Abstract
Ischemic preconditioning (IPC) protects brain against ischemic injury by activating specific mechanisms. Our goal was to determine if the inducible heme oxygenase 1 (HO1) is required for such protection. IPC before transient or permanent ischemia reduced cortical infarct volumes by 57.4% and 33.9%, respectively at 48 h in wildtype adult mice. Interestingly, IPC failed to protect the HO1 gene deleted mice against permanent ischemic brain injury. IPC also resulted in a significant increase in HO1 protein levels in the brain and correlated with reduced neurological deficits after permanent and transient brain ischemia. Our study demonstrates that neuroprotective effects of IPC are at least partially mediated via HO1. Elucidating the physiological/cellular role by which HO1 is protective against brain ischemia may aid the development of selective drugs to treat stroke and its associated neurological disorders.
Keywords: Heme oxygenase, Ischemic preconditioning, Middle cerebral artery occlusion, Neuroprotection, Permanent middle cerebral artery occlusion
Introduction
Endogenous protective mechanisms against various injuries have developed throughout evolution in living organisms. If such an injury is tolerable, it triggers cellular responses that precondition the body against more severe stimuli. Preconditioning stimuli are diverse in nature and, therefore, can activate various pathways, altered gene translations, and synthesis of proteins, resulting in a stronger phenotype. Ischemic preconditioning (IPC) is a phenomenon in which brief ischemic episodes result in resistance of an organ to later severe ischemic insult(s). In clinical practice, brief ischemic episodes are known as transient ischemic attacks (TIAs) and have been studied for over two decades (Murry et al., 1986; Wegener et al., 2004). In human brain, TIAs induce tolerance by raising the threshold of tissue vulnerability (Wegener et al., 2004), a response that is critical for neuroprotection and its underlying molecular mechanisms. Despite promising results obtained from animal studies and an abundance of experimental neuroprotective compounds, clinical practice still lacks effective methods of stroke therapy. Investigating the underlying mechanisms activated by TIAs, could lead to novel targets for treating or preventing stroke.
Studies of IPC have revealed that tolerance induced in the brain can be early (minutes to hours) or late (hours to days) and that various protective molecular pathways are activated at different time points after IPC (Steiger and Hanggi, 2007). In particular, expression of the heat shock protein heme oxygenase 1 (HO1) is activated 12 to 24 h after IPC and remains up-regulated for up to 7 days in newborn rat brains (Bergeron et al., 1997). HO1 is an inducible enzyme that degrades the heme molecule to biliverdin, carbon monoxide (CO), and iron; its expression and activity can be increased by various preconditioning stimuli in the brain and in the whole body. Many investigators have suggested HO1 to be potentially responsible for preconditioning-induced protection in vivo and in vitro. For example, HO1 was reported to be involved in long-term tolerance after hypoxic preconditioning in the retina of mice (Zhu et al., 2007). Also, cardioprotection in diabetic rats provided by pharmacologic preconditioning was found to be at least partially mediated by HO1 (Thirunavukkarasu et al., 2007). Similarly, HO1 plays a role in the protection of cultured spinal neurons exposed to hyperbaric preconditioning (Li et al., 2007). HO1 up-regulation after lipopolysacharide preconditioning was shown to protect intestinal and lung tissue (Tamion et al., 2001); and myocardial cells induce HO1 expression after ischemic preconditioning, resulting in protection against apoptotic-like cell death and oxidative stress (Jancso et al., 2007). Activation of HO1 by various stimuli is a common protective phenomenon for different organ systems after IPC, but mechanisms of HO1 activation and signaling that are unique to the brain still need to be differentiated.
The importance of HO1 in protection against excitotoxicity in the brain (Ahmad et al., 2006) and in prevention of cell death (Ferris et al., 1999) has been previously shown. However, the question of whether HO1 alone is sufficient to protect the brain against ischemia still remains to be fully addressed. The primary function of HO1 enzyme is to degrade the heme molecule to biliverdin, iron, and CO, but the antioxidant and intrinsic properties of these end-products and HO1 intracellular signaling can be equally important factors for neuroprotection. To determine whether IPC-induced HO1 contributes to the neuroprotection we subjected wildtype (WT) and HO1 knockout (HO1−/−) mice to transient and permanent focal ischemia models.
