Abstract
The induction of donor-specific tolerance remains a major goal in the field of transplantation immunology. Therapies that target costimulatory molecules can induce tolerance to heart and pancreatic islet allografts in mouse models, but fail to do so following transplantation of skin or intestinal allografts. We have proposed that organs colonized by commensal bacteria such as skin, lung and intestine may be resistant to such therapies as a result of bacterial translocation at the time of transplantation, which may promote antigen-presenting cell (APC) maturation and the production of pro-inflammatory cytokines, consequently enhancing responses of alloreactive T cells. Our results indicate that the inability to sense signaling by most toll-like receptors (TLRs), as well as by interleukin (IL)-1R and IL-18R, as a result of genetic ablation of myeloid differentiation factor 88 (MyD88) promotes the acceptance of skin allografts. Conversely, TLR signals and infections by a model bacterium, Listeria monocytogenes (LM), at the time of transplantation can prevent the induction of transplantation tolerance. The effects of the TLR9 agonist CpG are MyD88-dependent, while the pro-rejection capacity of LM depends on the intracellular sensing of LM and the production of type I interferon (IFN). Therefore, transiently targeting these innate, pro-inflammatory pathways may have therapeutic value to promote transplantation tolerance.
Keywords: Toll-like receptor, bacterial infections, type I IFN, acute rejection, tolerance
Microbial signals and alloresponses
Achieving cardiac allograft acceptance in the absence of chronic immunosuppression while retaining residual immune competence to non-allogeneic antigens remains a major goal in transplantation immunology. However, many hurdles hinder this goal. Microorganisms may interfere with transplantation tolerance at different levels. First, it has been shown that viral and parasitic infections prior to allograft transplantation can result in the development of memory T cells some of which may cross-react with alloantigens, a phenomenon sometimes referred to as heterologous immunity (1-4). Second, we have proposed that bacterial translocation after transplantation of organs colonized with commensal bacteria such as skin, lung and intestine, may explain the higher resistance of these organs to transplantation tolerance (5). Finally, pathogenic infections occurring at the time of surgical transplantation or later on in patients bearing stable allografts may prevent the induction of tolerance or break established tolerance, respectively (6). Most bacterial infections in transplant recipients occur within the first month after transplantation and include wound and catheter infections, and hospital-based infections (7, 8). Infections occurring later than six months post transplantation may be related to the immunosuppressive regimens administered to prevent graft rejection and commonly include community-based infections (8). Although bacterial infections have been associated with acute rejection of allografts in the clinic, it is not completely clear whether infections precede transplant rejection or are the consequence of increased immunosuppression to prevent or treat rejection episodes.
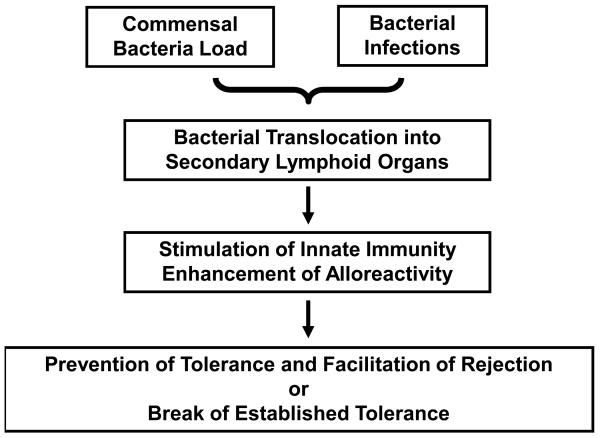
We propose a model, depicted in Figure 1, in which translocation of bacteria following transplantation of organs colonized by commensal bacteria or pathogenic infections result in the recognition of bacterial molecular patterns by receptors expressed on/in donor and recipient antigen-presenting cells (APCs), leading to enhanced presentation of alloantigen, increased alloresponses and relative resistance to otherwise effective doses of immunosuppressive treatments. Several types of receptors may be responsible for recognition of bacterial molecular patterns. These include both membrane-associated and cytosolic receptors. Toll-like receptors (TLRs), NODs and DAI are the 3 types of receptors described to span either plasma or endosomal membranes, whereas cytosolic receptors include RNA-sensing (MIG and MDA5) and putative DNA-sensing receptors (6). Engagement of these receptors can lead to increased expression of MHC and costimulatory molecules as well as the production of pro-inflammatory cytokines, including type I interferon (IFN), tumor necrosis factor (TNF), interleukin (IL)-6 and IL-12, which secondarily drive adaptive immunity.
