Abstract
For a number of disease entities, oxidative stress becomes a significant factor in the etiology and progression of cell dysfunction and injury. Therapeutic strategies that can identify novel signal transduction pathways to ameliorate the toxic effects of oxidative stress may lead to new avenues of treatment for a spectrum of disorders that include diabetes, Alzheimer's disease, Parkinson's disease and immune system dysfunction. In this respect, metabotropic glutamate receptors (mGluRs) may offer exciting prospects for several disorders since these receptors can limit or prevent apoptotic cell injury as well as impact upon cellular development and function. Yet the role of mGluRs is complex in nature and may require specific mGluR modulation for a particular disease entity to maximize clinical efficacy and limit potential disability. Here we discuss the potential clinical translation of mGluRs and highlight the role of novel signal transduction pathways in the metabotropic glutamate system that may be vital for the clinical utility of mGluRs.
Key words: Akt, Alzheimer's disease, amyotrophic lateral sclerosis, apoptosis, β-catenin, erythropoietin, forkhead, Huntington's disease, metabotropic, oxidative stress, Wnt
Oxidative Stress and Apoptotic Cell Injury
A number of mechanisms can account for the degeneration of cells in the body, but the generation of cellular oxidative stress represents a significant component for cell loss in several diseases. Oxidative stress occurs as a result of the development of reactive oxygen species (ROS) that consist of oxygen free radicals and other chemical entities. Oxygen consumption in organisms, or at least the rate of oxygen consumption in organisms, has intrigued a host of investigators and may have had some of its original origins with the work of Pearl. Pearl proposed that increased exposure to oxygen through an increased metabolic rate could lead to a shortened life span.1 Subsequent work by multiple investigators has furthered this hypothesis by demonstrating that increased metabolic rates could be detrimental to animals in an elevated oxygen environment.2 With current work, oxygen free radicals and mitochondrial DNA mutations have become associated with oxidative stress injury, aging mechanisms, and accumulated toxicity for an organism.3
Oxidative stress is a result of the release of ROS that include superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide, and peroxynitrite.4 Oxygen free radicals and mitochondrial DNA mutations have become associated with tissue injury, aging, and accumulated toxicity for an organism.5,6 Most ROS are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase, glutathione peroxidase, catalase, and small molecule substances, such as vitamins C, E, D37 and nicotinamide, the amide form of niacin or vitamin B3.60–63
Oxidative stress represents a significant mechanism for the destruction of cells that can involve apoptotic cell injury.8–10 It has recently been shown that genes involved in the apoptotic process are replicated early during processes that involve cell replication and transcription, suggesting a much broader role for these genes than originally anticipated.11 Apoptotic induced oxidative stress in conjunction with processes of mitochondrial dysfunction can contribute to a variety of disease states such as diabetes, ischemia, general cognitive loss, Alzheimer's disease, and trauma.12–16 Oxidative stress can lead to apoptosis in a variety of cell types that involve neurons, endothelial cells (ECs), cardiomyocytes, and smooth muscle cells through multiple cellular pathways.14,17–21
Membrane phosphatidylserine (PS) externalization is an early event during cell apoptosis22,23 and can become a signal for the phagocytosis of cells.10,24,25 The loss of membrane phospholipid asymmetry leads to the externalization of membrane PS residues and assists microglia to target cells for phagocytosis19,26–29 This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress,5,30 since blockade of PSR function in microglia prevents the activation of microglia.27,31 As an example, externalization of membrane PS residues occur in neurons during anoxia,32–34 nitric oxide exposure,35,36 and during the administration of agents that induce the production of reactive oxygen species, such as 6-hydroxydopamine.37 Membrane PS externalization on platelets also has been associated with clot formation in the vascular system.38
The cleavage of genomic DNA into fragments39–41 is considered to be a later event during apoptotic injury.42 Several enzymes responsible for DNA degradation have been differentiated and include the acidic, cation independent endonuclease (DNase II), cyclophilins, and the 97 kDa magnesium-dependent endonuclease.4,12 Three separate endonuclease activities are present in neurons that include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease.43,44
During oxidative stress, mitochondrial membrane transition pore permeability also is increased,19,45–47 a significant loss of mitochondrial NAD+ stores occurs, and further generation of superoxide radicals leads to cell injury.28,48 In addition, mitochondria are a significant source of superoxide radicals that are associated with oxidative stress.12,49 Blockade of the electron transfer chain at the flavin mononucleotide group of complex I or at the ubiquinone site of complex III results in the active generation of free radicals which can impair mitochondrial electron transport and enhance free radical production.4,30 Furthermore, mutations in the mitochondrial genome have been associated with the potential development of a host of disorders, such as hypertension, hypercholesterolemia, and hypomagnesemia.50,51 Reactive oxygen species also may lead to the induction of acidosis-induced cellular toxicity and subsequent mitochondrial failure.13 Disorders, such as hypoxia,52 diabetes53,54 and excessive free radical production44,55,56 can result in the disturbance of intracellular pH.
Metabotropic Glutamate Receptors (mGluRs) Structure and Functional Role
Glutamate, an excitatory neurotransmitter, plays an important role during both cellular function and cellular injury in the mammalian central nervous system (CNS). Until the mid 1980s, the actions of glutamate in the brain were believed to occur through the activation of glutamate-gated channels termed ionotropic glutamate receptors. Yet, further studies provided evidence for the existence of another family of glutamate receptors that was directly coupled to GTP-binding regulatory proteins. Initial work, such as the observation of glutamate induced phospholipase C generation in neurons, indicated that glutamate had more complex roles that could not be accounted for by only N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), or kainate receptor families.57 Subsequently, it became evident that a new class of glutamate receptors, termed metabotropic glutamate receptors (mGluRs), was coupled to effector systems through GTP-proteins (G-proteins).58–60
The first mGluR, now generally termed mGluR1a, was cloned in 1991 by a functional expression screening procedure.60,61 Since molecular cloning has preceded pharmacological characterization in the identification of novel mGluRs, the mGluRs are numbered following the order in which their cDNAs have been cloned. G-proteins consist of heterotrimeric proteins that contain three subunits termed α, β and γ. At least forty-six G-proteins have been identified with twenty-seven classified as Gα, five classified as Gβ, and fourteen classified as the Gγ. A variety of heterotrimeric combinations can be formed that may produce a broad spectrum of G-protein signaling.62 Activation of G-protein-coupled receptors results in the dissociation of the heterotrimer of the G-protein into its α and βγ subunits, which can then bind to a variety of effector molecules. A particular G-protein may be responsible for the modulation of a series of signal transduction pathways. The G-protein βγ has been associated with many effector molecules including adenylate cyclase (AC), phospholipase C-β (PLC-β), mitogen-activated protein kinases (MAPKs), and phosphoinositide 3 kinase (PI 3-K).63
G-protein-coupled receptors can be divided into three major subfamilies based on nucleotide and amino acid sequence similarity. Family A consists of the rhodopsin/adrenergic receptors and is characterized by the presence of a restricted number of conserved residues (Asp-Arg-Tyr). Family B consists of peptide hormone and neuropeptide receptors that are characterized by a large extracellular NH2 terminus containing six cysteine residues. Metabotropic glutamate receptors share a common molecular morphology with other G protein-linked receptors. The mGluRs are part of family C of G-protein-coupled receptors, which also includes gamma aminobutyric acid (GABA) receptors and ionotropic calcium receptor transmission. Unlike the other G-protein-coupled receptors families, mGluRs contain a long NH2 terminal chain and couple to G-proteins through their second intracellular loop rather than the third intracellular loop of the receptor.
The mGluRs also are classified into three major groups based on sequence homology, G-protein coupling specificity, and agonist selectivity. Group I mGluRs (including mGluR1 and mGluR5) couple preferentially to Gq to stimulate PLC-β. Activation of PLC-β results in the generation of two second messengers, inositol-1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG), to mobilize intracellular calcium and activate protein kinase C (PKC). Group I mGluRs also can activate AC via coupling to Gs to result in an increase in cAMP.64 In contrast to group I mGluRs, group II mGluRs (including mGluR2 and 3) and group III mGluRs (including mGluR4, 6, 7, and 8) are negatively coupled to AC to reduce the amount of intracellular cAMP. In addition, activation of group II/III can modulate activity of extracellular signal-regulated protein kinases (ERKs) and PI 3-K (Fig. 1).65 Yet, different subgroups of the mGluR system may employ pathways of ERKs. For example, group I mGluRs can increase the phosphorylation of ERK2 through PKC.66 In addition, ERKs may employ phosphorylation of the pro-apoptotic protein Bad and induction of pro-survival gene expression via the cAMP responsive element-binding (CREB) protein dependent pathway to lead to cellular protection.67 It is not entirely evident whether the protective mechanisms utilized by mGluRs also involve the cellular pathways of the MAP-kinases, since activation of mGluRs does not alter the activity of either p38 or c-Jun N-terminal kinase (JNK), suggesting that protection by mGluRs is independent or below the level of p38 activation.10,68
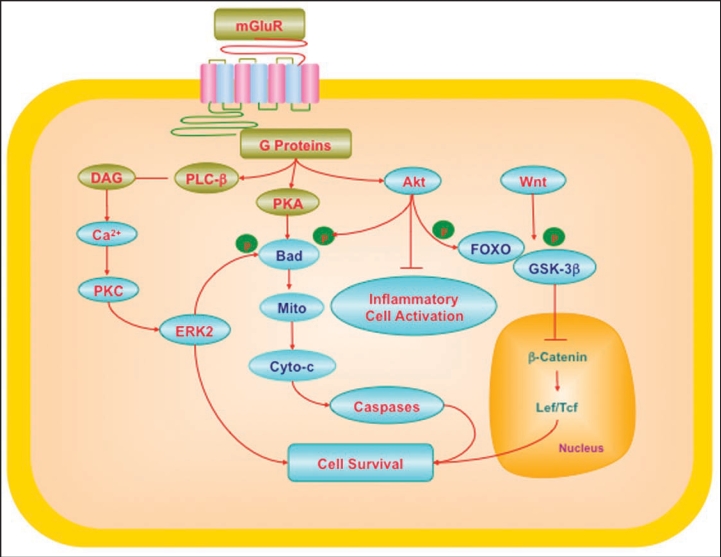
Figure 1.
