Abstract
Growth hormone (GH) plays a pivotal role in growth and metabolism, with growth promotion mostly attributed to generation of insulin-like growth factor I (IGF-I) in liver or at local sites of GH action, whereas the metabolic effects of GH are considered to be intrinsic to GH itself. To distinguish the effects of GH from those of IGF-I, we developed a Cre-lox-mediated model of tissue-specific deletion of the growth hormone receptor (GHR). Near total deletion of the GHR in liver (GHRLD) had no effect on total body or bone linear growth despite a >90% suppression of circulating IGF-I; however, total bone density was significantly reduced. Circulating GH was increased 4-fold, and GHRLD displayed insulin resistance, glucose intolerance, and increased circulating free fatty acids. Livers displayed marked steatosis, the result of increased triglyceride synthesis and decreased efflux; reconstitution of hepatic GHR signaling via adenoviral expression of GHR restored triglyceride output to normal, whereas IGF-I infusion did not correct steatosis despite restoration of circulating GH to normal. Thus, with near total absence of circulating IGF-I, GH action at the growth plate, directly and via locally generated IGF-I, can regulate bone growth, but at the expense of diabetogenic, lipolytic, and hepatosteatotic consequences. Our results indicate that IGF-I is essential for bone mineral density, whereas hepatic GH signaling is essential to regulate intrahepatic lipid metabolism. We propose that circulating IGF-I serves to amplify the growth-promoting effects of GH, while simultaneously dampening the catabolic effects of GH.
Growth hormone (GH),2 secreted by pituitary somatotrophs under neural, hormonal, and metabolic control (1), is a pivotal hormone in regulating postnatal growth and metabolism of carbohydrate, protein, and fat (2–6). According to the original somatomedin hypothesis (5), the growth-promoting effects of GH are primarily mediated by hepatic generation of insulin-like growth factor I (IGF-I), which, together with its major binding protein IGFBP-3 and the acid labile subunit (ALS), forms a ternary complex that circulates in plasma to permit controlled release of IGF-I at tissues where it exerts biological effects. Although GH exerts direct effects at local tissue sites to generate IGF-I for autocrine and paracrine actions (6), circulating IGF-I derives predominantly from hepatic generation (5–8), a process that begins with binding of GH to its cognate dimerized receptor (growth hormone receptor (GHR)) followed by a Janus kinase (JAK)/Stat-mediated signal transduction cascade that ultimately leads to the activation of nuclear transcription and gene expression (9). Defects in each of these steps of the action of growth hormone are associated with impaired growth, confirming the importance of this pathway in human biology (9). However, not all of the growth-promoting effects of GH can be explained by circulating IGF-I, and the precise direct contribution of GH as opposed to circulating IGF-I for normal growth remains to be determined (6–10). To distinguish between the effects of IGF-I as opposed to the direct effects of GH, investigators deleted these hormones or their receptor genes in the germline (10), producing various phenotypic effects and demonstrating that deletion of both GHR as well as IGF-I results in significantly greater growth impairment than either one alone (10). However, deletion of the igf-i gene in liver, which resulted in diminished circulating IGF-I by ∼75%, did not appreciably alter the growth of mice (7, 8, 11). In these mice, circulating GH concentrations were markedly increased, a result of the absence of the inhibitory effects of IGF-I on pituitary GH secretion (7, 8, 11–13), suggesting that GH must exert effects directly on tissues or via locally generated IGF-I (8, 13). Similar results were obtained after global deletion of the als gene (8).
The metabolic effects of GH include antagonism to insulin action, thereby predisposing to diabetes (2–4, 14), promotion of lipolysis (2), and enhancement of protein synthesis with decreased proteolysis (2, 15). In mice with liver-specific deletion of IGF-I, glucose intolerance was ascribed to the high circulating GH that could still act on liver to promote glucose production (2, 7) and concomitantly antagonize insulin action in muscle and fat tissues (14). However, the precise contribution of GH action in various tissues remains unknown because to date, tissue-specific deletion of GH action has not been described.
We developed a Cre-lox model to study the effects of tissue-specific deletion of the GHR on phenotype, biochemical, and molecular markers in specific tissues. Here, we report the effects of hepatic-specific deletion of the GHR.
EXPERIMENTAL PROCEDURES
Mice
Ghr exon 4 floxed mice were generated through standard gene-targeting methods as described (16). Both Flp recombinase-expressing mice (129S4/SvJSor-Gt(ROSA)26Sortm1(FLP1)Dym/J) and albumin-cre mice (6.Cg-Tg(Alb-cre)21Mgn/J) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen-free animal facility at Rangos Research Center, Pittsburgh, PA. All experiments were performed in accordance with institutional guidelines.
Blood Glucose and Insulin Levels
Blood glucose levels were monitored with the Ascensia Contour blood glucose monitoring system (Bayer HealthCare LLC, Mishawaka, IN). Blood insulin levels were measured with the Mercodia ultrasensitive mouse insulin ELISA kit (Mercodia) following the manufacturer's protocol.
