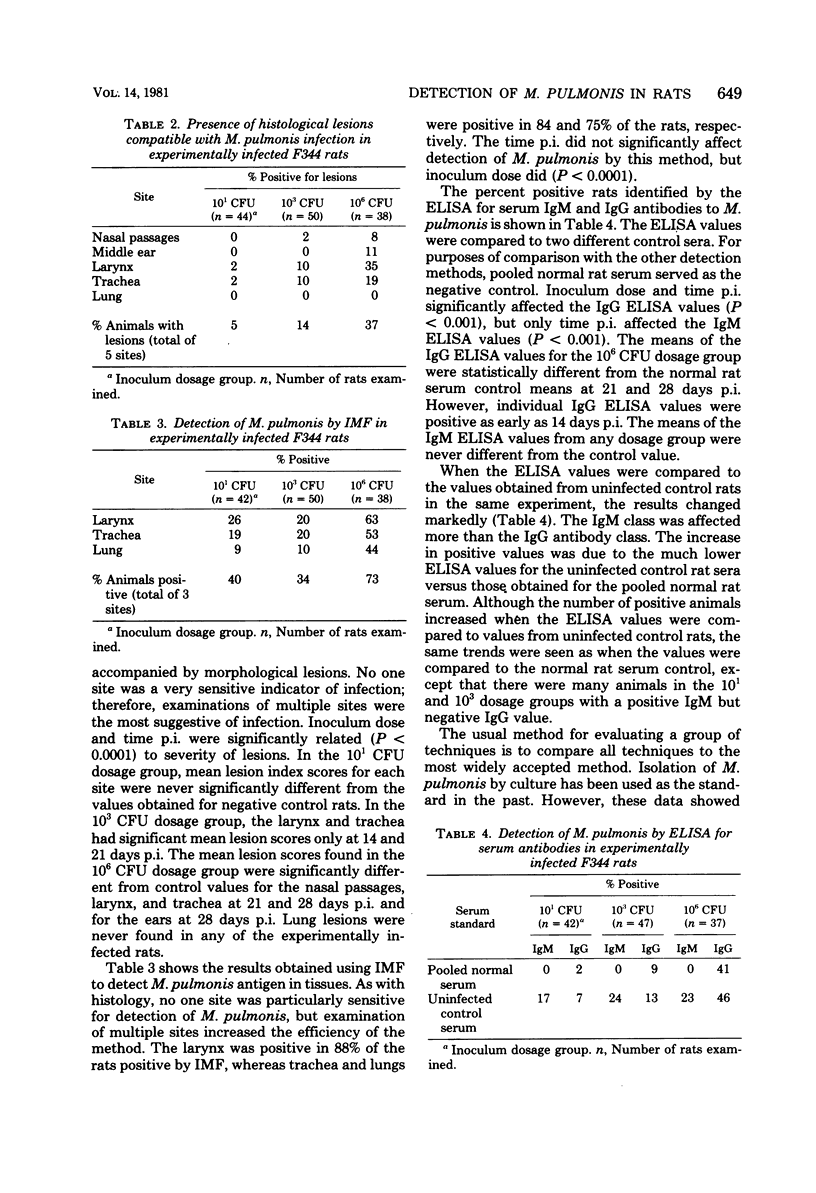
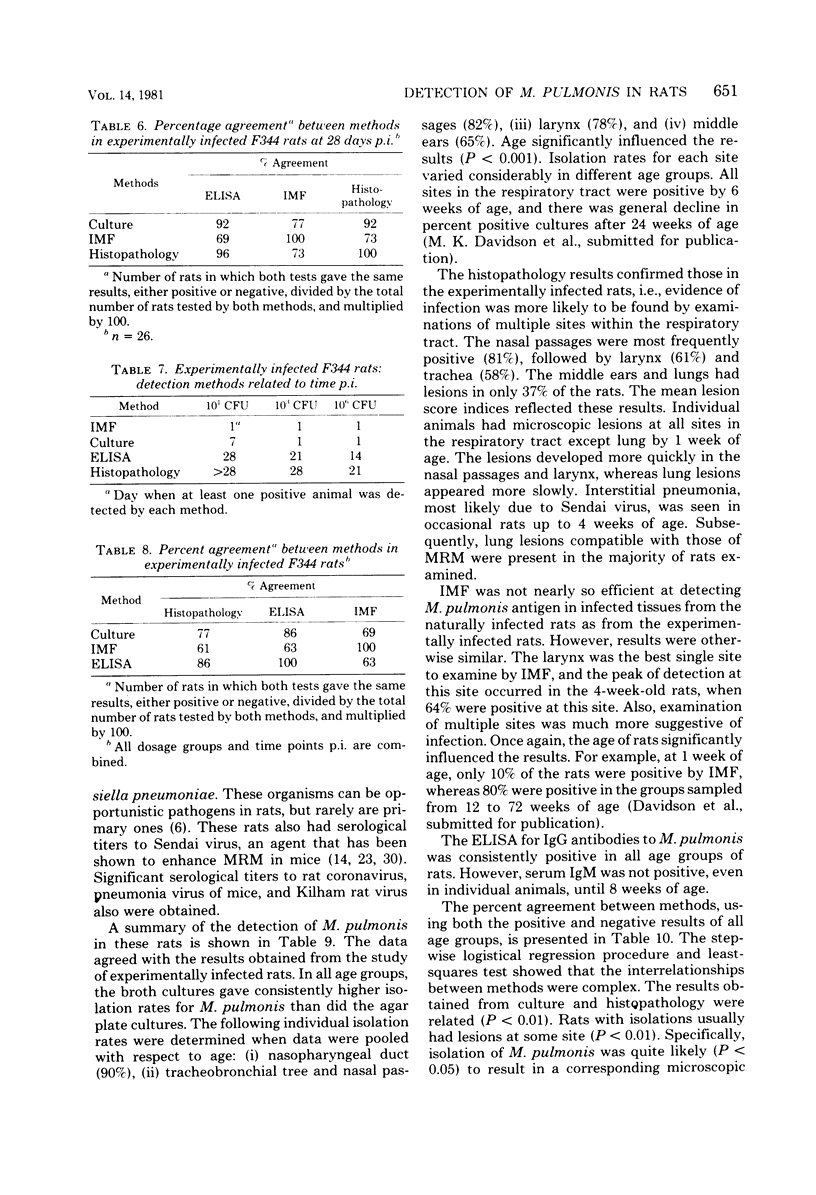
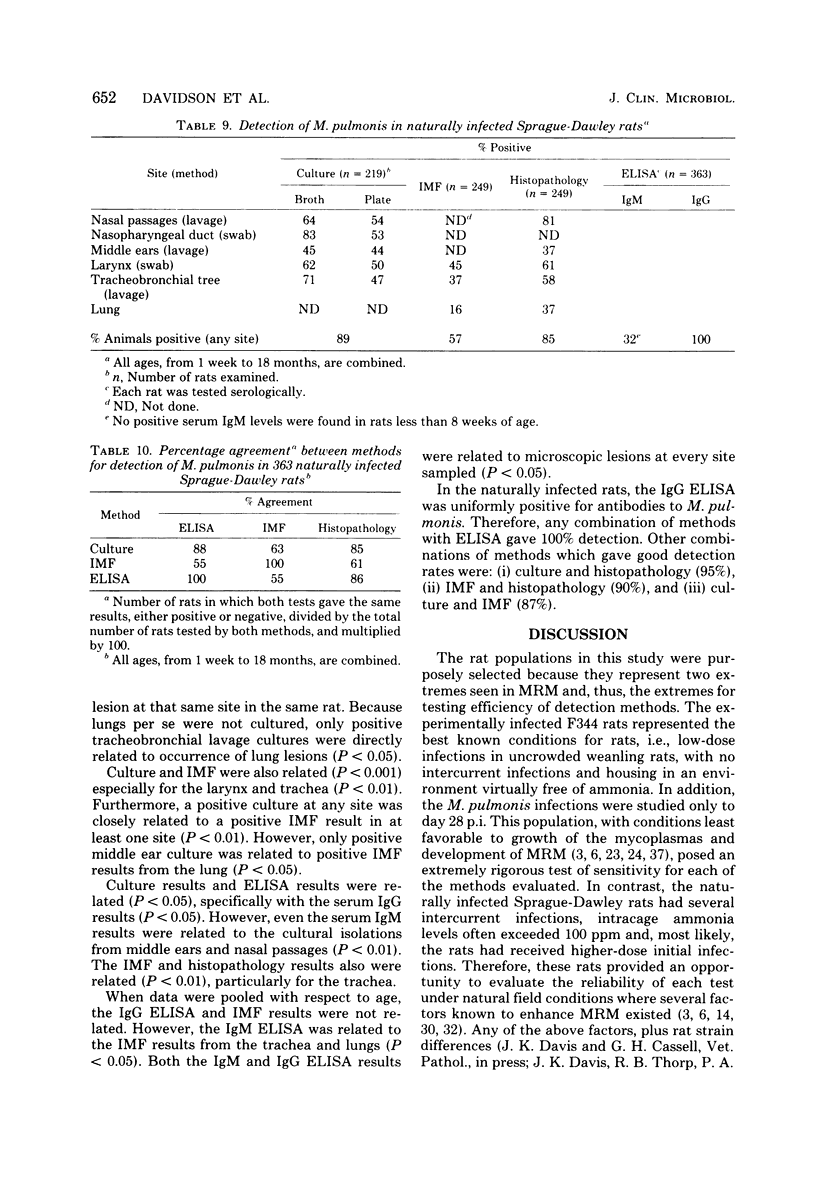
Abstract
Isolation, indirect immunofluorescence, an enzyme-linked immunosorbent assay (ELISA), and histopathological examination of tissues for characteristic lesions were evaluated for their efficiency in detecting Mycoplasma pulmonis infection in rats. Whereas all of the methods were efficient in naturally infected Sprague-Dawley rats, none of the methods consistently detected infection in F344 rats experimentally infected with low doses of the organism. In the experimental infections, however, the success rate of any method was directly related (P less than 0.05) to increasing inoculum dose and time postinoculation. Collectively, the data indicated that isolation of M. pulmonis was the most efficient single detection method and the nasopharyngeal duct was the best single site to culture, although sampling of multiple sites within the respiratory tract increased the rate of isolating the organism. The ELISA was understandably the least sensitive method in the low-dose, experimentally infected rats because of the time required for development of a detectable serum antibody response. Although each of the four methods identified a high percentage of naturally infected rats, the ELISA was the most efficient method in these animals as it was uniformly positive. The use of combinations of methods was found to increase the rate of detection of M. pulmonis infection in both experimentally and naturally infected rats.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Del Giudice R. A., Grabowski M. W., Hopps H. E. Media for the isolation of mycoplasma from biologic materials. Dev Biol Stand. 1974;23:128–133. [PubMed] [Google Scholar]