Cytoprotection through mGluRs may require the regulation of multiple cellular pathways. G-protein βγ in the mGluR system activates phospholipase β (PLC-β), diacylglycerol (DAG), and phosphoinositide 3 kinase (PI 3-K). These pathways lead to the activation of protein kinases A (PKA), B (Akt) and C (PKC) that can involve intracellular calcium (Ca2+). mGluRs can activate ERK2 through PKC. PKA can phosphorylate (p) Bad, a member of Bcl-2 protein family, which can prevent apoptotic cell injury. Akt provides an anti-apoptotic survival signal through the phosphorylation and inactivation of Bad and also interfaces with the Wnt and forkhead pathways. Wnt signaling can inhibit glycogen synthase kinase (GSK-3β). Inhibition of GSK-3β prevents phosphorylation (p) of β-catenin and leads to the accumulation of β-catenin. β-catenin subsequently translocates to the cell nucleus and contributes to the formation of Lef/Tcf lymphocyte enhancer factor/T cell factor (Lef/Tcf) and β-catenin complex that may cooperate with factors to target nuclear gene transcription and prevent cell injury. Interconnected pathways with Akt and Wnt involve the forkhead family members (FOXO), mitochondrial (Mito) membrane potential (ΔΨm), cytochrome c (Cyto-c), and caspases. If left unabated, these pathways converge to lead to apoptotic cell injury.
The G-protein-coupled receptor family is the largest family of cell-surface molecules. These receptors are activated by a wide variety of stimuli, including neurotransmitters, peptides, hormones, growth factors, ions, lipids and light. The mGluR system is one of the principal members in this receptor family and provides an important function as presynaptic auto-receptors that mediate feedback inhibition of glutamate release in a wide variety of brain regions. One of the mechanisms of glutamate inhibition is thought to result from the down-regulation of voltage-activated calcium channels which are necessary for synaptic vesicle exocytosis.69 The mGluR system also is a critical mediator for the modulation of intracellular signal cascades and physiological function. Interaction among each G-protein subunit, such as -α, -β and -γ subunits, can stimulate effector molecules including adenylyl/guanylyl cyclases, phosphodiesterases, phospholipases, and phosphoinositide 3-kinase (PI3-K) resulting in the modulation of other second messengers. Several second messenger systems, such as cAMP, cGMP, inositol (3,4,5)-trisphosphate (Ins(1,4,5)P3), arachidonic acid, phosphatidic acid, and calcium are active participants in these signal transduction cascades.70
Electrophysiological studies of mGluRs have shown that activation of mGluRs may lead to a range of cellular changes such as the inhibition of calcium and potassium currents, mediation of slow excitatory postsynaptic potential, and the presynaptic inhibition of transmitter release.69,71,72 In particular, activation of group I mGluRs can contribute to slow-onset potentiation in the hippocampal region of CA1.73 Activation of postsynaptic group I mGluRs also suppresses transmission at excitatory synapses onto CA1 pyramidal cells.74 In addition, group I mGluRs have been shown to alter calcium homeostasis and trigger calcium-sensitive gene transcription in striatal neurons.75 Group II mGluRs have a significant role in the modulation of GABA afferent inhibition in the ventrobasal thalamus that controls functions of sleep, arousal, and sensation.76 Both group I and II receptors may be required for the activity-dependent regulation of ribosomes during auditory function.77 High-frequency stimulation appears to be particularly dependent upon group I and group II metabotropic glutamate receptors.78 Group III mGluRs have a greater role in motor function through the inhibition of GABA and glutamate transmission in the substantia nigra, pars reticulata,79 and the periaqueductal grey area.80
The ubiquitous distribution of glutamatergic synapses in the nervous system offers a great potential for mGluRs to modulate global CNS function. In the spinal cord, Group I mGluRs can regulate slow excitatory synaptic transmission.81 In addition, behavioral and physiological studies have demonstrated that mGluRs can regulate fast synaptic transmission, changes in synaptic plasticity, and the modification of the calcium currents.39,82 During memory imprinting, group I mGluRs which are juxtaposition to NMDA receptors can modulate the potentiation of NMDA receptor activity to influence both long-term potentiation and long-term depression.73,83 Yet, multiple members of the mGluR system may be required for memory formation84 or memory restoration following an ischemic insult.85 Activation of mGluRs also can lead to depolarization-induced synapsin I phosphorylation, a process that may be involved in synaptic vesicle exocytosis in visceral sensory neurons.86 Furthermore, cognitive impairment and psychiatric disease such as with schizophrenia may be tied to mGluR expression and activation.87
mGluRs Expression, Progenitor Cell Differentiation and Cell Development
Metabotropic glutamate receptors are expressed throughout the mammalian CNS. In neuronal populations, the mGluR system participates in the processing of cognition, sensory, motor and olfactory information. For example, mGluRs are present in the cerebral cortex,88 cerebellar neurons,89 striatal neurons and in the spinal cord.90 In the hippocampus, a more restricted expression of the receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 has been demonstrated.91 The receptor subtype mGluR4 also has a distinct distribution in the thalamus, hypothalamus, and caudate nucleus.92 In the retina, mGluR6 is expressed.93 In contrast, mGluR3 is expressed throughout the brain with dense expression in neurons of the cerebral cortex, caudate-putamen, thalamus, and cerebellum.94
The mGluR receptors are distributed in specific subcellular regions and alter their expression during development of the nervous system. During embryonic development, group III mGluRs may prevent the self-renewal of undifferentiated neocortical progenitor cells95 while group I mGluRs can promote neuroblast proliferation.96 Group I mGluRs including mGluR1a and mGluR5 predominantly exist on the post-synaptic membranes of the glutamatergic synapse junctions.97 Yet, in the initial postnatal period, mGluR1a and mGluR5 can be found in proximal dendrites and the cell somata. With age, these receptors become densely distributed in the distal part of dendrites to participate in synaptic function.98 Group II mGluRs (mGluR2/3) are present primarily in astrocytes surrounding the neuronal somata and synapses. A less dense population of group II mGluRs is also located in presynaptic axon terminals. The distribution pattern of mGluR2/3 is believed to be consistently maintained during postnatal development.98 Of the group III mGluRs, mGluR6 is initially distributed in both the neuronal soma and dendrites in rat retinal bipolar cells, but later redistributes to postsynaptic sites.99 Presynaptic expression is more common for the mGluR7 subtypes.100 In group III mGluRs, mGluR4, mGluR6, and mGluR8 also have been identified in microglia and astrocytes.101 The presence of these receptors in a variety of cell types may be responsible for protection of neuronal cell populations.102 Redistribution of the expression of mGluRs appears to be necessary for proper nervous system development. For example, redistribution of mGluR6 in rat retinal bipolar cells occurs from somatic and dendritic sites to restricted localization at postsynaptic sites.99 In the mouse thalamus, subcellular relocalization of group I mGluRs also occurs during postnatal development.98 In addition to redistribution of receptors, functional changes in the mGluR system also may occur during development. The generation of second messengers, such as cAMP, has been reported to vary under mGluR control during critical periods of ocular dominance and plasticity.103
Less information is available for the role of the mGluR system in the vascular system, but new investigations are beginning to provide evidence for a vital function for the mGluR system in brain endothelial cells. Initial work has outlined the expression of mGluRs in cultured rat cerebrovascular ECs104 and in cardiac cells.105 Further studies have now demonstrated not only the expression of specific group I mGluRs in cerebral endothelial cells, but also the potential for the mGluR system to protect against apoptotic injury.26,28,106 In addition, mGluR1, mGluR2/3, mGluR4a, mGluR5 and mGluR7 have been demonstrated in the meningeal microvasculature.107 Interestingly, agents such as nicotine can inhibit the expression of mGluRs in cardiac tissue.108 More recent studies suggest that activation of mGluRs may control vascular tone.109
mGluRs and Disorders of the Nervous and Vascular Systems
mGluRs may be involved in diseases such as diabetes mellitus (DM) which is a significant health concern.110,111 Approximately 16 million individuals in the United States and more than 165 million individuals worldwide suffer from DM. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions.112 Type 2 DM represents at least 80% of all diabetics and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index.113 Type 1 insulin-dependent DM is present in 5–10% of all diabetics,111 but is increasing in adolescent minority groups.114 Furthermore, the incidence of undiagnosed diabetes, impaired glucose tolerance, and fluctuations in serum glucose in the young raises additional concerns.115 Patients with DM can develop severe neurological and vascular disease116,117 that can lead to an increased risk for cognitive decline.12,30,118 As a result, the development of insulin resistance and the complications of DM in the nervous and vascular systems are believed to be, at least in part, a result of cellular oxidative stress.110,111 Hyperglycemia can lead to increased production of ROS in endothelial cells, liver and pancreatic β-cells.119–122 Recent clinical correlates support these experimental studies to show that elevated levels of ceruloplasmin are suggestive of increased ROS.123 Furthermore, acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes.124
Several studies highlight the role of mGluRs during cellular metabolism and DM. In animal models of DM, the expression of mGluR1 and mGluR5 messenger RNA are significantly increased in all layers of the dorsal horn of the spinal cord, suggesting that mGluR expression and activity may be associated with the development of diabetic neuropathy.125 In addition, mGluRs are expressed in pancreatic islet cells and can impact upon the release of glucagon release. For example, the mGlu8 receptor is present in glucagon-secreting α-cells and intrapancreatic neurons and functions to inhibit glucagon release.126 Additional work has shown that mGluR3 activation can protect neurons from glucose related oxidative stress127 as well as prevent apoptotic injury in vascular cells.10
