Abstract
In mammals, nic o tin a mide phosphoribosyltransferase (NAMPT) and nic o tin a mide mononucleotide ad en y lyltransferase 1 (NMNAT-1) constitute a nuclear NAD+ salvage pathway which regulates the functions of NAD+-de pend ent enzymes such as the protein deacetylase SIRT1. One of the major functions of SIRT1 is to regulate target gene transcription through modification of chromatin-associated proteins. However, little is known about the molecular mechanisms by which NAD+ biosynthetic enzymes regulate SIRT1 activity to control gene transcription in the nucleus. In this study we show that stable short hairpin RNA-mediated knockdown of NAMPT or NMNAT-1 in MCF-7 breast cancer cells reduces total cellular NAD+ levels and alters global patterns of gene expression. Furthermore, we show that SIRT1 plays a key role in mediating the gene regulatory effects of NAMPT and NMNAT-1. Specifically, we found that SIRT1 binds to the promoters of genes commonly regulated by NAMPT, NMNAT-1, and SIRT1 and that SIRT1 histone deacetylase activity is regulated by NAMPT and NMNAT-1 at these promoters. Most significantly, NMNAT-1 interacts with, and is recruited to target gene promoters by SIRT1. Collectively, our results reveal a mechanism for the direct control of SIRT1 deacetylase activity at a set of target gene promoters by NMNAT-1. This mechanism, in collaboration with NAMPT-de pend ent regulation of nuclear NAD+ production, establishes an important pathway for transcription regulation by NAD+.
Nicotinamide adenine dinucleotide (NAD+), a coenzyme in metabolic processes and redox reactions, is an important signaling molecule. NAD+ is (i) a substrate for mono- and poly-ADP-ribosylation of proteins, (ii) required for NAD+-dependent protein deacetylation, and (iii) a precursor for calcium mobilizing agents (1). As a signaling molecule, NAD+ is consumed as a donor of ADP-ribose, releasing nicotinamide (NAM)2 as a byproduct. Consequently, resynthesis of NAD+ is crucial for maintaining the functions of a wide variety of NAD+-dependent enzymes in the cytoplasm and nucleus.
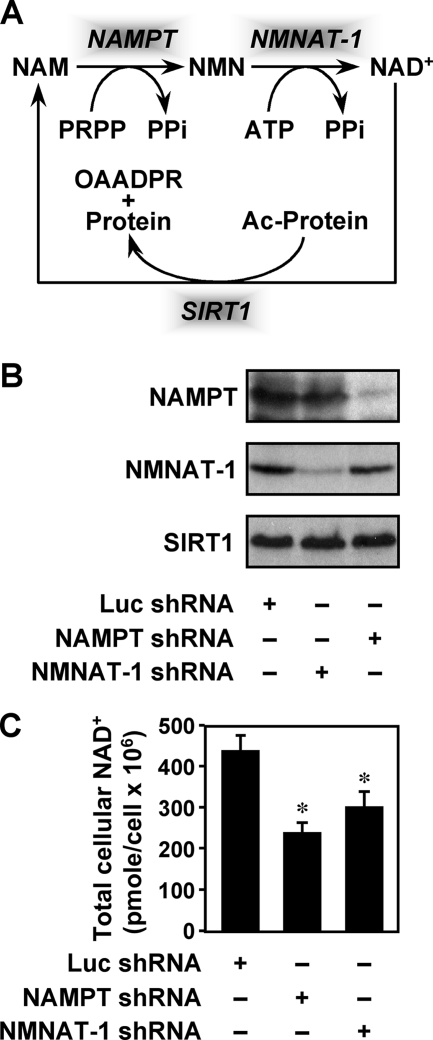
In mammalian cells the enzymes nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase (NMNAT) constitute an NAD+ salvage/recycling pathway using NAM as the precursor (see Fig. 1A) (2). NAMPT, a unique enzyme encoded by a single gene, catalyzes the conversion of NAM to nicotinamide mononucleotide (NMN). NAMPT localizes to both the cytosol and nucleus (3, 4).3 Interestingly, an extracellular form of NAMPT has also been described, although controversy exists regarding its function (5, 6). NMN produced by NAMPT is further converted into NAD+ by NMNAT. Three NMNAT enzymes encoded by distinct genes are found in mammals (7–10). Among them, NMNAT-1 is localized exclusively in the nucleus, whereas NMNAT-2 and NMNAT-3 are found in the Golgi and mitochondria, respectively (11). In the nucleus, NAMPT and NMNAT-1 form a nuclear NAD+ salvage pathway that supplies NAD+ as a substrate for a variety of NAD+-dependent enzymes, including the protein deacetylase SIRT1 and the poly(ADP-ribose) polymerase PARP-1 (Fig. 1A).
FIGURE 1.
Enzymes in the nuclear NAD+ salvage pathway regulate cellular NAD+ levels in MCF-7 cells. A, the nuclear NAD+ salvage pathway produces NAD+ for protein deacetylation by SIRT1. PRPP, phosphoribosylpyrophosphate; OAADPR, O-acetyl-ADP-ribose. B, stable shRNA-mediated knockdown of NMNAT-1 and NAMPT in MCF-7 cells. The Luc shRNA sequence was used as a control. NMNAT-1 and NAMPT protein levels were determined by Western blot analysis. C, total cellular NAD+ levels in control and NAMPT or NMNAT-1 knockdown cells. The concentrations of NAD+ in whole cell extracts were measured using a quantitative HPLC/mass spectrometry method with 18O standards. Error bars, S.E.; n = 8 independent biological replicates. *, significantly different from NAD+ levels in Luc control cells, p < 0.02 (Student's t test).
Many recent studies have examined the biological functions of NAMPT (12–18). These studies found that NAMPT expression is regulated by nutrients and stress in a number of human cell lines and primary rat tissues (13). Increased NAMPT levels protect cells against genotoxic stress through regulation of NAD+ levels and SIRT3 and SIRT4 functions in mitochondria (13). Nutrient restriction also stimulates NAMPT expression in skeletal myoblasts (17), leading to modulation of cellular NAD+/NADH ratio as well as NAM levels and to SIRT1-dependent impairment of myoblast differentiation (17). Similarly, NAMPT is up-regulated during human vascular smooth muscle cell maturation (15). This process is accompanied by increased cellular NAD+ levels and requires NAD+-dependent deacetylase activity (15). Intriguingly, NAMPT levels decline in aging smooth muscle cells, and ectopic expression of NAMPT delays smooth muscle cell senescence in a SIRT1-dependent pathway (14). Together, these studies illustrate a critical role of NAMPT in muscle cell differentiation, maturation, and senescence. Consistent with these observations, knock-out mouse studies indicate that NAMPT is essential for early embryo development (16) as well as lymphocyte differentiation (19). Recent studies have also shown that NAMPT is required to modulate circadian gene expression (20, 21).
In comparison to NAMPT, our knowledge of the physiological functions of NMNAT-1 is limited to observations made using the Wallerian degeneration slow (WldS) mouse model. The axonal protective phenotype of these mice results from overexpression of a chimeric nuclear protein WldS with NMNAT-1 activity (22, 23). Both mammalian NMNAT-1 and Drosophila NMNAT exhibit neuronal protective activity, although in some studies with reduced efficacy compared to WldS protein (24–30). Controversy exists over the mechanism of WldS action; in some experimental systems NMNAT enzymatic activity is critical for the neuronal protection phenotype (24, 25), whereas in other systems mechanisms independent of the enzymatic activity have been proposed (26–28, 31).