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Casillo S., Blackmore D. K. Uterine infections caused by bacteria and mycoplasma in mice and rats. J Comp Pathol. 1972 Oct;82(4):477–482. doi: 10.1016/0021-9975(72)90048-5. [DOI] [PubMed] [Google Scholar]
- GRAHAM W. R. RECOVERY OF A PLEUROPNEUMONIA-LIKE ORGANISM (P.P.L.O.) FROM THE GENITALIA OF THE FEMALE ALBINO RAT. Lab Anim Care. 1963 Oct;13:719–724. [PubMed] [Google Scholar]
- Ganaway J. R., Allen A. M., Moore T. D., Bohner H. J. Natural infection of germfree rats with Mycoplasma pulmonis. J Infect Dis. 1973 May;127(5):529–537. doi: 10.1093/infdis/127.5.529. [DOI] [PubMed] [Google Scholar]
- HALLIDAY R. The absorption of antibodies from immune sera by the gut of the young rat. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):408–413. doi: 10.1098/rspb.1955.0020. [DOI] [PubMed] [Google Scholar]
- Hill A. Transmission of Mycoplasma pulmonis between rats. Lab Anim. 1972 Sep;6(3):331–336. doi: 10.1258/002367772781006068. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Stott E. J., Taylor G. The effect of pneumonia induced in mice with Mycoplasma pulmonis on resistance to subsequent bacterial infection and the effect of a respiratory infection with Sendai virus on the resistance of mice to Mycoplasma pulmonis. J Gen Microbiol. 1978 Nov;109(1):79–87. doi: 10.1099/00221287-109-1-79. [DOI] [PubMed] [Google Scholar]
- Kohn D. F., Kirk B. E. Pathogenicity of Mycoplasma pulmonis in laboratory rats. Lab Anim Care. 1969 Jun;19(3):321–330. [PubMed] [Google Scholar]
