Abstract
Activation of the transcription factor NF-κB is a highly regulated multi-level process. The critical step during activation is the release from its inhibitor IκB, which as any other protein is under the direct influence of translation regulation. In this review, we summarize in detail the current understanding of the impact of translational regulation on NF-κB activation. We illustrate a newly developed mechanism of eIF2α kinase-mediated IκB depletion and subsequent NF-κB activation. We also show that the classical NF-κB activation pathways occur simultaneously with, and are complemented by, translational down regulation of the inhibitor molecule IκB, the importance of one or the other being shifted in accordance with the type and magnitude of the stressing agent or stimuli.
Keywords: Inhibitor of nuclear factor κB, Nuclear factor κB, Eukaryotic initiation factor 2, eIF2α kinase, IκB kinase
History
In 1986 David Baltimore’s laboratory discovered a nuclear protein in mature B cells that binds to a 10 nucleotide stretch of double-stranded DNA in the κ immunoglobulin light chain enhancer (GGGACTTTCC) [1]. It was soon proven that this nuclear factor had a role in the mediated expression of the κ light chain and that it’s localization in the nuclei is associated with different cellular stimuli [2]. Further studies have shown that NF-κB is involved in the regulation of the expressions of many genes that are mostly related to the immune and inflammatory response, along with genes determining developmental processes, cellular growth, and apoptosis [3, 4].
NF-κB family members
The mammalian NF-κB family is composed of five members, i.e., p65 (RelA), RelB, NF-κB1 (p50 and its precursor p105), c-Rel, and NF-κB2 (p52 and its precursor p100) [5, 6]. They all have in common a 300 amino acid Rel homology domain (RHD) located close to the N terminus of the protein [7]. However, while p65 and p50 were found to be universally present, the other three members (RelB, cRel, and p52) were suggested to be only expressed in lymphoid cells [8]. The RHD contains sequences are accountable for the homo- or hetero-dimerization of the family members. Of the five members, only three p65, RelB, and c-Rel contain a trans-activation domain (TAD), which is needed to promote transcription by facilitating the employment of activators and banishment of repressors [9]. Subsequently homodimers of the other two members, p52 and p50 are unable to activate transcription. Instead, they attenuate expression of target genes.
The role of IκB in regulation of NF-κB activation
The activity of NF-κB is regulated at multiple levels. The best known regulatory step is the cytoplasmic to nuclear transport of activated NF-κB p65:p50 heterodimer [10, 11]. Without stimulation, cytoplasmic compartmentalization of NF-κB in cells is due to binding through the RHD to a member from the family of proteins called inhibitor of NF-κB (IκB ). IκB family consists of IκBα, IκBβ, IκBε, IκBγ, BCL-3, and the two NF-κB precursors p100 and p105 [12, 13]. IκBα and IκBβ achieve the cytoplasmic localization by masking the nuclear localization sequence (NLS) of amino acids on the NF-κB p65 subunit [14–16]. Failure to mask the NLS of the p65 subunit in addition to the existence of a nuclear export sequence (NES) on IκBα and p65, results in the constant shuttling of IκBα:p65:p50 complexes between the cytoplasm and nucleus. On the other hand, IκBβ:p65:p50 complexes are restricted to the extra nuclear compartment, this phenomena adding to the complexity of NF-κB regulation.
The role of kinases in regulation of NF-κB activation
After removing IκB , a second level of regulation is conferred mainly by stimulus-induced phosphorylation of NF-κB [17]. A protein kinase A (PKA) phosphorylation site was identified on both p65 and c-Rel at Ser 276, located 25 amino acids from the NLS, inside the Rel homology domain (RHD) [18]. Over-expression of PKA leads to a higher DNA-binding activity of NF-κB. This is mainly due to the fact that phosphorylated Ser 276 inhibits intermolecular association with inhibitors, thus facilitating nuclearization and DNA binding [17, 19]. The same phosphorylation also promotes interaction with coactivator CREB binding protein (CBP/p300) [18]. A similar mechanism of NF-κB activation was identified during tumor necrosis factor α (TNFα) stimulation when p65 phosphorylation occurred at Ser 529 mediated by casein kinase II (CKII) [20, 21]. Also during TNFα stimulation another activating phosphorylation occurs at Ser 536 by none other than IKK [22]. It is worthy to note that the same catalytic activity of IKK is required for IκB phosphorylation followed by ubiquitination and NF-κB activation by direct phosphorylation, fact that adds to the complexity of IKK mediated NF-κB activation [23]. The activity of stimulated NF-κB is down regulated by a feedback pathway through the newly synthesized IκBα, one of the first genes activated by NF-κB. The re-synthesized IκBα enters the nucleus, binds to NF-κB and exports it to the cytosol, thus inhibiting its functionality [24, 25].
