Abstract
Using MLR, the effect of atorvastatin on proliferation of human and baboon PBMC and human CD4+T cells in response to wild-type (WT) and α1,3-galactosyltransferase gene-knockout (GTKO) porcine aortic endothelial cells (pAEC) was investigated. SLA class-II expression on pAEC before and after pIFN-γ stimulation, and the effect of atorvastatin on this expression was assessed. Added to the MLR, atorvastatin reduced (i) the human PBMC response to unstimulated (p<0.05) and (ii) the human and baboon PBMC responses to stimulated (p<0.05) WT and GTKO pAEC. Atorvastatin treatment of human PBMC before MLR reduced their response to stimulated WT (p<0.05) and GTKO (p<0.05) pAEC. Stimulation of pAEC with pIFN-γ increased SLA-II expression 20-60-fold, which was downregulated by atorvastatin. Atorvastatin treatment of stimulated pAEC before MLR reduced proliferation of human PBMC (p<0.05) and CD4+T cells (p<0.05). Atorvastatin downregulates the primate cellular xenoresponse, possibly through its antiproliferative effect on PBMC and reduction of SLA-II on pAEC.
Keywords: pig, primate, statins, swine leukocyte antigens class II, xenotransplantation
INTRODUCTION
Statins (3-hydroxyl-3-methyl coenzyme A [HMG-CoA] reductase inhibitors) are widely used to lower blood cholesterol levels. Clinical trials have demonstrated beneficial effects independent of their cholesterol-lowering ability. These effects indicate that statins have anti-inflammatory, anticoagulant, and immunomodulatory effects. Observations in heart transplant patients, together with several in vitro studies that have determined a potential mechanism for their immunosuppressive effect, have provided a principle for the use of statins in patients with organ allografts (1).
Failure of porcine xenografts in nonhuman primates has been attributed to several factors. The anti-inflammatory, anticoagulant, and immunomodulatory effects of statins may be beneficial in extending xenograft survival (2). We therefore investigated whether statins can modulate the primate cellular response to porcine antigens in vitro.
METHODS
Reagents
Atorvastatin was purchased from Pfizer Inc. (New York, NY), mevalonic acid (MVA) from Sigma (St. Louis, MO), porcine interferon gamma (pIFN-γ) from Serotec (Raleigh, NC), and mouse anti-SLA-DR (Cat# 553642) and FITC anti-mouse IgG2a/2b (Cat# 553399) antibodies from BD Pharmingen (San Diego, CA).
Cells
Human peripheral blood mononuclear cells (hPBMC) were isolated from buffy coats of blood type O (Institute for Transfusion Medicine, Pittsburgh, PA). Human CD4+T cells were isolated using CD4+T cell isolation kit II (cat# 130-091-155, Miltenyi Biotec, Auburn, CA). Baboon PBMC were collected from healthy baboons. Pig aortic endothelial cells (pAEC) were obtained from freshly-harvested wild-type (WT) and α1,3-galactosyltransferase gene-knockout (GTKO) porcine aortas; WT and GTKO pigs were of the same genetic background, cells were then cultured and used from passages 3 to 8.
Mixed Lymphocyte Reaction (MLR)
Human PBMC/CD4+T cells (or baboon PBMC) were used as responders (0.2×106 cells/well). pAEC were stimulated with pIFN-γ (500U/ml for 24h or 250U/ml for 48h). Irradiated pAEC, as stimulators (at responder-stimulator ratios 1:10 or 1:20), were incubated for 4-5 days. 3H-thymidine (1μCi/well) was added to each well during the last 16h of incubation. Cells were harvested on glass-fiber filter mats with a cell harvester, and were analyzed by beta-scintillation counting on a liquid scintillation counter (PerkinElmer, Waltham, MA). The mean of triplicate results was expressed as 3H-thymidine uptake.
Flow cytometry
Using LSR II flow, pAEC were assessed for swine leukocyte antigen class II (SLA-II) expression before and after stimulation with pIFN-γ. Data were analyzed using winMDI software.
Statistical analysis
Statistical analysis was carried out using the Student t-test. Values <0.05 were considered significant.