In regards to other disorders, the modulation of the mGluR system has been proposed for the treatment of bipolar disease since these receptors may modulate signal transduction during affective disorders.128 Furthermore, more recent work in animal models suggests that Group III mGluRs may be beneficial for the treatment of psychosis.129 For diseases such as trisomy 21, a congenital disorder with mental impairment, enhanced expression of the mGluR subtype 5 has been reported.130 Furthermore, dysfunctional signaling in the Group I mGluR system may be responsible for other types of abnormal cognitive development found in disorders such as fragile X mental retardation caused by the lack of fragile X mental retardation protein (FMRP).131
In progressive disorders such as amyotrophic lateral sclerosis (ALS), the mGluR1a receptor may be offer endogenous cellular protection, since surviving motor neurons from the spinal cord of ALS patients maintain mGluR1a at levels comparable to that from controls.132 Yet, other work indicates that mGluR inhibition may be required to protect neurons during ALS.133 In epilepsy, group I mGluR activation can lead to prolonged epileptiform discharges134 and enhanced expression of group II and III metabotropic receptors in the hippocampus of patients with epilepsy has been observed,135,136 However, some work supports a protective role for mGluRs in epilepsy, since activation of group II mGluRs has been shown to prevent seizures in experimental animal models.137 Furthermore, recent work illustrates that disruption between the mGluR7a receptor and its PDZ-interacting protein, protein interacting with C kinase 1 (PICK1), can result in behavioral changes and EEG recordings consistent with absence epilepsy, suggesting that maintenance of mGluR function can protect against epileptic disorders in some scenarios.138 Reported abnormal expression of mGluR1 also has been reported in Pick's disease.139 Modulation of synaptic and receptor activity by mGluRs during other chronic neuronal disorders, such as in Huntington's disease, has recently been suggested to alter cellular susceptibility to injury.140 In particular, huntingtin protein, which is associated with excitotoxic death of striatal neurons, can lead to the desensitization of mGluR1 that may promote cell death.141 Changes in expression patterns of group III mGluRs also have been observed during inflammatory disorders, such as multiple sclerosis,101,142 and during acute central143 or peripheral nerve trauma.144
mGluRs also appear to have a role in Parkinson's disease (PD). For example, mGluRs have been associated with the basal ganglia synaptic transmission through several mechanisms. Increased binding of mGluR5 in the striatum of parkinsonian primates has been demonstrated with PET scanning.145 Activation of group I mGluRs can invoke excitatory postsynaptic current in dopaminergic neurons,146 facilitate dopamine release from nigrostriatal terminals147,148 and modulate GABA release.149 In addition, group I mGluR activation can prevent amphetamine-induced rotational behavior in the unilateral 6-hydroxydopamine (6-OHDA) lesion rat model of PD.150 These results suggest that group I mGluRs agonists may have a therapeutic potential in PD. Yet, group I mGluRs in the subthalamic nucleus appear also to increase the excitation output in the basal ganglia. Group I mGluRs (mGluR5) can lead to an excitatory drive in subthalamic nucleus neurons.151 More specifically, stimulation of striatal group I mGluRs inhibits striatal projection neuronal activity, while stimulation of subthalamic metabotropic glutamate receptors increases subthalamic nucleus activity.
Another consideration for PD is the involvement of group II mGluRs. Group II mGluRs are presynaptically localized on subthalamic nucleus terminals. Activation of these receptors inhibits excitatory transmission at subthalamic nucleus synapses.152 As a result, selective agonists of group II mGluRs can reduce excitatory drive through an indirect pathway, which is enhanced in PD, and provide a different approach to the treatment of PD. For example, in a haloperidol-induced rat model of PD, a selective agonist of group II mGluRs, LY354740 ((+)-2-aminobicyclo[3.1.0.] hexane-2,6,-dicarboxylic acid), has been demonstrated to reverse parkinsonian muscle rigidity and catalepsy.152,153 In another model of PD through application of reserpine in rats, the parkinsonian akinesia was relieved by injection of the group II mGluR receptor agonist, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine.154 In addition, group II mGluR receptor agonists may protect through the release of trophic factors155 or potentially modulate extracellular glutamate uptake.156 Taken together, agonists for group II mGluRs may be promising agents in the management of PD.
Group III mGluRs also play an important role in synaptic transmission in basal ganglia circuits. Of these, mGluR7 is presynaptically localized in the striatum, the globus pallidus, and the substantia nigra pars reticulata (Kosinski et al., 1999) and modulates synaptic transmission through direct and indirect pathways. Activation of mGluR7 inhibits GABA as well as glutamate transmission in the substantia nigra pars reticulata.79 As a result, modulation of excitatory and inhibitory synaptic transmission by mGluR7 may yield no alteration in the output of the substantia nigra pars reticulata. In contrast to mGluR7, mGluR4 appears to be more selectively localized in striatopallidal synapses and inhibits synaptic activity.157 Consequently, the selective agonists for mGluR4 may provide an alternate therapy for the treatment of PD.
In addition to PD, other neurodegenerative disorders such as Alzheimer's disease (AD) have been linked to mGluRs. AD is characterized by two pathologic hallmarks that consist of extracellular plaques of amyloid-β peptide aggregates and intracellular neurofibrillary tangles composed of hyperphosphorylated microtubular protein tau.12,13 The β-amyloid deposition that constitutes the plaques is composed of a 39–42 amino acid peptide (Aβ), which is the proteolytic product of the amyloid precursor protein (APP).13 Large soluble fragments (APPs) that are the result from the cleavage of APP within its Aβ domain are secreted into the extracellular medium. Overexpression of APP can accelerate Aβ secretion which can form insoluble amyloid aggregates contributing to the development of AD. In AD, a downregulation of mGluR binding sites occurs.158 In addition, group I mGluRs are desensitized in the frontal cortex in AD patients and these modifications have been correlated with the progression of AD.159 Other work has shown that mGluR antagonists may control Aβ synaptic transmission.160 Interestingly, postsynaptic FMRP binds to and regulates the translation of amyloid precursor protein (APP) mRNA through metabotropic glutamate receptor activation to suggest a link between fragile X syndrome and AD.161
Metabotropic receptors also have been coupled to the acceleration of APP processing. Activation of mGluR1 in human glioma and neuroblastoma cells favors the processing of APP into nonamyloidogenic APPs resulting in the reduction of Aβ formation162 and an alteration in APP secretion.163 In hippocampal neurons, the release of APPs also is accelerated by stimulation of mGluRs, but not with ionotropic glutamate receptors.162 In brain cortical and hippocampal slices, the stimulation of group I and group II mGluRs by trans-(1S,3R)-1-amino-1,3-cyclopentane dicarboxylic acid (ACPD) can increase the release of APPs. The process can be blocked by the administration of (±)-α-methyl-4-carboxyphenylglycine, a non-selective antagonist of group I and group II mGluRs.164
The regulation of APP processing by mGluRs also appears to be dependent on the activation of protein kinase C (PKC). PKC consists of a family of serine-threonine kinases that are physiologically activated by a number of lipid cofactors and are considered to be important transducers in several agonist-induced signaling cascades. In the PKC family, at least 12 distinct serine/threonine kinase isoenzymes that have important actions in transmembrane signal transduction pathways regulating cell proliferation, differentiation, cytoskeletal functions, gene transcription, apoptosis, and drug resistance exist.165 Activation of PKC may be either pro-apoptotic or anti-apoptotic depending on the cell type.166–168 Studies have begun to define isoform-specific functions of PKC in the apoptotic pathway and the alterations of specific PKC isoforms during injury.33,167–170 For example, PKC isoforms that appear to be anti-apoptotic include PKC-α, PKC-βII, and PKC-ε and the atypical isoforms PKC-λ and PKC-ζ.171 During free radical exposure or anoxia, activation of mGluRs has been shown to protect neurons through pathways that can modulate PKC. Neuroprotection by the subtypes mGluR1a, mGluR2, and mGluR5 appears to be dependent on the direct modulation of PKC activity (Fig. 1).167 Furthermore, PKC may be able to phosphorylate mGluRs and lessen activation of these receptors.172 In regards to AD, inhibition of PKC activity can block alterations in secretion of APPs in response to the activation of mGluRs.162,164
Of equal importance to the functional preservation of cells during neurodegenerative processes is the role of mGluRs during cellular inflammation. In particular, one can consider the role of microglia in the brain that can lead to the phagocytic removal of both neurons and vascular cells.17,19,24 During inflammation, microglial cells require the activation of intracellular cytoprotective pathways18,25 to proliferate and remove injured cells.29,173 Subsequently, microglia can form a barrier for the removal of foreign microorganisms from the CNS and promote tissue repair during neuronal and vascular cell injury.18,174 Yet, microglia also may lead to cellular damage through the generation of reactive oxygen species49,175 and through the production of cytokines.176,177 Furthermore, microglial activation has been correlated with several neurodegenerative disorders that include AD with the co-localization of microglia and amyloid plaque development.178
Given the impact that inflammatory cells may have upon the progression or resolution of degenerative insults throughout the body, it becomes essential to consider whether mGluRs can control inflammatory pathways. In regards to mGluRs, activation of group I mGluRs can prevent neuronal membrane PS exposure and inhibit microglial activation by decreasing the expression of proliferating cell nuclear antigen (PCNA) and uptake of bromodeoxyuridine (BrdU) in microglia. Activation of group I mGluRs can block neuronal PS exposure and prevented subsequent neuronal cell engulfment by microglia seeking neurons with PS externalization.24 However, other subgroups of mGluRs may invoke a different response with microglial activation during cell injury. For example, during ischemic stroke in an animal model, activation of group II mGluRs may lead to microglial release of tumor necrosis factor-α (TNF-α) that can be toxic to neurons.179
mGluRs and Novel Signal Transduction Pathways
Protein kinases.