The classical NF-κB activation mechanism
Upon extra- or intracellular stimulation the IκBs are phosphorylated by an IκB kinase (IKK), ubiquitin targeted and undergo proteosomal degradation thus automatically exposing the NLS necessary for NF-κB nuclear localization [26, 27]. IKK is a 700 kDa protein complex consisting of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ or NEMO—NF-κB essential modulator) [28–30]. Activation of the catalytic subunits takes place by phosphorylation followed by intra- and intermolecular trans-autophosphorylation releasing their kinase domains. A host of NF-κB inducers have been recognized so far, they consist of but are not limited to proinflamatory cytokines (TNFα, IL-1, etc.), double-stranded RNA (dsRNA), viruses and a variety of cell stressors like ultraviolet light (UV), reactive oxygen species (ROS), and genotoxic agents [7, 31]. Some of NF-κB activators, such as cytokines, achieve activation through the classical activation mechanism (Fig. 1), while others like UV, ROS, heat shock, and hypoxia regulate NF-κB through much more branched and complex cellular pathways [32–35]. The common feature of these general inducers is that they cause translation inhibition as a defense cellular response through their noxious effects.
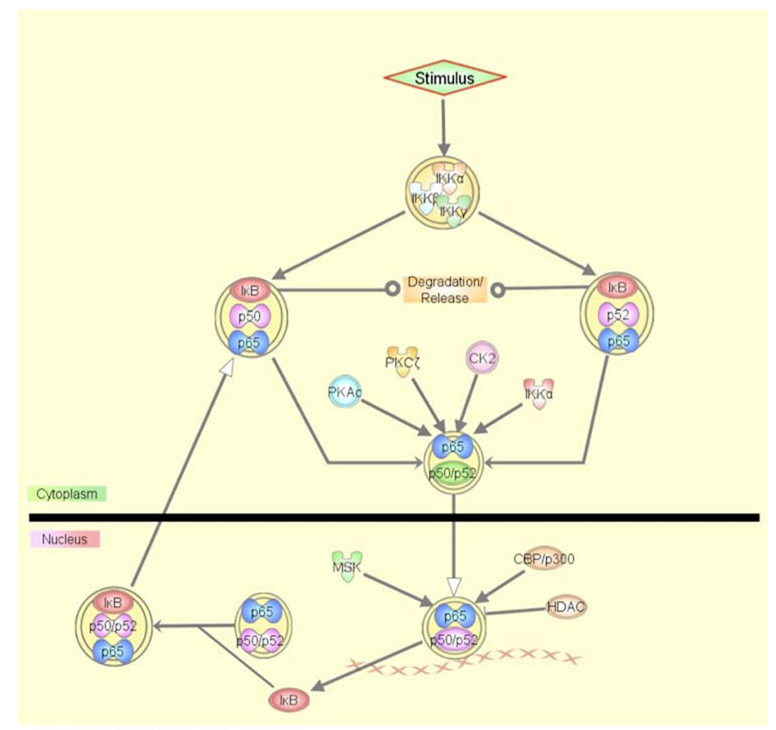
Fig. 1.
The classical NF-κB activation pathway. Stimulus induced IKK phosphorylates IκB inducing its degradation. Free NF-κB translocates to the nucleus and binds to the target DNA, while its transactivation efficiency and ability to recruit other activators is further regulated by different kinases. IκB provides feedback inhibition through expulsion of NF-κB from the nucleus. The graphical representation of the molecular network was generated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems). All lines are supported by at least one reference from the literature or from information of canonical pathways stored in the Ingenuity Pathways Knowledge Base
The impact of translation initiation on NF-κB activation
An entirely different approach to NF-κB activation is provided by translational regulation via the eukaryotic initiation factor 2 (eIF2). During the initiation step of translation, eIF2 forms a complex with GTP and Met-tRNA forming a ternary complex, which associated with the small ribosomal unit contributes to the selection of the start codon. The release from the ribosome is achieved at the expense of hydrolization of GTP to GDP. In order to restart the initiation cycle the guanine exchange factor eIF2B refreshes the eIF2-GDP to eIF2-GTP [36]. The phosphorylation on Ser 51 of the α subunit of eIF2 (eIF2α) stabilizes the eIF2-GDP-eIF2B initiation complex preventing GDP-GTP exchange, thus halting the translational initiation process [37, 38]. The eIF2α phosphorylation inhibits initiation of protein synthesis at a general level, allowing only the selective translation of some proteins that are required for mounting a stress response [39, 40].
Key players in translational regulation are a host of serine–threonine kinases that can phosphorylate the Ser 51 of eIF2α. Four eIF2α kinases (EIF2AKs) have been identified. While each of the EIF2AKs has its own specific inducers, some stimulus such as UV and hypoxia also activate one or more of the kinases (Fig. 2).