Experimental Procedures
Animals
WT C57BL/6 mice (Charles River Laboratories, Wilmington, MA), and HO1−/− mice (bred in our laboratory) were used at 8−10 weeks of age (20−25 g). Animals were housed at 22 ± 1°C with a 12 h:12 h light/dark cycle; water and food were available ad libitum. All protocols were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and were carried out in accordance with the guidelines of the National Institutes of Health.
Experimental design
Mice were exposed to anesthesia preconditioning alone by halothane or intraperitoneal injection of 250 mg/kg Avertin (2, 2-tribromoethanol; Sigma Co, St. Louis, MO) or IPC by bilateral common carotid artery occlusion (BCCAO) (Cho et al., 2005) before being subjected to either transient or permanent middle cerebral artery (MCA) occlusion (tMCAO and pMCAO, respectively). For the BCCAO, the mice were anesthetized before both common carotid arteries were exposed and ligated with 6−0 silk sutures three times for 1 min each. Between each ligation, the arteries were reopened for 5 min. Cerebral blood flow (CBF) was reduced by 90 ± 2% (mean ± SEM) during each occlusion and was restored to baseline during the 5-min reperfusions. Animals for which the CBF did not decrease by at least 80% were excluded from the study. All mice were allowed to recover in their cages for 24 h after preconditioning. A separate cohort of mice (n=6) was used to determine whether IPC by BCCAO alone induces brain infarction.
Transient focal ischemia (tMCAO)
Mice were anesthetized with 1.5% isoflurane in 25% oxygen-enriched air. Transient focal ischemia was induced by MCAO via the intraluminal suture technique (Shah et al., 2006; Zeynalov et al., 2006). A reduction in blood flow over the ipsilateral parietal cortex of 87−90% was used as confirmation of successful occlusion. Blood flow was monitored with a laser-Doppler flowmeter (Moor Instruments Ltd, England), and body temperatures were maintained at 37.0 ± 0.5°C in all groups during the experiment. The filament was removed from the lumen 90 min after occlusion to allow the blood to return to the ischemic region of the brain. All mice were allowed to recover from anesthesia before being returned to their cages.
Assessment of Neurological Deficit Score (NDS) after tMCAO
During the 24 h recovery period after IPC, and after 48 h of tMCAO, mice were tested for neurological deficits based on the following scoring system: 0 = normal motor function, 1 = flexion of torso and of contralateral forelimb on tail lift, 2 = circling to the contralateral side but normal posture at rest, 3 = leaning to contralateral side at rest, 4 = no spontaneous motor activity. Mice that had a neurological deficit score (NDS) ≤1 after completion of the tMCAO procedure and recovery from anesthesia were excluded from the study.
Permanent focal ischemia (pMCAO)
For the pMCAO model, the distal portion of the MCA was occluded according to a method described by Majid et al. (Majid et al., 2000). Mice were anesthetized with halothane, and a 1.0-cm vertical skin incision was made between the right eye and ear. The temporal muscle was moved aside, and the underlying temporal bone exposed. With the aid of a surgical microscope, a 2.0-mm burr hole was drilled just over the area of the MCA so that it was visible through the temporal bone. The main trunk of the distal MCA was occluded directly with a bipolar coagulator; complete interruption of blood flow at the occlusion site was confirmed by severance of the MCA. Core body temperature was maintained at 37.0 ± 0.5°C during and after the procedure, first with a heating blanket that was attached to the temperature probe for automatic temperature regulation, and then with a temperature-regulated incubator in which the mice recovered from the surgery. A successful occlusion was confirmed by placing the laser-Doppler probe above the temporal ridge to establish that blood flow into the region was terminated. Animals that did not circle toward the paretic side after the onset of ischemia and those that developed subarachnoid hemorrhage were not included in the study.
Assessment of NDS after pMCAO
To evaluate the neurological deficits caused by IPC or pMCAO, a 28-point score pattern was used (Wang et al., 2006). Forty-eight hours after the pMCAO procedure, an experimenter blinded to genotype scored all mice for neurological deficits. The tests included body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Each test was graded from 0 to 4, establishing a maximum deficit score of 28. Immediately after the evaluation, the mice were sacrificed for infarct volume analysis.
