both wild-type (WT) and Ephx2−/− DOCA-salt groups compared with their respective controls (n = 6/group *P < 0.05; A). MAP in the Ephx2−/− DOCA-salt group was significantly lower than WT DOCA-salt. MAP measurements were also made in WT and Ephx2−/− DOCA-salt groups treated with the soluble epoxide hydrolase (sEH) inhibitor tAUCB (n = 6/group; B). MAP was be significantly lower in WT and Ephx2−/− DOCA-salt+tAUCB groups compared with WT DOCA-salt, and there is no difference in MAP between Ephx2−/− and WT DOCA-salt tAUCB-treated groups. sEH protein expression was absent from the Ephx2−/− mice (C), and Cyp2c44 expression remained unchanged in both WT and Epx2−/− kidney homogenates (D).
To ensure that Ephx2−/− mice did not express sEH, Western blot analysis for sEH protein was performed on WT as well as Ephx2−/− kidney homogenates. Ephx2−/− mice did not express sEH protein (Fig. 1C). Since the Cyp2c44 protein is the major enzyme responsible for EET generation in the kidney of mice, Cyp2c44 expression was also examined in WT and Ephx2−/− mice kidneys, and expression remained similar in both groups (Fig. 1D).
Renal damage.
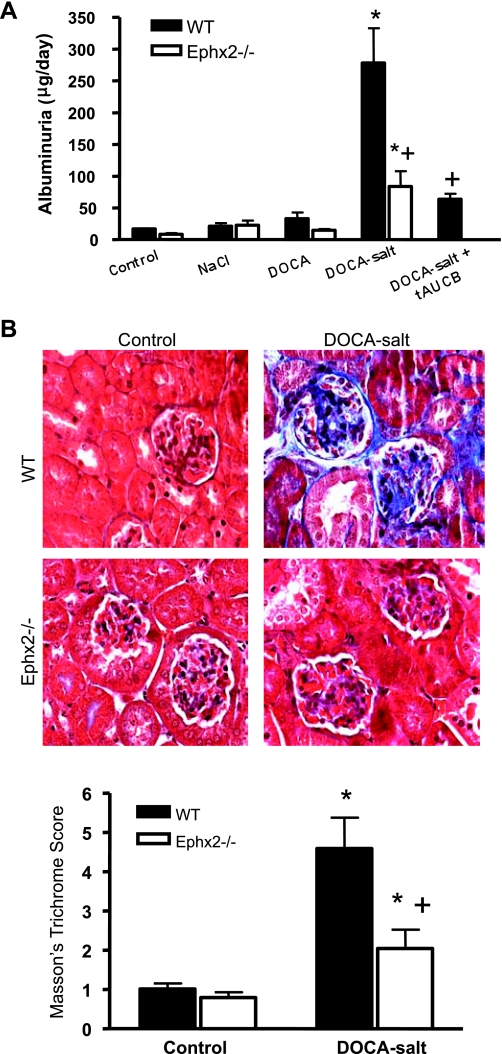
Albuminuria was measured to assess renal damage (Fig. 2A). Albuminuria increased significantly in the WT DOCA-salt compared with WT control (*P < 0.05), and levels were attenuated in the Ephx2−/− DOCA-salt group (+P < 0.05 vs. WT DOCA-salt). Albuminuria was also reduced in the WT DOCA-salt plus tAUCB group compared with WT DOCA-salt (+P < 0.05). Renal collagen deposition was assessed using Masson's trichrome staining (Fig. 2B). The intensity of Masson's trichrome stain increased significantly in the WT DOCA-salt group, while less was observed in the Ephx2−/− DOCA-salt group (P < 0.05, Fig. 2C).
Fig. 2.