Several cellular signal transduction pathways may impact upon the ultimate biological function of mGluRs. For example, the phosphatidylinositol 3-kinase (PI 3-K) pathway through protein kinase B (Akt) can determine cytoprotection through mGluRs.180 Phosphorylation of Akt by mGluRs leads to its activation and protects against genomic DNA degradation and membrane PS exposure.24,180,181 Upregulation of Akt activity during multiple injury paradigms, such as with matrix detachment,182 neuronal axotomy,183 N-methyl-D-aspartate toxicity,184 hypoxia,185,186 β-amyloid toxicity,187,188 and oxidative stress17,19,27 increases cell survival. Akt also can directly control microglial activation through the prevention of Bcl-xL degradation17 and the inhibition of caspase 1-, 3-, and 9-like activities.19,24,27 Activation of Akt by mGluRs also may contribute to new dendritic cell growth and protein synthesis in neurons.189 These observations are thought provoking since Akt is closely associated with a number of trophic factors such as erythropoietin (EPO).190–194 The ability of EPO to enhance cell survival during injury can depend upon the PI 3-K pathway through protein kinase B (Akt). Akt may be an essential component for EPO protection especially during disease processes such as diabetes, since inhibition of Akt activity blocks cellular protection and anti-inflammatory mechanisms by EPO.31,46,195 EPO has been shown to employ the PI 3-K/Akt pathway in a variety of experimental models of injury.25,31,185,195–203 Cytoprotection in these models can involve transcription factor regulation,197 maintenance of ΔΨm, prevention of cytochrome c release31,46,195 and blockade of caspase activity.31,46,185
Experimental studies have demonstrated that mGluRs can increase cellular survival during injury paradigms through the activation of the PI 3-K/Akt pathways26 (Fig. 1). In rat hippocampal neuronal cultures, application of the group I mGluR agonists prevent neuronal injury during oxidative stress through increased Akt activity.24,181 In addition, Akt activation through mGluRs may protect against amyloid toxicity.204 This enhancement of Akt activity by mGluR1 may proceed through the formation of a complex that includes Homer, an adaptor protein, and PI 3-K to prevent neuronal apoptosis.205 As a second possible protective mechanism, mGluR inhibition of caspase 3-like activity may serve to prevent the caspase 3 mediated cleavage of Akt and foster increased cellular survival through a prolonged half-life of Akt (Fig. 1).10,68,206 Furthermore, regulation of Akt activity appears closely linked to memory formation that involves mGluRs.207,208 Interestingly, mGluRs, such as mGluR1α, can loss the ability to be cytoprotective and activate Akt during NMDA receptor activation that may lead to the calpainmediated truncation of mGluR1α.209
In addition to Akt and PKC that have been previously discussed, protein kinase A (PKA) is another potential pathway that may offer control of synaptic plasticity as well as cytoprotection through the mGluR system (Fig. 1).168,210 The mGluR system employs PKA activation for the regulation of memory retrieval211,212 and long-term depression.213 In addition, postsynaptic depolarization of Purkinje neurons in the rat cerebellar cortex that leads to long-term potentiation of GABA receptor responsiveness termed rebound potentiation requires both mGluR1 and PKA.214 During paradigms of cellular injury, activation of PKA can prevent apoptosis in a number of cell types, including neurons, neutrophils, and smooth muscle cells.168,215,216 In addition, loss of PKA activity during toxic insults can lead to cell injury.168,217,218 Protection by PKA is believed to reside upstream from the inhibition of caspase 3-like activity.219 Furthermore, PKA has been shown to phosphorylate Bad, a member of Bcl-2 protein family, which can prevent the induction of cell injury.220 In the mGluR system, the subtype mGluR4 requires the activation of PKA to prevent cellular injury following acute neuro-degenerative insults.167
Forkhead transcription factors.
Several other novel pathways that may determine the cytoprotective capacity of mGluRs during oxidative stress are linked to Akt. For example, Akt is a central regulatory element for the mammalian forkhead transcription factor family that oversees processes that can involve cell metabolism, cell development, and apoptosis.117,221,222 Over one hundred forkhead genes and 19 human subgroups that range from FOXA to FOXS are now known to exist since the initial discovery of the fly Drosophila melanogaster gene forkhead.223 In particular, members of the mammalian forkhead transcription factors of the O class (FoxOs), FoxO1, FoxO3, FoxO4 and FoxO6, have emerged as important targets in the neuronal and vascular systems since they can modulate processes associated with angiogenesis, stem cell proliferation, cardiovascular injury, tumorigenesis, and vascular cell longevity. Forkhead proteins function as transcription factors to either inhibit or activate target gene expression. These proteins bind to DNA through the forkhead domain that relies upon fourteen protein-DNA contacts. Post-translational modification of forkhead proteins, such as phosphorylation or acetylation, alters the binding to DNA to prevent transcriptional activity. However, other mechanisms may influence DNA binding of forkhead proteins, such as variations in the N-terminal region of the DNA recognition helix, changes in electrostatic distribution, and the ability of forkhead proteins to be shuttled to the cell nucleus.222,224
Akt can phosphorylate and inactivate FoxO1, FoxO3a and FoxO4.221,222 During oxidative stress, Akt can prevent cellular apoptosis through the phosphorylation of FoxO proteins.225,226 Post-translational phosphorylation of FoxO proteins maintain FoxO transcription factors in the cytoplasm227 by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes.194,197 An exception in regards to the subcellular trafficking of FoxO proteins involves FoxO6. This FoxO protein usually resides in the nucleus of cells and is phosphorylated by Akt in the nucleus.222 Activation of Akt also controls the apoptotic pathways of the caspase family that may offer an alternative mechanism to regulate FoxO proteins.4,17 The caspases 1 and 3 have each been linked to the apoptotic pathways of genomic DNA cleavage, cellular membrane PS exposure, and activation of inflammatory cells.31,42,46 Caspase pathways may be tied to the forkhead transcription factor FoxO3a since increased activity of FoxO3a can result in cytochrome c release and caspase-induced apoptotic death.181,197,228,229 Pathways that can inhibit caspase 3 activity appear to offer a unique regulatory mechanism. For example, caspase 3 cleavage of FoxO3a can lead to pro-apoptotic amino-terminal (Nt) fragments that can lead to cell death. However, during caspase 3 inhibition, inactive phosphorylated FoxO3a remains intact and does not lead to apoptotic cell injury during oxidative stress.181,197,228
During periods of oxidative stress, FoxO transcription factors can lead to cell injury and apoptosis,190 since forkhead transcription factors such as FoxO1 and FoxO3a must be present for oxidative stress to result in apoptotic cell injury.230 In addition, FoxO3a in conjunction with c-Jun N-terminal kinase (JNK) has been shown to modulate an apoptotic ligand activating a Fas-mediated death pathway in cultured motoneurons,231 to lead to apoptosis through tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) and BH3-only proteins Noxa and Bim in neuroblastoma cells229 and to promote pro-apoptotic activity of p53.232 These studies correlate well with experimental work demonstrating that protein inhibition or gene knockdown of FoxO1 or FoxO3a can result in stroke reduction,233 enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress228 and provide trophic factor protection in the cardiovascular system with EPO197 and neurotrophins.234 However, some studies suggest that the loss of FoxO1, FoxO3a and FoxO4 protein expression may actually lead to an increase in free radical release that can be responsible for oxidative stress.235
In relation to mGluRs, inhibition of FoxO3a activity appears to be required to mediate cellular protection during oxidative stress (Fig. 1).181 During oxidative stress in primary hippocampal neurons, free radical exposure can increase the phosphorylation of FoxO3a within 6 hours, but over the course of a 12 hour period the expression of phosphorylated and total FoxO3a is lost, suggesting its destruction. In contrast, activation of the mGluR1 receptors maintains inhibitory phosphorylation of FoxO3a over both a 6 and 12 hour period to block FoxO3a activation.181 Furthermore, prevention of phosphorylated and total FoxO3a degradation by mGluR1 activation may assist with neuronal cytoprotection by inhibiting the formation of Nt fragments. The subsequent proteolysis of phosphorylated and total FoxO3a can lead to the generation of apoptotic amino-terminal (Nt) fragments.236
The proteolytic processing of FoxO3a also is linked to the induction of caspase 3 activity. FoxO3a has been shown to be a substrate for caspase 3-like proteases at the consensus sequence DELD304A (Fig. 1).236 Blockade of caspase 3-like activity prevents the destruction of phosphorylated FoxO3a in neurons during free radical exposure.181 Yet, inhibition of caspase 9-like activity does not offer a similar level of prevention for the degradation of phosphorylated FoxO3a since FoxO3a has a consensus sequence specific for caspase 3. Some protection by caspase 9 inhibition against FoxO3a degradation could be expected since caspase 3 activation is dependent upon initial caspase 9 activation.237 The results correlate well with other reports that illustrate that mGluR1 receptor activation can prevent caspase 3 and caspase 9 activity10,22,180 and that activation of group III mGluRs prevent caspase 3 activity during amyloid toxicity,238 suggesting that the mGluR receptors are able to block the degradation of FoxO3a as a result of the inhibition of caspase activity.
β-catenin, glycogen synthase kinase-3β and calcium pathways.