Histological analysis
All animals were euthanized at 48 h after tMCAO or pMCAO. A separate cohort of animals (n=6) was euthanized 24 h after IPC to determine whether the repeated BCCAO alone causes cerebral injury. Brains were harvested, sliced into five 2-mm thick coronal sections, mounted onto slides, and stained with 1% triphenyltetrazolium chloride (TTC, Sigma Co). Infarct areas of all slides were traced with the Image Analysis software (SigmaScan pro 5, Systat, Inc., Point Richmond, CA) The infarct area of the ipsilateral hemisphere of each brain was integrated into cubic millimeters and translated into a percentage of the contralateral hemisphere of the same brain with correction for swelling (Swanson et al, 1990).
Western blot analysis
Cortices of the preconditioned WT mice were dissected out and homogenized for Western blot analysis. Protein concentrations were determined by BCA kit (Pierce, Rockford, IL). Equivalent amounts of protein per sample were migrated through SDS-PAGE on 10% gels. After electrophoretic transfer of the proteins to a nitrocellulose membrane, the membrane was blocked for 1 h at 22°C with 5% dried milk and then exposed to primary antibodies overnight at 4°C and secondary antibodies in 5% dried milk for 1 h at 22°C. HO antibodies were purchased from Stressgen (Ann Arbor, MI), and actin antibody was from Sigma. Immunocomplexes were visualized by enhanced chemiluminescence (ECL) detection (Amersham, Piscataway, NJ).
Statistical analysis
Physiological parameters and differences in infarct volumes were analyzed by one-way ANOVA with Newman Keuls post-hoc test. NDSs were analyzed by the non-parametric Kruskal-Wallis test. Data are presented as mean ± SEM. A value of p < 0.05 was considered to be statistically significant.
Results
Beneficial effects of IPC against tMCAO
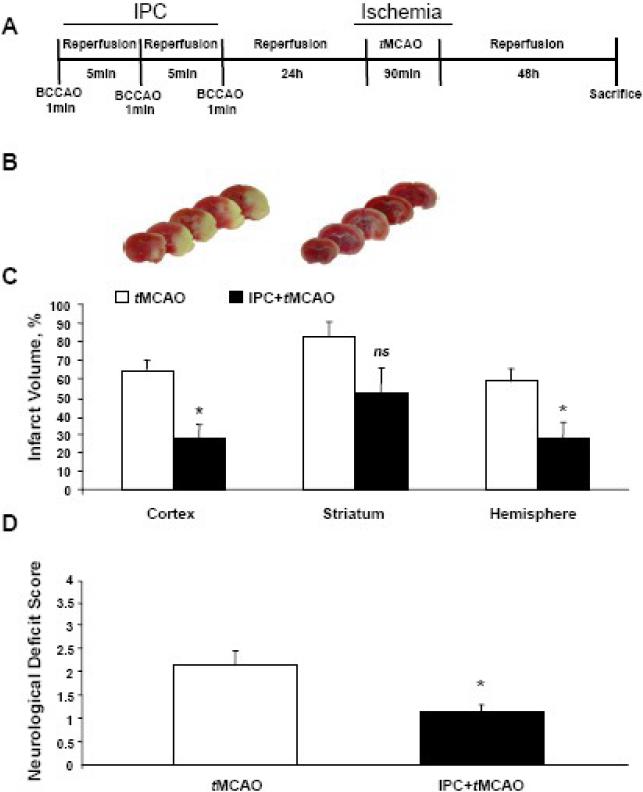
TTC staining of brain slices showed that IPC alone did not cause brain infarct injury in either WT or HO1−/− mice at 24 h (data not shown). Likewise, mice subjected only to IPC showed no signs of neurological deficit (data is not shown). Compared with WT mice that did not receive IPC (n=8), WT mice (n=7) that were exposed to IPC before tMCAO had smaller infarct volume in the cortex (64.1 ± 5.9% vs 27.3 ± 7.8%, p < 0.05); in the striatum (82.7 ± 7.3% vs 51.7 ± 13.9%, p = 0.07); and in the overall hemisphere (58.4 ± 6.8% vs 27.8 ± 8.6%, p<0.05; Fig. 1A–C). IPC also mitigated neurological deficits compared to mice that did not receive preconditioning (p < 0.05; Fig. 1D). HO1−/− mice subjected to tMCAO did not survive the first 48 h of reperfusion under this experimental protocol, whereas the mortality rate for WT mice subjected to tMCAO alone was 0/8 and was 1/8 for those that underwent IPC 24 h before tMCAO.
