Abstract
Adipose tissue (AT) can accumulate macrophages and secrete several inflammatory mediators. Despite its pivotal role in the progression of chronic inflammatory processes such as atherosclerosis, adaptive immunity’s role in obesity remains poorly explored.
Visceral AT of diet-induced obese C57BL/6 mice had higher numbers of both CD4+ and CD8+ T cells than lean controls, monitored by flow cytometry. When stimulated in vitro, T cells from obese AT produced more interferon-gamma (IFNγ) than those from controls. AT from obese animals also had more cells expressing I-Ab, a mouse class II histocompatibility marker implicated in antigen presentation, as determined by immunostaining. Differentiated 3T3-L1 cells stimulated with recombinant IFNγ or T helper-1 (Th1)-derived supernatant produced several chemokines and their mRNAs. Obese IFNγ-deficient animals had significantly reduced AT expression of mRNA-encoding inflammatory genes such as tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein-1 (MCP-1), decreased AT inflammatory cell accumulation, and better glucose tolerance than control animals consuming the same diet. Obese mice doubly deficient for IFNγ receptor and ApoE on a mixed 129SvEv/C57BL/6 (129/B6) genetic background, despite exhibiting similar AT mRNA levels of TNFα and MCP-1 as 129/B6-ApoE−/− controls, had decreased expression of important T cell related genes, such as interferon-gamma inducible protein (IP-10) and I-Ab, and lower plasma triglycerides and glucose.
These results indicate a role for T cells and IFNγ, a prototypical Th1 cytokine, in regulation of the inflammatory response that accompanies obesity.
Keywords: Inflammation, obesity, adipose tissue, T cell, IFNγ
Introduction
The recent development of a worldwide obesity pandemic has gained wide recognition1, 2. Approximately two-thirds of U.S. adults are either overweight or obese3. These alarming statistics portend a gigantic health burden, as excess adiposity entrains an array of atherogenic risk factors, including diabetes and dyslipidemia4. From a mechanistic perspective, both obesity and atherosclerosis involve chronic low-grade inflammation.
Obesity associates with macrophage accumulation in white adipose tissue (AT)5, 6, where these infiltrating cells interact with adipocytes and endothelial cells, comprising a local inflammatory network7–9. This crosstalk results in the production of multiple cytokines and chemokines, such as tumor necrosis factor-alpha (TNFα)5 and monocyte chemoattractant protein-1 (MCP-1)10, which can activate, propagate, and sustain the local inflammatory response in AT11.
Similarly, the inflammatory process within the arterial wall that characterizes atherosclerosis involves accumulation and activation of macrophages12–14. These phagocytes in atheromata also interact with local cells and secrete multiple mediators that modulate lesion evolution and complication. Other leukocytes also contribute to atherosclerosis. T cells orchestrate the inflammatory cascade evolution, depending on the set of cytokines they predominantly produce, T helper 1 (Th1) or T helper 2 (Th2)12, 13, 15. Interferon-gamma (IFNγ), the signature Th1 cytokine, elicits the production of macrophage mediators, induces leukocyte adhesion molecules, chemokines, and class II major histocompatibility antigens, and increases antigen-presenting capacity by macrophages and endothelial cells16–18. The enhanced antigen-presenting capacity of these cells can participate in adaptive immunity, an important contributor to the progression of atherosclerotic lesions18. Yet, the role of T cells in AT remains poorly explored. A previous study reported a greater number of T cells in white AT of mice fed a high-fat diet19, but the operation of Th1 cytokines such as IFNγ in the AT inflammatory network remains unknown.
The current study examined the participation of T cells, particularly of the signature Th1 cytokine IFNγ, in the setting of fat inflammation. In vitro data show that IFNγ modulates important functions of adipocytes. Our in vivo results affirm that IFNγ regulates the inflammatory response in obese AT and establish a novel mechanism by which mediators of adaptive immunity can contribute to the complications of obesity.
Methods
Diet-induced obesity in mice
Male C57BL/6J (Taconics) and IFNγ-deficient (IFNγ−/−) (The Jackson Laboratory) mice were fed ad libitum a standard low-fat diet (LF; PicoLab Rodent Chow 5053; 13% kcal from fat) after weaning until 6 weeks old. Mice were then switched to high-fat diet (HF; D12108 from Research Diets; 40% kcal from fat, 1.25% cholesterol, 0% cholate) and kept on this diet for 15 or 21 weeks. Lean controls were maintained on LF diet throughout the experiment.
Apolipoprotein E-deficient (ApoE−/−) (The Jackson Laboratory) and IFNγ-receptor-deficient ApoE−/− (IFNγR−/−ApoE−/−) mice were switched from LF to HF diet 8 weeks after weaning and kept on this diet for 8 weeks. The ApoE−/− and IFNγR−/−ApoE−/− animals used in the experiments all descended from the initial set of ApoE−/− and IFNγR−/−, in C57BL/6 and 129SvEv (129) genetic backgrounds, respectively. We crossed 129-IFNγR−/− and C57BL/6-ApoE−/− mice to generate F1 offspring heterozygous for both genes with a mixed 129/C57BL/6 background. From F1 interbreeding, we generated the 129/C57BL/6 F2 offspring. We subsequently crossed F2 × F2 and obtained 129/C57BL/6-ApoE−/− and 129/C57BL/6-IFNγR−/− ApoE−/−. In our experiments we used ApoE−/− and IFNγR−/−ApoE−/− animals from the same generation (F3) of siblings in an attempt to homogenize the genetic backgrounds. All genotyping was performed by PCR of DNA extracted from mouse tails.
On the day of harvesting, mice were anesthetized with 2,2,2-tribromoethanol (2.5mg/10g body weight [BW]) injected intraperitoneally (IP) and received heparin IP.
All experiments involving animals were performed according to a protocol approved by the Standing Committee on Animals of Harvard Medical School.