The most important mediators of NAMPT and NMNAT-1 actions identified so far are the sirtuin family of NAD+-dependent enzymes, especially SIRT1 (12–15, 17, 18, 24). SIRT1 is a nuclear NAD+-dependent deacetylase that connects cell metabolism to transcriptional regulation (32). SIRT1 regulates chromatin structure and gene transcription through modification of chromatin-associated proteins, including histones, transcription factors, and coregulators as well as components of the basal transcriptional machinery (33–35). SIRT1 directly interacts with DNA binding transcription factors and coregulators and is recruited to gene promoters through these interactions. Many of these same factors are direct targets of deacetylation by SIRT1, including p53, Ku70, NF-κB, the FOXO family of transcription factors, liver X receptor, estrogen receptor α, SOX9, PGC-1α, and p300 (18, 33, 34, 36). Once at gene promoters, SIRT1 can modify additional chromatin-associated proteins, including histones and other coregulators, to control the transcriptional outcome (18, 34). Transcription regulation by SIRT1 can be either activation or repression, depending upon the specific factors involved. Interestingly, a recent study has demonstrated that DNA damage induces global redistribution of SIRT1 on chromatin, leading to transcriptional deregulation of SIRT1-associated genes (37). Overall, SIRT1-dependent transcriptional regulation plays a key role in cell defense, survival, metabolism, and cellular signaling responses.
Mounting evidence suggests that NAD+ biosynthesis has a broad impact on cellular functions through transcription regulation. Of particular focus is the role of NAMPT in controlling SIRT1 activity through intracellular as well as systemic NAD+ production (5, 6). Many of the functions of NAMPT are mediated by SIRT1, presumably through changes in NAD+ and possibly NAM levels (12, 14, 15, 17). However, details on the regulatory events that occur at gene promoters in response to NAD+ production are not clear. In this study we focused on the target genes regulated by the mammalian NAD+ salvage pathway and explored the molecular mechanism that connects the NAD+ biosynthetic enzymes to SIRT1-dependent transcriptional regulation. Our results reveal a mechanism for the direct control of SIRT1 deacetylase activity at a set of target gene promoters by NMNAT-1, in collaboration with NAMPT.
EXPERIMENTAL PROCEDURES
Plasmids and Protein Expression
The human NAMPT cDNA variant 1 was obtained from American Type Culture Collection (ATCC catalog no. 10327682). The human NMNAT-1 cDNA was kindly provided by Dr. Mathias Ziegler (University of Bergen). The NAMPT and NMNAT-1 cDNAs were PCR-amplified with an amino-terminal FLAG tag and cloned into pQCXIP (BD Biosciences) for retrovirus-mediated expression in mammalian cells. Catalytic mutants and RNA interference-resistant mutants of NMNAT-1 and NAMPT were generated by site-directed mutagenesis following QuikChange protocol (Stratagene). The NMNAT-1 cDNA was also cloned into the following bacterial expression plasmids; 1) a modified pGEX-2TK vector for production of a GST-NMNAT-1 fusion protein and 2) a modified pET-15b vector with an amino-terminal His6 tag for production of a His6-NMNAT-1 fusion protein. The recombinant proteins were purified under standard non-denaturing conditions. All plasmid constructs derived from PCR products were confirmed by DNA sequencing. Recombinant mouse SIRT1 with an amino-terminal His6 tag was prepared as described previously (36).
Antibodies
Rabbit polyclonal antibodies were raised against purified human NMNAT-1 and mouse SIRT1 (Pocono Rabbit Farm and Laboratory). All other antibodies were from commercial sources: anti-NAMPT rabbit polyclonal antibody (Bethyl Laboratories), anti-FLAG M2 monoclonal antibody (Sigma), anti-acetyl-histone H4 (Lys-16) polyclonal antibody (Millipore), and anti-H3 polyclonal antibody (Abcam).
Short Hairpin RNAs (shRNAs)
shRNA sequences for RNA interference-mediated knockdown were designed using the web-based siDESIGN® Center software from Dharmacon and cloned into pSuper.Retro vectors (Oligoengine). Multiple target sequences were tested for each gene, and the most effective sequences were used for the studies described herein: NAMPT, 5′-GAGTGTTACTGGCTTACAA-3′ (#1) and 5′-TAACTTAGATGGTCTGGAA-3′ (#2); NMNAT-1, 5′-AACACAAGATTCTAGTCAA-3′ (#1) and 5′-AACTCACCTACTCTAGAAA-3′ (#2); SIRT1, 5′-TGAAGTGCCTCAGATATTA-3′ (#1) and 5′-AAGCGATGTTTGATATTGA-3′ (#2). An shRNA directed against firefly luciferase (Luc), 5′-GATATGGGCTGAATACAAA-3′, was used as a control (38).
Cell Culture, Ectopic Expression, and shRNA-mediated Knockdown
MCF-7 cells were kindly provided by Dr. Benita Katzenellenbogen (University of Illinois, Urbana-Champaign) and maintained in Eagle's minimum essential medium (Sigma) supplemented with 5% calf serum and antibiotics. Phoenix-Ampho retrovirus producer cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (Sigma) with 10% fetal bovine serum. Retroviruses were prepared following a standard transfection protocol using the Phoenix-Ampho cell line and the retroviral vectors described above (i.e. derivatives of pQCXIP, pSuper.Retro). The recombinant retroviruses were used to transduce MCF-7 cells, which were then selected with 1.0 μg/ml puromycin (Sigma) or 800 μg/ml G418 (Invitrogen) as appropriate for each vector. Expression and knockdown were screened by Western blotting.
NAD+ Measurements
The concentrations of NAD+ in whole cell extracts from MCF-7 cells were determined using a quantitative HPLC/mass spectrometry (MS) method (HPLC/matrix-assisted laser desorption ionization/MS) with 18O standards (13, 39).
Expression Microarray and Gene Ontology (GO) Analyses
To knockdown target factors for expression microarray analyses, the following shRNA sequences were used: NAMPT, shRNA #1; NMNAT-1, shRNA #1; SIRT1, shRNAs #1 and #2 (maintained under puromycin and G418 selection). For each factor studied, three independent isolates of MCF-7 cells recently (within 2–3 weeks) transduced with shRNA-expressing retroviruses were used. Studies on NMNAT-1, NAMPT, and SIRT1 were carried out independently, and each had its own matching Luc control. Total RNA was isolated from the various MCF-7 cell lines using Trizol reagent (Invitrogen) followed by RNeasy columns (Qiagen).
Sample labeling and hybridization to Affymetrix Human Genome U133 plus 2.0 and U133A 2.0 arrays were carried out under standard conditions at the Cornell Microarray Core Facility. The raw data were processed using Affymetrix GCOS software to obtain detection calls and signal values. Where applicable, the common probe sets of the two array platforms were normalized by scaling. The data sets were adjusted for batch effects using a parametric empirical Bayes method (40). After this normalization, all values <0.01 were adjusted to 0.01, and the data were log2-transformed, median-centered for each array, and median-centered for each gene. Only those probe sets having “present” calls in at least two of the three replicates were included for further analysis. A two-tailed Student's t test was applied to the normalized data matrix to identify genes differentially expressed between each knockdown condition and the matching Luc control. A p value cutoff of 0.05 was applied to define the differentially expressed gene set. A -fold change cutoff of log2 <−0.5 or >0.5 was applied to select significantly regulated gene sets. The gene lists were analyzed for enrichment of GO terms using the Functional Annotation Clustering and Functional Annotation Chart tools from the DAVID Bioinformatics Resources website (david.abcc.ncifcrf.gov). The expression microarray data sets can be accessed from the NIH GEO website (www.ncbi.nlm.nih.gov) using accession number GSE13577.
Reverse Transcription-Quantitative Real-time PCR (RT-qPCR) Assays
Total RNA samples were purified using Trizol Reagent (Invitrogen). cDNA samples were prepared by reverse transcription with an oligo(dT) primer and analyzed by SYBR Green real-time PCR under standard conditions. For data normalization, β-actin was used as the reference gene. The sequences of the primers used are available on request.
Chromatin Immunoprecipitation (ChIP)-Real-time PCR (qPCR) Assays
ChIP assays using MCF-7 cells were performed as described previously (41, 42). ChIP samples were analyzed by SYBR Green real-time PCR under standard conditions. The sequences of the primers used are available on request. Positive ChIP signals for all antibodies used in this study are at least 3-fold above no antibody controls. The ChIP data presented are values after subtraction of the corresponding no antibody control data.