Fig. 1.
Ischemic preconditioning (IPC) attenuated tMCAO-induced infarct volume and improved neurological deficits in wildtype mice. (A) Diagram of experimental protocol. IPC (1-min BCCAO repeated three times with 5-min reperfusion between occlusions) was carried out 24 h prior to tMCAO and 48 h of reperfusion. (B) Representative brain sections of mice after tMCAO only (left) and after IPC and tMCAO (right). (C) IPC attenuated infarct volume in ipsilateral cortex, striatum, and overall hemisphere. (D) IPC reduced tMCAO-induced neurological deficit compared to tMCAO alone. tMCAO, n=8 and IPC+tMCAO, n=7. *p < 0.05 vs. corresponding control; ns, not significant.
Beneficial effects of IPC against pMCAO
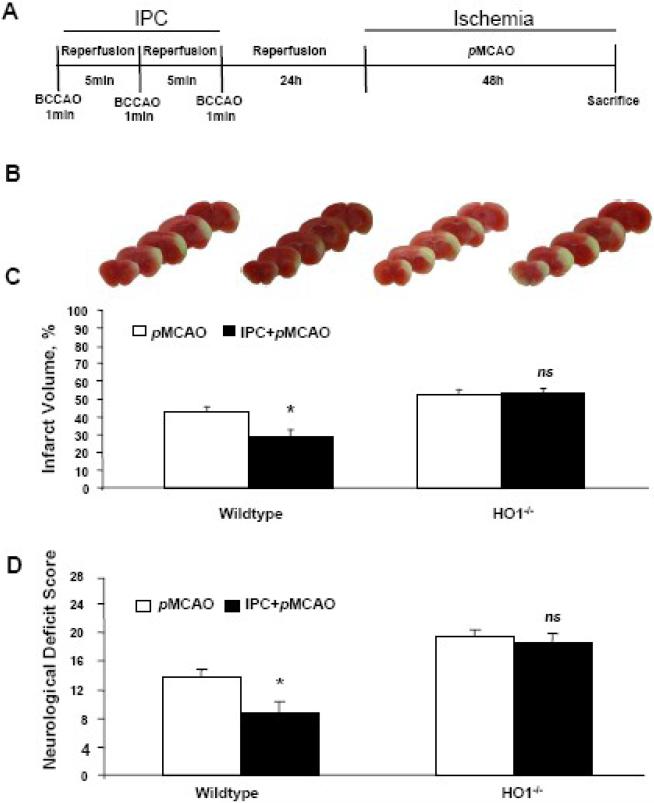
Compared with the control group, IPC significantly (p < 0.05) protected the brains of WT mice from the damage produced by pMCAO. Infarct volumes were 28.3 ± 4.2%, in the IPC group (n=10), but 42.8 ± 2.8% in the control group (n=9; Fig. 2A–C). Neurological deficit scores also were significantly lower (p < 0.01) in the IPC group (8.8±1.5) than in the control group (13.8 ± 1.0; Fig. 2D). HO1−/− mice subjected to the same experimental protocol did not receive any protective effects from IPC against the damage caused by pMCAO (Fig. 2B, C). Similarly, no differences were observed in NDS between the IPC (n=8) and control HO1−/− groups (n=9; Fig. 2 D). All mice survived the pMCAO protocol, possibly because it is a less invasive method than tMCAO.
Fig. 2.