Bone Micro-CT Analysis, X-ray Imaging, and Bone Volume Determination
Determination of bone mineral content via micro-CT analysis, imaging of the femurs via radiography, and measurement of bone volume in relation to total volume was performed as described previously in detail (17).
Serum Hormone Levels
Serum IGF-I, IGFBP-3, and ALS levels were measured with enzyme-linked immunosorbent assay (ELISA) developed in the laboratory of Dr. Pinchas Cohen (18). Growth hormone levels were measured with the mouse growth hormone ELISA kit (Diagnostic Systems Laboratories, Inc.) following the manufacturer's protocol.
RNA
The total RNA of liver samples was isolated using an RNA minikit according to the manufacturer's protocol (Qiagen). Real-time quantitative PCR was carried out on cDNA prepared from DNase I-treated RNA (Superscript III cDNA kit, Invitrogen) with the LightCycler FastStart DNA Master SYBR Green I kit and analyzed with the LightCycler 2 system (Roche Applied Science).
Histology
Livers were harvested, fixed in 4% paraformaldehyde for 3 h at 4 °C, and placed in 30% sucrose overnight. Cryosections 5 μm thick were cut and stained with GHR (L-15) antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For the detection of neutral lipid, liver cryosections were stained with the Oil Red O technique with 0.23% dye dissolved in 65% isopropyl alcohol for 10 min. To analyze the growth plate, tibia bones were harvested from 12-week-old male mice and fixed in 10% formalin followed by decalcification in 100 mm EDTA for 10 days.
Glucose and Insulin Tolerance Tests
Animals were fasted for 16 and 3 h before intraperitoneal injection of glucose (2 g/kg of body weight) and insulin (0.8 units/kg of body weight), respectively. Blood was obtained at intervals of 15 min from the tail vein, and glucose and insulin levels were analyzed as described above.
Free Fatty Acid and Triglyceride Assay
Both serum-free fatty acid levels and plasma triglyceride concentrations were measured using commercially available kits (NEFA-HR kit from Wako Diagnostics and Infinity triglyceride kit from Thermo Electron Corp., respectively). To measure hepatic triglyceride production rate, mice were injected with Triton WR-1339 (1 g/kg of body weight, Sigma) after 4 h of fasting, and plasma triglyceride levels was measured over a 3-hr period. The triglyceride production rate was calculated from the difference in plasma triglyceride levels over the first 90 min.
Whole Body Composition Analysis
Fat and lean (muscle) compositions of 16-week-old male mice were analyzed by an EchoMRI whole body composition analyzer (Echo Medical Systems, Houston, Texas).
Adenovirus-mediated GHR Reconstitution and IGF-I Infusion
Adenoviruses expressing either full-length mouse Ghr or full-length mouse LacZ were administrated to 12-week-old female Ghr liver-specific deletion (GHRLD) mice through the tail vein (109 infectious particles/mouse). Treated animals were subjected to experimental characterization 2 weeks after reconstitution. For the IGF-I infusion experiment, osmotic minipumps (DURECT Co., Cupertino, CA) loaded with either rhIGF-I or placebo (Tercica, Inc., Brisbane, CA) were implanted subcutaneously into the mid-back region of 16-week-old male GHRLD mice. Daily infusion dosage was 26.4 μg. After 2 weeks, the animals were sacrificed, and livers were harvested for analysis.
Statistical Analysis
In all experiments, unless specified, we used two-tail Student's t tests to assess statistical significance. p value < 0.05 was considered as significant.
RESULTS
Generation of the GHRLD Model
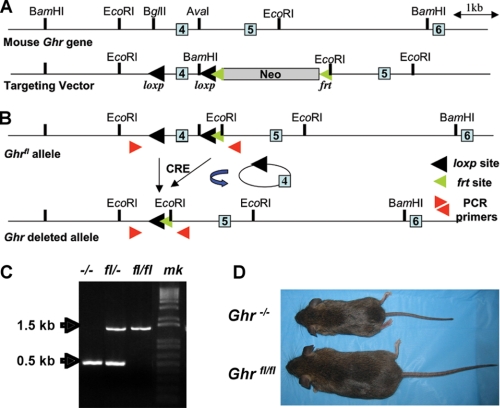
To enable the study of the specific effects of GH in a particular tissue, we flanked exon 4 of the mouse Ghr gene, which encodes a portion of the extracellular domain of the GH receptor, with two loxP sites and generated homozygous animals harboring the floxed Ghr alleles (Ghrfl/fl) (Fig. 1, A and B). Global deletion of Ghr obtained after crossing Ghrfl/fl to E2a-Cre mice resulted in progeny with severe postnatal growth failure, consistent with the phenotype of Laron mice (19) (Fig. 1, C and D), documenting that Cre-mediated removal of exon 4 of the Ghr gene is sufficient to abolish GHR action. To delete GHR expression specifically in hepatocytes, Ghrfl/fl mice were crossed to albumin-cre transgenic mice, resulting in Ghrfl/fl; Alb−cre mice, designated as GHRLD mice (Fig. 2A). In these mice, Ghr expression in either muscle or fat tissue remained unchanged, whereas liver Ghr mRNA levels were extinguished (Fig. 2B). Quantification of Ghr mRNA expression revealed greater than 90% deletion in the GHRLD liver; the residual Ghr transcripts may represent Ghr mRNA in endothelial cells and/or Kupfer cells that would not be affected by the albumin-driven Cre (Fig. 2C). When analyzed by immunohistochemistry, hepatocyte GHR protein expression in GHRLD mice was absent (Fig. 2D).