Analysis of AT-derived stromal vascular cells (SVCs) by flow cytometry
Peri-epididymal fat from lean and obese C57BL/6J mice (15 weeks of LF or HF diet, respectively) was digested as described in the supplementary section. AT-derived SVCs were washed with Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS), counted, labeled with conjugated antibodies for F4/80 (Caltag), CD3 (eBioscience), CD4, CD8, CD11c, and B220 (BD Pharmingen), or their respective isotype controls, and analyzed with a FACScan.
Analysis of inflammatory cells in AT by immunohistochemistry
Peri-epididymal AT (from mice on LF or HF diet for 21 weeks) was fixed as described previously20, and embedded in paraffin. Section staining was performed for rat anti-mouse CD45, Mac-3, I-Ab (BD Pharmingen) and CD3 (Abcam) as described in the supplementary section. Positive cells were counted in 10 consecutive visual fields at the same magnification.
Intracellular staining of AT-derived stromal vascular cells
Equal numbers of stromal vascular cells from lean and obese mice were incubated at 37°C in media with 10 μg/ml of brefeldin A (BD Biosciences) with or without 10 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma) and 1 μg/ml of ionomycin (Sigma). After 4 h of incubation, cells were labeled with conjugated anti-CD3 (eBioscience), fixed and permeabilized with BD Cytofix/Cytoperm kit (BD Biosciences), labeled with conjugated anti-IFNγ (BDPharmingen), and analyzed with a FACScan.
Culture and differentiation of 3T3-L1 cells
Murine 3T3-L1 pre-adipocytes were cultivated and differentiated for 11 days as described in the supplementary section. At day 11, cells were stimulated with recombinant mouse IFNγ (Chemicon) at 10, 50, or 100 U/ml, and harvested 24 h after stimulation.
Organ culture
Peri-epididymal AT (350mg) was extracted from C57BL/6 animals on HF diet, minced and incubated with media alone or media containing 100 U/ml of Murine recombinant IFNγ. Media were collected after 6 h of incubation at 37°C.
Effects of T cells on mature adipocytes
Splenic CD4+ T cells were positively selected from C57BL/6J male mice and cultured and activated in vitro as described in the supplementary section. After seven days, conditioned media were used to stimulate differentiated 3T3-L1 cells for 24 h in the presence or absence of a neutralizing anti-IFNγ antibody at 10 μg/ml.
Cytokine and metabolic determinations
Interferon-gamma-inducible protein (IP-10), monokine induced by interferon-gamma (MIG), and adiponectin were measured using Quantikine ELISA kits (R&D Systems). Plasma levels of leptin were also measured with an ELISA kit (Crystal Chem). MCP-1, TNFα, interleukin-6 (IL-6), interleukin-2 (IL-2), and interleukin-12 (IL-12) were measured by BD Cytometric Bead Array (BD Biosciences). Total plasma cholesterol, triglycerides, and glucose were determined by enzymatic colorimetric methods (Wako).
For glucose-tolerance tests, mice were deprived of food for 12 h and then injected IP with glucose (1 mg/g of BW). Blood from tail vein was used for glucose measurement by a blood glucose meter (OneTouch Ultra, LifeScan) at time 0, and 20, 40, 60, 90, and 120 minutes after glucose administration.
Microarray analysis
Differentiated 3T3-L1 cells were treated or not with 100 U/ml of IFNγ for 4 and 24 h. Total RNA was isolated from 5–6 different plates with RNeasy Mini Kit (Qiagen) and used (10 μg) for microarray screening on Mouse Expression Array 430 2.0 Affymetrix chip. Data were captured using the Affymetrix GeneChip Laboratory Information Management System. Criteria for differential regulation by IFNγ treatment were set as t-statistic >2 at a probability value of <0.05. Genes with chemotactic activity were clustered by dChip application and ranked according to their p values at 24 h21.
Quantification of gene expression by reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from up to 500 mg of AT with RNeasy Lipid Tissue Midi Kit (Qiagen), and equal amounts were reverse transcribed by Superscript II (Invitrogen) according to the manufacturer’s instructions. Quantitative PCR was performed in a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). The sequences of mouse primers used are described in the supplementary section. The mRNA levels of the various genes tested were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used as an internal control in all experiments.
Results
AT from obese mice contains more T cells than that from their lean counterparts
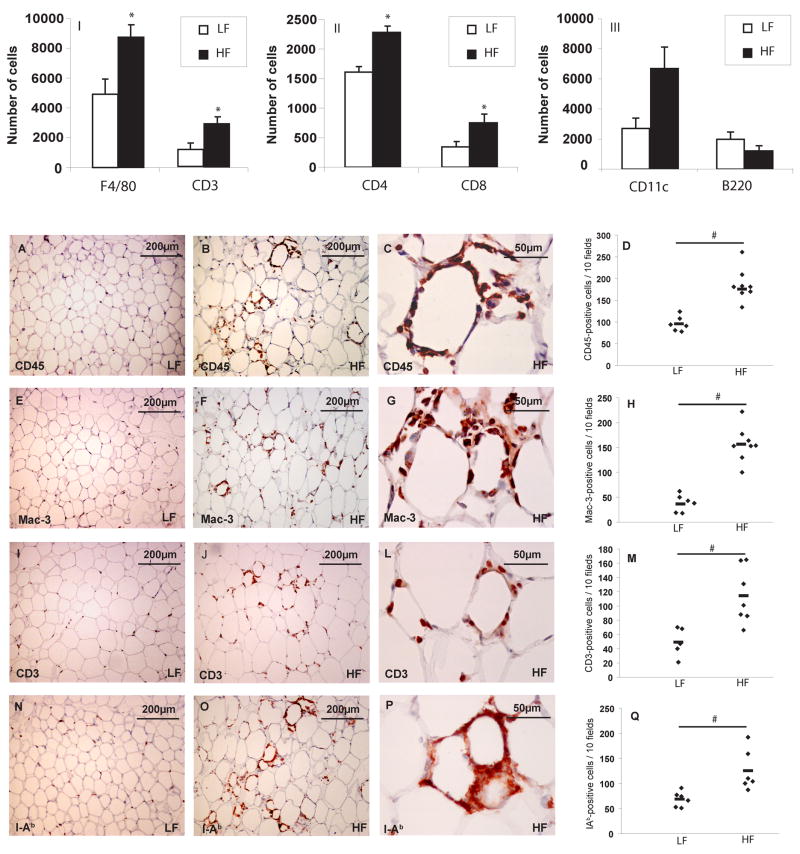
As expected, C57Bl/6J mice that consumed a HF diet had higher BW and more peri-epididymal AT than controls (mean BW after 15 weeks of LF or HF diet: 32.7 g vs 46.3 g, respectively; after 21 weeks: 31.7 g vs 44.4 g, respectively; p value<0.01 in both time points). In accord with previous studies, white AT from obese animals contained more macrophages (F4/80-positive cells) than that from lean ones, as determined by flow cytometry (figure 1–I). AT from obese animals also contained more T lymphocytes bearing CD3, a pan-T cell marker (figure 1–I), including cells of both CD4 and CD8 T cell subsets (figure 1–II). The obese group also tended to have more cells bearing CD11c, a dendritic cell marker, but the difference did not reach statistical significance (figure 1–III). B cell numbers did not differ between the two groups (figure 1–III).