ChIP-Western Assays
ChIP assays were carried out as described above. After washing samples in ChIP wash buffer, the protein A/G beads were resuspended in 2× SDS loading dye and boiled for 10 min. Samples were resolved on 10% SDS-PAGE for standard Western blot analysis.
GST-NMNAT-1 Interaction Assays
GST and GST-NMNAT-1 were expressed in bacteria and purified using standard glutathione-agarose affinity chromatography. The purified proteins were quantified by Coomassie Blue staining on SDS-PAGE gels using BSA as a standard. HeLa cell nuclear extract was incubated with immobilized GST or GST-NMNAT-1, the samples were washed, and the specifically bound proteins were analyzed by Western blotting.
RESULTS
NAMPT and NMNAT-1 Regulate NAD+ Biosynthesis and Gene Expression in MCF-7 Breast Cancer Cells
NAMPT and NMNAT-1 catalyze sequential reactions in the nuclear NAD+ salvage pathway that re-synthesizes NAD+ using NAM (Fig. 1A). To determine the functions of NAMPT and NMNAT-1 in gene regulation, we first examined their roles in NAD+ synthesis and gene expression in MCF-7 breast cancer cells. shRNA-mediated stable knockdown reduced NAMPT and NMNAT-1 protein levels to ∼10–20% of the levels in control (Luc) knockdown cells (Fig. 1B). Total cellular NAD+ levels in extracts from control and knockdown cells were measured using a quantitative HPLC/mass spectrometry method with 18O-labeled standards (13, 39). Using this sensitive method, we detected a 30–45% decrease in the total cellular NAD+ levels upon knockdown of either NAMPT or NMNAT-1 (Fig. 1C). These results indicate that both NAMPT and NMNAT-1 contribute to NAD+ production in MCF-7 cells. Considering that there are two other NMNAT enzymes in cells, one in Golgi (NMNAT-2) and one in mitochondria (NMNAT-3) (11), our results implicate NMNAT-1 as a major contributor to cellular NAD+ production.
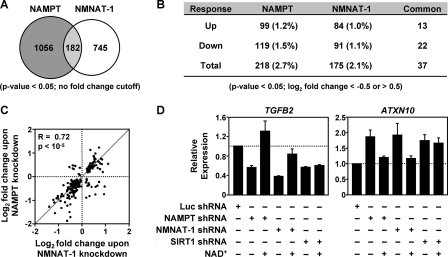
Expression microarray analyses using the same MCF-7 knockdown cell models indicate that both NAMPT and NMNAT-1 have broad effects on gene expression, each regulating as much as ∼10% of the MCF-7 cell transcriptome at 95% confidence (two-tailed Student's t test) or as much as ∼3% with a log2 <−0.5 or >0.5-fold change cutoff (Fig. 2, A and B). Overall, the extent and magnitude of expression regulation by NAMPT and NMNAT-1 in our study are in agreement with previously reported data from samples ectopically expressing these enzymes (12, 43).
FIGURE 2.
NAMPT and NMNAT-1 regulate gene expression in MCF-7 cells. A, Venn diagram showing number of genes significantly regulated by NAMPT and NMNAT-1 knockdown (p < 0.05, Student's t test) without a -fold change cutoff, as determined by expression microarray analysis. B, number of genes significantly regulated by NAMPT or NMNAT-1 knockdown (p < 0.05, Student's t test) after applying a -fold change cutoff (log2-fold change <−0.5 or >0.5). Numbers in parentheses indicate the percentage of total expressed genes. Each gene set is divided into up- and down-regulated groups. Note that two common genes are differentially regulated by NAMPT and NMNAT-1. C, scatter plot showing correlation of the expression profiles of 182 genes commonly regulated by NAMPT- and NMNAT-1 (i.e. the overlap in panel A). Spearman's rank correlation coefficient (r) and p value are indicated. D, effect of NAMPT, NMNAT-1, or SIRT1 knockdown with or without exogenously added NAD+ (1 mm) on the expression of NAMPT- and NMNAT-1-regulated genes. The target genes are selected based on the expression microarray analyses. Gene expression levels were determined by RT-qPCR using β-actin as the reference gene. Data are presented as expression levels relative to that in the Luc control cells. Error bars, S.E.; n ≥ 3 independent biological replicates.
Interestingly, we only observed an ∼15–20% overlap between the NAMPT- and NMNAT-1-regulated gene sets (Fig. 2, A and B), perhaps reflecting differences between the two enzymes in subcellular localization, interacting factors, and the metabolic intermediates accumulated as a result of enzyme depletion. The regulation of the overlapping genes, however, generally occurred in the same direction (up or down), with similar magnitude and was highly correlated (Spearman correlation coefficient 0.72; p value <10−5) (Fig. 2C). GO analyses support the same observation. The NAMPT- and NMNAT-1-regulated genes have overlapping, yet distinct cellular and molecular functions (supplemental Table S1). For the commonly regulated NAMPT and NMNAT-1 target genes, GO analysis revealed an enrichment of terms related to neuronal differentiation, cell signaling, and cellular membrane functions (supplemental Table S1), suggesting that these functions are particularly sensitive to perturbation of the nuclear NAD+ salvage pathway.
To confirm that expression regulation by the specific shRNAs used in our microarray analyses is not due to off-target effects, we tested an additional shRNA each for NAMPT and NMNAT-1 (supplemental Fig. S1A). In gene-specific RT-qPCR analysis, the two shRNAs targeting each factor showed similar effects on the expression of 38 genes (supplemental Fig. S1B). Furthermore, we observed a good agreement between the efficiency of protein knockdown and the effects on expression by the different shRNAs (supplemental Fig. S1). These results suggest that off-target effects were not a major influence on the identification of target genes in our expression analyses using the NAMPT and NMNAT-1 shRNAs.
For the purposes of this study, we focused on the commonly regulated NAMPT and NMNAT-1 target genes with a log2 <−0.5 or >0.5-fold change cutoff (37 genes total; Fig. 2B) as they are most likely to represent genes regulated by the intact NAMPT/NMNAT-1 salvage pathway rather than independent actions of NAMPT and NMNAT-1. As expected, gene-specific RT-qPCR analyses confirmed the NAMPT- and NMNAT-1-regulated expression of most of these genes, including TGFB2 (down-regulated by NAMPT or NMNAT-1 knockdown) and ATXN10 (up-regulated by NAMPT or NMNAT-1 knockdown) (Fig. 2D; see also Fig. 4). To test directly if this regulation requires NAD+, we examined the effect of adding exogenous NAD+ to the cell culture medium, as described previously (44). Treatment with 1 mm NAD+ for 24 h restored the expression of TGFB2 and ATXN10 in the NAMPT and NMNAT-1 knockdown cells (Fig. 2D). For some genes (e.g. CAV1), the addition of exogenous NAD+ was not able to restore expression in the NAMPT and NMNAT-1 knockdown cells (supplemental Fig. S2), suggesting NAD+-independent functions of NAMPT and NMNAT-1 in the regulation of some target genes.
FIGURE 4.
Regulation of target gene expression by NAMPT, NMNAT-1, and SIRT1 in MCF-7 cells. RT-qPCR confirmation of expression microarray data. Data are presented as expression levels relative to that in the luciferase control cells. The shaded area indicates the boundary for -fold change <1.414 (i.e. log2-fold change < 0.5) or -fold change >0.707 (i.e. log2-fold change >−0.5). Error bars, S.E.; n ≥ 3 independent biological replicates.
Focusing on the genes responsive to NAD+ treatment, we further tested if NAMPT and NMNAT-1 enzymatic activity is required for the regulation of these genes. When RNA interference-resistant forms of the NAD+ biosynthetic enzymes were re-expressed in the corresponding knockdown cell lines, the wild type enzymes restored the expression of TGFB2 and ATXN10 in the knockdown cells (supplemental Fig. S3). In contrast, the catalytic mutants had no effect (supplemental Fig. S3). This result demonstrates the essential role of NAD+ synthesis in the regulation of these genes. Overall, our NAD+ add back, and enzyme re-expression studies indicate that, for a subset of the NAMPT- and NMNAT-1-responsive genes, cellular NAD+ level plays a direct role in the regulation of their expression.