Renal injury in DOCA-salt hypertension groups. Albuminuria increased significantly in WT DOCA-salt compared with WT control (n = 6/group; A). Ephx2−/− DOCA-salt as well as WT DOCA-salt plus tAUCB mice displayed less albuminuria compared with WT DOCA-salt mice (+P < 0.05; A). B: Masson's trichrome staining in kidney histological sections. C: Masson's trichrome score (n = 6/group). Collagen deposition as assessed by the intensity of Masson's trichrome stain was increased in WT DOCA-salt compared with control mice (*P < 0.05). Masson's trichrome score was reduced in Ephx2−/− DOCA-salt compared with WT DOCA-salt (+P < 0.05).
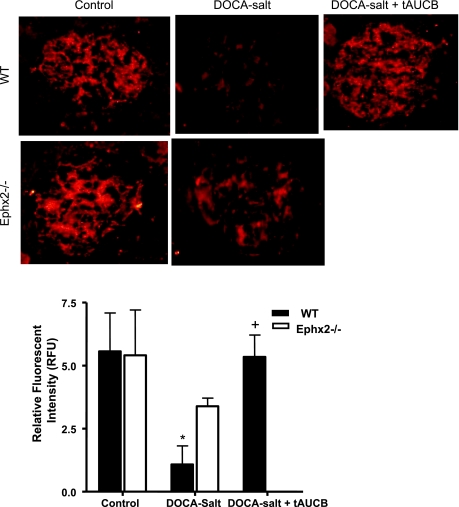
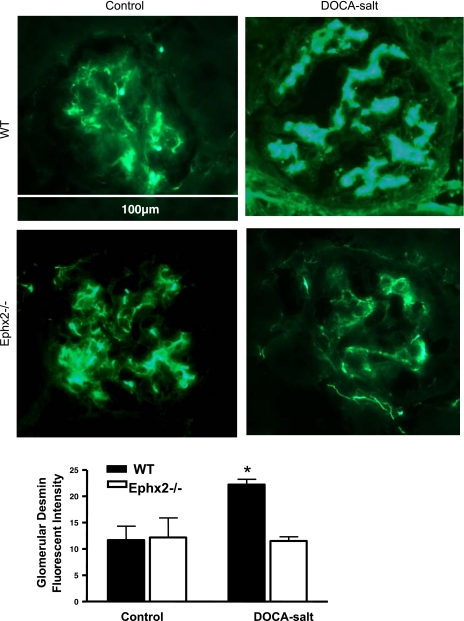
Glomerular nephrin immunofluorescence was also examined as an index of renal damage. Nephrin fluorescence decreased significantly in the WT DOCA-salt group compared with WT control, which indicates glomerular barrier injury (P < 0.05, Fig. 3). Fluorescent nephrin intensity did not change between Ephx2−/− control and Ephx2−/− DOCA-salt, but did increase in the WT DOCA-salt plus tAUCB group (P < 0.05 vs. WT DOCA-salt). Glomerular desmin has a reciprocal relationship with nephrin in that increased intensity is indicative of glomerular barrier injury (Fig. 4). Glomerular desmin intensity was increased in the WT DOCA-salt compared with WT control (P < 0.05). Fluorescent desmin intensity did not change between Ephx2−/− control and Ephx2−/− DOCA-salt mice.
Fig. 3.
Glomerular nephrin immunofluorescence (A) and levels of relative fluorescent intensity (B) between DOCA-salt groups (n = 6/group). Glomerular nephrin fluorescent intensity decreased significantly in WT DOCA-salt (*P < 0.05 vs. WT control), while no change was seen in Ephx2−/− DOCA-salt mice (+P < 0.05 vs. Ephx2−/− control). Intensity in the WT DOCA-salt plus tAUCB increased significantly compared with WT DOCA-salt mice (+P < 0.05).
Fig. 4.
Glomerular desmin expression in DOCA-salt hypertension groups. Glomerular desmin immunofluorescence (A) and levels of relative fluorescent intensity (B) between DOCA-salt groups (n = 6/group) are shown. Glomerular desmin fluorescent intensity increased significantly in WT DOCA-salt compared with WT control group (*P < 0.05), while no change was noted between Ephx2−/− DOCA-salt and control groups.