IPC attenuated pMCAO-induced infarct volume and reduced neurological deficit scores in wildtype (WT) but not HO1−/− mice. (A) Diagram of experimental protocol. IPC (1-min BCCAO repeated three times with 5-min reperfusion between occlusions) was carried out 24 h prior to pMCAO; mice were sacrificed after 48 h. (B) Representative brain sections of WT and HO1−/− mice after pMCAO with or without IPC. (C) IPC reduced ischemic brain injury in the ipsilateral cortex after pMCAO in WT but not HO1−/− mice. (D) Neurological deficit was less severe in the WT mice that underwent IPC than in those that did not receive IPC; no improvements were observed in HO1−/− mice. WT mice: pMCAO, n=9, and IPC+pMCAO, n=10; HO1−/− mice: pMCAO, n=9, and IPC+pMCAO, n=8. *p < 0.05 vs. corresponding control; ns, not significant.
HO1 induction after IPC
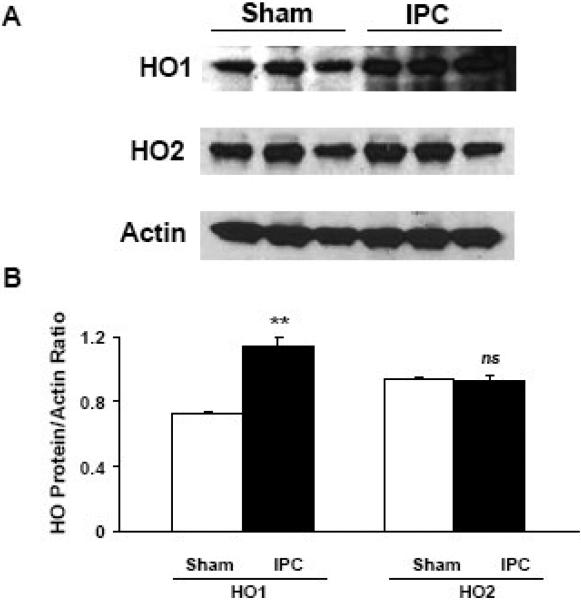
To determine whether HO1 protein levels are increased following IPC, we exposed WT mice to a sham procedure or IPC only and then sacrificed them after 24 h. Western blot analysis showed that HO1 protein expression was significantly greater in the IPC group than in the sham group after 24 h (p < 0.01; Fig. 3). Because HO2 and actin are constitutively expressed in the brain, we used them as controls. Expression levels of HO2 and actin were unchanged in the IPC group compared to the sham group, a finding that supports changes only in the inducible form of HO, HO1 (Fig. 3). The Western blot shown is a representative of at least three separate experiments.
Fig. 3.
Ischemic preconditioning (IPC) increased HO1 protein expression levels after 24 h in the brain cortex of wildtype mice. (A) Western blots were performed to measure the protein expression of HO1 and HO2. The expression of actin was used as a loading control. HO2, which is known to be constitutively expressed in the brain, was used as a control and, as expected, was unaffected. (B) The histograms show the ratio of density captured from HO1 and HO2 to that of actin. Values shown are means ± SEM from three independent sets of experiments. **p < 0.01 vs. corresponding control; ns, not significant.
Discussion
To demonstrate IPC-induced neuroprotection, we used the model of brief repeated BCCAO followed by transient or permanent focal ischemia. We showed that in WT mice, IPC induces neuroprotection against experimental transient and permanent brain ischemia. Neurological deficit reduction in WT mice correlated with decreased infarct volumes after both stroke paradigms. To determine the role of HO1 in the IPC-induced protection, we repeated the same protocols in HO1−/− mice. Most interestingly, we found that the protective effects of IPC were abolished in HO1−/− mice subjected to pMCAO, as neither infarct volume nor NDS were different from that of the control mice. Furthermore, quantification of protein levels in brain cortical homogenates revealed that only HO1 levels were increased after IPC, while HO2 levels remained stable.
In our study, significant reduction of infarct volumes in the cortex and overall hemisphere in combination with improved NDS demonstrated that IPC ameliorates stroke-induced brain damage, as depicted in Fig. 1. Permanent or transient brain ischemia results in a loss of vascular reactivity (Bolanos-Jimenez et al., 1995), a burst of local and systemic inflammation (Yin et al., 2007), and an increase in apoptotic-like cell death (Steiger and Hanggi, 2007); these pathophysiologic responses to stroke are paradoxically alleviated when animals are subjected to IPC. TIAs, the clinical correlate of experimental brain IPC, also mobilize protective mechanisms (Wegener et al., 2004) through reprogramming of cellular responses and activating endogenous forces against stroke.