SIRT1 Mediates NAMPT- and NMNAT-1-dependent Gene Regulation
Given the NAD+ dependence for the expression of TGFB2, ATXN10, and other NAMPT- and NMNAT-1-regulated genes, we considered the possible involvement of SIRT1, an NAD+-dependent nuclear deacetylase. SIRT1 regulates a variety of cellular processes, including stress responses, metabolism, and cell differentiation, maturation, and survival (33). In MCF-7 cells, reduced SIRT1 activity promotes re-expression of epigenetically silenced tumor suppressor genes, impairs activation of mitogen-activated protein kinase pathways, and induces senescence-like growth arrest (45, 46). SIRT1 NAD+-dependent enzymatic activity can mediate the deacetylation of histones and other nuclear proteins, including a variety of transcription factors (33). Previous studies have shown that SIRT1 regulates cellular events downstream of NAMPT and NMNAT-1 (12, 14, 17, 18, 24). Interestingly, in MCF-7 cells SIRT1 depletion had similar effects on the expression of ATXN10 and TGFB2 as depletion of NAMPT and NMNAT-1 (Figs. 2D and 4). Furthermore, although exogenous NAD+ rescued the expression of TGFB2 and ATXN10 in NAMPT- or NMNAT-1-depleted cells, it had little effect on these genes in SIRT1-depleted cells (Fig. 2D). These results indicate that SIRT1 is required for the NAD+-dependent regulation of these genes.
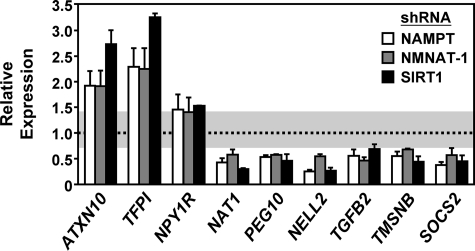
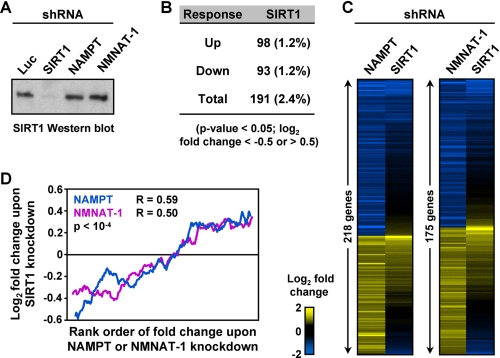
To further explore the relationship between gene regulation by SIRT1 and gene regulation by NAMPT or NMNAT-1, we performed an expression microarray analysis upon SIRT1 knockdown versus a control (Luc) knockdown in MCF-7 cells. Depletion of SIRT1 significantly altered the expression of ∼200 genes (log2 <−0.5 or >0.5-fold change cutoff), with approximately equal numbers of up-regulated and down-regulated genes (Fig. 3, A and B). In gene-specific RT-qPCR analyses, the two shRNA constructs used in our SIRT1 knockdown studies produced similar effects on gene expression (supplemental Fig. S1), suggesting that off-target effects were not a major influence on the identification of SIRT1 target genes in our expression analyses. GO analysis revealed important functions of the SIRT1-regulated genes in cell signaling and metabolism (supplemental Table S1). Many of the NAMPT- or NMNAT-1-regulated genes described above are similarly regulated (i.e. magnitude and direction of regulation) by SIRT1 knockdown, and the expression profiles show significant correlation in the knockdown cells (Fig. 3, C and D). Further analysis of the 37 genes commonly regulated by NMNAT-1 and NAMPT (log2 <−0.5 or >0.5-fold change cutoff) revealed extensive co-regulation by SIRT1 (supplemental Fig. S4), an observation that we confirmed by RT-qPCR (Fig. 4). Together, these results suggest that SIRT1 is a key regulator of NAMPT- and NMNAT-1-responsive genes.
FIGURE 3.
Many NAMPT- and NMNAT-1-regulated genes show similar patterns of regulation by SIRT1 in MCF-7 cells. A, stable shRNA-mediated knockdown of SIRT1 in MCF-7 cells. SIRT1 protein levels were determined by Western blot analysis. The shRNA constructs that knockdown NAMPT and NMNAT-1 (Fig. 1B) have no effect on SIRT1 protein levels. B, number of genes significantly regulated by SIRT1 knockdown (p < 0.05, Student's t test) after applying a -fold change cutoff (log2-fold change <−0.5 or >0.5) as determined by expression microarray analysis. Numbers in parentheses indicate the percentage of total expressed genes. The gene set is divided into up- and down-regulated groups. C, expression profiles of the NAMPT- and NMNAT-1-regulated genes (Fig. 2B) in response to SIRT1 knockdown. D, correlation analysis of the gene expression profiles shown in panel C. The plots show a 30-gene-moving average. Spearman's rank correlation coefficient (R) and p value are indicated.
The genes commonly regulated by NAMPT, NMNAT-1, and SIRT1 are significantly enriched in GO terms including “metabolism” and “cellular membrane” (supplemental Table S1), highlighting the cellular functions that are most critically controlled by the NAD+-dependent SIRT1 activity. Careful examination reveals that many of these genes play roles in cell signaling and/or neuronal functions (Table 1). For example, TGFβ2, SOCS2, and PEG10 function in cytokine signaling pathways, but PEG10 and SOCS2 also have neuronal functions (see Table 1 and the references therein). Furthermore, NPY1R and NELL2 have predominantly neuron-related signaling functions. Other factors, including ATXN10, NAT1, TFPI, and TMSNB, are associated with different pathological aspects of the nervous system. These results suggest that the NAD+-salvage pathway and NAD+-dependent SIRT1 activity may be important regulators of cell signaling and neuronal functions. Interestingly, neuronal protective functions have been reported for NMNAT and SIRT1 (24–30). The functions of these factors in gene regulation in a neuronal cell model is currently under investigation.
TABLE 1.
Genes with signaling and/or neuronal functions that are commonly regulated by NAMPT, NMNAT-1, and SIRT1 in MCF-7 cells
| Gene symbol | Gene name | Functions |
|---|---|---|
| ATXN10 | Ataxin 10 | Pentanucleotide repeat expansions in the ATXN10 gene lead to spinocerebellar ataxia type 10 (SCA10) (57). |
| NAT1 | N-Acetyltransferase 1 | NAT1 protein is a subunit of an N-acetyltransferase complex that co-localizes with microtubules in dendrites and regulates dendrite development (58). The same complex also associates with ß-amyloid precursor protein and regulates amyloid ß-protein generation (59). |
| NELL2 | NEL-like 2 | NELL2 encodes a neuron-specific epidermal growth factor-like protein that promotes neuronal differentiation (60). |
| NPY1R | Neuropeptide Y receptor type Y1 | NPY1R protein is a receptor for neuropeptide Y, one of the most abundant neuropeptides in the mammalian nervous system (61). |
| PEG10 | Paternally expressed 10 | PEG10 is an imprinted gene (62). PEG10 protein functions in part by interacting with members of the transforming growth factor β receptor family. PEG10 transcript is abundant in the brain. Overexpression of PEG10 and activin receptor-like kinase 1 (Alk1) in different cell types induces a neuronal-like morphology (63). |
| SOCS2 | Suppressor of cytokine signaling 2 | SOCS2 protein suppresses cytokine signaling through interactions with the cytoplasmic domain of insulin-like growth factor-1 receptor (64). In the nervous system, SOCS2 regulates neuronal differentiation and neurite outgrowth (65). |
| TFPI | Tissue factor pathway inhibitor | TFPI encodes a protease inhibitor that regulates the tissue factor-dependent pathway of blood coagulation (66). TFPI protein is elevated in frontal cortex samples from Alzheimer disease brains and associates with amyloid ß-containing senile plaques (67). |
| TGFB2 | Transforming growth factor β2 | TGFB2 encodes a cytokine that regulates many functions, e.g. tumor-suppression, cell invasion, immune regulation, and microenvironment modification (68). |
| TMSNB | Thymosin β identified in neuroblastoma cells | TMSNB was first identified in neuroblastoma cells. It binds to and sequesters actin monomers and, therefore, inhibits actin polymerization (69). |
NAMPT and NMNAT-1 Regulate SIRT1 Activity at Target Gene Promoters
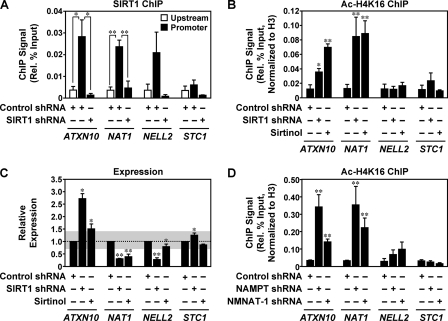
Next, we examined potential functional interplay between NAMPT, NMNAT-1, and SIRT1 at commonly regulated gene promoters. We restricted our analysis to these genes because they are more likely targets of regulation by the NAD+ salvage pathway as well as NAD+-dependent SIRT1 activity. Previous studies have reported the binding of SIRT1 to target gene promoters, with subsequent deacetylation of specific target proteins including histones and transcription factors (18, 33, 34, 37). We also observed binding of SIRT1 to the target genes that we identified in our analyses in MCF-7 cells. As shown in the ChIP assays presented in Fig. 5A, SIRT1 bound at the promoters but not upstream regions (∼−10 kilobases) of ATXN10, NAT1, and NELL2. A similar level of SIRT1 binding was not observed, however, at the promoter of a non-target gene, STC1 (Fig. 5, A and C). The SIRT1 ChIP signal was reduced to background levels upon SIRT1 knockdown, confirming the specificity of the signal in this assay (Fig. 5A).