FIGURE 1.
Genetic modification of the mouse Ghr gene. A, schematic view of the mouse Ghr gene. The targeting vector contains two engineered loxp sites, flanking exon 4, as well as an frt-tagged neo cassette for positive selection. Light blue boxes: exons. B, schematic view of the loxp-tagged Ghr allele (Ghrfl) after excision of the neo cassette by Flp recombinase. Subsequent Cre-mediated excision of loxp sites produces the exon 4-deleted Ghr allele (Ghr−). C, E2a-Cre-mediated total deletion of the mouse Ghr gene results in the dwarfism phenotype of the Laron mouse. PCR analysis of pups derived from Ghrfl/− × Ghrfl/− mating shows genotyping results for total knock-out (−/−), heterozygous (fl/−), and homozygous floxed (fl/fl) mice. mk, DNA marker. D, photograph of 12-week-old Ghr total deletion (Ghr−/−) and homozygous floxed (Ghrfl/fl) mice.
FIGURE 2.
Liver-specific deletion of the mouse Ghr gene. A, schematic view for generating the GHRLD mice. Transgenic animals carrying the Alb-Cre transgene were bred with the Ghrfl/fl mice to generate the Ghrfl/fl/+; Alb−cre mice, which were backcrossed further to obtain the GHRLD mice (Ghrfl/fl; Alb−cre). B, RT-PCR analysis of Ghr expression in different tissues, showing efficient deletion of liver Ghr in GHRLD mice. Control signifies littermates without the Alb-Cre transgene (Ghrfl/fl) unless otherwise specified. C, quantification of Ghr expression in liver by real-time quantitative PCR analysis showing greater than 90% reduction in GHRLD mice. Error bars indicate mean ± S.E. D, immunohistochemical analysis of GHR protein levels in liver sections. The white arrows in the inset show the membrane expression of GHR in liver sections of control animals. GHR is stained in red, and nucleus is stained blue.
Effects of GHRLD on Circulating GH and the Ternary Complex
Analysis of serum samples from GHRLD mice revealed that all components of the circulating ternary complex were markedly diminished: IGF-I by ∼95%, IGFBP-3 by ∼80%, and ALS levels by ∼85%. GHRLD mice had ∼4-fold higher plasma GH levels, secondary to the loss of negative regulation of pituitary GH secretion from the low circulating IGF-I levels (7, 11) (Table 1).
TABLE 1.
Serum IGF-I ternary complex and GH levels
| IGF-I | IGFBP-3 | ALS | Growth hormone | |
|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | ng/ml | |
| Control (n = 15) | 395.9 ± 33.1 | 1083.2 ± 125.1 | 1992.6 ± 330.2 | 9.9 ± 0.7 |
| GHRLD (n = 14) | 23.9 ± 4.2 | 220 ± 23.5 | 330.2 ± 41.7 | 33.0 ± 6.3 |
| p value | <10−10 | <10−5 | <10−9 | <10−4 |
Effects of GHRLD on Growth
Length, Weight, and Bone Growth
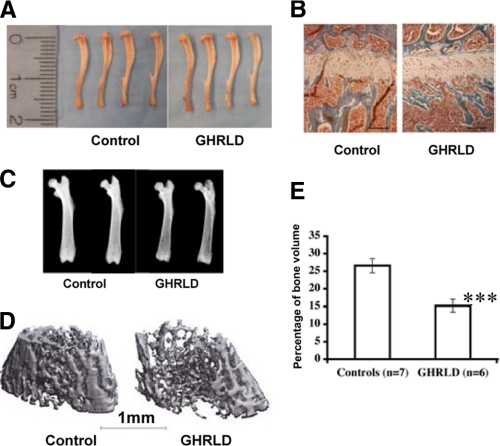
Despite the marked reduction of circulating IGF-I, GHRLD mice, in contrast to the Laron mice, exhibit normal postnatal growth with body weight and body length similar to controls (Table 2). Consistent with these measurements, tibia lengths, reflecting longitudinal bone growth, were identical between GHRLD and control mice (Table 2 and Fig. 3A), as were the size of the growth plates (Fig. 3B). However, bone density was markedly diminished in the GHRLD mice, as demonstrated both by x-ray and by micro-CT analysis (Fig. 3, C and D). Quantitative analysis of bone volume as a fraction of the total volume revealed a highly significant difference between the control and GHRLD mice at 16 weeks (Fig. 3E).
TABLE 2.