FoxO proteins are associated with additional pathways that modulate cellular survival during oxidative stress. One pathway involves proteins derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes. The Wnt proteins are secreted cysteine-rich glycosylated proteins that can control cell proliferation, differentiation, survival, and tumorigenesis.5,6 More than eighty target genes of Wnt signaling pathways have been demonstrated in human, mouse, Drosophila, Xenopus and zebrafish. These genes are present in several cellular populations, such as neurons, cardiomyocytes, endothelial cells, cancer cells and pre-adipocytes.239 At least nineteen of twenty-four Wnt genes that express Wnt proteins have been identified in the human.5,6,173 Wnt proteins are divided into functional classes based on their ability to induce a secondary body axis in Xenopus embryos and to activate certain signaling cascades that consist of the Wnt1 class and the Wnt5a class.6,239 One Wnt pathway involves intracellular calcium release and is termed the non-canonical or Wnt/calcium pathway consisting primarily of Wnt4, Wnt5a and Wnt11. The non-canonical system functions through non-β-catenin-dependent pathways and also includes the planar cell polarity (PCP) pathway or the Wnt-calcium-dependent pathways.5,6,173 A second pathway controls target gene transcription through β-catenin, generally referred to as the canonical pathway that involves Wnt1, Wnt3a and Wnt8.
The β-catenin pathway ties FoxO proteins and Wnt signaling together. For example, in relation to Alzheimer's disease, amyloid is toxic to cells187,240 and is associated with the phosphorylation of FoxO1 and FoxO3a that can be blocked with ROS scavengers.241 A common denominator in the pathways linked to amyloid toxicity involves Wnt signaling through β-catenin. β-catenin may increase FoxO transcriptional activity and competitively limit β-catenin interaction with members of the lymphoid enhancer factor/T cell factor family242 and β-catenin also has been demonstrated to be necessary for protection against amyloid toxicity in neuronal cells.240 With the mGluR system, activation of group I mGluRs can modulate the phosphorylation of β-catenin and its intracellular translocation from the cytoplasm to the cell nucleus (Fig. 1). During oxidative stress, phosphorylation of β-catenin is increased that can lead to its degradation and subsequent cell injury, but group I mGluR activation blocks phosphorylation of β-catenin within 6 hours following free radical exposure.181 The blockade of β-catenin phosphorylation is associated with its translocation from the cytoplasm to the nucleus to allow it to assist with known cytoprotective pathways. In addition, the ability of mGluR activation to control the phosphorylation and activity of β-catenin has been shown to be dependent upon Akt1, since gene silencing of Akt1 expression leads to phosphorylation of β-catenin during oxidative stress.181
Glycogen synthase kinase-3β (GSK-3β) also plays a role in these pathways, since Wnt binds to its receptors and the co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP-5/6) to inhibit GSK-3β. Pathways that block GSK-3β activity such as through Wnt appear to be critical for neuronal protection. For example, the neuroprotective attributes of Wnt1 against β-amyloid toxicity are lost during gene silencing of Wnt1 protein expression. More importantly, Wnt 1 protection is dependent upon Akt activity and the inhibition of GSK-3β with the cellular translocation β-catenin to the nucleus 240. Modulation of GSK-3β activity also can regulate progenitor cell proliferation and differentiation,243,244 promote midbrain differentiation,245 control cardiac hypertrophy246,247 and increase cell survival during oxidative stress, such as during neurofibrillary pathology248 and cardiac injury.249 GSK-3β activity also can influence inflammatory cell survival and activation.25,240,250 As a result, GSK-3β is considered to be an important treatment strategy for several degenerative disorders.12,18,251,252 Recently, additional work has shown that mGluR activation blocks GSK-3β activity to promote translocation of β-catenin to the nucleus through an Akt dependent mechanism (Fig. 1).253
Other Wnt pathways also pertain to the control of intracellular calcium (Ca2+) release. These involve the non-canonical or Wnt/Ca2+ pathway consisting primarily of Wnt4, Wnt5a and Wnt11 that functions through non-β-catenin-dependent pathways and also include the planar cell polarity (PCP) pathway254 and the Wnt-Ca2+-dependent pathways.254,255 In the Wnt-Ca2+-dependent pathways, calcium dependent kinases are activated through G-protein signaling that leads to elevations in intracellular Ca2+ either through cGMP or phospholipase activation.255–257
Interestingly during the development of the nervous system, G-protein signaling with the mGluR system is required for the modulation of intracellular calcium homeostasis. Immature and developing neurons require higher intracellular calcium concentrations than their mature counterparts to facilitate neuronal survival, synapse formation, dendrite growth, and other cellular functions.258 As a result, the modulation of intracellular calcium by the mGluR system has proven to be necessary for neuronal development, such in the cochlear nucleus magnocellular neurons259 and in maturing hippocampal neurons.39 Furthermore, group I mGluR1 facilitates L-type voltage-dependent calcium channels currents through PKC 260 and group II mGluRs can control calcium flux in the suprachiasmatic nucleus that may oversee circadian function.261 In astrocytes, both group I and group II mGluRs have been associated with the generation of calcium oscillations.262
Modulation of intracellular calcium by the mGluR system also may be vital for cytoprotection (Fig. 1). For example, preservation of cellular energy reserves during oxidative stress is dependent upon the maintenance of mitochondrial integrity.263 Group I mGluRs can preserve mitochondrial membrane potential in endothelial cells10,26 and neurons181,264 to prevent cell injury during oxidative stress. The precise mechanisms by which the mGluRs employ to preserve mitochondrial integrity are not clear, but may involve the modulation of intracellular calcium stores and intracellular pH. Reduction in mitochondrial intracellular calcium stores and free radical generation has been suggested to promote the maintenance of mitochondrial membrane potential and integrity.265 Activation of group I mGluRs can regulate the release of intracellular calcium from both Ins (1,4,5) P3-sensitive and ryanodine-sensitive calcium stores.39 In addition, group I mGluRs can modulate free radical signal transduction cascades in both neuronal and endothelial cell populations.10,22,266 Furthermore, intracellular acid-base levels may be involved in these protective mechanisms since calcium as well as pH control cellular endonuclease activity. For example, activation of mGluRs prevents cellular injury through the modulation of endonuclease activity that is linked to changes in intracellular pH.44
Considerations for the Future
As members of the broad G-protein receptor family, mGluRs play a diverse role in multiple processes that include cell development, cell signaling, and cell survival. Effective treatment for a number of disease entities such as amyotrophic lateral sclerosis, psychiatric disease, Parkinson's disease, and Alzheimer's disease may ultimately rely upon mGluR modulation and targeting novel signal transduction pathways of metabotropic receptors during oxidative stress. In particular, pathways tied to Akt, forkhead transcription factors, β-catenin, and intracellular calcium release may prove to be vital for the development of new therapeutic strategies.
Yet, the role of the mGluR activation during cell injury is not always clear and can differ among cell systems. In models of demyelinating disease, only activation of specific mGluRs has the capacity to protect oligodendrocyte progenitor cells.267 mGluRs also can have adverse consequences or ineffective results.268 In rat cerebellar granule cells and mouse cortical neurons, trophic deprivation can result in increased mGluR1 expression that is correlated with eventual cell death.269 In other scenarios, activation of the mGluR system may potentiate activity of the capsaicin receptor and contribute to hyperalgesia.270 However, downregulation of the mGluR system has been suggested to lead to the generation of post-injury pain following spinal cord injury during both pharmacological271 and in knockdown studies.272
Some studies also suggest that antagonism of mGluRs may be beneficial. For example, inhibition of mGluR activity during the progression of a toxic insult in some experimental models may subsequently improve neuronal survival.273,274 Acute versus chronic application of mGluR antagonists also may generate different therapeutic efficacy. In a rat model of PD, it is chronic rather than acute treatment with a mGluR5 antagonist that can reverse akinetic deficits.275 Furthermore, changes in the cellular environment, such as decreased intracellular calcium release, may allow antagonism of the mGluR system to exert cytoprotection.39 In addition, the nature and extent of the cellular injury may determine whether activation or inhibition of the mGluR system is ultimately required for cellular protection. Since the role mGluRs play in the body is not straightforward, future investigations are necessary to further define the unique signal transduction pathways controlled by the metabotropic system for the effective translation of mGluRs into new therapeutic avenues.