Figure 1. Visceral AT from obese mice contains more inflammatory cells than that from lean controls.
SVCs from peri-epididymal AT from lean and obese C57BL/6J animals were labeled with conjugated antibodies to F4/80, CD3 (I), CD4, CD8 (II), CD11c, and B220 (III), and quantified by flow cytometry. The graphs represent differences between mice on LF (lean) and HF (obese) diets, calculated by Student’s t-test. *p<0.05 vs LF.
Immunostaining of peri-epididymal fat tissue with rat anti-mouse CD45 (A–C), Mac-3 (E–G), CD3 (I–L), and I-Ab (N-P) antibodies was performed as described. Graphs (D, H, M, Q) represent the numbers of positive cells in each group, and the differences were calculated by Student’s t-test. Original magnification: x100 for A–B, E–F, I–J, N–O; x400 for C, G, L, and P. #p<0.05 vs LF; n=5–8.
Quantitative analysis of inflammatory cells by immunohistochemistry in C57BL/6J mice after 21 weeks of HF or LF diet yielded findings compatible with those of flow cytometry. AT from obese mice had more cells bearing CD45 (a pan-leukocyte marker), Mac-3 (macrophage marker), or CD3 than AT from lean congenic mice (figures 1A–M). The AT of the obese group also contained more cells expressing I-Ab, a mouse major histocompatibility class II antigen (figures 1N–Q), indicating a greater state of immune activation in fat from obese compared to lean mice.
IFNγ expression in AT increases with diet-induced obesity in mice
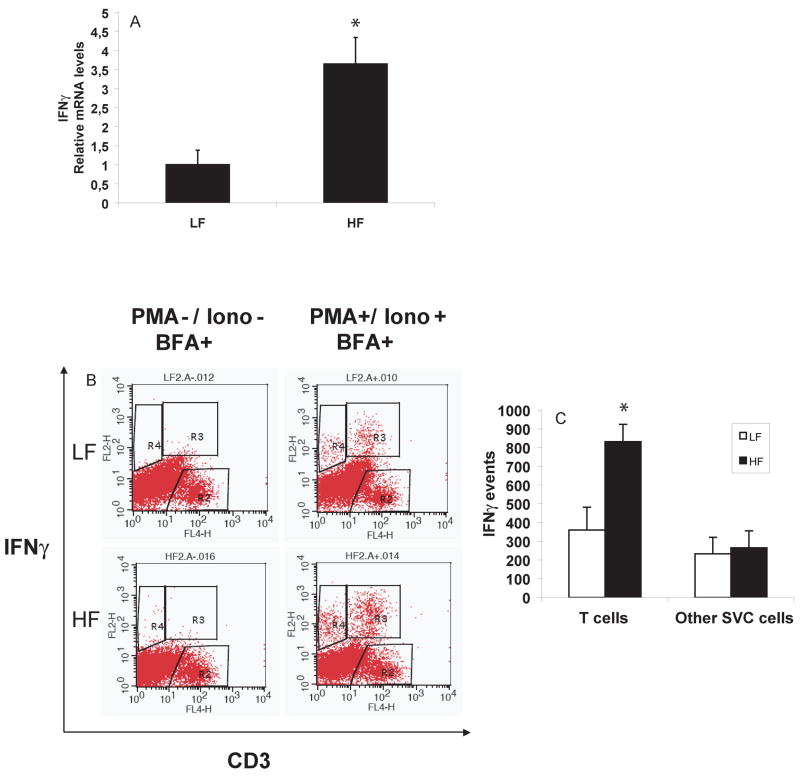
Extracts of AT from obese animals had higher levels of IFNγ mRNA than lean controls after 21 weeks of HF or LF diet, respectively (figure 2A). AT stromal cells isolated from lean and obese mice had negligible intracellular levels of IFNγ protein shown by flow cytometry after brefeldin A treatment to block secretion. However, after stimulation with PMA and ionomycin, T cells from AT of obese animals produced significantly more IFNγ than those from lean controls (figures 2B and C). The number of other IFNγ-producing cells did not differ between the two groups of mice.
Figure 2. IFNγ expression in AT increases with diet-induced obesity in mice.
RNA was extracted from visceral AT from C57BL/6J mice on LF or HF diet for 21 weeks. RT-qPCR measured mRNA levels of IFNγ over GAPDH in both groups (n=10 in each) (2A). SVCs isolated from AT from C57BL/6J mice fed a LF or HF diet for 15 weeks were labeled with CD3 and IFNγ antibodies after incubation with (2B, right) or without (2B, left) PMA and ionomycin. The graph (2C) represents the difference of IFNγ production by T cells and other cells between lean and obese groups, calculated by Student’s t-test. *p<0.05 vs LF.