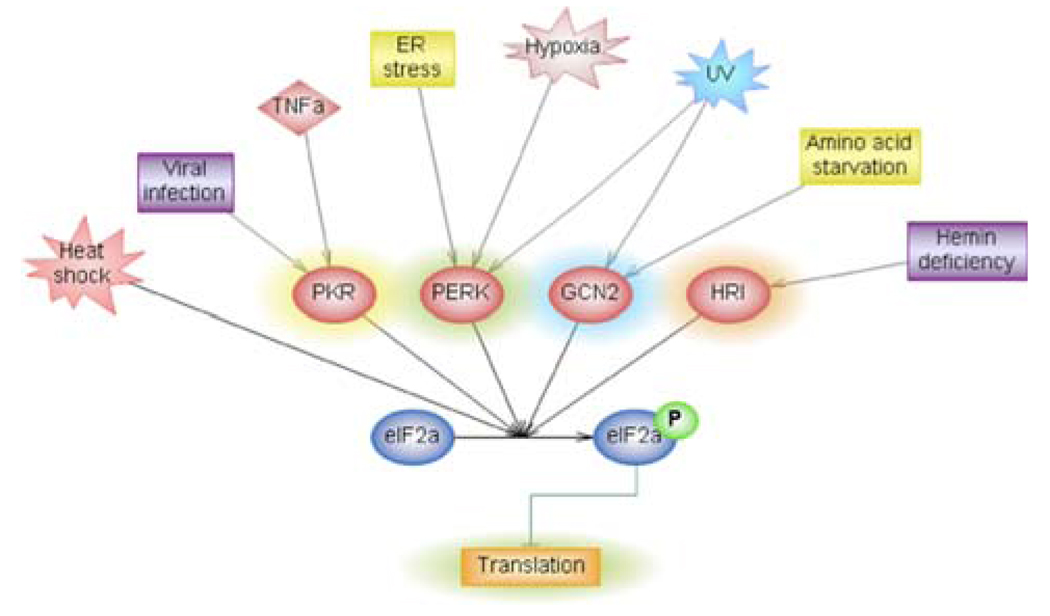
Fig. 2.
The eIF2 kinase regulated signaling pathways. A number of stimuli achieve eIF2α phosphorylation through the four known eIF2α kinases. The graphical representation of the molecular network was generated through the use of Pathway Studio 6 (Ariadne Genomics®). All lines are supported by at least one reference from previously published literature stored in the Pathway Studio database
EIF2AK1, known as the heme-regulated inhibitor kinase (HRI), is a critical component during erythroid maturation that regulates the stoichiometric ratio of hemoglobin components, i.e., α-globin, β-globin, and heme [41]. Two separate heme binding sites were identified in HRI [42]. HRI is activated by heme deficiency in multi-stages through series of auto-phosphorylations [43]. The phosphorylation of HRI first stabilizes its monomer that lacks eIF2α kinase activity, but has first heme-binding site occupied. Further phosphorylation of HRI induces the dimerization and confers heme sensitivity [44]. During high heme concentrations, heme binds to the second binding site inhibiting HRI kinase activity thus allowing for protein and implicitly hemoglobin translation [45]. In the situation of insufficient heme accumulation the second heme-binding site remains unoccupied, which leads to the induction of HRI kinase activity and inhibition of translation by eIF2α phosphorylation [46]. While heme deficiency leads to activation of NF-κB, there is no direct evidence yet to show that the translation inhibition is involved in the activation of signaling pathways. Besides heme deficiency, other NF-κB activator, such as arsenite-induced oxidative stress and heat shock were also found to activate HRI [47].
EIF2AK2, known as the interferon-induced double-stranded (ds) RNA-dependent protein kinase (PKR), plays a critical role in anti-viral defense [48]. The binding of the dsRNA exposes an ATP-binding site inducing dimerization and subsequent auto-phosphorylation leading to an active form of PKR [49–51].Avariety of stimuli, like growth factors and cytokines, activate PKR independently of dsRNA through PKR-associated activator proteins [52, 53]. Initially, PKR was suggested to directly phosphorylate IκB [54]. However, the hypothesis was challenged by results showing that kinase inactivated PKR is still capable of activating NF-κB [55]. Furthermore, co-immunoprecipitation analysis demonstrated that PKR forms a complex with IKK independent of its ability of activation of NF-κB [55, 56]. Based on these findings, it was proposed that PKR binds to the IKK complex or acts upstream facilitating IKK to phosphorylate IκBα at serines 32 and 36 [55–58]. Conversely, it has also been reported by others that PKR mutants that are unable to activate NF-κB still preserve their ability to co-immunoprecipitate with IKK [56]. While the roles of PKR and its catalytic activity in NF-κB activation remain controversial [55–58], several PKR activators, such as dsRNA and interferon γ (IFNγ) have been shown to induce NF-κB activation. Regardless of PKRs’ inability to activate NF-κB independently of its kinase function, activated PKR does nevertheless phosphorylate eIF2α thus inhibiting global translation and potentially can decrease IκB synthesis.
EIF2AK3, also known as the PKR like endoplasmic reticulum (ER) related kinase (PERK), is an ER membrane localized kinase [59–61]. Its inactive monomer state is stabilized by an ER chaperone immunoglobulin (Ig) heavy chain binding protein (BiP). Under ER-stress, BiP releases PERK, which undergoes dimerization, trans-phosphorylation and sequentially activation [60–63]. Outside or inside perturbations negatively affect protein-folding process in ER resulting in an accumulation of malfolded proteins, which triggers the unfolded protein response (UPR). While UPR transcriptionally activates the expression of ER chaperone to facilitate the folding process, it translationally inhibits general protein synthesis through phosphorylating eIF2α to reduce the accumulation of newly synthesized proteins in ER [64]. The converging point between the accumulation of unfolded proteins and global translation inhibition by eIF2α phosphorylation was determined to be PERK [59, 65]. The PERK-mediated eIF2α phosphorylation and translation inhibition was shown to be directly involved in ER-stress-mediated NF-κB activation upon various stimuli, such as hypoxia, UV, and thapsigargin [33, 34, 66–69].