HO1 is one such endogenous force that can provide protection when induced by different preconditioning stimuli in various organs. HO1 has been reported to be induced in the liver after IPC (Lai et al., 2006) and has been shown to suppress inflammation, potentially through inhibition of IL-1 and involvement of nitric oxide (NO) (Franco-Gou et al., 2006). Up-regulation of HO1 after pharmacological preconditioning protects rat liver against ischemia-reperfusion injury (Heiman et al., 2006), and skeletal muscles subjected to ischemic preconditioning develop tolerance against later ischemia (Dungey et al., 2006). In the small intestine, IPC provided cytoprotection and improved villous microcirculation via a pathway that involved HO (Mallick et al., 2005). In the brain, erythropoietin preconditioning induced the HO1 gene and contributed to protection against ischemic injury (Diaz et al., 2005). But the question of how HO1 protects against ischemia remains unanswered.
A variety of possibilities could explain how IPC-induced HO1 stimulation might lead to protection against brain ischemia. IPC triggers multiple mechanisms that eventually dictate cellular responses to more severe injury. For example, the transcriptional factor hypoxia inducible factor 1△ (HIF-1△) activates HO1 to further regulate early (minutes to hours) and delayed (days to weeks) protective mechanisms (Loor and Schumacker, 2008). HIF-1△-induced HO1 up-regulation after ischemia-reperfusion injury improves cell survival, activates angiogenesis pathways, including collateral vessel development, but also occurs in a tissue-specific manner (Loor and Schumacker, 2008). Induction of HO1 after ischemia leads to increased degradation of heme and generation of biliverdin/bilirubin and CO, which in turn have antioxidant and anti-inflammatory properties (Loor and Schumacker, 2008). Furthermore, injuries or brief ischemic stresses stimulate release of free heme from heme-containing proteins, such as hemoglobin, myoglobin, neuroglobin, cytochromes, cyclooxygenase, and others. After an injury, over-expressed HO1 metabolizes toxic levels of the pro-oxidant heme in the cell (Balla et al., 1993) to produce CO, antioxidants and free radical scavengers biliverdin/bilirubin, and iron. These products of HO1 can attenuate inflammatory (Wagener et al., 2003) and apoptotic (Wang et al., 2007) responses after ischemic insult. CO production by HO1 is a physiologically regulated response to injury in endothelial cells (Soares et al., 1998) and provides protection by suppressing apoptosis and inflammation through TNFα and transcription factor NFκB (Brouard et al., 2002). We have recently shown that exposure of mice to a low level of exogenous CO is partially protective against transient focal ischemia (Zeynalov and Doré, 2009). All of these isolated facts can be integrated into future comprehensive studies and applied in our paradigm to learn more about the neuroprotective role of IPC-induced HO1.
We found that WT mice that underwent pMCAO exhibited a pattern of neuroprotection similar to that produced by tMCAO. We also observed a slight trend toward larger infarct volumes after pMCAO in HO1−/− mice than in WT mice (Fig 2B), but the difference was not statistically significant. Using pMCAO enabled us to obtain reproducible and consistent results to support our hypothesis that HO1 plays an important role in IPC-induced protection against brain ischemia. We found that in HO1−/− mice, IPC failed to protect cortex from ischemic injury (Fig 2C). The increase in HO1 protein expression in brain cortex of WT mice is an indication that HO1 protein is up-regulated by three repeated sub-lethal stimuli, i.e. IPC. Constitutively expressed HO2 remained unchanged after IPC stimuli. The results indicate that HO1 is required for the IPC-induced protection against ischemic brain injury. It remains to be determined if the IPC-induced HO1 can protect brain against ischemia at time points beyond 48 h.