RESULTS
Primate cellular response to WT and GTKO pAEC
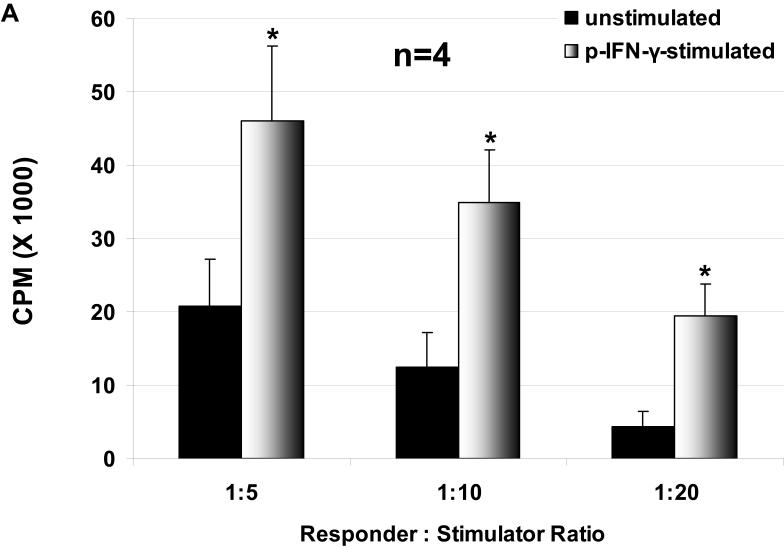
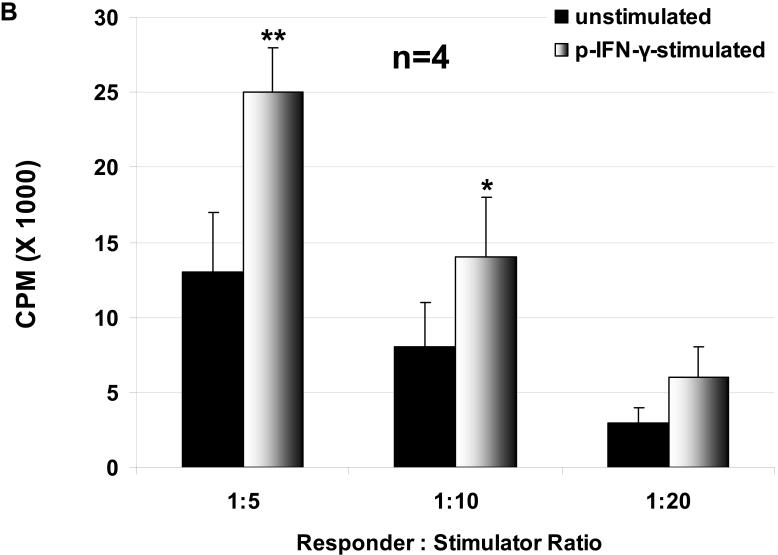
The proliferation of hPBMC in response to unstimulated and stimulated WT and GTKO pAEC was assessed, where pAEC were stimulated with pIFN-γ before the MLR. The hPBMC response to stimulated pAEC was significantly higher than that to unstimulated pAEC for both WT and GTKO (Figure 1 A, B). The proliferation of hPBMC in response to pAEC was significantly higher for WT than for GTKO.
Figure 1.
The proliferative response of hPBMC to WT and GTKO pAEC before and after stimulation with pIFN-γ: The response to pAEC was significantly higher to WT (A) than to GTKO (B) (unstimulated p<0.05; pIFN-γ-stimulated p<0.001). Data represent the mean (+/- SEM) response of PBMC isolated from four different healthy volunteers. (*p<0.05, **p<0.01, versus unstimulated). (Note the different scales between A and B)
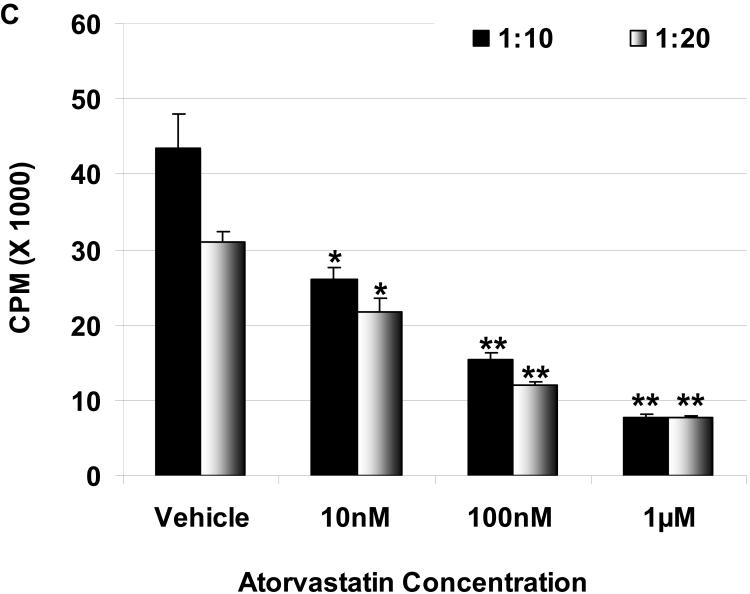
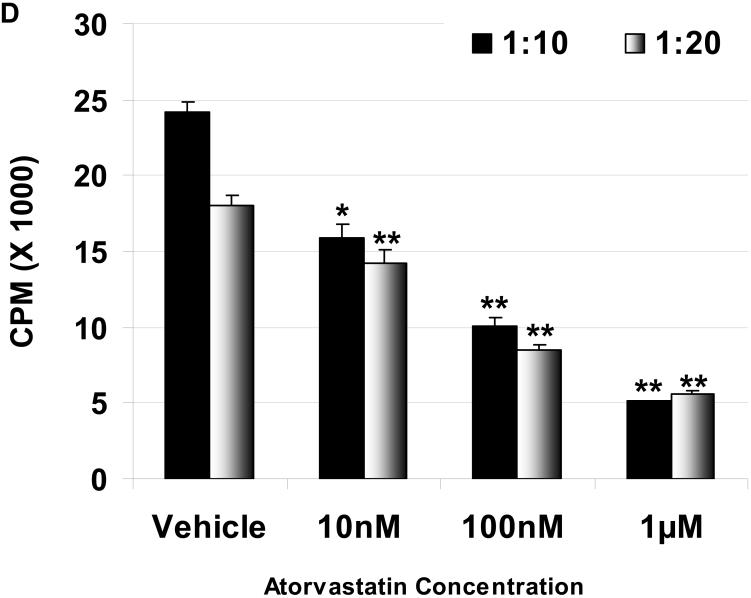
Suppression of the human cellular response to pAEC by atorvastatin in MLR: The hPBMC proliferative responses to pIFN-γ-stimulated (250U/ml for 48h) WT (C) and GTKO (D) pAEC in the presence or absence of atorvastatin (at responder:stimulator ratios of 