EIF2AK4 is also known as the amino acid starvation dependent general control of amino acid biosynthesis kinase (GCN2) [70, 71]. It is an amino acid abundance controlled eIF2α kinase, which is activated during amino acid starvation. Its specific role is to halt protein translation while activating the translation of factors that are needed in amino acid synthesis [61, 72]. The activation mechanism involves a histidyl-tRNA synthase (HisRS) homologous sequence, where the excess of uncharged tRNAs bind during amino acid deprivation [73]. A C-terminal RNA binding region is also required for its dimerization, activation, and association with ribosome [74, 75]. The GCN2-mediated eIF2α phosphorylation and translation inhibition was shown to be directly involved in amino acid starvation induced NF-κB activation [66]. Besides nutritional stresses, the HisRS similar sequence also allows for activation by other stresses, such as UV and proteosome inhibition [71, 72]. While there is no evidence yet to show that GCN2 is directly involved in NF-κB activation upon proteosome inhibition, it has been demonstrated that GCN2 mediates UV-induced NF-κB activation [76].
Besides eIF2α another initiation factor was recently also found to regulate NF-κB. This is the eukaryotic initiation factor 4E (eIF4E), which facilitates translation by binding to the 5′ cap structure of the mRNA. Although for now the studies fall short of providing any details for the activation mechanism, they offer other possible alternatives to the classical NF-κB activation pathway [77].
Regulation of IκB turnover
NF-κB is stranded in the cytoplasm bound by its inhibitor protein IκB. Even though both IκBα and IκBβ are able to inhibit NF-κB, it is IκBα that bears the major role in regulating its activation [78]. NF-κB is a transcription factor that has a fast response time in order to react promptly to cellular stress. In order to achieve this fast activation, the IκB levels are tightly and rapidly regulated [79]. While activation of receptor signaling cascade, such as TNFα and interleukin-1 (IL-1), often leads to phosphorylation, ubiquitination, and proteolysis of IκB , the more general cellular stimulus, such as UV and hypoxia, also possess the ability to induce translational inhibition of IκB synthesis.
IκB degradation occurs through two mechanisms, i.e., a signal-dependent and signal-independent (basal degradation) process [80]. IκB turnover is tightly linked to its structural domains. The centrally located ankyrin repeats are necessary for NF-κB binding and the two terminal regions are implicated in the degradation of IκB . The N-terminal sequence contains two IKK phosphorylation sites Ser 32 and 36 [81–84] and two ubiquitination sites Lys 21 and 22 [85, 86]. The phosphorylation and ubiquitination of these sites promote IκB degradation in the 26S proteosome. The C-terminal region contains a PEST domain (Pro, Glu, Asp, Ser, and Thr rich regions), which is associated in general with high turnover proteins [87]. The PEST site in addition to multiple casein kinase II (CKII) phosphorylation sites on the C-terminal region are needed for both signal-induced degradation [84, 88, 89] and basal turnover of IκB [17, 82, 90, 91].
The rate of degradation of IκB is also very much influenced by its association with NF-κB. Free IκB has a 30–40 min half-life, but the NF-κB associated one has a fivefold longer degradation time [92–95]. Free IκB constitutes only a 15% fraction of the total cellular IκB [92], and is a weak substrate for IKK phosphorylation [96]. The basal turnover of free IκB requires the CKII phosphorylation sites while the signal-dependent degradation is induced by IKK phosphorylation. For the NF-κB associated IκB , the basal turnover is also regulated by CKII phosphorylation, while the signal-induced degradation is regulated by both CKII and IKK phosphorylation [80, 95, 97–99] (Table 1).
Table 1.
Role of kinases in regulation of IκB turnover
| IκB state | Basal turnover | Signal-induced degradation |
|---|---|---|
| Free | CKII | IKK |
| NF-κB associated | CKII | IKK + CKII |
While IKK and CKII regulate the removal rate of IκB , the EIF2AKs determine the synthetic rate of IκB . The phosphorylation of eIF2α by EIF2AK leads to the inhibition of global protein synthesis, including IκB. Since IκB has a relatively high basal turnover rate [79], the inhibition of new IκB synthesis results in a rapid depletion of IκB thus shifting the dynamic balance from NF-κB associated IκB toward free NF-κB and IκB (Fig. 3).