Renal inflammation.
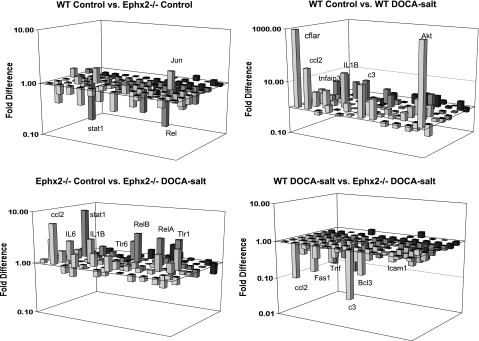
Superarray analysis of inflammatory gene expression was examined and is shown in Fig. 5. DOCA-salt hypertension caused an increase in the expression of 25 proinflammatory genes in WT mice (P < 0.05 vs. WT control, Fig. 5 and supplemental Table 1; all supplementary material for this article can be found on the journal web site). DOCA-salt hypertension also caused a significant increase in the expression of 13 proinflammatory genes in Ephx2−/− (P < 0.05 vs. Ephx2−/− control). In addition, 24 inflammatory genes were significantly reduced in the Ephx2−/− DOCA-salt group compared with the WT DOCA-salt group (P < 0.05). These data demonstrate that DOCA-salt hypertension increases renal inflammatory gene expression and that Ephx2 gene deficiency has an anti-inflammatory action.
Fig. 5.
Renal inflammatory gene expression between DOCA-salt hypertension groups. Gene expression between WT and Ephx2−/− control groups (A) and gene expression between WT control and WT DOCA-salt groups (B) are shown. Gene expression between Ephx2−/− control and Ephx2−/− DOCA-salt groups (C) and gene expression between WT and Ephx2−/− DOCA-salt groups (D) are also shown. mRNA expression of proinflammatory cytokines increased within WT as well as Ephx2−/− DOCA-salt compared with control groups, while Ephx2−/− DOCA-salt expression of inflammatory genes was less than WT DOCA-salt mice.
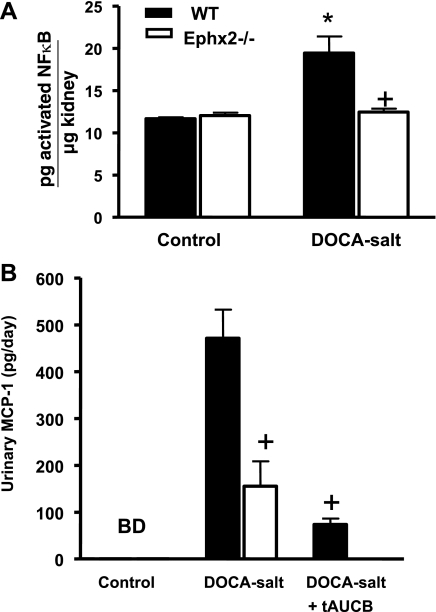
Kidney sections were stained with ED-1 that recognizes CD68 to evaluate macrophage infiltration as an inflammatory indicator. The number of CD68-positive cells were counted and found to be increased significantly in the WT DOCA-salt compared with WT control group (346 ± 31, n = 6 vs. 136 ± 22 cell/mm2, n = 5; P < 0.05). The increase in CD68-positive cells was significantly less in Ephx2−/− mice and averaged 131 ± 21 cell/mm2 (n = 6) in Ephx2−/− control and 233 ± 54 cell/mm2 (n = 5) in Ephx2−/− DOCA-salt mice. To further examine inflammation at the protein level, renal NF-κB activity was determined and is shown in Fig. 6A. Renal NF-κB activity increased significantly in the WT DOCA-salt group compared with WT control (*P < 0.05) whereas no change was noted between Ephx2−/− control and Ephx2−/− DOCA-salt groups. In addition, renal NF-κB activity was significantly reduced in the Ephx2−/− DOCA-salt compared with WT DOCA-salt group (+ P < 0.05). Another index of renal inflammation that was measured was urinary MCP-1 excretion (Fig. 6B). Control WT and Ephx2−/− mice displayed undetectable MCP-1 levels, and Ephx2−/− DOCA-salt mice excreted significantly lower levels of MCP-1 than WT DOCA-salt mice (+P < 0.05). Urinary MCP-1 excretion was also examined in the WT DOCA-salt plus tAUCB group and was significantly lower than the WT DOCA-salt mice (+P < 0.05). Taken together, these data demonstrate that inhibition the hydrolase domain of the sEH enzyme has renal anti-inflammatory actions in DOCA-salt hypertension.