FIGURE 5.
NAMPT and NMNAT-1 regulate SIRT1 deacetylase activity at target gene promoters in MCF-7 cells. A, ChIP-qPCR analysis of SIRT1 localization at promoter and upstream (∼−10 kilobase) regions of target genes in MCF-7 cells. SIRT1 localization was also examined at the target gene promoters in SIRT1 knockdown cells. Statistical significance was determined by a two-tailed Student's t test (*, p < 0.05; **, p < 0.01). B and D, ChIP-qPCR analysis of acetylated H4K16 (Ac-H4K16) levels at target gene promoters in SIRT1, NAMPT, or NMNAT-1 knockdown cells or in control cells with or without sirtinol (50 μm) treatment. Ac-H4K16 levels were normalized for H3 occupancy. Statistical significance for differences between Luc control samples and other samples was determined by a two-tailed Student's t test (*, p < 0.05; **, p < 0.01). C, effect of sirtinol on the expression of SIRT1-regulated genes. RT-qPCR data are presented as expression levels relative to those in the control cells. The shaded areas indicate the boundaries for -fold change <1.414 (i.e. log2-fold change < 0.5) or -fold change >0.707 (i.e. log2-fold change >−0.5). Statistical significance for differences between Luc control samples and other samples was determined by two-tailed Student's t test (*, p < 0.05; **, p < 0.01). All panels: error bars, S.E.; n ≥ 3 independent biological replicates.
One outcome of SIRT1 activity is the deacetylation of histone H4 acetylated at lysine 16 (Ac-H4K16), a modification targeted exclusively by sirtuin family members (47). Previous studies have shown that Ac-H4K16 levels peak at gene promoters, although they may be somewhat elevated in the coding regions of genes as well (48). Our ChIP-qPCR analysis and previous ChIP-chip analyses indicates that SIRT1 is also found in peaks at target gene promoters (supplemental Fig. S5) (37). Based on these observations, we focused our analysis of Ac-H4K16 levels at promoter regions of target genes as an indicator of SIRT1 activity. shRNA-mediated knockdown of SIRT1 or inhibition of SIRT1 enzymatic activity with the chemical inhibitor sirtinol (49) increased the levels of Ac-H4K16 (normalized to total histone H3 content) at the promoters of the common target genes ATXN10 and NAT1 (Fig. 5B). The same treatment conditions increased expression for ATXN10 and decreased expression for NAT1 (Fig. 5C), suggesting different modes of SIRT1-dependent regulation for these two genes. Interestingly, knockdown of NAMPT or NMNAT-1 had similar effects on ATXN10 and NAT1 with respect to the levels of promoter-directed Ac-H4K16 (Fig. 5D) and expression (Fig. 4). In contrast, NELL2, another gene regulated by SIRT1 knockdown, showed (i) no requirement for SIRT1 enzymatic activity for expression (as determined by sirtinol treatment; Fig. 5C), (ii) no effect of SIRT1 knockdown or sirtinol treatment on the levels of promoter-directed Ac-H4K16 (Fig. 5B), and (iii) no effect of NAMPT or NMNAT-1 knockdown on the levels of promoter-directed Ac-H4K16 (Fig. 5D). As expected, the non-target gene STC1 was largely unaffected by all of the experimental conditions tested (Fig. 5, B–D). As a control, recruitment of SIRT1 protein to these gene promoters was not affected by knockdown of NAMPT or NMNAT-1 (supplemental Fig. S6). Thus, NAMPT and NMNAT-1 control SIRT1-dependent deacetylation of Ac-H4K16 and transcriptional regulation of target genes requiring SIRT1 enzymatic activity (e.g. ATXN10 and NAT1). At target genes regulated independently of SIRT1 enzymatic activity (e.g. NELL2); however, NAMPT and NMNAT-1 may control gene expression through mechanisms other than NAD+ production. Alternatively, other NAD+-dependent enzymes such as PARP-1 (50) may mediate NAD+-dependent transcription regulation of these genes. Together, these results indicate that NAMPT and NMNAT-1 can directly regulate molecular outcomes at some target genes by regulating SIRT1 catalytic activity through the production of NAD+.
NMNAT-1 Is Recruited to Gene Promoter Regions through Interaction with SIRT1
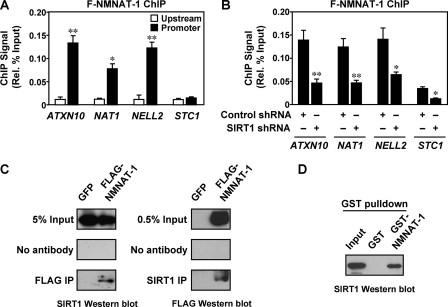
Our results suggest a mechanism in which NAD+ production by NAMPT and NMNAT-1 regulates gene expression through modulation of SIRT1 activity at target gene promoters. Given that both NAMPT and NMNAT-1 are found in the nucleus (3, 4, 11), we considered the possibility that they might be recruited to the promoters of target genes for localized actions in a manner similar to SIRT1. To test this hypothesis, we examined the binding of NAMPT and NMNAT-1 to target gene promoters in MCF-7 cells expressing FLAG-tagged NAMPT or NMNAT-1. Like SIRT1, NMNAT-1 bound at the promoters of the commonly regulated target genes (i.e. ATXN10, NAT1, and NELL2) but not at upstream and downstream regions of the same genes or at the promoter of a non-target gene (i.e. STC1) (Fig. 6A and supplemental Fig. S5). Under the same assay conditions, we were unable to detect NAMPT binding at the same promoters despite the fact that the FLAG antibody can immunoprecipitate FLAG-NAMPT under our ChIP assay conditions (data not shown). The difference in the chromatin association of NMNAT-1 and NAMPT may mark an important distinction between their cellular functions.
FIGURE 6.