Two types of IPC models are available and commonly used for an in vivo simulation of TIA: 1) BCCAO (Cho et al., 2005), also known as global ischemia, and 2) repetitive short focal occlusions of the MCA (Atochin et al., 2003). BCCAO was our first choice to simulate IPC in this study because the method is less invasive, takes less time, and affects whole brain. Unlike IPC associated with BCCAO, the IPC produced by MCAO is limited to the area of the brain within the vascular pool of the MCA. The BCCAO model of IPC has been well described by Cho et al (Cho et al., 2005), and as we observed by TTC staining, did not itself induce cell death (data not shown). Anesthetics we used during the IPC protocol, Avertin and halothane, did not offer any benefit or protection when used alone without IPC, under our experimental paradigm (unpublished observations). We have also investigated whether the handling of the animals could be stressful enough to lead to an IPC effect, but found none (unpublished observations).
Under our experimental protocols, we observed that HO1 gene deletion causes mice to be highly vulnerable to damage from the tMCAO. In contrast, HO1−/− mice survived the pMCAO protocol for longer time periods. This observation further indicates that HO1 is important and potentially critical for survival after severe ischemic brain injury. As mentioned, while the pMCAO is considered to be a less invasive method than tMCAO, possible pathophysiological differences between permanent and transient ischemia could account for such observations. Some differences between the two focal ischemic models need to be highlighted and likely further investigated. In our study, 90-min tMCAO caused large ischemic brain lesions encompassing up to 58.4% of the whole hemisphere, including physiologically important regions such as striatum, cortex, and others (Fig 1B), whereas, pMCAO affected selectively 42.8% of the cortex (Fig 2B). In addition, unlike pMCAO, tMCAO includes a reperfusion phase. It is well established that brain ischemia-reperfusion causes a surge in free radicals and blood-brain barrier disruption, during which leukocytes, inflammatory molecules and heme can infiltrate the brain interstitial space. In the absence of HO1, toxic heme can remain longer unmetabolized. This excess heme and lack of antioxidant and anti-inflammatory agents (biliverdin/bilirubin, CO) may greatly increase cellular and tissue damage and reduce chances for survival in HO1−/− mice.
Together, our results indicate that IPC requires HO1 to facilitate neuroprotection against ischemic brain injury. It remains to be determined whether HO1 induces protection via heme degradation pathways and whether CO, as a physiologic product of heme degradation, plays a role in this paradigm. The ability of CO to dilate cerebral arteries and increase CBF and brain perfusion rate after ischemia would be a potential benefit in clinical practice, and improvements in collateral circulation and vascular supply as well as an increase in thrombolytic activity have been observed in patients as a result of IPC (Wegener et al., 2004). Other investigators reported that in animal studies, IPC was associated with increased CBF at reperfusion after MCAO (Zhao and Nowak, 2006), suggesting that cerebral vasodilatory capacity could potentially be improved by preconditioning as well. However, the involvement of endothelial, neuronal, and inducible nitric oxide synthase-derived NO (Atochin et al., 2003; Cho et al., 2005) should be considered as well in IPC-induced protection. In this study, using laser-Doppler flowmetry, we did not observe differences in relative CBF between the cohorts with and without IPC. Previously, we compared regional CBF in WT and HO1−/− mice using 14C-iodoantipyrine autoradiography but failed to find a difference (Saleem et al., 2008). Conducting a comprehensive study of regional CBF changes after IPC in the brain is the next step in investigating the possible impact of HO1 induction and potentially CO generation in IPC.
Conclusion
In summary, we have shown that IPC induces protection against transient and permanent brain ischemia. Our results also suggest that induction of HO1 by IPC might be a key factor in attenuating brain infarct volume and improving NDS after permanent brain ischemia. This finding indicates that HO1 could be critical in protection against various stroke paradigms, and in IPC-induced tolerance.
Acknowledgements
This work was supportedby grants from the National Institutes of Health AT004025 and NS046400 (SD); GM075774−02 (EZ), and AT004197 (ZAS) The authors would like to thank Claire Levine for her assistance in the manuscript preparation and all members of the Doré lab for their active participation.
Abbreviations
- IPC
ischemic preconditioning
- MCA
middle cerebral artery
- tMCAO
transient middle cerebral artery occlusion
- pMCAO
permanent distal middle cerebral artery occlusion
- HO1
heme oxygenase 1
- TIA
transient ischemic attack
- WT
wildtype
- HO1−/− mice
heme oxygenase 1 knockout mice
- CO
carbon monoxide
- BCCAO
bilateral common carotid artery occlusion
- CBF
cerebral blood flow
- NDS
neurological deficit score
- TTC
1% triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some of the data were previously presented at the 2006 Annual Meeting of the Society for Neuroscience.