IFNγ stimulates the expression of chemokines by 3T3-L1 cells in vitro
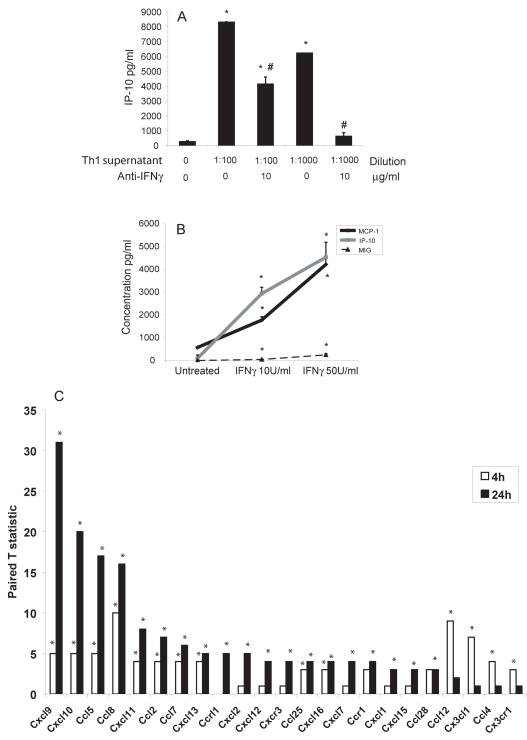
The presence of T cells and IFNγ in AT suggested the operation of T cell chemoattractants. Our prior studies showed the presence of T-cell-tropic chemokines in atheromatous tissue22. We therefore tested whether differentiated 3T3-L1 adipocytes could produce such mediators in vitro. Supernatants of activated Murine CD4 cells stimulated expression of the prototypical T-cell chemoattractant IP-10 from differentiated 3T3-L1 cells (figure 3A). Blocking antibodies against IFNγ limited IP-10 release from the 3T3-L1 adipocytes (figure 3A). Adipocytic 3T3-L1 cells stimulated with Murine recombinant IFNγ, even at levels as low as 10 U/ml, elaborated significantly increased levels of the T cell chemoattractants IP-10 and MIG, as well as the monocyte chemoattractant MCP-1 (figure 3B). Interleukin-6, IL-10, IL-12, and TNFα levels changed little or not at all after IFNγ stimulation of 3T3-L1 adipocytes (not shown). These results suggested that IFNγ regulates inflammatory gene expression selectively in adipocytes. Therefore, we undertook a broader analysis of the effects of IFNγ on gene expression in adipocytic cells by transcriptional profiling of 3T3-L1 cells treated with 100 U/ml of IFNγ for 4 and 24 hours. Compared to controls, the IFNγ-stimulated adipocytes significantly increased the production of several chemokines from both the CC and CXC chemokine families (figure 3C and supplementary table 1). Consistent with our previous results, IFNγ stimulation significantly increased expression not only of the T-cell chemoattractants MIG, IP-10, and interferon-inducible T cell α chemoattractant (I-TAC), but also of MCP-1, MCP-2, and regulated upon activation, normal T cell expressed and secreted (RANTES).
Figure 3. IFNγ stimulates the expression of chemokines in 3T3-L1 cells.
In 3A, 3T3-L1 cells were incubated with different dilutions of conditioned media from Th1 cells, with or without 10 μg/ml of anti-IFNγ. Levels of IP-10 were measured in the supernatants. Differences were calculated in pairs by Student’s t-test. *p<0.05 vs control (Th1 0/anti-IFNγ 0); #p<0.05 vs preceding bar. In 3B, 3T3-L1 cells were stimulated with different concentrations of mouse recombinant IFNγ. MCP-1, IP-10, and MIG protein levels were measured in the supernatants. In 3C, 3T3-L1 cells were stimulated with 100 U/ml of IFNγ or left untreated. After 4 and 24 h, cells were harvested and mRNA was used in a microarray screening. The white bars represent the T statistic values after 4 h and the black bars, after 24 h of stimulation with IFNγ, relative to expression in untreated cells; n=5–6; *p<0.05 vs untreated.
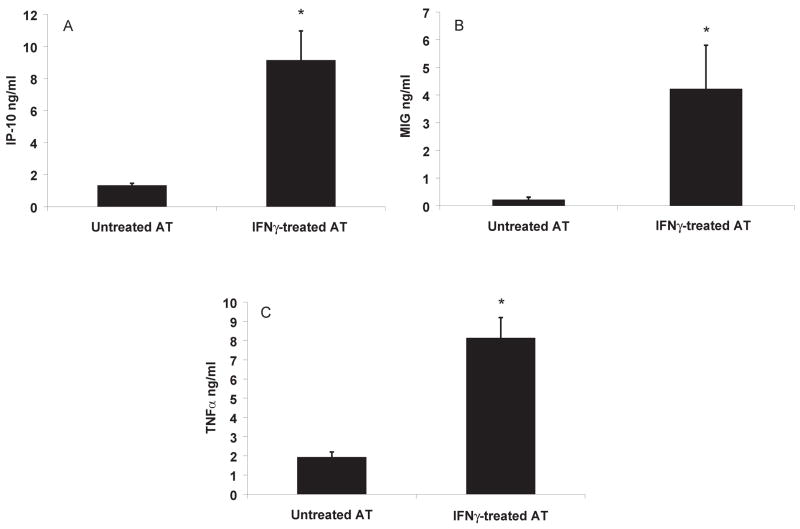
Similar to the results of 3T3-L1 stimulation with IFNγ, incubation of mouse peri-gonadal AT with this cytokine in culture significantly increased the secretion of IP-10 and MIG (figures 4A–B). Interestingly, media from IFNγ-treated AT had higher TNFα levels than untreated controls (figure 4C), in contrast with the experiments with differentiated 3T3-L1. These different results most probably occur because AT contains not only adipocytes, but also inflammatory cells such as macrophages, considered the most important source of TNFα in the AT.