NMNAT-1 is recruited to target gene promoters by SIRT1. A, FLAG-based ChIP-qPCR analysis of NMNAT-1 localization at promoter and upstream (∼−10 kilobase) regions of target genes. MCF-7 cells expressing FLAG-NMNAT-1 were used for the FLAG ChIP assay. Error bars, S.E.; n ≥ 3 independent biological replicates. Statistical significance for differences between ChIP signals at upstream and promoter regions of the same gene was determined by a two-tailed Student's t test (*, p < 0.05; **, p < 0.01). B, FLAG-based ChIP-qPCR analysis of NMNAT-1 localization at target gene promoters in control or SIRT1 knockdown cells. Error bars, S.E.; n ≥ 3 independent biological replicates. Statistical significance for differences between ChIP signals in control and SIRT1 knockdown cells was determined by a two-tailed Student's t test (*, p < 0.05; **, p < 0.01). C, ChIP-Western analysis of interaction between SIRT1 and NMNAT-1. MCF-7 cells expressing GFP or FLAG-NMNAT-1 were used for FLAG and SIRT1 ChIP assays. The immunoprecipitated (IP) material was subjected to SIRT1 and FLAG Western blot analysis, respectively. Gels shown are representative of three independent experiments. D, GST-NMNAT-1 interaction assay with native SIRT1 from nuclear extract. SIRT1 bound to glutathione-agarose resin was detected by Western blot analysis. The gel shown is representative of three independent experiments.
Given the colocalization of NMNAT-1 and SIRT1 to the same promoters, we considered the possibility that SIRT1 might recruit NMNAT-1 to specific sites on chromatin. Indeed, knockdown of SIRT1 reduced the binding of NMNAT-1 to target gene promoters (Fig. 6B). Furthermore, under our ChIP assay conditions SIRT1 co-immunoprecipitated with FLAG-NMNAT-1 and vice versa (Fig. 6C), demonstrating in vivo interaction between the two proteins. In an in vitro binding assay, native SIRT1 in nuclear extracts specifically bound to GST-NMNAT-1 but not GST alone (Fig. 6D). Together, these results indicate that SIRT1 binds to and recruits NMNAT-1 to specific sites on chromatin.
DISCUSSION
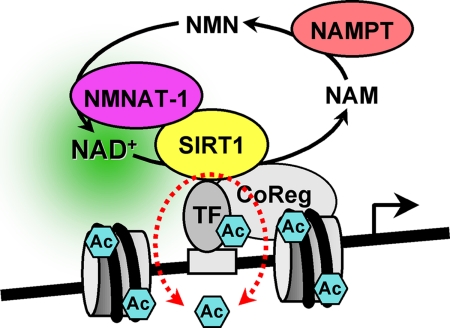
In this study we examined the gene regulatory function of two enzymes in the nuclear NAD+ biosynthetic pathway, NAMPT and NMNAT-1, as well as the client nuclear NAD+-dependent protein deacetylase, SIRT1. Using shRNA-mediated knockdown and expression microarray analyses, we identified a set of genes commonly regulated by NAMPT, NMNAT-1, and SIRT1 in MCF-7 cells. Using ChIP assays, we showed that SIRT1 binds specifically to the promoter regions of these genes. Although we have not examined the mode of SIRT1 recruitment in our studies, previous studies have shown that SIRT1 can be recruited to gene promoters through direct interaction with sequence-specific transcription factors (e.g. FOXO family members, NF-κB, p53, SOX9) as well as transcription coregulators (e.g. p300, NCoR, SMRT) (18, 34). In our study we showed that the deacetylase activity of SIRT1 at target gene promoters is controlled by NAMPT and NMNAT-1, establishing one possible mechanism for NAMPT and NMNAT-1-dependent transcription regulation. Interestingly, we found that NMNAT-1 is recruited to target gene promoters through interaction with SIRT1. This colocalization of NMNAT-1 and SIRT1 on chromatin may provide a unique mechanism for NAD+-dependent transcriptional regulation (Fig. 7).
FIGURE 7.
A model for the regulation of SIRT1 activity at target gene promoters by NAMPT and NMNAT-1. SIRT1 binds to target gene promoters and regulates the acetylation status of transcription factors, histones, and other chromatin-associated proteins in an NAD+-dependent manner. Both NAMPT and NMNAT-1 localize to the nucleus and constitute an NAD+ recycling pathway. Nuclear NAD+ production by NAMPT and NMNAT-1 supports SIRT1 deacetylase activity. In addition, NMNAT-1 interacts with SIRT1 and is recruited to SIRT1 target gene promoters. Interactions between NMNAT-1 and SIRT1 on chromatin may underlie novel mechanisms for transcriptional regulation by SIRT1. TF, transcription factor. CoReg, coregulator.
A somewhat surprising observation from our expression microarray analysis is that the gene sets regulated by NAMPT or NMNAT-1 show only a moderate overlap (15–20%, Fig. 2, A and B). The enzymes are in a linear NAD+ biosynthetic pathway, and their knockdown have similar effects on cellular NAD+ levels (Fig. 1C). Therefore, genes that are regulated by nuclear NAD+ levels are likely to respond to both enzymes in a similar way. However, both NAMPT and NMNAT-1 may have gene regulatory activities independent of NAD+ production in the nucleus. NAMPT is found in cytoplasm, including mitochondria (3, 4, 13), where it can modulate NAD+ levels and may impact gene expression independent of the nuclear NAD+ pathway. Importantly, one consequence of NAMPT enzymatic activity is to remove NAM (17), an inhibitor of sirtuin and PARP activities. Direct measurement of NAM failed to show any changes in total cellular NAM levels in our NAMPT or NMNAT-1 knockdown cells (data not shown). However, we cannot rule out the possibility that some effects of NAMPT on gene expression are mediated through NAM removal in a microenvironment. NMNAT-1 is an exclusively nuclear protein and is recruited to gene promoters (Fig. 6A). It interacts with nuclear proteins such as SIRT1 (Fig. 6, C and D) and PARP-1 (51).4 The unique localization and interaction partners of NMNAT-1 may contribute to gene regulation independent of nuclear NAD+ production. Based on these considerations, for the mechanistic studies we focused on a subset of genes that are commonly regulated by NAMPT, NMNAT-1, and SIRT1 and are, therefore, most likely targets of transcription regulation by both the NAD+ salvage pathway and SIRT1.
Previous studies have shown that the cellular functions of SIRT1 are regulated by NAMPT and NMNAT-1 (12, 14, 15, 17, 18, 24). In the case of NAMPT, its actions have been linked to increases in cellular NAD+ production (12–14, 17, 19). Additionally, NAMPT can reduce cellular NAM levels (17). Both of these actions of NAMPT can positively stimulate SIRT1 activity. Relatively little was known, however, about the molecular mechanisms by which NAMPT and NMNAT-1 affects SIRT1-dependent regulation of target gene promoters. NAMPT can enhance the repressive actions of SIRT1 in a reporter gene assay (12). In human chondrocytes, SIRT1 is recruited to the promoter of a cartilage-specific gene through interactions with SOX9 (18). SIRT1 recruitment is accompanied by cofactor binding and histone modification, leading to transcription activation. NAMPT stimulates SIRT1 activity and target gene expression through NAD+ production (18). Our results build upon these previous studies to present a detailed molecular mechanism for the regulation of SIRT1 activity at endogenous target gene promoters by nuclear NAD+ biosynthetic enzymes. Both NAMPT and NMNAT-1 regulate cellular NAD+ production as well as SIRT1 activity at the promoter of common target genes, highlighting the central role of NAD+ production in SIRT1-dependent transcriptional regulation. More importantly, colocalization of NMNAT-1 and SIRT1 at target gene promoters suggests novel regulatory mechanisms dependent upon the interaction between NMNAT-1 and SIRT1.
Why might SIRT1 recruit an NAD+-producing enzyme such as NMNAT-1 to target gene promoters for localized actions? Given the very rapid diffusion rate of small molecules in cells, the need for localized NAD+ production seems unnecessary, and the ability to accumulate promoter-localized pools of elevated NAD+ seems unlikely. Colocalization of NMNAT-1 and SIRT1 at target gene promoters may, however, regulate SIRT1 activity in several ways. For example, close proximity of NMNAT-1 and SIRT1 may facilitate more efficient NAD+ utilization by SIRT1, perhaps through a substrate channeling mechanism (52). In this regard, Grubisha et al. (53) have hypothesized that NAD+ biosynthetic enzymes may form a complex with SIRT1 and channel NAD+ directly to SIRT1, creating a microdomain of high NAD+ concentration for regulation of SIRT1 activity. Our results provide compelling evidence in support of this hypothesis. Interestingly, our results demonstrate that a 30–45% decrease in total cellular NAD+ levels upon NAMPT or NMNAT-1 knockdown (Fig. 1C) can lead to as much as a 10-fold increase in H4K16 acetylation levels at SIRT1 target gene promoters (Fig. 5D). One explanation for this observation is that the changes in nuclear NAD+ levels upon NAMPT or NMNAT-1 knockdown may well exceed those observed in the cell as a whole. Grubisha et al. (53) have previously proposed that localized NAD+ production at the site of SIRT1 function, rather than total cellular NAD+ levels, may play a more significant role in controlling SIRT1 activity.