Fig. 6.
Renal NF-κB activity and urinary MCP-1 excretion between DOCA-salt hypertension groups. A: renal NF-κB activity increased in the WT DOCA-salt (n = 6/group, *P < 0.05 vs. WT control) and was significantly reduced in Ephx2−/− DOCA-salt mice (n = 6/group, +P < 0.05 vs. WT DOCA-salt). B: urinary MCP-1 excretion in Ephx2−/− DOCA-salt as well as in WT DOCA-salt plus tAUCB was significantly reduced compared with WT DOCA-salt (n = 6/group, P < 0.05).
DISCUSSION
Chronic hypertension is a major risk factor for end-stage renal disease (1, 12). In addition, the Western diet includes a high salt intake, which in turn can exacerbate the disease condition (1, 5, 13). With the onset of hypertension, activation of the inflammatory pathway also occurs, which itself can possess adverse effects (15, 43, 54).
Interestingly, the major finding of the current study is that deletion of the Ephx2−/− gene provides renal protection by reducing renal injury and inflammation within the setting of salt-sensitive hypertension. Specifically, renal injury was attenuated as seen by reduced albuminuria, desmin, and increased nephrin levels. In addition, renal inflammation is reduced as seen by decreased macrophage infiltration, proinflammatory cytokine mRNA, as well as reduced renal NF-κB activity. These experimental findings are similar to previous findings in rat angiotensin salt-sensitive hypertension where urinary albumin excretion decreased and macrophage infiltration was reduced (20). The current study extends these findings to an angiotensin-independent animal model of salt-sensitive hypertension. Although blood pressure could be a factor contributing to the decrease in renal inflammation and injury, it is becoming clear in a number of renal and cardiovascular disease models that end-organ protection occurs independently of lowering blood pressure. In a recent study, sEH inhibition provided renal protection but did not lower blood pressure in diabetic rats that had salt-sensitive hypertension induced (40). Cisplatin-induced renal injury is also decreased by sEH inhibitor treatment independently of blood pressure changes (41). Experimental studies in Ephx2−/− mice have also demonstrated decreased inflammation and end-organ protection that is independent of blood pressure regulation (25, 35, 45). Since the Ephx2 gene is made up of a C-terminal hydrolase and N-terminal phosphatase domain, it remained unclear whether deletion of the phosphatase domain in Ephx2−/− mice was contributing to the renal protection. By utilizing an inhibitor that is selective for the C-terminal hydrolase domain of sEH, the current study demonstrates that renal protection with salt-sensitive hypertension is due solely to inhibition of the hydrolase domain.
Maintenance of the filtration barrier is vital for proper renal structure and function. A reciprocal relationship exists between the podocyte proteins nephrin and desmin in that a decrease in nephrin and a rise in desmin correlate to renal damage (28, 53). This finding is further supported by a previous study from our laboratory that also examined these two proteins as indicators of renal damage (23). In addition, albuminuria increased significantly, which also supports that damage has occurred to the filtration barrier. Therefore, the current study demonstrates that deletion of the Ephx2−/− gene ameliorates these indices of renal damage.