Figure 4. IFNγ induces expression of cytokines by AT in culture.
Minced peri-epididymal AT was incubated with media alone or with 100U/ml of IFNγ for 6 h at 37°C and protein levels of IP-10, MIG and TNFα (A–C) were measured; n=3; *p≤0.01 vs untreated AT, calculated by Student’s t-test.
IFNγ deficiency in mice limits inflammatory gene expression in AT and improves insulin sensitivity in vivo
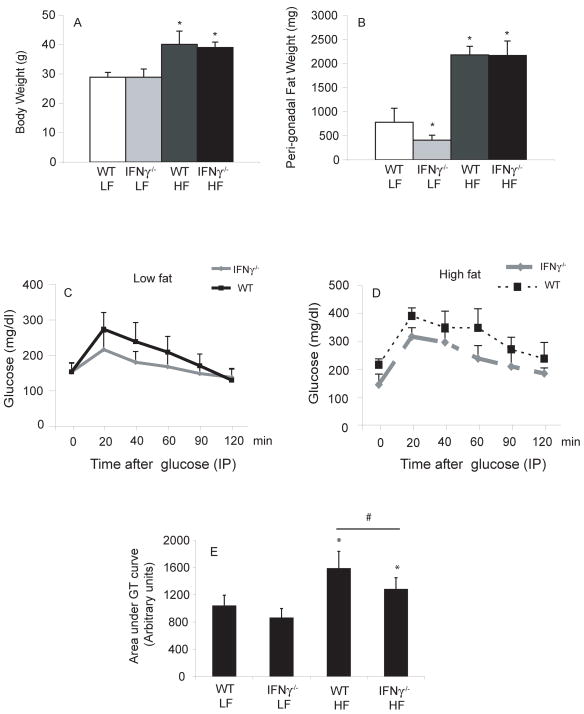
In vivo studies sought correlates of the in vitro effects described above. C57BL/6J and IFNγ-deficient male mice consumed a LF or HF diet for 15 weeks. The groups on HF diet had significantly higher BW than those on LF diet, but weight did not differ between wild type (WT) and IFNγ-deficient animals on the same diet (figure 5A). Mice on HF diet had heavier visceral fat pads than those consuming chow (figure 5B). Interestingly, within the lean group, the IFNγ-deficient animals had significantly smaller peri-epididymal fat pads than controls. IFNγ-deficient and WT animals consuming chow diet had similar glucose tolerance curves (figure 5C). On HF diet, however, IFNγ-deficient animals had better glucose tolerance than controls (figure 5D–E).
Figure 5. IFNγ deficiency does not change total body weight, but improves glucose tolerance (GT) in obese mice.
5A–B: the bars represent average numbers of each group, and differences were calculated by Student’s t-test.
In 5C, average GT curves from both groups under LF diet and in 5D, from groups under HF diet. Area under the GT curve was calculated for each mouse and the average of each group represented in 5E. Differences between groups were calculated by Student’s t-test. *p≤0.05 vs WT/LF; #p≤0.05.; n=5–6.
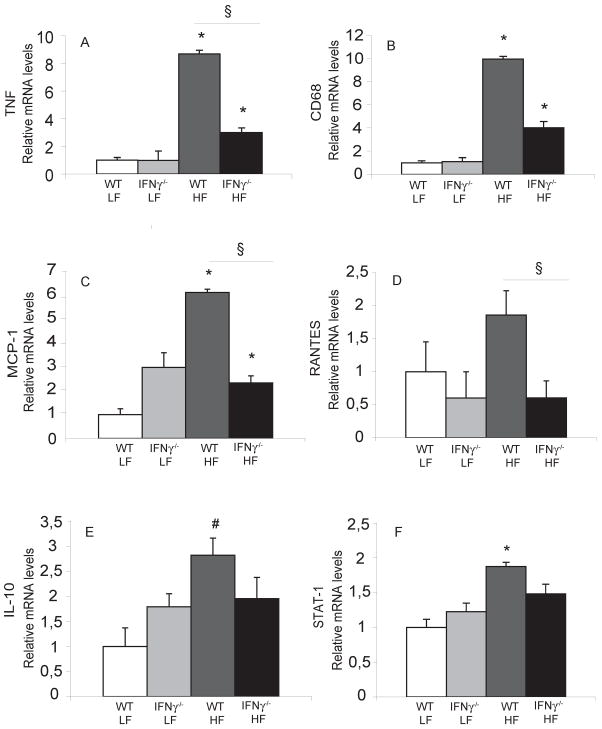
As demonstrated by others5, 6, diet-induced obesity yielded increased expression of inflammatory genes in AT. For example, mRNAs encoding CD68, a macrophage marker, and TNFα increased in AT of obese WT mice compared to lean controls, as previously shown5, 6. Interestingly, the IFNγ-deficient obese group had lower expression of these messages compared to the obese WT group (figures 6A–B), despite their similar BWs. The obese IFNγ-deficient animals had significantly reduced expression of chemokines such as MCP-1 (figure 6C) compared to obese controls. These animals also had decreased levels of RANTES (figure 6D), another important chemokine positively regulated by IFNγ in the transcription profiling study, compared to their WT counterparts. Interestingly, compared to their controls, obese IFNγ-deficient animals also had significantly reduced levels of interleukin-10 (IL-10) (Figure 6E), a cytokine that can exert anti-inflammatory actions. This observation raises the possibility of a counter-regulatory mechanism also operating in obesity. T regulatory cells (T regs) comprise one major source of IL-10 in several inflammatory conditions, including atherosclerosis. RNA levels of FoxP3, a forkhead family transcription factor and a marker for T regs, did not differ between obese IFNγ-deficient and obese WT animals or between either of these groups and lean WT controls (not shown), suggesting that local recruitment of T regs does not play a role in the IL-10 increase in obese AT.