Another way in which the colocalization of NMNAT-1 and SIRT1 at target gene promoters may regulate SIRT1 activity is to promote allosteric interactions that enhance the enzymatic activity of either or both enzymes, as shown for NMNAT-1 and PARP-1 (51). Finally, interactions between NMNAT-1 and SIRT1 may allow for regulation by cellular signaling inputs, providing an additional level of regulatory control.
With respect to the latter point, both NAD+ production and SIRT1 activity are regulated by a wide array of extracellular signals (32, 54, 55). Stress, nutrient availability, and cellular differentiation regulate the expression of NAMPT (13–15, 17), a rate-limiting enzyme in the NAD+ recycling pathway (12). Consequently, these signals can control NMNAT-1-dependent NAD+ synthesis through regulation of NMN production. Additionally, signal inputs from protein kinases may control the interaction between NMNAT-1 and NAD+-utilizing enzymes (51), leading to dynamic regulation of NMNAT-1 recruitment to specific sites on chromatin. Our results suggest that the integrated input from these signaling pathways is likely to be an important factor in determining NAD+ production and SIRT1-dependent transcriptional regulation at target gene promoters.
The production of small molecule substrates by nuclear metabolic enzymes for use by chromatin-modifying or transcription-regulating enzymes is an emerging theme. For example, acetyl-CoA production by a nuclear acetyl-CoA synthetase in the yeast Saccharomyces cerevisiae has been shown to regulate the activity of histone acetyltransferases (56). The extent to which acetyl-CoA synthetase and other substrate-producing metabolic enzymes are, like NMNAT-1, recruited to target gene promoters has yet to be determined, but this mode of action may be a general and relatively unexplored mechanism for transcriptional control.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK069710 (NIDDK, to W. L. K.) and R01 DK73466 (NIDDK, to A. A. S.). This work was also supported by grants from the Endocrine Society (to W. L. K.) and the Ellison Medical Foundation (to A. A. S.), postdoctoral fellowships from the New York State Health Research Science Board (to T. Z.) and the American Heart Association (AHA; to M. J. G.), and predoctoral fellowships from the Alfred P. Sloan Foundation (to J. G. B.) and the AHA (to K. M. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S6.
K. M. Frizzell and W. L. Kraus, unpublished information.
T. Zhang and W. L. Kraus unpublished data.
- NAM
- nicotinamide
- ChIP
- chromatin immunoprecipitation
- NAMPT
- nicotinamide phosphoribosyltransferase
- NMN
- nicotinamide mononucleotide
- NMNAT-1
- nicotinamide mononucleotide adenylyltransferase 1
- qPCR
- quantitative real-time PCR
- RT
- reverse transcription
- PARP
- poly(ADP-ribose) polymerase
- GO
- gene ontology
- GST
- glutathione S-transferase
- shRNA
- short hairpin RNA
- HPLC
- high performance liquid chromatography
- Luc
- luciferase.
REFERENCES
- 1.Berger F., Ramírez-Hernández M. H., Ziegler M. (2004) Trends Biochem. Sci. 29, 111–118 [DOI] [PubMed] [Google Scholar]
- 2.Rongvaux A., Andris F., Van Gool F., Leo O. (2003) BioEssays 25, 683–690 [DOI] [PubMed] [Google Scholar]
- 3.Rongvaux A., Shea R. J., Mulks M. H., Gigot D., Urbain J., Leo O., Andris F. (2002) Eur. J. Immunol. 32, 3225–3234 [DOI] [PubMed] [Google Scholar]
- 4.Kitani T., Okuno S., Fujisawa H. (2003) FEBS Lett. 544, 74–78 [DOI] [PubMed] [Google Scholar]
- 5.Imai S. I. (2009) Cell Biochem. Biophys. 53, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garten A., Petzold S., Körner A., Imai S., Kiess W. (2009) Trends Endocrinol. Metab. 20, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanuelli M., Carnevali F., Saccucci F., Pierella F., Amici A., Raffaelli N., Magni G. (2001) J. Biol. Chem. 276, 406–412 [DOI] [PubMed] [Google Scholar]
- 8.Raffaelli N., Sorci L., Amici A., Emanuelli M., Mazzola F., Magni G. (2002) Biochem. Biophys. Res. Commun. 297, 835–840 [DOI] [PubMed] [Google Scholar]
- 9.Schweiger M., Hennig K., Lerner F., Niere M., Hirsch-Kauffmann M., Specht T., Weise C., Oei S. L., Ziegler M. (2001) FEBS Lett. 492, 95–100 [DOI] [PubMed] [Google Scholar]
- 10.Yalowitz J. A., Xiao S., Biju M. P., Antony A. C., Cummings O. W., Deeg M. A., Jayaram H. N. P. (2004) Biochem. J. 377, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger F., Lau C., Dahlmann M., Ziegler M. (2005) J. Biol. Chem. 280, 36334–36341 [DOI] [PubMed] [Google Scholar]
- 12.Revollo J. R., Grimm A. A., Imai S. (2004) J. Biol. Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- 13.Yang H., Yang T., Baur J. A., Perez E., Matsui T., Carmona J. J., Lamming D. W., Souza-Pinto N. C., Bohr V. A., Rosenzweig A., de Cabo R., Sauve A. A., Sinclair D. A. (2007) Cell 130, 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Veer E., Ho C., O'Neil C., Barbosa N., Scott R., Cregan S. P., Pickering J. G. (2007) J. Biol. Chem. 282, 10841–10845 [DOI] [PubMed] [Google Scholar]
- 15.van der Veer E., Nong Z., O'Neil C., Urquhart B., Freeman D., Pickering J. G. (2005) Circ. Res. 97, 25–34 [DOI] [PubMed] [Google Scholar]
- 16.Revollo J. R., Körner A., Mills K. F., Satoh A., Wang T., Garten A., Dasgupta B., Sasaki Y., Wolberger C., Townsend R. R., Milbrandt J., Kiess W., Imai S. (2007) Cell Metab. 6, 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulco M., Cen Y., Zhao P., Hoffman E. P., McBurney M. W., Sauve A. A., Sartorelli V. (2008) Dev. Cell 14, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvir-Ginzberg M., Gagarina V., Lee E. J., Hall D. J. (2008) J. Biol. Chem. 283, 36300–36310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rongvaux A., Galli M., Denanglaire S., Van Gool F., Drèze P. L., Szpirer C., Bureau F., Andris F., Leo O. (2008) J. Immunol. 181, 4685–4695 [DOI] [PubMed] [Google Scholar]
- 20.Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. (2009) Science 324, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey K. M., Yoshino J., Brace C. S., Abrassart D., Kobayashi Y., Marcheva B., Hong H. K., Chong J. L., Buhr E. D., Lee C., Takahashi J. S., Imai S., Bass J. (2009) Science 324, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conforti L., Tarlton A., Mack T. G., Mi W., Buckmaster E. A., Wagner D., Perry V. H., Coleman M. P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11377–11382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack T. G., Reiner M., Beirowski B., Mi W., Emanuelli M., Wagner D., Thomson D., Gillingwater T., Court F., Conforti L., Fernando F. S., Tarlton A., Andressen C., Addicks K., Magni G., Ribchester R. R., Perry V. H., Coleman M. P. (2001) Nat. Neurosci. 4, 1199–1206 [DOI] [PubMed] [Google Scholar]