1:10 and 1:20) are shown. Data represent the response of hPBMC from a single healthy volunteer, and are representative of three different experiments. Values represent the mean of triplicates (+/- SD). (*p<0.05, **p<0.01, versus vehicle only).
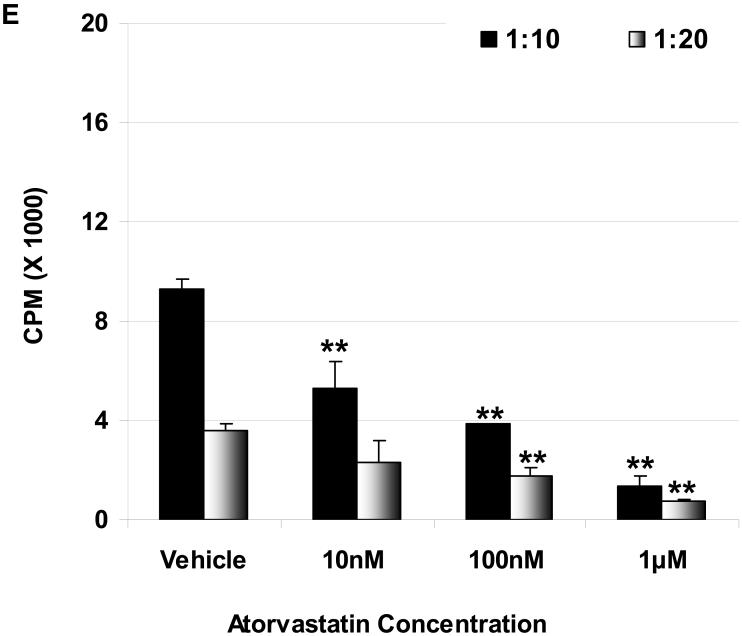
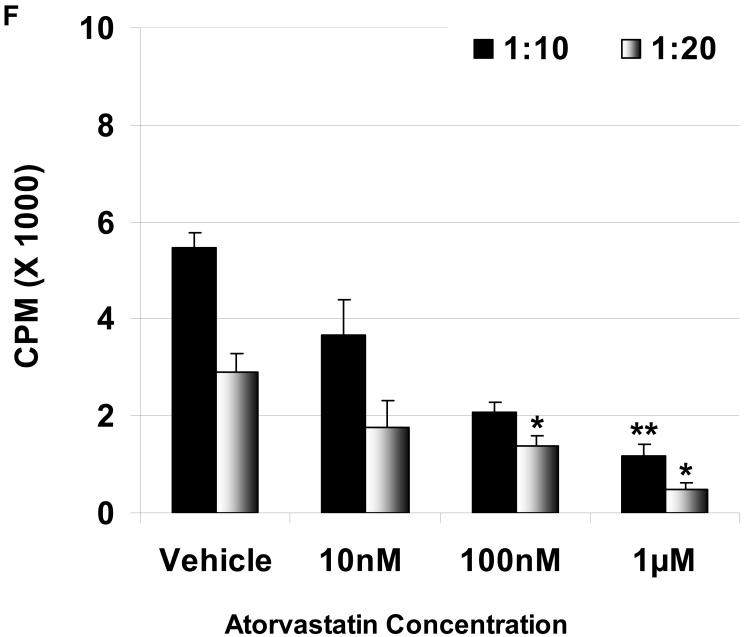
Suppression of the baboon cellular response to pAEC in MLR: The baboon PBMC proliferative responses to pIFN-γ-stimulated (250U/ml for 48h) WT (E) and GTKO (F) pAEC in the presence or absence of atorvastatin (at responder:stimulator ratios of 1:10 and 1:20) are shown. Data represent the response of PBMC from a single baboon, and are representative of two different experiments. Values represent the mean of triplicates (+/- SD). (*p<0.05, **p<0.01, versus vehicle only).
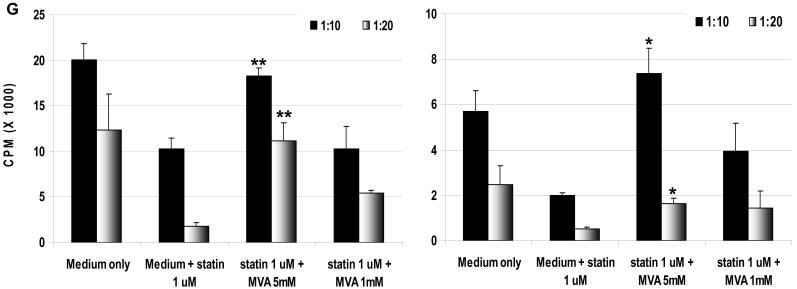
Mevalonic acid (MVA) was added to the MLR in the presence of atorvastatin (G): MVA (at 5mM concentration) restored the proliferative response of hPBMC to WT (left) and GTKO (right) pAEC in the presence of atorvastatin (1μM). Data are representative of three different experiments. Values represent the mean of triplicates (+/- SD). (*p<0.05, **p<0.01, versus atorvastatin only).
Atorvastatin suppresses the primate cellular response to WT and GTKO pAEC
The proliferation of hPBMC against unstimulated and stimulated pAEC was measured in the presence or absence of atorvastatin. Atorvastatin was added to the MLR at 10nM, 100nM, and 1μM concentrations, and suppressed hPBMC proliferation in a dose-dependent manner. At 1μM, the proliferation of hPBMC in response to unstimulated WT and GTKO pAEC was reduced by 40% and 48%, respectively; however, this reduction was not significant (not shown). When pAEC were stimulated with pIFN-γ, the increased hPBMC proliferative response was significantly reduced by atorvastatin at 1μM concentration by 76% for WT and 73% for GTKO (Figure 1C, D).
Since pig-to-baboon xenotransplantation is a common experimental model, whether atorvastatin had the same effect on baboon PBMC was tested. In a dose-dependent manner, atorvastatin significantly reduced proliferation of baboon PBMC in response to stimulated WT and GTKO pAEC (Figure 1E, F).
The conversion HMG-CoA by HMG-CoA reductase to MVA is the rate-limiting step reaction in cholesterol synthesis. Adding MVA to the MLR with atorvastatin counters its inhibitory action. The proliferative response of hPBMC to pAEC was restored by adding MVA, indicating that this suppression was statin-specific (Figure 1G).