Figure 1. Model for enhancement of alloresponses by bacteria.
Bacterial translocation may occur following transplantation of organs colonized by commensal bacteria or in patients experiencing active infections. Bacterial molecular patterns may activate donor and recipient antigen-presenting cells (APCs) resulting in APC maturation and production of pro-inflammatory cytokines. Alloreactive T cells stimulated in this context may develop augmented responses leading to prevention of, or breach in, transplantation tolerance.
TLR/MyD88-dependent signals prevent transplantation tolerance
In support of a role for commensal bacteria in the prevention of transplantation tolerance, we and others have reported that the genetic deficiency in both donor and recipient mice of myeloid differentiation factor 88 (MyD88), an adaptor essential for signaling by most TLRs, as well as by IL-1R and IL-18R, results in the ability of anti-CD154 and donor-specific transfusion (DST) to promote long-term acceptance of skin allografts, a tissue that is otherwise rejected if MyD88 is expressed in either the donor or the host (5, 9). TLR signals elicited following skin transplantation have been reported to promote the migration of donor APCs into recipient draining lymphoid organs and promote Th1 alloreactivity, thereby facilitating acute rejection (9-12).
Confirming the possible antagonistic effect of TLR signals on tolerance induction, we and others have shown that ligation of TLRs by administration of exogenous TLR agonists results in prevention of graft acceptance in different systems (5, 13, 14). In our model of cardiac transplantation, peri-operative injection of the TLR9 agonist CpG or of the TLR2 ligand Pam3CysK4 prevented prolonged allograft acceptance by anti-CD154 or tolerance induction by anti-CD154+DST (5). This pro-rejection effect of CpG correlated with reduced accumulation of Tregs in cardiac allografts and diminished the intra-graft ratio of Tregs:Teffectors. Current experiments are investigating whether prevention of tolerance by CpG is due to MyD88 signaling in donor or recipient cells and whether it targets cells of hematopoietic or non-hematopoietic origin. In addition, we are investigating the role of inflammatory cytokines induced by CpG in its ability to prevent transplantation tolerance. Of relevance, it has been recently reported that the ability of the TLR4 agonist LPS and of the TLR3 ligand poly I:C to prevent skin allograft acceptance by anti-CD154+DST is due to the induction by these TLR signals of type I IFN production (15). The capacity of CpG to drive acute rejection of bm12 heart allografts by C57Bl/6 mice, a single MHC class II mismatch combination, has been recently reported to be IL-6-independent, suggesting that not all innate proinflammatory cytokines lead to acute rejection or can prevent the induction of transplantation tolerance (14).
Consequences of bacterial infections on transplantation tolerance
To determine if infections with live bacteria would also prevent transplantation tolerance, we have used LM as a model pathogen, since immune responses to this bacterium are well studied. Our results indicate that administration of LM at the time of transplantation effectively antagonizes the tolerogenic effect of anti-CD154+DST both in cardiac and skin allograft models (16). This is due to early signals delivered by LM as antibiotic eradication of LM infection on day 2 post-transplantation abolishes the ability of LM to prevent transplantation tolerance. The pro-rejection effects of LM require that it expresses listeriolysin O (LLO), a pore forming molecule enabling LM to escape phagosomes and invade macrophages. Although similar pro-rejection effects of CpG are MyD88-dependent, the ability of LM to trigger rejection is independent of MyD88 expression by both donor and recipient cells. Instead, it is exquisitely dependent on IFNα/β signaling in recipient cells as LM failed to prevent tolerance to skin and cardiac allografts in mice deficient in IFNαRI, a common receptor for IFNα and β signaling (16). Indeed, LM infection resulted in detectable serum levels of IFNβ and systemic injection of recombinant IFNβ was sufficient to prevent anti-CD154+DST-mediated allograft acceptance. These findings may be clinically important, as type I IFN treatment can be used to treat recurrence of hepatitis C or lupus flares in some patients, and thus, such treatment may have the capacity to promote acute allograft rejection in the clinic.