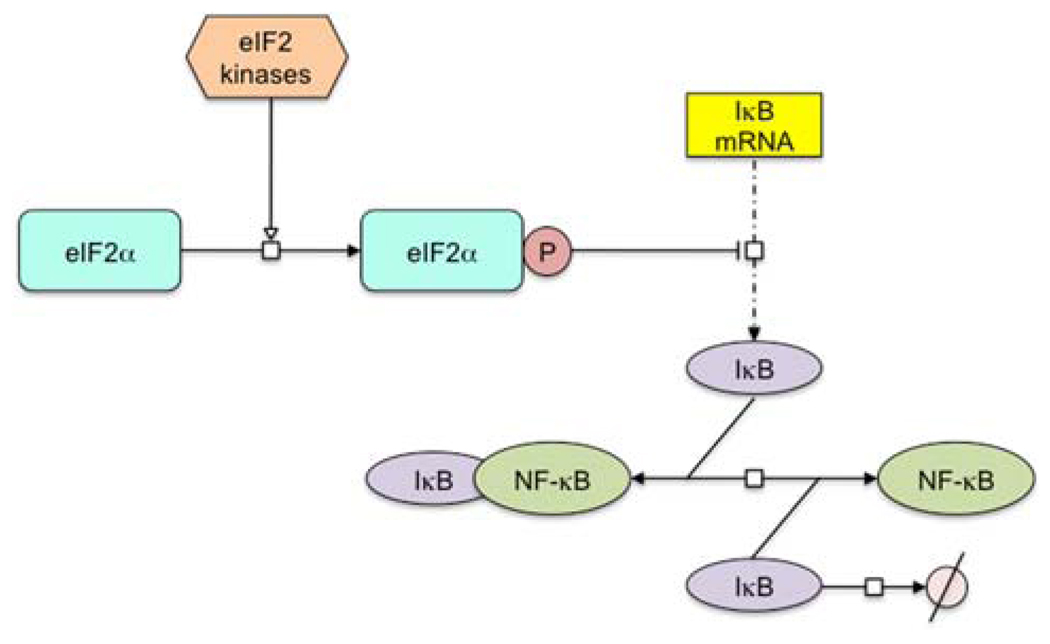
Fig. 3.
Model for translation regulation of NF-κB activation. The eIF2 kinases phosphorylate the α subunit of eIF2, which results in the translation inhibition of IκB synthesis. The reduction of IκB leads to the dissociation of IκB-NF-κB complex and subsequent NF-κB activation. The molecular network was depicted with CellDesigner® diagram editor
Targeting NF-κB for therapeutic development
NF-κB plays an important role in regulation of the process of innate and adaptive immune responses. Its ability to activate transcription of genes encoding cytokines (e.g., TNFα, IL-1, IL-2, and IL-6), chemokines, adhesion molecules (e.g., ICAM, VCAM, and E-selectin), inducible enzymes (e.g., iNOS and COX-2), and antimicrobial peptides (β defensine) gives it a central role in the overall process of immune response [78]. NF-κB also regulates genes outside the immune system presumably having an anti-apoptotic effect that would give an opportunity to the cell to repair DNA damage. Deregulation of these genes may lead to many diseases, such as cancer, atherosclerosis, arthritis, AIDS, etc. [3]. NF-κB has been a target for the development of therapeutics for many diseases [100]. Since eIF2α phosphorylation also impacts NF-κB activation, compounds that affect eIF2α phosphorylation through the aforementioned kinases will be potential therapeutics for treatment of various diseases. Indeed, several ER-stress inducing drugs are already in the spotlight for their ability to induce apoptosis in malignant cells. The chemotherapeutic agents doxorubicin and cisplatin, although known to mainly target DNA, were also shown to induce ER-stress and activate PERK [101–104]. Interferon and TNF-α are both antiviral proteins that have been used in combination with chemo- and radiation therapy and that possess the ability to activate PKR [105, 106]. In addition, the potential for successful use of proteasome inhibitors for cancer treatment may be granted by the ability of these compounds to induce apoptosis through the blocking of protein degradation, which implicitly leads to ER-stress. The anti-multiple myeloma drug Velcade (PS-341), for example, which is a proteasome inhibitor, inhibits IκB degradation. In fact, Velcade was also shown to disrupt protein folding in the ER resulting in ER-stress [107–109]. In summary, elucidating the role of EIF2AK in mediation of NF-κB activation may lead us to a better understanding of the mechanisms of current NF-κB targeting drugs and development of new therapeutics to treat diseases related to deregulation of NF-κB.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 CA86926 (to S. W.) and R56 CA086928 (to S. W.).