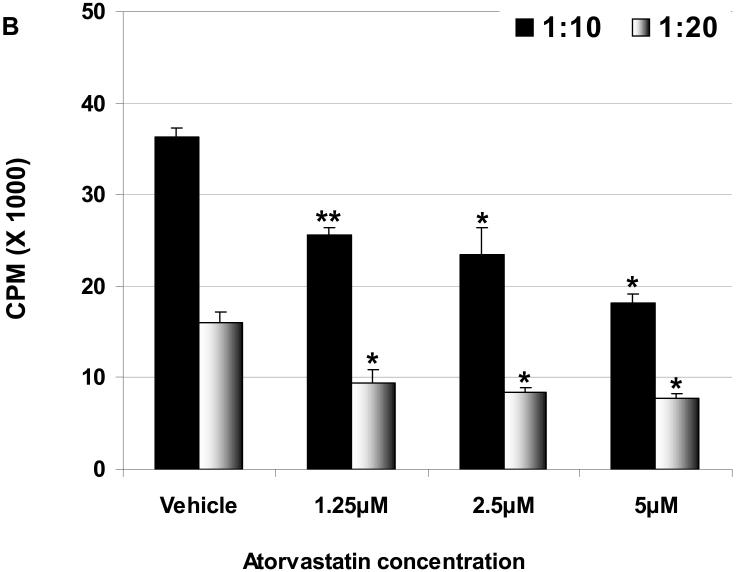
Atorvastatin treatment of hPBMC before MLR downregulates their proliferation
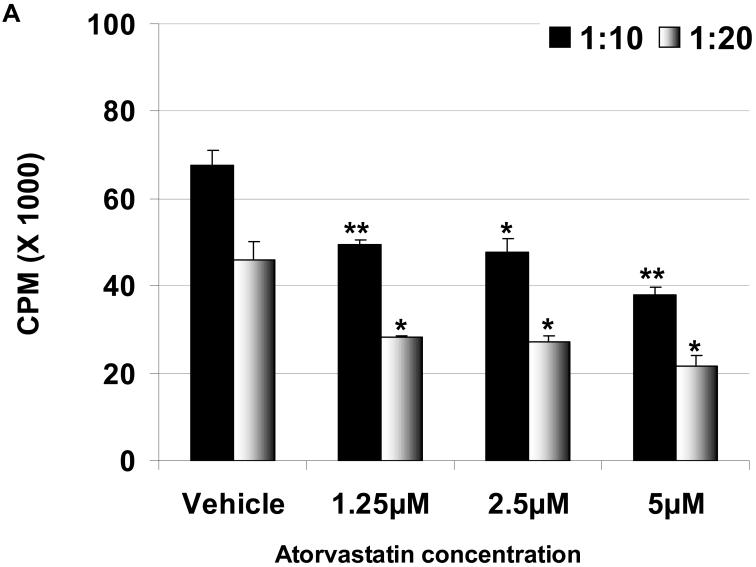
To determine whether the effect of atorvastatin was on hPBMC, pAEC, or both, only hPBMC were treated for 72h with atorvastatin (at 1.25μM, 2.5μM, and 5μM concentrations) before the MLR. The hPBMC response to stimulated WT and GTKO pAEC was significantly reduced by atorvastatin at all concentrations (Figure 2A, B). The same response was seen by atorvastatin-treated hPBMC to unstimulated pAEC (not shown). These data suggest that prior treatment of hPBMC with atorvastatin can reduce their proliferative capacity in response to pAEC.
Figure 2.
When exposure is prior to MLR, atorvastatin treatment downregulates the human proliferative response to WT (A) and GTKO (B) pAEC. hPBMC were treated with atorvastatin at different concentrations (5, 2.5, and 1.25 μM for 72h). No atorvastatin was added to the MLR. At responder:stimulator ratios of 1:10 and 1:20, atorvastatin-treated hPBMC proliferation against pIFN-γ-stimulated (250U/ml for 48h) pAEC was significantly reduced at all concentrations. Data are representative of three different experiments. Values represent the mean of triplicates (+/- SD). (*p<0.05, **p<0.01, versus vehicle only).