Figure 6. IFNγ regulates inflammatory gene expression in visceral fat of obese mice.
mRNA levels of TNFα, CD68, MCP-1, RANTES, IL-10, and STAT-1 (A–F, respectively) in peri-epididymal AT were quantified by RT-qPCR and normalized to GAPDH. Fold change was calculated relative to WT/LF. *p<0.01 vs WT/LF; #p<0.05 vs WT/LF; §p<0.05; n=5–6.
Signal transducer and activation of transcription (STAT) proteins can regulate transcription in inflammation and host defense. STAT-1 in particular mediates the effects of IFNs23, 24. As expected, our transcriptional profiling experiments showed increased expression of this transcription factor in IFNγ-stimulated adipocytes25 (not shown). The AT of obese WT animals also had increased STAT-1 mRNA levels compared to that from lean mice. AT from obese IFNγ-deficient mice, however, did not show elevated levels of STAT-1 mRNA (figure 6F).
IFNγ deficiency changed leptin and total cholesterol levels
The various groups of animals had similar plasma levels of adiponectin, except the IFNγ-deficient animals consuming a chow diet, which had lower adiponectin levels than the others (supplementary figure 1A). As expected, the obese mice had significantly higher leptin levels than their lean counterparts. Interestingly, both lean and obese IFNγ-deficient groups had lower plasma leptin levels than WT controls (supplementary figure 1B). Both obese groups had higher plasma total cholesterol levels than lean mice. Although WT and IFNγ-deficient animals had similar total cholesterol levels on a chow diet, obese IFNγ-deficient mice had lower cholesterol levels than WT controls (supplementary figure 1C).
IFNγ deficiency reduces inflammatory cell accumulation in white AT
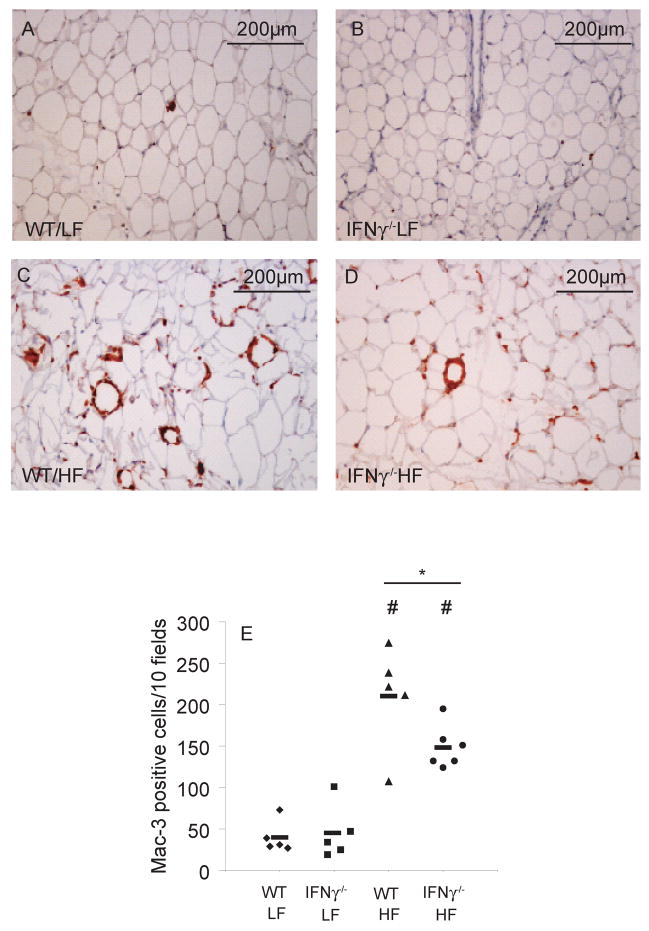
Macrophage infiltration indicates local inflammation in white AT. We therefore sought in vivo evidence for a net proinflammatory effect of IFNγ in AT by assessing its macrophage content. Lean animals from both WT and IFNγ-deficient groups had similar low numbers of Mac-3-positive cells in their white AT (figure 7A–B). Obese mice, as demonstrated before, had more macrophages in their AT than the lean ones, but fat from the IFNγ-deficient group showed fewer of these cells than obese controls (figure 7C–D).
Figure 7. IFNγ deficiency limits inflammatory cell accumulation in obese visceral AT.
Fixed and paraffin-embedded AT was stained with anti-Mac-3 antibody, and positive cells were counted in 10 consecutive fields in each slide. A representative picture from each group is shown (7A–D). Numbers from each group were plotted in 7E. Differences were calculated by Student’s t-test. #p<0.05 vs WT/LF; *p<0.05; n=5–6..
Infiltrating inflammatory cells in obese white AT typically assume a crown-like distribution, arrayed around adipocytes. Interestingly, AT from the obese IFNγ-deficient group not only had significantly fewer isolated macrophages than the obese WT group, but also fewer “crown” formations (supplementary figure 2).