There is growing support for the hypothesis that salt-sensitive hypertension is an inflammatory disease and therefore treatments that are anti-inflammatory can slow the progression of renal injury to end-stage renal disease (4, 49, 54). In the current study, we found that renal inflammation is enhanced both at the mRNA as well as protein level. The real-time PCR array clearly demonstrates that a significant inflammatory response occurs with DOCA-salt hypertension. In particular, ccr2, the receptor for MCP-1, increased significantly in the WT DOCA-salt group. Our urine analysis further corroborates this finding by showing increased urinary MCP-1 excretion with DOCA-salt hypertension in WT mice. Also, ICAM-1 and IL-1β mRNA were shown to be upregulated and have been previously found to be elevated with salt sensitive hypertension (31, 47, 54). Other inflammatory cytokines that increased with DOCA-salt hypertension include TNF-α as well as IL-6, and past studies have also shown these inflammatory markers to be elevated in other models of salt-sensitive hypertension (6–8, 31, 36). Interestingly, the real-time PCR array indicates that there is less inflammatory cytokine mRNA activated in the Ephx2−/− DOCA-salt compared with WT DOCA-salt hypertension, which supports the notion that inhibition of epoxide breakdown by sEH provides renal protection.
To examine posttranslational activation of the inflammatory process, we measured renal NF-κB activity. Past studies have shown that EETs are anti-inflammatory and that EETs prevent the activation of NF-κB (9, 38). Acute inflammation induced by lipopolysaccharide injection is also decreased by sEH inhibition in mice (44). We have shown here that renal NF-κB activity is blunted in the Ephx2−/− DOCA-salt compared with WT DOCA-salt mice. We have also shown that renal macrophage infiltration is reduced in Ephx2−/− DOCA-salt compared with WT DOCA-salt mice. Hence, by preventing the degradation of epoxides by sEH, renal inflammation is blunted and may help restore renal structure with DOCA-salt hypertension.
The sEH enzyme is a homodimer, and each subunit is composed of two domains. The 35-kDa C-terminal domain imparts sEH activity, while the 25-kDa N-terminal domain has been classified as a phosphatase domain (37). Both domains have been well characterized, and evidence supports the notion that the C-terminal hydrolase domain plays a significant role in blood pressure regulation; however, a specific role of the N-terminal domain remains elusive (11, 17). To eliminate any involvement of the phosphatase domain in the blood pressure, anti-inflammatory, and renal-protective effects of Ephx2−/− gene deletion, a sEH inhibitor that selectively inhibits the C-terminal hydrolase domain was administered to WT and Ephx2−/− DOCA-salt mice. Our data demonstrate that it is the hydrolase domain that provides renal protection and that the phosphatase domain is not participating in this protection. Hence, silencing of the phosphatase domain in the Ephx2−/− mice does not contribute to the blood pressure-lowering effect and subsequent renal protection of sEH inhibition. Moreover, we observed no differences in MAP in Ephx2−/− DOCA-salt mice and WT DOCA-salt mice treated with the sEH inhibitor. Therefore, we have demonstrated that renal protection in the setting of salt-sensitive hypertension with total Ephx2 gene deletion is due to the C-terminal hydrolase domain.
The effects of Ephx2 gene deletion have also been examined in other vascular disease states, and these studies support the notion that sEH inhibitor effects are a consequence of inhibiting the activity of the C-terminal epoxide hydrolase domain (30, 32, 35, 45, 51). For instance, a recent study has identified Ephx2 as a heart failure susceptibility gene in the spontaneously hypertensive heart failure rat, which corresponds to human hypertension-associated heart failure (32). In addition, this study also tested the cardioprotective effects of Ephx2 gene deletion with angiotensin II administration as well as with pressure overload. Ephx2−/− mice, while similar to WT mice at baseline, were protected from cardiac arrhythmias following angiotensin II and demonstrated reduced ejection fraction decline with pressure overload (32). Ephx2−/− mice are also protected from end-organ damage that occurs with cerebral and cardiac ischemia and provided evidence that this protection was due to silencing the hydrolase domain of the sEH enzyme (25, 35, 45, 51). Taken as a whole, there is overwhelming evidence in experimental cardiovascular disease models that inhibition of the sEH enzyme hydrolase domain can provide significant protection from end-organ damage.