Normal growth of GHRLD mice
| Body weight | Body lengtha | Tibia length | |
|---|---|---|---|
| g | cm | cm | |
| Control (n = 8) | 25.00 ± 0.86 | 9.07 ± 0.07 | 1.80 ± 0.02 |
| GHRLD (n = 6) | 23.45 ± 0.67 | 8.88 ± 0.11 | 1.82 ± 0.03 |
a Snout to tail base.
FIGURE 3.
Effects of liver-specific Ghr deletion on growth. A, similar tibia lengths were observed in GHRLD mice (right) when compared with littermate controls (left). Shown here are tibias harvested from 16-week-old female mice. Control signifies littermates without the Alb-Cre transgene (Ghrfl/fl) unless otherwise specified. B, histological analysis of growth plate of tibia bones isolated from GHRLD mice (right) and littermate controls (left), showing similar size. C, x-ray analysis of femur bones harvested from GHRLD mice and control littermates, showing that GHRLD mice have less bone density. D, examples of micro-CT images of femurs from GHRLD mice and controls. E, quantification of bone volume of GHRLD femurs based on micro-CT image analysis. ***, p < 0.002. Error bars indicate mean ± S.E.
Organ Weights and Tissue Composition
Significant differences were observed in liver and kidney weights between GHRLD and control mice; in GHRLD mice, liver was heavier (4.71 ± 0.18% versus 4.0 ± 0.19% of total body weight, p < 0.01) and kidneys were smaller (0.49 ± 0.02% versus 0.62 ± 0.02%, p < 0.01), whereas other organs were unchanged (Table 3). Magnetic resonance imaging analysis showed that GHRLD and control mice had similar body composition in regard to total fat (14.58 ± 1.59%, n = 7 versus 15.04 ± 0.75%, n = 12 of total body weight, respectively) and lean (muscle) percentage (71.28 ± 2.34%, n = 7 versus 68.01 ± 0.82%, n = 12 of total body weight, respectively), further substantiating the overall normal growth of GHRLD mice.
TABLE 3.
Organ weights of GHRLD mice
| % of Total body weight |
|||||
|---|---|---|---|---|---|
| Liver | Kidney (left) | Kidney (right) | Heart | Lung | |
| Control (n = 8) | 4.09 ± 0.19 | 0.59 ± 0.02 | 0.62 ± 0.02 | 0.65 ± 0.03 | 0.64 ± 0.01 |
| GHRLD (n = 6) | 4.71 ± 0.18a | 0.46 ± 0.02a | 0.49 ± 0.02a | 0.63 ± 0.05 | 0.67 ± 0.03 |
ap < 0.05.
Effects of GHRLD on Metabolism
Carbohydrate
Non-fasting glucose levels were normal, but plasma insulin levels were 2–3 times elevated when compared with controls, indicative of insulin resistance (Fig. 4, A and B), which was confirmed by an intraperitoneal insulin tolerance test (Fig. 4C). An intraperitoneal glucose tolerance test demonstrated glucose intolerance in the GHRLD mice at 4–6 weeks of age (Fig. 4D). Quantitative real-time PCR analysis of liver mRNA revealed that there were no significant alterations in the steady state abundance of mRNAs for three key gluconeogenic enzymes, Fbp1, G6Pase, and Pck1, suggesting that the impaired glucose disposal in GHRLD mice was not due to increased hepatic gluconeogenesis (Fig. 4E).
FIGURE 4.
GHRLD mice display insulin resistance and glucose intolerance. A, plasma glucose levels of 6–8-week-old GHRLD mice in the fed state. Control, littermates without the Alb-Cre transgene (Ghrfl/fl). Error bars indicate mean ± S.E. B, plasma insulin levels of 6–8-week-old GHRLD in random fed mice. C, intraperitoneal insulin tolerance test on 6-week-old GHRLD mice. ***, p < 0.0001 (two-way ANOVA test). D, intraperitoneal glucose tolerance test on 6-week-old GHRLD mice. ***, p < 0.0001 (two-way ANOVA test). E, real-time analysis of gluconeogenic enzyme mRNA expression in livers from control and GHRLD mice. Data are presented as mean ± S.E. No appreciable changes were detected. G6Pase, glucose-6-phosphatase; Fbp1, fructose-1,2-bisphosphatase; Pck1, phosphoenolpyruvate kinase 1.
Lipid
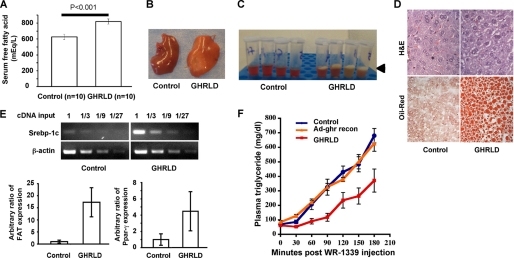
GH is lipolytic, and high levels of GH in blood act to increase lipolysis in adipocytes and enhance triglyceride secretion from the liver (2). Serum free fatty acid levels were 30% higher in GHRLD mice when compared with controls (Fig. 5A). Livers were glistening, suggesting increased lipid content (Fig. 5B), which was confirmed by demonstrating a creamy layer on gravitational sedimentation of liver homogenate extracts (Fig. 5C). Histochemical analysis revealed hepatic steatosis (Fig. 5D). Fat accumulation in liver arose in part by enhanced lipogenesis from increased free fatty acid flux with ∼10-fold up-regulation of Srebp-1c expression, ∼4–5-fold up-regulation of peroxisome proliferator-activated receptor-γ (PPARγ), as well as ∼20-fold increase of fatty acid translocase/CD36 (Fat), key regulators of hepatic triglyceride synthesis (20) (Fig. 5E).