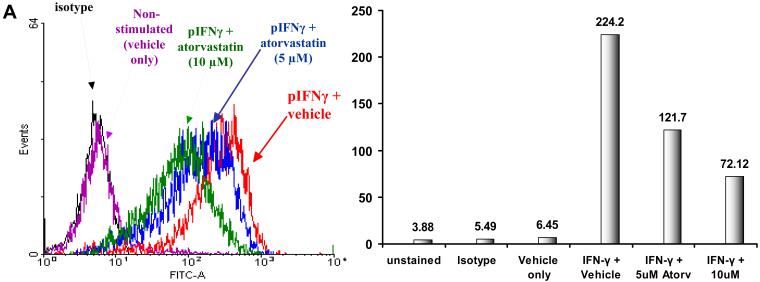
Atorvastatin downregulates SLA II-expression on pAEC
Unstimulated pAEC (both WT and GTKO) expressed no or minimal SLA-II. pAEC stimulation with pIFN-γ resulted in upregulation of SLA-II by 20-60-fold (depending on the incubation period and the dose of pIFN-γ). This increased SLA II expression was variable; however, the mean percentage of increase was comparable between both WT and GTKO pAEC. When pAEC were stimulated with pIFN-γ (500U/ml for 24h) in the presence of atorvastatin, the upregulation of SLA-II was reduced (Figure 3A). At 5μM and 10μM, atorvastatin reduced SLA-II expression on pIFN-γ-stimulated pAEC (WT by 33% and 48%, respectively, and GTKO by 22% and 66%, respectively).
Figure 3.
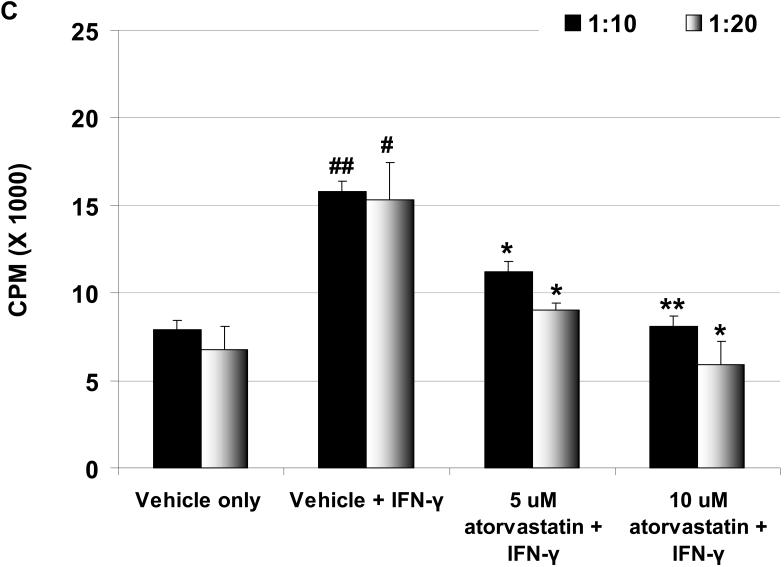
(A) Downregulation of SLA-II by atorvastatin. GTKO pAEC were stimulated with pIFN-γ (500U/ml) for 24h in the presence or absence of atorvastatin (5μM and 10μM) (left). Mean fluorescent Intensity (MFI) values are also shown (right). Similar results were obtained with WT pAEC (not shown). Data are representative of three different experiments. (Although the results were almost identical, these experiments are different from those previously published [2].) (B) MLR was performed to assess the hPBMC response to atorvastatin-treated pAEC (using the same GTKO pAEC as in A). WT and GTKO pAEC were treated with atorvastatin (5μM and 10μM) in addition to pIFN-γ stimulation before the MLR (at a responder:stimulator ratio of 1:20). No atorvastatin was added to the MLR. Data are representative of three different experiments. Values represent the mean of triplicates (+/- SD). (*p<0.05, **p<0.01, versus vehicle + IFN-γ). (C) MLR was performed with MACS-purified human CD4+T cells as responders (at responder:stimulator ratios of 1:10 and 1:20). Purity of CD4+T cells was >90% in all cases. GTKO pAEC were incubated with atorvastatin (5μM and 10μM) for 48h, and stimulated with pIFN-γ (100U/ml) during the last 24h of incubation. No atorvastatin was added to the MLR. Upregulation of SLA-II by pIFN-γ and its downregulation in the presence of atorvastatin was confirmed by flow cytometry (not shown). Data are representative of three different experiments. Values represent the mean of triplicates (+/- SD). (##p<0.01, #p<0.05, versus vehicle only, *p<0.05 versus vehicle + IFN-γ).