IFNγ deficiency regulates visceral fat inflammation and affects lipid and glucose metabolism in obese ApoE−/− mice
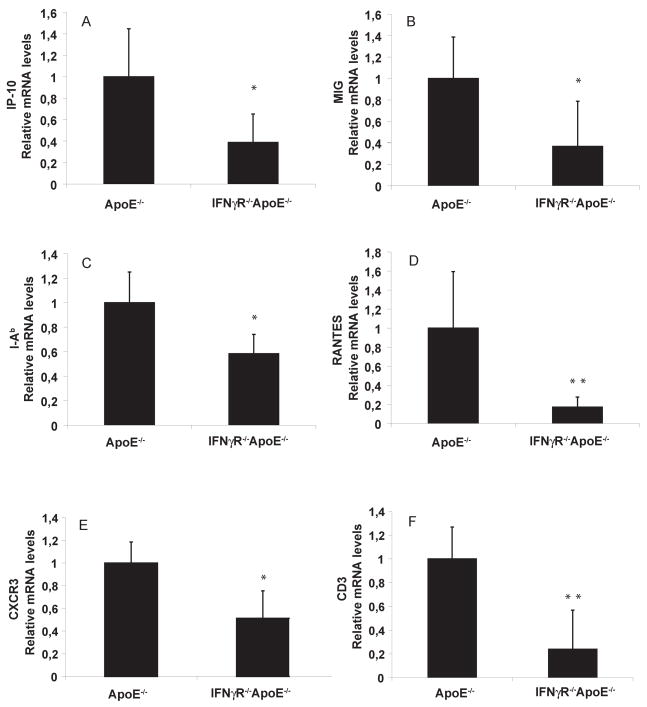
Abundant evidence links inflammation to atherosclerosis. The foregoing findings favoring a role for IFNγ in the fat inflammatory network in C57BL/6J animals stimulated further exploration of the effects of impaired IFNγ signaling in AT of atherosclerosis-prone mice. Others have analyzed atherosclerotic lesions in mice with impaired IFNγ signaling26. Mice doubly deficient for IFNγ receptor and ApoE (IFNγR−/−ApoE−/−) and their matching ApoE−/− controls consumed a HF diet. After 2 months, visceral AT of IFNγR−/−ApoE−/− had lower levels of mRNAs encoding various inflammatory genes compared to single-deficient ApoE−/− controls (figure 8), despite their similar BWs (not shown). The visceral fat of those animals had reduced levels of IP-10, MIG, I-Ab, RANTES, and C-X-C receptor-3 (CXCR3) mRNA (figure 8A–E), each encoding a mediator with important roles in the adaptive immune response of atherosclerosis. The decreased expression of CD3 (figure 8F) agreed with these findings, indicating a reduced T cell content in AT of doubly deficient animals compared to controls. Levels of MCP-1, CD68, and TNFα mRNA, on the other hand, did not decrease in the doubly deficient animals compared to controls (not shown), in contrast to the reduced expression of these genes in obese non-atherosclerotic IFNγ-deficient animals described above. Absence of IFNγ signaling in obese ApoE−/− animals also affected lipid and glucose metabolism. Although the two groups had the same total cholesterol levels (supplementary figure 3A), IFNγR-deficient animals had significantly reduced plasma triglycerides (supplementary figure 3B) and glucose (supplementary figure 3C) in fed state.
Figure 8. IFNγ deficiency regulates inflammation in visceral fat of obese ApoE −/− mice.
mRNA levels of IP-10, MIG, I-Ab, RANTES, CXCR3, and CD3 (A–F) in peri-epididymal AT were quantified by RT-qPCR and normalized to GAPDH. Fold change was calculated relative to ApoE−/−. *p<0.05 vs ApoE−/− **p<0.01 vs ApoE−/−; n=5–8.
Discussion
Our results demonstrated the importance of the prototypical Th1 cytokine, IFNγ, in white AT inflammation in mice with diet-induced obesity. In agreement with a previous study19, our work showed that T cells, both CD4 and CD8 subtypes, exist in greater numbers in AT of diet-induced obese mice than in lean controls. Immunolocalization generally showed T lymphocytes in clusters with other T cells and macrophages, forming crown-like structures around adipocytes.
Beyond their mere presence, the magnitude of I-Ab expression by antigen-presenting cells (APCs) such as dendritic cells and macrophages indicates their level of immune activation. Encountering APCs bearing class II major histocompatibility antigens can trigger adaptive immune responses mediated by T cells. The overall increase of I-Ab expression in visceral AT of obese, but not lean mice, observed here indicates local T cell activation and operation of IFNγ, an important regulator of I-Ab.
Macrophages and dendritic-like cells in AT have recently received considerable attention5, 6, 27. Yet, the participation of T cells and their cytokines in obesity and its metabolic consequences remains poorly explored. The local predominance of Th1 over Th2 cytokines in other chronic inflammatory conditions such as atherosclerosis12 focused our attention on IFNγ, a Th1 prototype. Indeed, IFNγ mRNA expression in AT increases in diet-induced obese animals fed a HF diet for 21 weeks compared to lean controls. Interestingly, T cells extracted from fat tissue of obese mice and stimulated in vitro also produced higher amounts of this cytokine than the ones extracted from lean animals. This result suggests that obesity primes T cells from AT toward a Th1 slant.
IFNγ participates in the progression of atherosclerotic plaque, where it affects importantly all local cell types: endothelial cells, smooth muscle cells, and macrophages12. IFNγ might also play an important role in growing AT, where adipocytes respond to inflammatory products derived from infiltrating macrophages such as TNFα7. Indeed, when stimulated by IFNγ, differentiated 3T3-L1 cells secrete various inflammatory mediators, including the T cell chemoattractants IP-10 and MIG. These results suggest the potential of a positive feedback loop that amplifies T cell recruitment to AT during obesity. Our study shows that, while quantitatively less prominent than macrophages, T cells may decisively influence the biology of AT in obesity. Thus, in addition to the innate immune response now well documented in obesity, adaptive immunity may also participate in this condition and its metabolic consequences.
The transcription profiling of IFNγ-stimulated 3T3-L1 adipocytes enabled a broader view of the actions of this mediator on adipocytes. Besides the T cell chemoattractants mentioned previously, stimulation of adipocytes with IFNγ increased the expression of an array of other chemokines and chemokine receptors implicated in atherogenesis. The abundance and variety of AT-derived chemoattractants, many of them regulated by IFNγ, likely result in a certain degree of redundancy, suggesting a collective operation, rather than selective contributions of individual chemokines to local inflammatory cell recruitment. Interestingly, adipocytes stimulated with IFNγ also demonstrated differential expression of important genes related to glucose and fat metabolism (not shown), emphasizing the idea that, similar to other cytokines, IFNγ may produce direct effects on metabolic pathways in adipocytes. These results suggest that the T cells in AT, while morphologically and numerically modest, may have major regulatory roles not only locally, but systemically.