Acknowledgments
We apologize to our colleagues whose work we were unable to cite as a result of article space limitations. This research was supported by the following grants (K.M.): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: www.landesbioscience.com/journals/oximed/article/6842
References
- 1.Pearl R. The rate of living. London: University of London Press; 1928. [Google Scholar]
- 2.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Yui R, Matsuura ET. Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat Res. 2006;594:155–161. doi: 10.1016/j.mrfmmm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regulska M, Leskiewicz M, Budziszewska B, Kutner A, Jantas D, Basta-Kaim A, Kubera M, Jaworska-Feil L, Lason W. Inhibitory effects of 1,25-dihydroxyvitamin D(3) and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol Rep. 2007;59:393–401. [PubMed] [Google Scholar]
- 8.Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 10.Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SM, Cordeiro-Stone M, Kaufman DG. Early replication and the apoptotic pathway. J Cell Physiol. 2007;213:434–439. doi: 10.1002/jcp.21156. [DOI] [PubMed] [Google Scholar]
- 12.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 16.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 17.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 20.Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: protection by alpha-lipoic acid. Faseb J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 21.Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62:257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- 24.Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- 27.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 28.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 29.Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16:633–644. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- 33.Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15:440–449. doi: 10.1038/jcbfm.1995.55. [DOI] [PubMed] [Google Scholar]
- 34.Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999;47:661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- 35.Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003;23:561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem. 1997;68:710–714. doi: 10.1046/j.1471-4159.1997.68020710.x. [DOI] [PubMed] [Google Scholar]
- 37.Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 38.Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006;4:2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- 39.Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 42.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 43.Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999;246:290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- 44.Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp Neurol. 1999;155:79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- 45.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 46.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 47.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- 50.Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- 51.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts E, Jr, Chih CP. The influence of age of pH regulation in hippocampal slices before, during, and after anoxia. J Cereb Blood Flow Metab. 1997;17:560–566. doi: 10.1097/00004647-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Cardella F. Insulin therapy during diabetic ketoacidosis in children. Acta Biomed. 2005;76(Suppl 3):49–54. [PubMed] [Google Scholar]
- 54.Kratzsch J, Knerr I, Galler A, Kapellen T, Raile K, Korner A, Thiery J, Dotsch J, Kiess W. Metabolic decompensation in children with type 1 diabetes mellitus associated with increased serum levels of the soluble leptin receptor. Eur J Endocrinol. 2006;155:609–614. doi: 10.1530/eje.1.02261. [DOI] [PubMed] [Google Scholar]
- 55.Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95:2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- 56.Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J Neurobiol. 1999;40:171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Sladeczek F, Pin J-P, Recasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature (Lond) 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- 58.Yuzaki M, Mikoshiba K. Pharmacological and immunocytochemical characterization of metabotropic glutamate receptors in cultured Purkinje cells. J Neurosci. 1992;12:4253–4263. doi: 10.1523/JNEUROSCI.12-11-04253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prezeau L, Manzoni O, Homburger V, Sladeczek F, Curry K, Bockaert J. Characterization of a metabotropic glutamate receptor: direct negative coupling to adenylyl cyclase and involvement of a pertussis toxin-sensitive G protein. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8040–8044. doi: 10.1073/pnas.89.17.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O'Hara PJ, Mulvihill ER, Almers W, Hagen FS. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 61.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 62.Albert PR, Robillard L. G protein specificity. Traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 63.Hur EM, Kim KT. G protein-coupled receptor signalling and cross-talk. Achieving rapidity and specificity. Cell Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 64.Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;273:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- 65.Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C. Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- 66.Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–3773. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choe ES, Wang JQ. Group I metabotropic glutamate receptor activation increases phosphorylation of cAMP response element-binding protein, Elk-1, and extracellular signal-regulated kinases in rat dorsal striatum. Brain Res Mol Brain Res. 2001;94:75–84. doi: 10.1016/s0169-328x(01)00217-0. [DOI] [PubMed] [Google Scholar]
- 68.Lin SH, Maiese K. Group I metabotropic glutamate receptors prevent endothelial programmed cell death independent from MAP kinase p38 activation in rat. Neurosci Lett. 2001;298:207–211. doi: 10.1016/s0304-3940(00)01766-3. [DOI] [PubMed] [Google Scholar]
- 69.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 70.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 71.Losonczy A, Somogyi P, Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol. 2003;89:1910–1919. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- 72.White AM, Kylanpaa RA, Christie LA, McIntosh SJ, Irving AJ, Platt B. Presynaptic group I metabotropic glutamate receptors modulate synaptic transmission in the rat superior colliculus via 4-AP sensitive K+ channels. Br J Pharmacol. 2003 doi: 10.1038/sj.bjp.0705570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watabe AM, Carlisle HJ, O'Dell TJ. Postsynaptic Induction and Presynaptic Expression of Group 1 mGluR- Dependent LTD in the Hippocampal CA1 Region. J Neurophysiol. 2002;87:1395–1403. doi: 10.1152/jn.00723.2001. [DOI] [PubMed] [Google Scholar]
- 75.Mao L, Wang JQ. Group I metabotropic glutamate receptor-mediated calcium signalling and immediate early gene expression in cultured rat striatal neurons. Eur J Neurosci. 2003;17:741–750. doi: 10.1046/j.1460-9568.2003.02495.x. [DOI] [PubMed] [Google Scholar]
- 76.Salt TE, Turner JP. Modulation of sensory inhibition in the ventrobasal thalamus via activation of group II metabotropic glutamate receptors by 2R,4R- aminopyrrolidine-2,4-dicarboxylate. Exp Brain Res. 1998;121:181–185. doi: 10.1007/s002210050450. [DOI] [PubMed] [Google Scholar]
- 77.Nicholas AH, Hyson RL. Group I and II metabotropic glutamate receptors are necessary for the activity-dependent regulation of ribosomes in chick auditory neurons. Brain Res. 2004;1014:110–119. doi: 10.1016/j.brainres.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 78.Ene FA, Kullmann PH, Gillespie DC, Kandler K. Glutamatergic calcium responses in the developing lateral superior olive: receptor types and their specific activation by synaptic activity patterns. J Neurophysiol. 2003;90:2581–2591. doi: 10.1152/jn.00238.2003. [DOI] [PubMed] [Google Scholar]
- 79.Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of Group III mGluRs Inhibits GABAergic and Glutamatergic Transmission in the Substantia Nigra Pars Reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- 80.Marabese I, de Novellis V, Palazzo E, Mariani L, Siniscalco D, Rodella L, Rossi F, Maione S. Differential roles of mGlu8 receptors in the regulation of glutamate and gamma-aminobutyric acid release at periaqueductal grey level. Neuropharmacology. 2005;49(Suppl):157–166. doi: 10.1016/j.neuropharm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Galik J, Youn DH, Kolaj M, Randic M. Involvement of group I metabotropic glutamate receptors and glutamate transporters in the slow excitatory synaptic transmission in the spinal cord dorsal horn. Neuroscience. 2008;154:1372–1387. doi: 10.1016/j.neuroscience.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 82.Dietrich D, Beck H, Kral T, Clusmann H, Elger CE, Schramm J. Metabotropic glutamate receptors modulate synaptic transmission in the perforant path: pharmacology and localization of two distinct receptors. Brain Res. 1997;767:220–227. doi: 10.1016/s0006-8993(97)00579-9. [DOI] [PubMed] [Google Scholar]
- 83.Chen J, Heinke B, Sandkuhler J. Activation of group I metabotropic glutamate receptors induces long- term depression at sensory synapses in superficial spinal dorsal horn. Neuropharmacology. 2000;39:2231–2243. doi: 10.1016/s0028-3908(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 84.Holscher C, Gigg J, O'Mara SM. Metabotropic glutamate receptor activation and blockade: their role in long-term potentiation, learning and neurotoxicity. Neurosci Biobehav Rev. 1999;23:399–410. doi: 10.1016/s0149-7634(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 85.Wisniewski K, Car H. (S)-3,5-DHPG: a review. CNS Drug Rev. 2002;8:101–116. doi: 10.1111/j.1527-3458.2002.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hay M, Hoang CJ, Hasser EM, Price EM. Activation of metabotropic glutamate receptors inhibits synapsin I phosphorylation in visceral sensory neurons. J Membr Biol. 2000;178:195–204. doi: 10.1007/s002320010027. [DOI] [PubMed] [Google Scholar]
- 87.Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Bendito G, Shigemoto R, Fairen A, Lujan R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- 89.Berthele A, Platzer S, Laurie DJ, Weis S, Sommer B, Zieglgansberger W, Conrad B, Tolle TR. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport. 1999;10:3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
- 90.Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience. 2001;105:509–520. doi: 10.1016/s0306-4522(01)00181-6. [DOI] [PubMed] [Google Scholar]
- 91.Blumcke I, Behle K, Malitschek B, Kuhn R, Knopfel T, Wolf HK, Wiestler OD. Immunohistochemical distribution of metabotropic glutamate receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 in human hippocampus. Brain Res. 1996;736:217–226. doi: 10.1016/0006-8993(96)00697-x. [DOI] [PubMed] [Google Scholar]
- 92.Makoff A, Lelchuk R, Oxer M, Harrington K, Emson P. Molecular characterization and localization of human metabotropic glutamate receptor type 4. Brain Res Mol Brain Res. 1996;37:239–248. doi: 10.1016/0169-328x(95)00321-i. [DOI] [PubMed] [Google Scholar]