Polymorphisms of Ephx2 have been studied within the patient population and have been correlated to the incidences of such conditions as ischemic stroke, plasma cholesterol modification, and coronary vascular disease (14, 24, 26). In vitro studies by Koerner et al. (24) tested polymorphisms in the human Ephx2 gene and demonstrated that an alteration in sEH activity can affect neuronal survival postischemic injury. The Atherosclerosis Risk In Communities study identified a significant association between polymorphisms of Ephx2 and the incidence of coronary heart disease in Caucasian patients (26). These studies demonstrate that the Ephx2 gene has an important role in the development of cardiovascular disease, and therefore the potential therapeutic advantage of inhibiting this enzymatic pathway within cardiovascular disease states warrants further investigation.
Our study has demonstrated that renal injury and inflammation that occurs from salt-sensitive hypertension can be ameliorated by preventing the degradation of the epoxides and that this protection is due to the hydrolase domain of the Ephx2 gene. The protective effects of Ephx2 gene deletion include a reduction in blood pressure as well as an attenuation of glomerular injury which is correlated to a significant reduction in the inflammatory process. Therefore, the potential therapeutic benefit of using sEH inhibitors may have a twofold protection, from blood pressure plus inflammation, that could alleviate the progression to end-stage renal disease associated with salt-sensitive hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL-59699, HL-074167, and DK-38226 and an American Heart Association Established Investigator Award to J. D. Imig.
REFERENCES
- 1.Barri YM Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep 10: 39–45, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci 21: 125–127, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 28: 158–167, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Chiolero A, Wurzner G, Burnier M. Renal determinants of the salt sensitivity of blood pressure. Nephrol Dial Transplant 16: 452–458, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II-salt hypertension. Hypertension 50: 1069–1076, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47: 557–562, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK. 11,12-Epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett 13: 4011–4014, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Fleming I Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med 18: 20–25, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Franco M, Sanchez-Lozada LG, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Pathophysiology of salt-sensitive hypertension: a new scope of an old problem. Blood Purif 26: 45–48, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Muller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke 39: 1593–1596, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hilgers KF, Hartner A, Porst M, Veelken R, Mann JF. Angiotensin II type 1 receptor blockade prevents lethal malignant hypertension: relation to kidney inflammation. Circulation 104: 1436–1440, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289: F496–F503, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev 24: 169–188, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ channels. Microcirculation 15: 137–150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45: 759–765, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem 47: 2110–2122, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension 51: 352–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci 27: 4642–4649, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci 13: 2833–2841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet 15: 1640–1649, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol 287: R1394–R1398, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys 47: 87–98, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem 282: 2891–2898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manhiani MM, Quigley JE, Socha MJ, Motamed K, Imig JD. IL6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney Blood Press Res 30: 195–202, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet 40: 529–537, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA 96: 8849–8854, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311–333, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol 295: H2128–H2134, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA 100: 1558–1563, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Gαs mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem 276: 15983–15989, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci 116: 61–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parrish AR, Chen G, Burghardt RC, Watanabe T, Morisseau C, Hammock BD. Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol Toxicol 25: 217–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roman RJ P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Mezzano S, Egido J. Renin-angiotensin system and renal damage: emerging data on angiotensin II as a proinflammatory mediator. Contrib Nephrol 123–137, 2001. [DOI] [PubMed]
- 44.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 102: 9772–9777, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Socha MJ, Manhiani M, Said N, Imig JD, Motamed K. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol 171: 1104–1112, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073–2078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15: 1244–1253, 2004. [PubMed] [Google Scholar]
- 53.Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS, Liu ZH. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int 74: 596–612, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Zoccali C, Mallamaci F, Tripepi G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int Suppl: S105–S110, 2003. [DOI] [PubMed]