FIGURE 5.
Effects of loss of hepatic GHR signaling on lipid metabolism. A, GHRLD mice exhibited elevated serum free fatty acid levels. Control signifies littermates without the Alb-Cre transgene (Ghrfl/fl) unless otherwise specified. B, representative photographs of liver lobes harvested from 8-week-old male control and GHRLD mice. Note the pale glistening appearance of the GHRLD. C, a creamy fat layer (arrowhead) was found in liver extracts from GHRLD mice following homogenization and centrifugation, indicating excessive fat deposition in liver. D, histological analysis of GHRLD liver sections. Upper panel, hematoxylin and eosin (H&E) staining. Lower panel, Oil Red O staining of lipid droplets. E, RT-PCR analysis of hepatic expression of key regulators of lipogenesis and lipid homeostasis. Upper panel, semiquantified PCR analysis of liver sterol regulatory element-binding protein 1c (Srebp-1c) expression. The relative amount of cDNA provided as template is shown on the top of the gel photographs, indicating close to 10-fold up-regulation of Srebp-1c expression in GHRLD mice. Lower panel, -fold changes of Fat/CD36 and PPARγ expression in GHRLD livers (n = 4), in comparison with controls (n = 4), were analyzed by quantitative PCR using the comparative ΔΔCt method. Hprt was used as endogenous control. p < 0.0005 for both. Error bars indicate mean ± S.E. F, hepatic triglyceride output assay. Inhibition of peripheral lipoprotein lipase activity with Triton WR-1339 reveals a lower output rate of hepatic triglyceride in GHRLD mice (red line, n = 8) when compared with controls (blue line, n = 8). Reconstitution (recon) of hepatic Ghr expression via tail-vein injection of Ghr-expressing adenovirus (Ad-ghr) corrected the triglyceride output defect in GHRLD mice (orange line, n = 4). p < 0.0001 between either GHRLD and control or GHRLD and Ad-ghr reconstituted mice (two-way ANOVA test).
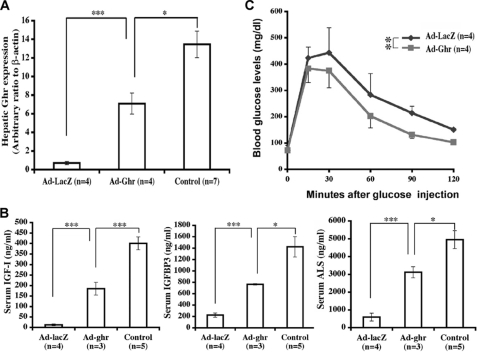
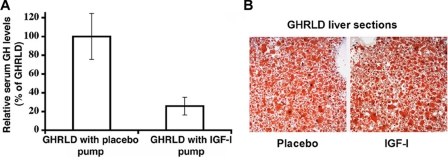
Although the expression of microsomal triglyceride transfer protein (Mttp) and apolipoprotein B (ApoB) was unchanged, triglyceride secretion from the liver was markedly impaired (1.1 mg/dl/min versus controls of 3.7 mg/dl/min, p < 0.001) (Fig. 5F) when measured by triglyceride concentration in blood during the 3 h after administration of the lipoprotein lipase inhibitor Triton WR-1339 (21). Rescue of GHR signaling in livers of GHRLD mice via adenovirus-mediated GHR expression completely restored hepatic triglyceride output to normal, as well as restoring glucose tolerance (Figs. 5F and 6). By contrast, infusion of rhIGF-I (26.4 μg/day for 14 days) via subcutaneously implanted mini Alzet pumps failed to correct hepatic steatosis, although circulating GH was restored to normal (Fig. 7).
FIGURE 6.
Reconstitution of hepatic Ghr expression via adenoviral expression vectors. A, 12-week-old female GHRLD mice were infused with adenovirus expressing either full-length Ghr (Ad-ghr) or full-length lacZ (Ad-lacZ). Hepatic Ghr expression levels were evaluated by quantitative PCR analysis and normalized to endogenous control (β-actin). As shown from the arbitrary ratios, Ad-ghr-treated GHRLD mice expressed approximately one-half of the amounts of Ghr found in control animals. Control signifies littermates without the Alb-Cre transgene (Ghrfl/fl) unless otherwise specified. B, reconstitution of hepatic Ghr expression partially restored serum IGF-I ternary complex levels. As shown, serum IGF-I, IGFBP3 and ALS levels in Ad-ghr-treated mice were all restored to ∼50% of the levels of controls. In both A and B, data are presented as mean ± S.E. (error bars) *, p < 0.05; **, p < 0.01; ***, p < 0.005. C, GHRLD mice treated with Ad-ghr adenovirus (red line, Ad-ghr, n = 4) displayed improved glucose tolerance as demonstrated by an intraperitoneal glucose tolerance test. In contrast, GHRLD mice treated with Ad-lacZ remained glucose intolerant (blue line, n = 4). Each of the data points is presented as mean ± S.E. Data were analyzed by two-way ANOVA test.