- 93.Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 94.Makoff A, Volpe F, Lelchuk R, Harrington K, Emson P. Molecular characterization and localization of human metabotropic glutamate receptor type 3. Brain Res Mol Brain Res. 1996;40:55–63. doi: 10.1016/0169-328x(96)00037-x. [DOI] [PubMed] [Google Scholar]
- 95.Nakamichi N, Yoshida K, Ishioka Y, Makanga JO, Fukui M, Yoneyama M, Kitayama T, Nakamura N, Taniura H, Yoneda Y. Group III metabotropic glutamate receptor activation suppresses self-replication of undifferentiated neocortical progenitor cells. J Neurochem. 2008;105:1996–2012. doi: 10.1111/j.1471-4159.2008.05289.x. [DOI] [PubMed] [Google Scholar]
- 96.Gandhi R, Luk KC, Rymar VV, Sadikot AF. Group I mGluR5 metabotropic glutamate receptors regulate proliferation of neuronal progenitors in specific forebrain developmental domains. J Neurochem. 2008;104:155–172. doi: 10.1111/j.1471-4159.2007.04955.x. [DOI] [PubMed] [Google Scholar]
- 97.Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 98.Liu XB, Munoz A, Jones EG. Changes in subcellular localization of metabotropic glutamate receptor subtypes during postnatal development of mouse thalamus. J Comp Neurol. 1998;395:450–465. doi: 10.1002/(sici)1096-9861(19980615)395:4<450::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 99.Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 100.Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 101.Geurts JJ, Wolswijk G, Bo L, Redeker S, Ramkema M, Troost D, Aronica E. Expression patterns of Group III metabotropic glutamate receptors mGluR4 and mGluR8 in multiple sclerosis lesions. J Neuroimmunol. 2005;158:182–190. doi: 10.1016/j.jneuroim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Yao HH, Ding JH, Zhou F, Wang F, Hu LF, Sun T, Hu G. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J Neurochem. 2005;92:948–961. doi: 10.1111/j.1471-4159.2004.02937.x. [DOI] [PubMed] [Google Scholar]
- 103.Reid SN, Daw NW, Gregory DS, Flavin H. cAMP levels increased by activation of metabotropic glutamate receptors correlate with visual plasticity. J Neurosci. 1996;16:7619–7626. doi: 10.1523/JNEUROSCI.16-23-07619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krizbai IA, Deli MA, Pestenacz A, Siklos L, Szabo CA, Andras I, Joo F. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res. 1998;54:814–819. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 105.Gill SS, Pulido OM, Mueller RW, McGuire PF. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull. 1999;48:143–146. doi: 10.1016/s0361-9230(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 106.Maiese K, Chong ZZ, Kang J. Transformation into treatment: Novel therapeutics that begin within the cell. In: Maiese K, editor. Neuronal and Vascular Plasticity: Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. Norwell, MA: Kluwer Academic Publishers; 2003. pp. 1–26. [Google Scholar]
- 107.Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461:317–332. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- 108.Hu D, Cao K, Peterson-Wakeman R, Wang R. Altered profile of gene expression in rat hearts induced by chronic nicotine consumption. Biochem Biophys Res Commun. 2002;297:729–736. doi: 10.1016/s0006-291x(02)02280-5. [DOI] [PubMed] [Google Scholar]
- 109.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 113.Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. 2001;249:225–235. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 114.Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 115.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 117.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008;6:281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 119.Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45:471–477. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- 120.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 121.Ling PR, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metabolism. 2003;52:868–874. doi: 10.1016/s0026-0495(03)00057-x. [DOI] [PubMed] [Google Scholar]
- 122.Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9:111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- 123.Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 124.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 125.Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 126.Tong Q, Ouedraogo R, Kirchgessner AL. Localization and function of group III metabotropic glutamate receptors in rat pancreatic islets. Am J Physiol Endocrinol Metab. 2002;282:E1324–E1333. doi: 10.1152/ajpendo.00460.2001. [DOI] [PubMed] [Google Scholar]
- 127.Berent-Spillson A, Russell JW. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J Neurochem. 2007;101:342–354. doi: 10.1111/j.1471-4159.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- 128.Quiroz JA, Singh J, Gould TD, Denicoff KD, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: clues from the molecular pathophysiology. Mol Psychiatry. 2004;9:756–776. doi: 10.1038/sj.mp.4001521. [DOI] [PubMed] [Google Scholar]
- 129.Palucha-Poniewiera A, Klodzinska A, Stachowicz K, Tokarski K, Hess G, Schann S, Frauli M, Neuville P, Pilc A. Peripheral administration of group III mGlu receptor agonist ACPT-I exerts potential antipsychotic effects in rodents. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 130.Oka A, Takashima S. The up-regulation of metabotropic glutamate receptor 5 (mGluR5) in Down's syndrome brains. Acta Neuropathol (Berl) 1999;97:275–278. doi: 10.1007/s004010050985. [DOI] [PubMed] [Google Scholar]
- 131.Wang H, Wu LJ, Zhang F, Zhuo M. Roles of calcium-stimulated adenylyl cyclase and calmodulin-dependent protein kinase IV in the regulation of FMRP by group I metabotropic glutamate receptors. J Neurosci. 2008;28:4385–4397. doi: 10.1523/JNEUROSCI.0646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valerio A, Ferrario M, Paterlini M, Liberini P, Moretto G, Cairns NJ, Pizzi M, Spano P. Spinal cord mGlu1a receptors: possible target for amyotrophic lateral sclerosis therapy. Pharmacol Biochem Behav. 2002;73:447–454. doi: 10.1016/s0091-3057(02)00835-3. [DOI] [PubMed] [Google Scholar]
- 133.Anneser JM, Chahli C, Borasio GD. Protective effect of metabotropic glutamate receptor inhibition on amyotrophic lateral sclerosis-cerebrospinal fluid toxicity in vitro. Neuroscience. 2006;141:1879–1886. doi: 10.1016/j.neuroscience.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 134.Wong RK, Bianchi R, Chuang SC, Merlin LR. Group I mGluR-induced Epileptogenesis: Distinct and Overlapping Roles of mGluR1 and mGluR5 and Implications for Antiepileptic Drug Design. Epilepsy Curr. 2005;5:63–68. doi: 10.1111/j.1535-7597.2005.05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang FR, Lee WL. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol. 2001;30:137–143. doi: 10.1023/a:1011939223872. [DOI] [PubMed] [Google Scholar]
- 136.Tang FR, Lee WL, Yang J, Sim MK, Ling EA. Metabotropic glutamate receptor 8 in the rat hippocampus after pilocarpine induced status epilepticus. Neurosci Lett. 2001;300:137–140. doi: 10.1016/s0304-3940(01)01579-8. [DOI] [PubMed] [Google Scholar]
- 137.Folbergrova J, Druga R, Otahal J, Haugvicova R, Mares P, Kubova H. Seizures induced in immature rats by homocysteic acid and the associated brain damage are prevented by group II metabotropic glutamate receptor agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate. Exp Neurol. 2005;192:420–436. doi: 10.1016/j.expneurol.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 138.Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat Neurosci. 2008;11:940–948. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dalfo E, Albasanz JL, Rodriguez A, Martin M, Ferrer I. Abnormal group I metabotropic glutamate receptor expression and signaling in the frontal cortex in Pick disease. J Neuropathol Exp Neurol. 2005;64:638–647. doi: 10.1097/01.jnen.0000171649.86718.f2. [DOI] [PubMed] [Google Scholar]
- 140.Benn CL, Slow EJ, Farrell LA, Graham R, Deng Y, Hayden MR, Cha JH. Glutamate receptor abnormalities in the YAC128 transgenic mouse model of Huntington's disease. Neuroscience. 2007;147:354–372. doi: 10.1016/j.neuroscience.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anborgh PH, Godin C, Pampillo M, Dhami GK, Dale LB, Cregan SP, Truant R, Ferguson SS. Inhibition of metabotropic glutamate receptor signalling by the huntingtin binding protein optineurin. J Biol Chem. 2005 doi: 10.1074/jbc.M504508200. [DOI] [PubMed] [Google Scholar]
- 142.Mouzaki A, Tselios T, Papathanassopoulos P, Matsoukas I, Chatzantoni K. Immunotherapy for Multiple Sclerosis: Basic insights for new clinical strategies. Curr Neurovasc Res. 2004;1:325–340. doi: 10.2174/1567202043362180. [DOI] [PubMed] [Google Scholar]
- 143.Mills CD, Fullwood SD, Hulsebosch CE. Changes in metabotropic glutamate receptor expression following spinal cord injury. Exp Neurol. 2001;170:244–257. doi: 10.1006/exnr.2001.7721. [DOI] [PubMed] [Google Scholar]
- 144.Anneser JM, Berthele A, Borasio GD, Castro-Lopes JM, Zieglgansberger W, Tolle TR. Axotomy of the sciatic nerve differentially affects expression of metabotropic glutamate receptor mRNA in adult rat motoneurons. Brain Res. 2000;868:215–221. doi: 10.1016/s0006-8993(00)02332-5. [DOI] [PubMed] [Google Scholar]
- 145.Sanchez-Pernaute R, Wang JQ, Kuruppu D, Cao L, Tueckmantel W, Kozikowski A, Isacson O, Brownell AL. Enhanced binding of metabotropic glutamate receptor type 5 (mGluR5) PET tracers in the brain of parkinsonian primates. Neuroimage. 2008;42:248–251. doi: 10.1016/j.neuroimage.2008.04.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shen KZ, Johnson SW. A slow excitatory postsynaptic current mediated by G-protein-coupled metabotropic glutamate receptors in rat ventral tegmental dopamine neurons. Eur J Neurosci. 1997;9:48–54. doi: 10.1111/j.1460-9568.1997.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 147.Campusano JM, Abarca J, Forray MI, Gysling K, Bustos G. Modulation of dendritic release of dopamine by metabotropic glutamate receptors in rat substantia nigra. Biochem Pharmacol. 2002;63:1343–1352. doi: 10.1016/s0006-2952(02)00870-5. [DOI] [PubMed] [Google Scholar]
- 148.Shimazoe T, Doi Y, Arai I, Yoshimatsu A, Fukumoto T, Watanabe S. Both metabotropic glutamate I and II receptors mediate augmentation of dopamine release from the striatum in methamphetamine-sensitized rats. Jpn J Pharmacol. 2002;89:85–88. doi: 10.1254/jjp.89.85. [DOI] [PubMed] [Google Scholar]
- 149.Saransaari P, Oja SS. Metabotropic glutamate receptors modulate GABA release from mouse hippocampal slices. Neurochem Res. 2001;26:175–180. doi: 10.1023/a:1011055014357. [DOI] [PubMed] [Google Scholar]
- 150.Agari T, Yasuhara T, Matsui T, Kuramoto S, Kondo A, Miyoshi Y, Shingo T, Borlongan CV, Date I. Intrapallidal metabotropic glutamate receptor activation in a rat model of Parkinson's disease: behavioral and histological analyses. Brain Res. 2008;1203:189–196. doi: 10.1016/j.brainres.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 151.Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J Neurosci. 2000;20:3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wolfarth S, Konieczny J, Lorenc-Koci E, Ossowska K, Pilc A. The role of metabotropic glutamate receptor (mGluR) ligands in parkinsonian muscle rigidity. Amino Acids. 2000;19:95–101. doi: 10.1007/s007260070038. [DOI] [PubMed] [Google Scholar]