References
- 1.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [PubMed] [Google Scholar]
- 2.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. doi: 10.1016/0092-8674(86)90807-X. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Investig. 2001;107:3–6. doi: 10.1172/JCI11891. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 5.Duckett CS, Perkins ND, Kowalik TF, et al. Dimerization of NF-KB2 with RelA(p65) regulates DNA binding, transcriptional activation, and inhibition by an I kappa B-alpha (MAD-3) Mol Cell Biol. 1993;13:1315–1322. doi: 10.1128/mcb.13.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm S, Baeuerle PA. The inducible transcription factor NF-kappa B: structure–function relationship of its protein subunits. Biochem J. 1993;290(Pt 2):297–308. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 8.Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–429. doi: 10.1128/CMR.15.3.414-429.2002. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H, May MJ, Jimi E, et al. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. doi: 10.1016/S1097-2765(02)00477-X. [DOI] [PubMed] [Google Scholar]
- 10.Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988;53:211–217. doi: 10.1016/0092-8674(88)90382-0. doi: 10.1016/0092-8674 (88)90382-0. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 12.Naumann M, Nieters A, Hatada EN, et al. NF-kappa B precursor p100 inhibits nuclear translocation and DNA binding of NF-kappa B/rel-factors. Oncogene. 1993;8:2275–2281. [PubMed] [Google Scholar]
- 13.Naumann M, Wulczyn FG, Scheidereit C. The NF-kappa B precursor p105 and the proto-oncogene product Bcl-3 are I kappa B molecules and control nuclear translocation of NF-kappa B. EMBO J. 1993;12:213–222. doi: 10.1002/j.1460-2075.1993.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huxford T, Huang DB, Malek S, et al. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. doi: 10.1016/S0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 15.Malek S, Huxford T, Ghosh G. Ikappa Balpha functions through direct contacts with the nuclear localization signals and the DNA binding sequences of NF-kappaB. J Biol Chem. 1998;273:25427–25435. doi: 10.1074/jbc.273.39.25427. doi: 10.1074/jbc.273.39.25427. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. doi: 10.1016/S0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 17.Naumann M, Scheidereit C. Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. doi: 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki T, Sakon S, Sasazuki T, et al. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem Biophys Res Commun. 2003;300:807–812. doi: 10.1016/s0006-291x(02)02932-7. doi: 10.1016/S0006-291X(02)02932-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Baldwin AS., Jr Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Westerheide SD, Hanson JL, et al. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai H, Chiba H, Miyoshi H, et al. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the trans-activation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 23.Tergaonkar V, Bottero V, Ikawa M, et al. IkappaB kinase-independent IkappaBalpha degradation pathway: functional NF-kappaB activity and implications for cancer therapy. Mol Cell Biol. 2003;23:8070–8083. doi: 10.1128/MCB.23.22.8070-8083.2003. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arenzana-Seisdedos F, Thompson J, Rodriguez MS, et al. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown K, Park S, Kanno T, et al. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek S, Chen Y, Huxford T, et al. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J Biol Chem. 2001;276:45225–45235. doi: 10.1074/jbc.M105865200. doi: 10.1074/jbc.M105865200. [DOI] [PubMed] [Google Scholar]
- 27.Malek S, Huang DB, Huxford T, et al. X-ray crystal structure of an IkappaBbeta × NF-kappaB p65 homodimer complex. J Biol Chem. 2003;278:23094–23100. doi: 10.1074/jbc.M301022200. doi: 10.1074/jbc.M301022200. [DOI] [PubMed] [Google Scholar]
- 28.Huang TT, Feinberg SL, Suryanarayanan S, et al. The zinc finger domain of NEMO is selectively required for NF-kappa B activation by UV radiation and topoisomerase inhibitors. Mol Cell Biol. 2002;22:5813–5825. doi: 10.1128/MCB.22.16.5813-5825.2002. doi: 10.1128/MCB.22.16.5813-5825. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothwarf DM, Zandi E, Natoli G, et al. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 30.Zandi E, Rothwarf DM, Delhase M, et al. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. doi: 10.1016/S0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 31.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 32.Laszlo CF, Wu S. Mechanism of UV-induced IkappaBalpha-independent activation of NF-kappaB. Photochem Photobiol. 2008;84:1564–1568. doi: 10.1111/j.1751-1097.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Tan M, Hu Y, et al. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J Biol Chem. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 34.Koumenis C, Naczki C, Koritzinsky M, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curry HA, Clemens RA, Shah S, et al. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J Biol Chem. 1999;274:23061–23067. doi: 10.1074/jbc.274.33.23061. doi: 10.1074/jbc.274.33.23061. [DOI] [PubMed] [Google Scholar]
- 36.Hershey JW, Asano K, Naranda T, et al. Conservation and diversity in the structure of translation initiation factor EIF3 from humans and yeast. Biochimie. 1996;78:903–907. doi: 10.1016/s0300-9084(97)86711-9. doi: 10.1016/S0300-9084(97)86711-9. [DOI] [PubMed] [Google Scholar]
- 37.Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 38.Sudhakar A, Ramachandran A, Ghosh S, et al. Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry. 2000;39:12929–12938. doi: 10.1021/bi0008682. doi: 10.1021/bi0008682. [DOI] [PubMed] [Google Scholar]