FIGURE 7.
Infusion of GHRLD with IGF-I failed to rescue hepatic steatosis. A, mini osmotic pumps filled with either IGF-I or placebo were implanted subcutaneously into 16-week-old male GHRLD mice. As shown, IGF-I infusion (26.4 μg/day) efficiently normalized the serum GH levels (approximately one-quarter of the GHRLD mice treated with placebo). Error bars indicate mean ± S.E. B, histological sections of livers harvested from GHRLD mice treated with either placebo (left) or IGF-I (right) for 2 weeks were stained with Oil Red O. Similar lipid contents were observed under both conditions, indicating that IGF-I infusion did not correct hepatic steatosis despite restoration of circulation GH concentration to normal.
DISCUSSION
Our model of liver-specific GHR deletion demonstrates that circulating IGF-I is derived primarily from hepatic production and that circulating IGF-I is not essential for normal postnatal growth in the mouse. Our results also establish a novel and critical role for hepatic GH signaling in maintenance of lipid homeostasis.
The fundamental difference between the current model of inducing deficiency of circulating IGF-I via tissue-specific deletion of GHR in liver with total abrogation of growth hormone signaling in liver and previously reported models of IGF-I deficiency secondary to deletion of the igf-i gene in liver is that with the exception of IGF-I generation, GHR signaling is intact in these latter models. Previous studies wherein the igf-i gene was deleted in liver or ALS was knocked out globally, and which resulted in an ∼65–80% reduction of circulating IGF-I, also reported that growth was unaltered (11, 12, 22). However, it was considered possible that the remaining 20–35% of circulating IGF-I was sufficient to allow normal growth in those models, as suggested by the authors (11, 12, 22). By demonstrating virtual total suppression of circulating IGF-I (∼95%), our study leads to the conclusion that circulating growth hormone alone is sufficient to promote normal growth, providing that GHR, along with its intact post-receptor signaling cascade, is expressed in the remaining tissues outside of the liver, specifically at the growth plate (23). Another possible explanation for normal growth in our model would be that free IGF-I levels remained sufficient to sustain bone growth via circulating IGF-I. However, deletion of GHR expression in all tissues of our mouse model leads to recapitulation of the Laron mouse with markedly impaired growth, as described previously (19), and lends further support to the proposition that growth hormone must act at local tissues for both direct effects as well as the generation of local IGF-I-mediated growth (24). Because total deletion of IGF-I also results in impaired fetal growth and survival and causes developmental abnormalities of brain, lung, muscle, and bone, as well as postnatal growth retardation and infertility, IGF-I is clearly critical for growth and development (10). The most likely explanation for retention of normal postnatal growth in our model is that the higher circulating growth hormone concentrations, the result of the lack of inhibition by circulating IGF-I on pituitary growth hormone secretion, have direct effects to promote bone and tissue growth as well as local generation of IGF-I, which mediates growth via paracrine-autocrine effects (5–8, 11, 12, 22). Alternatively, even normal GH concentrations may have resulted in normal growth in the absence of circulating IGF-I. The data suggest, however, that higher GH concentrations compensated for the lack of IGF-I because long term observation of the GHRLD mice did not reveal augmented growth despite the 4-fold increase in circulating GH levels. Hepatic-derived IGF-I is critical for normal bone architecture, as shown by the reduced bone mineral density in our animals. In the model of combined liver-specific IGF-I deletion together with global ALS knock-out, both circulating IGF-I from the liver, and local IGF-I signaling were impaired due to the global absence of ALS, resulting in impaired growth and a smaller growth plate (8). A major difference between this model and ours, however, is that the availability of ALS at the bone environment may contribute to IGF activity in our model because the als gene itself was not disrupted. The kidneys of the GHRLD animals were significantly lighter than controls, documenting that unlike bone growth, hepatic-derived IGF-I has an important role in kidney growth, as suggested previously (25, 26).