Evaluating the relevance of IFNγ in the AT inflammatory network in vivo entailed the use of IFNγ-deficient and control animals fed a LF or HF diet. Indeed, IFNγ-deficient animals demonstrated significant decreases in expression of inflammatory genes, including chemokines and cytokines. The reduced accumulation of leukocytes in AT of IFNγ-deficient animals affirmed the functional consequences in vivo of the increased mRNA levels of these proinflammatory cytokines mediated by IFNγ. Interestingly, obese IFNγ-deficient mice also had lower plasma levels of leptin and total cholesterol and had greater glucose tolerance, supporting the systemic consequences of altered adaptive immunity in obesity.
The significant suppression of several inflammatory genes in visceral fat tissue of IFNγR−/−ApoE−/− mice compared to ApoE−/− agreed with these results. The disruption of IFNγ signaling in those animals significantly reduced the expression of genes with established roles in T cell chemotaxis and adaptive immune response in atherosclerosis, such as IP-1022, MIG22, RANTES28, 29, and I-Ab 12, 13. Importantly, IFNγ stimulation augmented expression of all these genes in differentiated adipocytes in our microarray data, again suggesting an interaction between the Th1 arm of the immune response and adipocytes. IFNγR−/−ApoE−/− also had reduced CD3 content in visceral AT compared to ApoE−/−, supporting the operation of an autocrine loop where T cells can perpetuate their own presence in AT through IFNγ. The suppressed inflammatory response in visceral AT of doubly deficient mice relative to their controls may also strengthen the link between fat inflammation and atherosclerosis development, as a previous study already demonstrated reduced atherosclerotic lesion size associated with IFNγ receptor deficiency in ApoE null mice26. The abrogation of IFNγ signaling in obese ApoE−/− mice related to changes in lipid and glucose metabolism, as discussed with C57BL/6 mice. The ApoE−/− and IFNγR−/−ApoE−/− animals used in our experiments were all F3 siblings derived from the offspring of a single cross between the original pair of 129-IFNγR−/− and C57BL/6-ApoE−/−. However, we cannot exclude the possibility of differences in the mixed (129/C57BL/6) genetic background of the final mice and rule out the influence of this possible heterogeneity on our findings.
Previous animal and human studies corroborate the aforementioned effects of IFNγ on the endocrine system30, 31. Interestingly, previous in vitro observations supported a direct stimulatory effect of IFNγ on lipolysis and increased supply of nonesterified fatty acids (NEFA) to the liver32, 33. The importance of this cytokine in host defenses and its influence on fuel mobilization bear evolutionary plausibility and reaffirm the link between inflammatory responses and metabolic disturbances also observed with other cytokines, such as TNFα.
Recognized for decades as pathogenic partners in other chronic inflammatory diseases including atherosclerosis, our results point to macrophage-T cell cooperation in obesity, another long-term inflammatory condition. The I-Ab positivity of macrophages in AT supports crosstalk between these prototypic cells of the innate and adaptive immune responses12, 13. In addition to the known interaction between infiltrating macrophages and adipocytes within the obese AT, T cells also appear to participate in this network. T cells do not exclusively produce IFNγ, despite its classification as a classic Th1 cytokine. Further studies, such as conditional disruption of the IFNγ gene in T cells, would be necessary to precisely establish the source of this cytokine in the AT environment.
Although not previously considered a Th1-associated condition, our current data establish that the key cytokine secreted by this T cell subset, IFNγ, can promote inflammation in fat tissue. These results highlight the importance of further exploration of adaptive immunity and the role of T cells and their products in obesity.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jesus San Roman and Dr. Yuri Sheikine for their help with statistical analysis, Elissa Simon-Morrissey, Eugenia Shvartz, and Gihan Suliman for skillful technical assistance, and Joan Perry for excellent editorial assistance.
Sources of Funding
This work was supported by the Donald W. Reynolds Foundation and a grant from NHLBI, HL-34636 (to Dr. Libby), CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior) BEX 1594 04/4 (to Dr. Rocha), American Heart Association Scientist Development grant (to Dr. Shimizu), and a grant award from the Roche Organ Transplantation Research Foundation (to Dr. Shimizu).
Footnotes
Disclosures
None.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 4.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha VZ, Libby P. The multiple facets of the fat tissue. Thyroid. 2008;18:175–183. doi: 10.1089/thy.2007.0296. [DOI] [PubMed] [Google Scholar]
- 10.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 14.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 16.Geng YJ, Hansson GK. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992;89:1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988;63:712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- 18.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 20.Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–320. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, Wang SJ, Tsai CA, Lin CJ. Selection of differentially expressed genes in microarray data analysis. Pharmacogenomics J. 2007;7:212–220. doi: 10.1038/sj.tpj.6500412. [DOI] [PubMed] [Google Scholar]
- 22.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 24.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 25.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 29.Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 30.Memon RA, Feingold KR, Moser AH, Doerrler W, Grunfeld C. In vivo effects of interferon-alpha and interferon-gamma on lipolysis and ketogenesis. Endocrinology. 1992;131:1695–1702. doi: 10.1210/endo.131.4.1396316. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein D, Gockerman J, Krishnan R, Ritchie J, Jr, Tso CY, Hood LE, Ellinwood E, Laszlo J. Effects of gamma-interferon on the endocrine system: results from a phase I study. Cancer Res. 1987;47:6397–6401. [PubMed] [Google Scholar]
- 32.Patton JS, Shepard HM, Wilking H, Lewis G, Aggarwal BB, Eessalu TE, Gavin LA, Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986;83:8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feingold KR, Doerrler W, Dinarello CA, Fiers W, Grunfeld C. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology. 1992;130:10–16. doi: 10.1210/endo.130.1.1370149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.