References
- Ahmad AS, Zhuang H, Dore S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–8. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A. 1993;90:9285–9. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Ferriero DM, Vreman HJ, Stevenson DK, Sharp FR. Hypoxia-ischemia, but not hypoxia alone, induces the expression of heme oxygenase-1 (HSP32) in newborn rat brain. J Cereb Blood Flow Metab. 1997;17:647–58. doi: 10.1097/00004647-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Bolanos-Jimenez F, Manhaes de Castro R, Sarhan H, Prudhomme N, Drieu K, Fillion G. Stress-induced 5-HT1A receptor desensitization: protective effects of Ginkgo biloba extract (EGb 761). Fundam Clin Pharmacol. 1995;9:169–74. doi: 10.1111/j.1472-8206.1995.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–61. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- Diaz Z, Assaraf MI, Miller WH, Jr., Schipper HM. Astroglial cytoprotection by erythropoietin pre-conditioning: implications for ischemic and degenerative CNS disorders. J Neurochem. 2005;93:392–402. doi: 10.1111/j.1471-4159.2005.03038.x. [DOI] [PubMed] [Google Scholar]
- Dungey AA, Badhwar A, Bihari A, Kvietys PR, Harris KA, Forbes TL, Potter RF. Role of heme oxygenase in the protection afforded skeletal muscle during ischemic tolerance. Microcirculation. 2006;13:71–9. doi: 10.1080/10739680500466228. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–7. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Franco-Gou R, Rosello-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, Bartrons R, Peralta C. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208:62–73. doi: 10.1002/path.1859. [DOI] [PubMed] [Google Scholar]
- Heiman J, Wallin M, Gustafsson BI, Friman S, Delbro D. Pharmacological preconditioning of rat liver by up-regulation of heme oxygenase 1. Transplant Proc. 2006;38:2705–7. doi: 10.1016/j.transproceed.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Jancso G, Cserepes B, Gasz B, Benko L, Borsiczky B, Ferenc A, Kurthy M, Racz B, Lantos J, Gal J, Arato E, Sinayc L, Weber G, Roth E. Expression and protective role of heme oxygenase-1 in delayed myocardial preconditioning. Ann N Y Acad Sci. 2007;1095:251–61. doi: 10.1196/annals.1397.029. [DOI] [PubMed] [Google Scholar]
- Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–7. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- Li Q, Li J, Zhang L, Wang B, Xiong L. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–93. doi: 10.1016/j.lfs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008;15:686–90. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke. 2000;31:2707–14. doi: 10.1161/01.str.31.11.2707. [DOI] [PubMed] [Google Scholar]
- Mallick IH, Yang W, Winslet MC, Seifalian AM. Protective effects of ischemic preconditioning on the intestinal mucosal microcirculation following ischemia-reperfusion of the intestine. Microcirculation. 2005;12:615–25. doi: 10.1080/10739680500301631. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Saleem S, Zhuang H, Biswal S, Christen Y, Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–96. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Doré S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–8. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–7. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Hanggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- Tamion F, Richard V, Bonmarchand G, Leroy J, Lebreton JP, Thuillez C. Induction of heme-oxygenase-1 prevents the systemic responses to hemorrhagic shock. Am J Respir Crit Care Med. 2001;164:1933–8. doi: 10.1164/ajrccm.164.10.2010074. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–71. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhuang H, Doré S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis. 2006;22:473–6. doi: 10.1016/j.nbd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282:1718–26. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–21. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Yin W, Signore AP, Iwai M, Cao G, Gao Y, Johnnides MJ, Hickey RW, Chen J. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke. 2007;38:1017–24. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–7. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeynalov E, Nemoto M, Hurn PD, Koehler RC, Bhardwaj A. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26:414–20. doi: 10.1038/sj.jcbfm.9600196. [DOI] [PubMed] [Google Scholar]
- Zhao L, Nowak TS., Jr. CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2006;26:1128–40. doi: 10.1038/sj.jcbfm.9600269. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr., Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48:1735–43. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]