- 24.Araki T., Sasaki Y., Milbrandt J. (2004) Science 305, 1010–1013 [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Zhai Q., Chen Y., Lin E., Gu W., McBurney M. W., He Z. (2005) J. Cell Biol. 170, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conforti L., Fang G., Beirowski B., Wang M. S., Sorci L., Asress S., Adalbert R., Silva A., Bridge K., Huang X. P., Magni G., Glass J. D., Coleman M. P. (2007) Cell Death Differ. 14, 116–127 [DOI] [PubMed] [Google Scholar]
- 27.Zhai R. G., Zhang F., Hiesinger P. R., Cao Y., Haueter C. M., Bellen H. J. (2008) Nature 452, 887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai R. G., Cao Y., Hiesinger P. R., Zhou Y., Mehta S. Q., Schulze K. L., Verstreken P., Bellen H. J. (2006) PLoS Biol. 4, e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald J. M., Beach M. G., Porpiglia E., Sheehan A. E., Watts R. J., Freeman M. R. (2006) Neuron 50, 869–881 [DOI] [PubMed] [Google Scholar]
- 30.Hoopfer E. D., McLaughlin T., Watts R. J., Schuldiner O., O'Leary D. D., Luo L. (2006) Neuron 50, 883–895 [DOI] [PubMed] [Google Scholar]
- 31.Fainzilber M., Twiss J. L. (2006) Neuron 50, 819–821 [DOI] [PubMed] [Google Scholar]
- 32.Guarente L. (2000) Genes Dev. 14, 1021–1026 [PubMed] [Google Scholar]
- 33.Michan S., Sinclair D. (2007) Biochem. J. 404, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feige J. N., Auwerx J. (2008) Curr. Opin. Cell Biol. 20, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang T., Fu M., Pestell R., Sauve A. A. (2006) Trends Endocrinol. Metab. 17, 186–191 [DOI] [PubMed] [Google Scholar]
- 36.Kim M. Y., Woo E. M., Chong Y. T., Homenko D. R., Kraus W. L. (2006) Mol. Endocrinol. 20, 1479–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberdoerffer P., Michan S., McVay M., Mostoslavsky R., Vann J., Park S. K., Hartlerode A., Stegmuller J., Hafner A., Loerch P., Wright S. M., Mills K. D., Bonni A., Yankner B. A., Scully R., Prolla T. A., Alt F. W., Sinclair D. A. (2008) Cell 135, 907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. (2004) Nat. Biotechnol. 22, 326–330 [DOI] [PubMed] [Google Scholar]
- 39.Yang T., Sauve A. A. (2006) AAPS J. 8, E632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson W. E., Li C., Rabinovic A. (2007) Biostatistics 8, 118–127 [DOI] [PubMed] [Google Scholar]
- 41.Kininis M., Chen B. S., Diehl A. G., Isaacs G. D., Zhang T., Siepel A. C., Clark A. G., Kraus W. L. (2007) Mol. Cell. Biol. 27, 5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnakumar R., Gamble M. J., Frizzell K. M., Berrocal J. G., Kininis M., Kraus W. L. (2008) Science 319, 819–821 [DOI] [PubMed] [Google Scholar]
- 43.Gillingwater T. H., Wishart T. M., Chen P. E., Haley J. E., Robertson K., MacDonald S. H., Middleton S., Wawrowski K., Shipston M. J., Melmed S., Wyllie D. J., Skehel P. A., Coleman M. P., Ribchester R. R. (2006) Hum. Mol. Genet 15, 625–635 [DOI] [PubMed] [Google Scholar]
- 44.Billington R. A., Travelli C., Ercolano E., Galli U., Roman C. B., Grolla A. A., Canonico P. L., Condorelli F., Genazzani A. A. (2008) J. Biol. Chem. 283, 6367–6374 [DOI] [PubMed] [Google Scholar]
- 45.Ota H., Tokunaga E., Chang K., Hikasa M., Iijima K., Eto M., Kozaki K., Akishita M., Ouchi Y., Kaneki M. (2006) Oncogene 25, 176–185 [DOI] [PubMed] [Google Scholar]
- 46.Pruitt K., Zinn R. L., Ohm J. E., McGarvey K. M., Kang S. H., Watkins D. N., Herman J. G., Baylin S. B. (2006) PLoS Genet. 2, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaquero A., Sternglanz R., Reinberg D. (2007) Oncogene 26, 5505–5520 [DOI] [PubMed] [Google Scholar]
- 48.Wang Z., Zang C., Rosenfeld J. A., Schones D. E., Barski A., Cuddapah S., Cui K., Roh T. Y., Peng W., Zhang M. Q., Zhao K. (2008) Nat. Genet. 40, 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grozinger C. M., Chao E. D., Blackwell H. E., Moazed D., Schreiber S. L. (2001) J. Biol. Chem. 276, 38837–38843 [DOI] [PubMed] [Google Scholar]
- 50.Kraus W. L. (2008) Curr. Opin. Cell Biol. 20, 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger F., Lau C., Ziegler M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3765–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srere P. A. (1987) Annu. Rev. Biochem. 56, 89–124 [DOI] [PubMed] [Google Scholar]
- 53.Grubisha O., Smith B. C., Denu J. M. (2005) FEBS J. 272, 4607–4616 [DOI] [PubMed] [Google Scholar]
- 54.Sauve A. A. (2008) J. Pharmacol. Exp. Ther. 324, 883–893 [DOI] [PubMed] [Google Scholar]
- 55.Sauve A. A., Wolberger C., Schramm V. L., Boeke J. D. (2006) Annu. Rev. Biochem. 75, 435–465 [DOI] [PubMed] [Google Scholar]
- 56.Takahashi H., McCaffery J. M., Irizarry R. A., Boeke J. D. (2006) Mol. Cell 23, 207–217 [DOI] [PubMed] [Google Scholar]
- 57.Lin X., Ashizawa T. (2005) Cerebellum 4, 37–42 [DOI] [PubMed] [Google Scholar]
- 58.Ohkawa N., Sugisaki S., Tokunaga E., Fujitani K., Hayasaka T., Setou M., Inokuchi K. (2008) Genes Cells 13, 1171–1183 [DOI] [PubMed] [Google Scholar]
- 59.Asaumi M., Iijima K., Sumioka A., Iijima-Ando K., Kirino Y., Nakaya T., Suzuki T. (2005) J. Biochem. 137, 147–155 [DOI] [PubMed] [Google Scholar]
- 60.Nelson B. R., Claes K., Todd V., Chaverra M., Lefcort F. (2004) Dev. Biol. 270, 322–335 [DOI] [PubMed] [Google Scholar]
- 61.Lin S., Boey D., Herzog H. (2004) Neuropeptides 38, 189–200 [DOI] [PubMed] [Google Scholar]
- 62.Ono R., Kobayashi S., Wagatsuma H., Aisaka K., Kohda T., Kaneko-Ishino T., Ishino F. (2001) Genomics 73, 232–237 [DOI] [PubMed] [Google Scholar]
- 63.Lux A., Beil C., Majety M., Barron S., Gallione C. J., Kuhn H. M., Berg J. N., Kioschis P., Marchuk D. A., Hafner M. (2005) J. Biol. Chem. 280, 8482–8493 [DOI] [PubMed] [Google Scholar]
- 64.Rico-Bautista E., Flores-Morales A., Fernández-Pérez L. (2006) Cytokine Growth Factor Rev. 17, 431–439 [DOI] [PubMed] [Google Scholar]
- 65.Turnley A. M. (2005) Pediatr. Endocrinol. Rev. 2, 366–371 [PubMed] [Google Scholar]
- 66.Crawley J. T., Lane D. A. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 233–242 [DOI] [PubMed] [Google Scholar]
- 67.Hollister R. D., Kisiel W., Hyman B. T. (1996) Brain Res. 728, 13–19 [PubMed] [Google Scholar]
- 68.Massagué J. (2008) Cell 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama M., Nishi Y., Yoshii J., Okubo K., Matsubara K. (1996) DNA Res. 3, 311–320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.