- 39.Deng J, Lu PD, Zhang Y, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wek RC. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem Sci. 1994;19:491–496. doi: 10.1016/0968-0004(94)90136-8. doi: 10.1016/0968-0004(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 41.Chen JJ, London IM. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. doi: 10.1016/S0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- 42.Chefalo PJ, Oh J, Rafie-Kolpin M, et al. Heme-regulated eIF-2alpha kinase purifies as a hemoprotein. Eur J Biochem. 1998;258:820–830. doi: 10.1046/j.1432-1327.1998.2580820.x. doi: 10.1046/j.1432-1327.1998.2580820.x. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell CR, Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969;35:79–85. doi: 10.1016/0006-291x(69)90485-9. doi: 10.1016/0006-291X(69)90485-9. [DOI] [PubMed] [Google Scholar]
- 44.Bauer BN, Rafie-Kolpin M, Lu L, et al. Multiple autophosphorylation is essential for the formation of the active and stable homodimer of heme-regulated eIF2alpha kinase. Biochemistry. 2001;40:11543–11551. doi: 10.1021/bi010983s. doi: 10.1021/bi010983s. [DOI] [PubMed] [Google Scholar]
- 45.Rafie-Kolpin M, Chefalo PJ, Hussain Z, et al. Two heme-binding domains of heme-regulated eukaryotic initiation factor-2alpha kinase. N terminus and kinase insertion. J Biol Chem. 2000;275:5171–5178. doi: 10.1074/jbc.275.7.5171. doi: 10.1074/jbc.275.7.5171. [DOI] [PubMed] [Google Scholar]
- 46.Rafie-Kolpin M, Han AP, Chen JJ. Autophosphorylation of threonine 485 in the activation loop is essential for attaining eIF2alpha kinase activity of HRI. Biochemistry. 2003;42:6536–6544. doi: 10.1021/bi034005v. doi: 10.1021/bi034005v. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green SR, Mathews MB. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev. 1992;6:2478–2490. doi: 10.1101/gad.6.12b.2478. doi: 10.1101/gad.6.12b.2478. [DOI] [PubMed] [Google Scholar]
- 49.Galabru J, Katze MG, Robert N, et al. The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur J Biochem. 1989;178:581–589. doi: 10.1111/j.1432-1033.1989.tb14485.x. doi: 10.1111/j.1432-1033.1989.tb14485.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291–1296. doi: 10.1074/jbc.272.2.1291. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Rehemtulla A, Gupta NK, et al. A eukaryotic translation initiation factor 2-associated 67 kDa glycoprotein partially reverses protein synthesis inhibition by activated double-stranded RNA-dependent protein kinase in intact cells. Biochemistry. 1996;35:8275–8280. doi: 10.1021/bi953028+. doi: 10.1021/bi953028+ [DOI] [PubMed] [Google Scholar]
- 52.Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 53.Patel RC, Sen GC. Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J Biol Chem. 1992;267:7671–7676. [PubMed] [Google Scholar]
- 54.Kumar A, Haque J, Lacoste J, et al. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii T, Kwon H, Hiscott J, et al. Activation of the I kappa B alpha kinase (IKK) complex by double-stranded RNA-binding defective and catalytic inactive mutants of the interferon-inducible protein kinase PKR. Oncogene. 2001;20:1900–1912. doi: 10.1038/sj.onc.1204267. doi: 10.1038/sj.onc.1204267. [DOI] [PubMed] [Google Scholar]
- 56.Gil J, Rullas J, Garcia MA, et al. The catalytic activity of dsRNA-dependent protein kinase, PKR, is required for NF-kappaB activation. Oncogene. 2001;20:385–394. doi: 10.1038/sj.onc.1204109. doi: 10.1038/sj.onc.1204109. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet MC, Weil R, Dam E, et al. PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol. 2000;20:4532–4542. doi: 10.1128/mcb.20.13.4532-4542.2000. doi: 10.1128/MCB.20.13.4532-4542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gil J, Alcami J, Esteban M. Activation of NF-kappa B by the dsRNA-dependent protein kinase, PKR involves the I kappa B kinase complex. Oncogene. 2000;19:1369–1378. doi: 10.1038/sj.onc.1203448. doi: 10.1038/sj.onc.1203448. [DOI] [PubMed] [Google Scholar]
- 59.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 60.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 61.Sonenberg N, Hershey JWB, Mathews M. Translational control of gene expression. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. p x, 1020 pp. [Google Scholar]
- 62.Brostrom CO, Bocckino SB, Brostrom MA. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983;258:14390–14399. [PubMed] [Google Scholar]
- 63.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 64.Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. doi: 10.1016/S0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y, Vattem KM, Sood R, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang HY, Wek SA, McGrath BC, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imaizumi K, Tohyama M. The regulation of unfolded protein response by OASIS, a transmembrane bZIP transcription factor, in astrocytes. Nippon Yakurigaku Zasshi. 2004;124:383–390. doi: 10.1254/fpj.124.383. doi: 10.1254/fpj.124.383. [DOI] [PubMed] [Google Scholar]
- 68.Wu S, Hu Y, Wang JL, et al. Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2002;277:18077–18083. doi: 10.1074/jbc.M110164200. doi: 10.1074/jbc.M110164200. [DOI] [PubMed] [Google Scholar]
- 69.Pahl HL, Baeuerle PA. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 70.Berlanga JJ, Santoyo J, De Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem. 1999;265:754–762. doi: 10.1046/j.1432-1327.1999.00780.x. doi: 10.1046/j.1432-1327.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 71.Sood R, Porter AC, Olsen D, et al. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimball SR, Antonetti DA, Brawley RM, et al. Mechanism of inhibition of peptide chain initiation by amino acid deprivation in perfused rat liver. Regulation involving inhibition of eukaryotic initiation factor 2 alpha phosphatase activity. J Biol Chem. 1991;266:1969–1976. [PubMed] [Google Scholar]