- Kohn D. F. Sequential pathogenicity of Mycoplasma pulmonis in laboratory rats. Lab Anim Sci. 1971 Dec;21(6):849–855. [PubMed] [Google Scholar]
- Lentsch R. H., Wagner J. E., Owens D. R. Comparison of techniques for primary isolation of respiratory Mycoplasma pulmonis from rats. Infect Immun. 1979 Nov;26(2):590–593. doi: 10.1128/iai.26.2.590-593.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebich H. G. Zum Bau der oberen Luftwege der weissen Ratte (Mus rattus norvegicus, var. albinos) Anat Anz. 1975;138(3):170–179. [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Saito M., Nakagawa M., Kinoshita K., Imaizumi K. Etiological studies on natural outbreaks of pneumonia in mice. Nihon Juigaku Zasshi. 1978 Jun;40(3):283–290. doi: 10.1292/jvms1939.40.283. [DOI] [PubMed] [Google Scholar]
- Sparrow S. The microbiological and parasitological status of laboratory animals from accredited breeders in the United Kingdom. Lab Anim. 1976 Oct;10(10):365–373. doi: 10.1258/002367776780956845. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Rask-Nielsen R. Mycoplasma in leukemic and nonleukemic mice. Ann N Y Acad Sci. 1967 Jul 28;143(1):345–352. doi: 10.1111/j.1749-6632.1967.tb27674.x. [DOI] [PubMed] [Google Scholar]
- Whittlestone P., Lemcke R. M., Olds R. J. Respiratory disease in a colony of rats. II. Isolation of Mycoplasma pulmonis from the natural disease, and the experimental disease induced with a cloned culture of this organism. J Hyg (Lond) 1972 Sep;70(3):387–407. doi: 10.1017/s0022172400062975. [DOI] [PMC free article] [PubMed] [Google Scholar]