In contrast to the lack of effects on bone growth, deletion of GHR expression in liver revealed a novel and essential role of hepatic GH signaling for the maintenance of normal metabolism with primary effects focused on hepatic lipid metabolism and secondary effects focused on peripheral lipolysis, insulin resistance, and glucose intolerance. Although the effects of excessive GH action in inducing insulin resistance and glucose intolerance were anticipated (7, 11), the effects on hepatic lipid metabolism were unexpected. Peripheral insulin resistance with hyperinsulinemia and increased free fatty acid existed in other models of circulating IGF-I deficiency with increased circulating GH concentrations. However, hepatic steatosis did not occur in the originally reported Laron mouse (16), nor in our global deletion model, and was not reported in the LID mouse where hepatic GH-GHR signaling remained intact (7). It must be emphasized that increased circulating GH concentrations with peripheral insulin resistance, hyperinsulinemia, and increased free fatty acid existed in these models of circulating IGF-I deficiency. Thus, GH signaling appears to be essential for intrahepatic lipid metabolism because restoration of GHR signaling via the adenoviral GHR construct totally restored hepatic triglyceride output to normal. Hepatic steatosis has recently been described in mice in which STAT5b has been abrogated in liver (27). In these animals, IGF-I was reduced by only 50% and triglyceride output was not reported. Because the STAT5b is an essential component of the canonical signaling cascade for the GHR receptor, our current studies are the first to uncover a direct link between a circulating ligand and the liver phenotype secondary to hepatic STAT5b deficiency. Thus, the lack of GH signaling in liver is associated with hepatic steatosis and raises the possibility that the hepatic steatosis observed in obesity may be caused, in part, by the state of decreased growth hormone secretion and signaling that occurs in obesity (28).
The peripheral metabolic defects observed in our animals are likely to be the direct result of increased pituitary growth hormone secretion and action in tissues in which growth hormone receptor expression remained unaltered (29). In addition to its lipolytic effects, growth hormone induces a state of insulin resistance (2–4), manifest in our animals by a raised plasma concentration of insulin, resistance to insulin-mediated hypoglycemia, and glucose intolerance. Although growth hormone is also reported to enhance hepatic gluconeogenesis (3), this mechanism was excluded because of the absence of hepatic GH signaling in our animals and because there were no changes in the expression of the three key gluconeogenic enzymes, G6Pase, Fbp1, and Pck1, in the liver. Recently, it has been suggested that GH regulates p85α expression and phosphatidylinositol 3-kinase activity in white adipose tissue and that this mechanism is responsible for insulin resistance and reduced fat mass observed in mice with growth hormone excess (14). Because growth hormone was increased in our animals and growth hormone signaling in fat tissue remained intact, this is a possible mechanism for the observed insulin resistance. Additionally, the increased circulating free fatty acid likely contributed to insulin resistance through direct or indirect generation of metabolites that alter the insulin-signaling cascade in various tissues (30).
The lack of appreciable effect of circulating IGF-I on growth challenges the classic somatomedin hypothesis (5), which postulates that growth is mediated by a “second messenger,” eventually named IGF-I, generated from the liver. Our studies suggest that hepatic-derived circulating IGF-I signaling is essential for normal kidney growth and normal bone density, which cannot be compensated by elevated GH alone, consistent with findings in the Laron mouse and human (19, 25, 26). Recently, it has been proposed that the combined effects of the GH-IGF-I system serve to augment the anabolic actions of GH, while concurrently counteracting its potential harmful effects in inducing insulin resistance and diabetes mellitus and limiting its lipolytic effects to prevent depletion of lipid stores (5). This hypothesis is consistent with our findings because despite high GH concentrations, growth was normal, but not excessive as found in acromegaly. Thus, in the presence of normal hepatic GH signaling and hepatic IGF-I generation, normal growth is attained at approximately one-quarter of the circulating GH concentration required to maintain growth in our model and without inducing insulin resistance, diabetes, and excessive lipolysis.
In conclusion, the ability to delete GHR and its signaling cascade in liver demonstrates that circulating IGF-I is not an essential mediator of bone growth, although it is essential for normal bone mineral accretion. Notably, GH signaling in liver is essential for normal intrahepatic lipid metabolism. The ability to delete GHR signaling in specific tissues such as fat, muscle, and bone will permit a clearer delineation of the specific direct effects of growth hormone and its indirect effects on local tissue generated IGF-I for growth and provide an opportunity to identify the tissue sites and signaling mechanisms by which growth hormone induces insulin resistance.
Supplementary Material
Acknowledgments
We thank H. H. Dong (University of Pittsburgh) for critical reading of the manuscript and insightful discussions. We thank Tercica for providing the rhIGF-I used in our studies. We thank J. R. Chaillet and B. Shaffer for assistance in the early phase of this work.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-AR052746-01 (to T. C. and M. A. S.) and RO1-DK049845-11A2 (to R. K. M. and M. A. S.). This work was also supported in part by a grant from the Henry Hillman Endowment Chair in Pediatric Immunology and by Department of Defense Grant W81XWH-06-1-0317 (to M. T.).
This article was selected as a Paper of the Week.
- GH
- growth hormone
- GHR
- growth hormone receptor
- GHRLD
- Ghr liver-specific deletion
- IGF-I
- insulin-like growth factor I
- rhIGF-I
- recombinant human IGF-I
- ALS
- acid labile subunit
- Stat
- signal transducers and activators of transcription
- micro-CT
- micro-computed tomography
- ELISA
- enzyme-linked immunosorbent assay
- ANOVA
- analysis of variance.