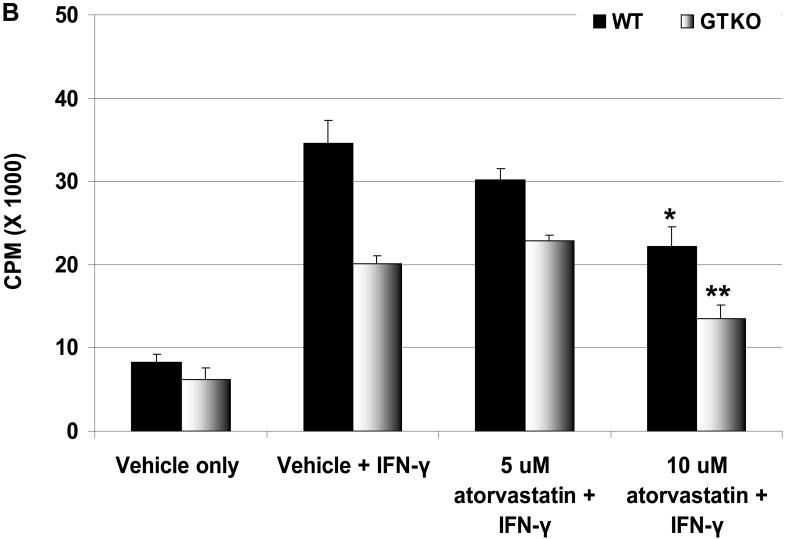
Proliferation of human PBMC in response to atorvastatin-treated pAEC is reduced
To assess whether atorvastatin treatment of pAEC would modulate the proliferative response of hPBMC in association with the downregulation of SLA-II expression, pAEC were treated with atorvastatin for 24h before the MLR. The hPBMC response to unstimulated pAEC treated with atorvastatin (at different concentrations) was reduced only at 10μM concentration and was not statistically significant (not shown). When pAEC were stimulated with pIFN-γ (500U/ml for 24h) in the presence of 10μM atorvastatin, the hPBMC proliferation was reduced (by 35% for WT and by 35% for GTKO) (Figure 3B).
Human CD4+T cell proliferation is reduced in response to atorvastatin-treated pAEC
Human CD4+T cells respond to the upregulation of SLA-II on pAEC (3). We tested whether the downregulation of SLA-II by atorvastatin treatment of stimulated WT and GTKO pAEC before MLR would be associated with a reduction in proliferation of CD4+T cells. When GTKO pAEC were stimulated with pIFN-γ (500U/ml for 24h), the human CD4+T cell proliferative response was significantly higher than that to unstimulated pAEC (Figure 3C). However, when GTKO pAEC were stimulated in the presence of 5μM or 10μM atorvastatin, the proliferative response was significantly reduced (p<0.05), suggesting that the downregulation of SLA-II on pAEC by atorvastatin was associated with a reduced CD4+T cell response.
DISCUSSION
Although it has been shown that statins can suppress NK cell cytotoxicity against pAEC (4), to our knowledge there has been virtually no investigation of the effect of statins on the human immune cellular response to a xenograft. In the experiments reported here, although the exact mechanism of downregulation of the cellular response to pAEC was not fully investigated, there was clear reduction of the cellular response in the presence of atorvastatin, which probably results from several mechanisms as reported by others. Statins can modulate the human immune response through, for example, inhibition of LFA-1-mediated adhesion and costimulation (5), Th1 to Th2 shift (6, 7), and decreased T cell proliferation (8) which is not related to lowering cholesterol levels (9).
The primate cellular response to a porcine organ almost certainly plays a significant role in xenograft failure, as suggested by the prolongation of xenograft survival associated with immunosuppression directed towards the cellular response. Whether the absence of Gal1,3Gal (Gal) antigen expression has any effect on modifying the cellular response has not been fully elucidated, although it has been reported that a reduction of Gal expression on pAEC can reduce T cell proliferation (10). This is supported by the observations in the present study whereby proliferation of PBMC (both human and baboon) in response to pAEC was significantly higher to WT than to GTKO (whether unstimulated or pIFN-γ stimulated). As resting pAEC (WT or GTKO) showed no or minimal expression of SLA II, and the increase in expression on stimulation was comparable irrespective of the source of the cells, our conclusion is that the significantly greater proliferation in response to WT pAEC was not related to a difference in SLA II expression. The absence of Gal would appear to account for the reduced response on MLR in some other way, but we do not yet understand the mechanism.
Atorvastatin was able to downregulate the human proliferative response against pAEC. This was achieved through an effect on both pAEC and hPBMC. Treating pAEC with atorvastatin at a high concentration (10μM) for 72h before the MLR reduced the proliferative response. This high concentration might not be achieved in vivo, although a lower dose administered to the organ-source pig for a prolonged period of time might have a similar effect, as demonstrated by Geissler et al (12). At a clinically-relevant therapeutic level (1μM) in the MLR, atorvastatin significantly reduced the hPBMC proliferative response against pAEC. We therefore believe that treatment of both the organ-source pig pre-transplantation and of the primate recipient both pre- and post-transplantation might be beneficial. However, it is unlikely that statin treatment alone would be sufficient to control the cellular response, but it is likely to have an additive effect used in conjunction with immunosuppressive agents.