- 73.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. doi: 10.1042/BST0340007. [DOI] [PubMed] [Google Scholar]
- 74.Qiu H, Garcia-Barrio MT, Hinnebusch AG. Dimerization by translation initiation factor 2 kinase GCN2 is mediated by interactions in the C-terminal ribosome-binding region and the protein kinase domain. Mol Cell Biol. 1998;18:2697–2711. doi: 10.1128/mcb.18.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramirez M, Wek RC, Hinnebusch AG. Ribosome association of GCN2 protein kinase, a translational activator of the GCN4 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3027–3036. doi: 10.1128/mcb.11.6.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang HY, Wek RC. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochem J. 2005;385:371–380. doi: 10.1042/BJ20041164. doi: 10.1042/BJ20041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenwald IB, Koifman L, Savas L, et al. Expression of the translation initiation factors eIF-4E and eIF-2* is frequently increased in neoplastic cells of Hodgkin lymphoma. Hum Pathol. 2008;39:910–916. doi: 10.1016/j.humpath.2007.10.021. doi: 10.1016/j.humpath.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 78.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 79.Mathes E, O’Dea EL, Hoffmann A, et al. NF-kappaB dictates the degradation pathway of IkappaBalpha. EMBO J. 2008;27:1357–1367. doi: 10.1038/emboj.2008.73. doi: 10.1038/emboj.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krappmann D, Wulczyn FG, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-kappaB inhibitor IkappaB alpha in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 81.Brockman JA, Scherer DC, McKinsey TA, et al. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whiteside ST, Ernst MK, LeBail O, et al. N- and C-terminal sequences control degradation of MAD3/I kappa B alpha in response to inducers of NF-kappa B activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Traenckner EB, Pahl HL, Henkel T, et al. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benkowski LA, Ravel JM, Browning KS. mRNA binding properties of wheat germ protein synthesis initiation factor 2. Biochem Biophys Res Commun. 1995;214:1033–1039. doi: 10.1006/bbrc.1995.2389. doi: 10.1006/bbrc.1995.2389. [DOI] [PubMed] [Google Scholar]
- 85.Scherer DC, Brockman JA, Chen Z, et al. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baldi L, Brown K, Franzoso G, et al. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of I kappa B-alpha. J Biol Chem. 1996;271:376–379. doi: 10.1074/jbc.271.1.376. doi: 10. 1074/jbc.271.1.376. [DOI] [PubMed] [Google Scholar]
- 87.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 88.Sun S, Elwood J, Greene WC. Both amino- and carboxyl-terminal sequences within I kappa B alpha regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aoki T, Sano Y, Yamamoto T, et al. The ankyrin repeats but not the PEST-like sequences are required for signal-dependent degradation of IkappaBalpha. Oncogene. 1996;12:1159–1164. [PubMed] [Google Scholar]
- 90.Barroga CF, Stevenson JK, Schwarz EM, et al. Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proc Natl Acad Sci USA. 1995;92:7637–7641. doi: 10.1073/pnas.92.17.7637. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mellits KH, Hay RT, Goodbourn S. Proteolytic degradation of MAD3 (I kappa B alpha) and enhanced processing of the NF-kappa B precursor p105 are obligatory steps in the activation of NF-kappa B. Nucleic Acids Res. 1993;21:5059–5066. doi: 10.1093/nar/21.22.5059. doi: 10.1093/nar/21.22.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rice NR, Ernst MK. In vivo control of NF-kappa B activation by I kappa B alpha. EMBO J. 1993;12:4685–4695. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henkel T, Machleidt T, Alkalay I, et al. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 94.Miyamoto S, Chiao PJ, Verma IM. Enhanced I kappa B alpha degradation is responsible for constitutive NF-kappa B activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pando MP, Verma IM. Signal-dependent and -independent degradation of free and NF-kappa B-bound IkappaBalpha. J Biol Chem. 2000;275:21278–21286. doi: 10.1074/jbc.M002532200. doi: 10.1074/jbc.M002532200. [DOI] [PubMed] [Google Scholar]
- 96.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 97.Schwarz EM, Van Antwerp D, Verma IM. Constitutive phosphorylation of IkappaBalpha by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IkappaBalpha. Mol Cell Biol. 1996;16:3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato T, Jr, Delhase M, Hoffmann A, et al. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. doi: 10.1016/S1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 99.Alvarez-Castelao B, Castano JG. Mechanism of direct degradation of IkappaBalpha by 20S proteasome. FEBS Lett. 2005;579:4797–4802. doi: 10.1016/j.febslet.2005.07.060. doi: 10.1016/j.febslet.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 100.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 101.Jang YM, Kendaiah S, Drew B, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 102.Mandic A, Hansson J, Linder S, et al. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- 103.Ranganathan AC, Zhang L, Adam AP, et al. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fribley AM, Evenchik B, Zeng Q, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 105.Meurs E, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. doi: 10.1016/0092-8674(90)90374-N. [DOI] [PubMed] [Google Scholar]
- 106.Yeung MC, Liu J, Lau AS. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 108.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee AH, Iwakoshi NN, Anderson KC, et al. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci USA. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]