REFERENCES
- 1.Goldenberg N., Barkan A. (2007) Endocrinol. Metab. Clin. North Am. 36, 37–55 [DOI] [PubMed] [Google Scholar]
- 2.LeRoith D., Yakar S. (2007) Nat. Clin. Pract Endocrinol. Metab. 3, 302–310 [DOI] [PubMed] [Google Scholar]
- 3.Sherwin R. S., Schulman G. A., Hendler R., Walesky M., Belous A., Tamborlane W. (1983) Diabetologia 24, 155–161 [DOI] [PubMed] [Google Scholar]
- 4.Clemmons D. R. (2002) Pituitary 5, 181–183 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan S. A., Cohen P. (2007) J. Clin. Endocrinol Metab. 92, 4529–4535 [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Zhou J., Cheng C. M., Kopchick J. J., Bondy C. A. (2004) J. Enodcrinol. 180, 247–255 [DOI] [PubMed] [Google Scholar]
- 7.Liu J. L., Yakar S., LeRoith D. (2000) Proc. Soc. Exp. Biol. Med. 223, 344–351 [DOI] [PubMed] [Google Scholar]
- 8.Yakar S., Rosen C. J., Beamer W. G., Ackert-Bicknell C. L., Wu Y., Liu J. L., Ooi G. T., Setser J., Frystyk J., Boisclair Y. R., LeRoith D. (2002) J. Clin. Invest. 110, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld R. G., Belgorosky A., Camacho-Hubner C., Savage M. O., Wit J. M., Hwa V. (2007) Trends Endocrinol Metab. 18, 134–141 [DOI] [PubMed] [Google Scholar]
- 10.Lupu F., Terwilliger J. D., Lee K., Segre G. V., Efstratiadis A. (2001) Dev. Biol. 229, 141–162 [DOI] [PubMed] [Google Scholar]
- 11.Sjögren K., Liu J. L., Blad K., Skrtic S., Vidal O., Wallenius V., LeRoith D., Törnell J., Isaksson O. G., Jansson J. O., Ohlsson C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjögren K., Jansson J. O., Isaksson O. G., Ohlsson C. (2002) Endocrine 19, 249–256 [DOI] [PubMed] [Google Scholar]
- 13.LeRoith D. (2008) Pediatr. Endocrinol. Rev. 5, Suppl. 2, 739–743 [PubMed] [Google Scholar]
- 14.del, Rincon J. P., Iida K., Gaylinn B. D., McCurdy C. E., Leitner J. W., Barbour L. A., Kopchick J. J., Friedman J. E., Draznin B., Thorner M. O. (2007) Diabetes 56, 1638–1646 [DOI] [PubMed] [Google Scholar]
- 15.Kostyo J. L., Hotchkiss J., Knobil E. (1959) Science 130, 1653–1654 [DOI] [PubMed] [Google Scholar]
- 16.Fan Y., Melhem M. F., Chaillet J. R. (1999) Developmental Biology 210, 481–496 [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Bruxvoort K. J., Zylstra C. R., Liu J., Cichowski R., Faugere M. C., Bouxsein M. L., Wan C., Williams B. O., Clemens T. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang D. L., Lee P. D., Cohen P. (2008) Growth Horm IGF Res. 18, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Xu B. C., Maheshwari H. G., He L., Reed M., Lozykowski M., Okada S., Cataldo L., Coschigamo K., Wagner T. E., Baumann G., Kopchick J. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimano H. (2007) J. Mol. Med. 85, 437–444 [DOI] [PubMed] [Google Scholar]
- 21.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. (2005) J. Lipid Res. 46, 2023–2028 [DOI] [PubMed] [Google Scholar]
- 22.Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson A., Isgaard J., Lindahl A., Dahlström A., Skottner A., Isaksson O. G. (1986) Science 233, 571–574 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson O., Marino R., De Luca F., Phillip M., Baron J. (2005) Hormone Res. 64, 157–165 [DOI] [PubMed] [Google Scholar]
- 25.Svensson J., Tivesten A., Sjögren K., Isaksson O., Bergström G., Mohan S., Mölne J., Isgaard J., Ohlsson C. (2007) J. Endocrinol. 193, 359–366 [DOI] [PubMed] [Google Scholar]
- 26.Imberti B., Morigi M., Tomasoni S., Rota C., Corna D., Longaretti L., Rottoli D., Valsecchi F., Benigni A., Wang J., Abbate M., Zoja C., Remuzzi G. (2007) J. Am. Soc. Nephrol. 18, 2921–2928 [DOI] [PubMed] [Google Scholar]
- 27.Cui Y., Hosui A., Sun R., Shen K., Gavrilova O., Chen W., Cam M. C., Gao B., Robinson G. W., Hennighausen L. (2007) Hepatology 46, 504–513 [DOI] [PubMed] [Google Scholar]
- 28.Johannsson G., Bengtsson B. A. (1999) J. Endocrinol. Invest. 22, 41–46 [PubMed] [Google Scholar]
- 29.Yakar S., Setser J., Zhao H., Stannard B., Haluzik M., Glatt V., Bouxsein M. L., Kopchick J. J., LeRoith D. (2004) J. Clin. Invest. 113, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delarue J., Magnan C. (2007) Curr. Opin. Clin. Nutr. Metab. Care 10, 142–148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.