Statins have been shown to reduce “inducible” MHC II expression, thus suppressing T cell activation, which was related to inhibition of CIITA in human endothelial cells and monocytes/macrophages (11). Furthermore, an in vivo study, where pigs were given statins at 3mg/kg/day for 16 days, showed that SLA-II expression was reduced on endothelial cells (12). It has been shown that blocking of SLA II in xenogeneic MLR can down-regulate the human cellular xenoresponse (13). In the present in vitro study, the downregulation of SLA-II by atorvastatin (resulting from only 24h exposure at high concentration) was associated with a reduction in the proliferation of both hPBMC and human CD4+T cells. This would suggest a role for statins in modulating antigen presentation by pAEC through downregulation of SLA-II, as has been demonstrated by others (3, 14). The down-regulatory effect of statins on ‘inducible’ MHC II on human antigen-presenting cells (monocytes/macrophages) and on pAEC SLA II could reduce the cellular response to both direct and indirect antigen presentation.
In conclusion, these data suggest that statin treatment of the donor (pre-transplant) and of the recipient (pre- and post-transplant) might reduce the primate cellular response to a porcine xenograft.
ACKNOWLEDGEMENTS
Work in our laboratory is supported in part by NIH grants #U01AI068642 and R21-A1074844 and by a Sponsored Research Agreement between the University of Pittsburgh and Revivicor, Inc, Blacksburg, VA.
ABBREVIATIONS
- Gal
Galα1,3Gal
- GTKO
α1,3-galactosyltransferase gene-knockout
- HMG-CoA
3-hydroxyl-3-methyl coenzyme A reductase inhibitors
- MVA
mevalonic acid
- pAEC
porcine aortic endothelial cells
- PBMC
peripheral blood mononuclear cells
- SLA II
swine leukocyte antigens class II
- WT
wild-type
REFERENCES
- 1.Kobashigawa JA. Statins in solid organ transplantation: Is there an immunosuppressive effect? Am J Transplant. 2004;4:1013–1018. doi: 10.1111/j.1600-6143.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 2.Ezzelarab M, Cooper DK. Xenotransplantation. 2007;14:100–103. doi: 10.1111/j.1399-3089.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.Yun S, Rose ML, Fabre JW. The induction of major histocompatibility complex class II expression is sufficient for the direct activation of human CD4+T cells by porcine vascular endothelial cells. Transplantion. 2000;69:940–944. doi: 10.1097/00007890-200003150-00046. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Porter CM, Horvath-Arcidiacono JA, Bloom ET. Lipophilic statins suppress cytotoxicity by freshly isolated natural killer cells through modulation of granule exocytosis. Int Immunol. 2007;19:163–173. doi: 10.1093/intimm/dxl133. [DOI] [PubMed] [Google Scholar]
- 5.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 6.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 7.Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–1530. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 9.Blank N, Schiller M, Krienke S, Busse F, Schätz B, Ho A, et al. Atorvastatin inhibits T cell activation through 3-hydroxy-3-methylglutaryl coenzyme A reductase without decreasing cholesterol synthesis. J Immunol. 2007;179:3613–3621. doi: 10.4049/jimmunol.179.6.3613. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Li T, Ma Y. Reactivity of human peripheral blood lymphocyte to alpha-Gal on porcine arotic endothelial cell. Chinese J Rep Rec Surgery. 1998;12:359–362. [PubMed] [Google Scholar]
- 11.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 12.Geissler I, Collins L, Schofield R, Fabre JW. In vivo suppression of major histocompatibility complex class II expression on porcine vascular endothelial cells by an HMG-CoA reductase inhibitor. Transplantation. 2006;81:922–926. doi: 10.1097/01.tp.0000179154.17329.68. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;11:5249–56. [PubMed] [Google Scholar]
- 14.Sumitran-Holgersson S, Brevig T, Widner H, Holgersson J. Activated porcine embryonic brain endothelial cells induce a proliferative human T-lymphocyte response. Cell Transplant. 2003;12:637–646. doi: 10.3727/000000003108747118. [DOI] [PubMed] [Google Scholar]