Therefore at least 2 pathways, MyD88 and type I IFN, can independently oppose transplantation tolerance. It is clear that not all bacteria induce production of type I IFNs (our own unpublished observations), and whether additional pathways may interfere with transplantation tolerance remains to be established. Of great clinical importance is the question of whether bacterial or viral infections occurring after establishment of transplantation tolerance can reverse established tolerance. Clearly, bacterial infections do occur in long-term stable transplant recipients (8), but prospective clinical trials are lacking to determine whether this type of infections can precipitate acute or chronic rejection. Likewise, experimental models testing whether infections of any kind can break established tolerance are lacking. Our unpublished data indicate that administration of CpG after cardiac or skin allografts are stably accepted is not sufficient to elicit rejection. However, bacterial infections can induce several simultaneous pro-inflammatory pathways that could cooperate to reverse established tolerance.
Conclusions
Our data indicate that TLR signaling and type I IFNR signaling can both prevent the induction of transplantation tolerance by costimulation-targeting therapies. It may be of interest to consider these pathways as therapeutic targets to facilitate transplantation tolerance to organs colonized by commensal bacteria. However, inhibiting such pathways should proceed with caution as such interventions may result in serious infectious complications. For instance, mice lacking IFN signaling are exquisitely sensitive to viral infections that become rapidly lethal (17). In addition, TLR signals have been described in some cases to potentiate Treg function (18), although they can also inhibit regulation (19), such that antagonism of this pathway may prevent the induction of regulation, a tolerance mechanism important in both the induction and maintenance of tolerance by anti-CD154 (20). Elimination of bacterial load from colonized donor organs may be an alternative approach to facilitate transplantation tolerance, although the efficacy of this intervention remains to be demonstrated in animal models.
We conclude that TLR signals and bacterial infections can interfere with the induction of transplantation tolerance and that closer attention may need to be paid to peri-operative infections in the clinic, with respect to their capacity to promote transplant rejection. Future prospective clinical trials should help determine whether specific types of bacterial infections can precipitate acute or chronic rejection.
Footnotes
This work was supported by American Heart Association Fellowship 0620026Z to LC, NIAID RO1 AI071080 to MLA, NIAID R01 AI072630 and ROTRF 280559271 to AC and AST branch out grant to AC and CRW.
References
- 1.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr Opin Immunol. 2004;16:558. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 3.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 4.Welsh RM, Markees TG, Woda BA, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Wang T, Zhou P, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed EM, Alegre ML, Chong A. The role of bacterial infections in allograft rejection. Exp Rev Clin Immunol. 2008 doi: 10.1586/1744666X.4.2.281. in press. [DOI] [PubMed] [Google Scholar]
- 7.Rubin RH. Temporal aspects of transplant infectious disease. Transpl Infect Dis. 2003;5:63. doi: 10.1034/j.1399-3062.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 9.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J Immunol. 2006;177:5307. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 10.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur J Immunol. 2006;36:1994. doi: 10.1002/eji.200636249. [DOI] [PubMed] [Google Scholar]
- 11.Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant. 2004;4:1429. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111:1571. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornley TB, Brehm MA, Markees TG, et al. TLR Agonists Abrogate Costimulation Blockade-Induced Prolongation of Skin Allografts. J Immunol. 2006;176:1561. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porrett PM, Yuan X, LaRosa DF, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179:6620. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Chen L, Ahmed EM, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 18.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 20.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]