- 154.Dawson L, Chadha A, Megalou M, Duty S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br J Pharmacol. 2000;129:541–546. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matarredona ER, Santiago M, Venero JL, Cano J, Machado A. Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J Neurochem. 2001;76:351–360. doi: 10.1046/j.1471-4159.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- 156.Yang YL, Meng CH, Ding JH, He HR, Ellsworth K, Wu J, Hu G. Iptakalim hydrochloride protects cells against neurotoxin-induced glutamate transporter dysfunction in in vitro and in vivo models. Brain Res. 2005;1049:80–88. doi: 10.1016/j.brainres.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 157.Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- 158.Dewar D, Chalmers DT, Graham DI, McCulloch J. Glutamate metabotropic and AMPA binding sites are reduced in Alzheimer's disease: an autoradiographic study of the hippocampus. Brain Res. 1991;553:58–64. doi: 10.1016/0006-8993(91)90230-s. [DOI] [PubMed] [Google Scholar]
- 159.Albasanz JL, Dalfo E, Ferrer I, Martin M. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer's disease and dementia with Lewy bodies correlates with stage of Alzheimer's-disease-related changes. Neurobiol Dis. 2005;20:685–693. doi: 10.1016/j.nbd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 160.Chin JH, Ma L, MacTavish D, Jhamandas JH. Amyloid beta protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. J Neurosci. 2007;27:9262–9269. doi: 10.1523/JNEUROSCI.1843-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee RK, Wurtman RJ, Cox AJ, Nitsch RM. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 1995;92:8083–8087. doi: 10.1073/pnas.92.17.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Croucher MJ, Patel H, Walsh DT, Moncaster JA, Gentleman SM, Fazal A, Jen LS. Up-regulation of soluble amyloid precursor protein fragment secretion in the rat retina in vivo by metabotropic glutamate receptor stimulation. Neuroreport. 2003;14:2271–2274. doi: 10.1097/00001756-200312020-00027. [DOI] [PubMed] [Google Scholar]
- 164.Ulus IH, Wurtman RJ. Metabotropic glutamate receptor agonists increase release of soluble amyloid precursor protein derivatives from rat brain cortical and hippocampal slices. J Pharmacol Exp Ther. 1997;281:149–154. [PubMed] [Google Scholar]
- 165.Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- 167.Maiese K, Swiriduk M, TenBroeke M. Cellular mechanisms of protection by metabotropic glutamate receptors during anoxia and nitric oxide toxicity. J Neurochem. 1996;66:2419–2428. doi: 10.1046/j.1471-4159.1996.66062419.x. [DOI] [PubMed] [Google Scholar]
- 168.Maiese K, Boniece IR, Skurat K, Wagner JA. Protein kinases modulate the sensitivity of hippocampal neurons to nitric oxide toxicity and anoxia. J Neurosci Res. 1993;36:77–87. doi: 10.1002/jnr.490360109. [DOI] [PubMed] [Google Scholar]
- 169.Maiese K. Protein kinase C modulates the protective ability of peptide growth factors during anoxia. J Auton Nerv Syst. 1994;49:S187–S193. doi: 10.1016/0165-1838(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 170.Yoshida K. Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends Mol Med. 2008;14:305–313. doi: 10.1016/j.molmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 171.Lin WW, Wang CW, Chuang DM. Effects of depolarization and NMDA antagonists on the role survival of cerebellar granule cells: a pivotal role for protein kinase C isoforms. J Neurochem. 1997;68:2577–2586. doi: 10.1046/j.1471-4159.1997.68062577.x. [DOI] [PubMed] [Google Scholar]
- 172.Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 175.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- 176.Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 177.Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 178.Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol (Berl) 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- 179.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rytomaa M, Lehmann K, Downward J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- 183.Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 185.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol. 2007;292:H2573–H2581. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]
- 187.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Martin D, Salinas M, Lopez-Valdaliso R, Serrano E, Recuero M, Cuadrado A. Effect of the Alzheimer amyloid fragment Abeta(25-35) on Akt/PKB kinase and survival of PC12 cells. J Neurochem. 2001;78:1000–1008. doi: 10.1046/j.1471-4159.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 189.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 190.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 194.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 196.Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, Kirsch T, de Groot K, Laudeley R, Niemczyk E, Guler F, Menne J, Haller H, Fliser D. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- 197.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- 199.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 201.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 202.Um M, Lodish HF. Antiapoptotic Effects of Erythropoietin in Differentiated Neuroblastoma SH-SY5Y Cells Require Activation of Both the STAT5 and AKT Signaling Pathways. J Biol Chem. 2006;281:5648–5656. doi: 10.1074/jbc.M510943200. [DOI] [PubMed] [Google Scholar]
- 203.Wu Y, Shang Y, Sun S, Liu R. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur J Pharmacol. 2007;564:47–56. doi: 10.1016/j.ejphar.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 204.Liu F, Gong X, Zhang G, Marquis K, Reinhart P, Andree TH. The inhibition of glycogen synthase kinase 3beta by a metabotropic glutamate receptor 5 mediated pathway confers neuroprotection to Abeta peptides. J Neurochem. 2005;95:1363–1372. doi: 10.1111/j.1471-4159.2005.03474.x. [DOI] [PubMed] [Google Scholar]
- 205.Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- 206.Maiese K, Vincent AM. Group I metabotropic receptors down-regulate nitric oxide induced caspase-3 activity in rat hippocampal neurons. Neurosci Lett. 1999;264:17–20. doi: 10.1016/s0304-3940(99)00199-8. [DOI] [PubMed] [Google Scholar]
- 207.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 210.Maiese K, Boniece I, DeMeo D, Wagner JA. Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J Neurosci. 1993;13:3034–3040. doi: 10.1523/JNEUROSCI.13-07-03034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vianna MR, Coitinho AS, Izquierdo I. Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr Neurovasc Res. 2004;1:55–60. doi: 10.2174/1567202043480170. [DOI] [PubMed] [Google Scholar]
- 212.Szapiro G, Izquierdo LA, Alonso M, Barros D, Paratcha G, Ardenghi P, Pereira P, Medina JH, Izquierdo I. Participation of hippocampal metabotropic glutamate receptors, protein kinase A and mitogen-activated protein kinases in memory retrieval. Neuroscience. 2000;99:1–5. doi: 10.1016/s0306-4522(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 213.Huang LQ, Rowan MJ, Anwyl R. Role of protein kinases A and C in the induction of mGluR-dependent long-term depression in the medial perforant path of the rat dentate gyrus in vitro. Neurosci Lett. 1999;274:71–74. doi: 10.1016/s0304-3940(99)00561-3. [DOI] [PubMed] [Google Scholar]
- 214.Sugiyama Y, Kawaguchi SY, Hirano T. mGluR1-mediated facilitation of long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. Eur J Neurosci. 2008;27:884–896. doi: 10.1111/j.1460-9568.2008.06063.x. [DOI] [PubMed] [Google Scholar]
- 215.Orlov SN, Thorin-Trescases N, Dulin NO, Dam TV, Fortuno MA, Tremblay J, Hamet P. Activation of cAMP signaling transiently inhibits apoptosis in vascular smooth muscle cells in a site upstream of caspase-3. Cell Death Differ. 1999;6:661–672. doi: 10.1038/sj.cdd.4400539. [DOI] [PubMed] [Google Scholar]
- 216.Rossi AG, Cousin JM, Dransfield I, Lawson MF, Chilvers ER, Haslett C. Agents that elevate cAMP inhibit human neutrophil apoptosis. Biochem Biophys Res Commun. 1995;217:892–899. doi: 10.1006/bbrc.1995.2855. [DOI] [PubMed] [Google Scholar]
- 217.Findik D, Song Q, Hidaka H, Lavin M. Protein kinase A inhibitors enhance radiation-induced apoptosis. J Cell Biochem. 1995;57:12–21. doi: 10.1002/jcb.240570103. [DOI] [PubMed] [Google Scholar]
- 218.Nishio E, Watanabe Y. Nitric oxide donor-induced apoptosis in smooth muscle cells is modulated by protein kinase C and protein kinase A. Eur J Pharmacol. 1997;339:245–251. doi: 10.1016/s0014-2999(97)01368-x. [DOI] [PubMed] [Google Scholar]
- 219.Parvathenani LK, Buescher ES, Chacon-Cruz E, Beebe SJ. Type I cAMP-dependent protein kinase delays apoptosis in human neutrophils at a site upstream of caspase-3. J Biol Chem. 1998;273:6736–6743. doi: 10.1074/jbc.273.12.6736. [DOI] [PubMed] [Google Scholar]
- 220.Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 2000;349:547–557. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 221.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 224.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 226.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 227.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 229.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 230.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281(1–2):47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 231.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci U S A. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 234.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 236.Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, Deckert M. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- 237.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 238.Zhao L, Qian ZM, Zhang C, Wing HY, Du F, Ya K. Amyloid beta-peptide 31-35-induced neuronal apoptosis is mediated by caspase-dependent pathways via cAMP-dependent protein kinase A activation. Aging Cell. 2008;7:47–57. doi: 10.1111/j.1474-9726.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 239.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, Finkel T, Kusiak JW. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with {beta}-Catenin Inhibits {beta}-Catenin/T Cell Factor Activity. J Biol Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 243.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. beta-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 244.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 245.Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci. 2004;117:5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- 246.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 248.Wagner U, Brownlees J, Irving NG, Lucas FR, Salinas PC, Miller CC. Overexpression of the mouse dishevelled-1 protein inhibits GSK-3beta-mediated phosphorylation of tau in transfected mammalian cells. FEBS Lett. 1997;411:369–372. doi: 10.1016/s0014-5793(97)00733-3. [DOI] [PubMed] [Google Scholar]
- 249.van de Schans VA, van den Borne SW, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, Wynshaw-Boris A, Smits JF, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 250.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007;12:1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- 253.Ortega F, Perez-Sen R, Miras-Portugal MT. Gi-coupled P2Y-ADP receptor mediates GSK-3 phosphorylation and beta-catenin nuclear translocation in granule neurons. J Neurochem. 2008;104:62–73. doi: 10.1111/j.1471-4159.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- 254.Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 256.Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281:30990–41001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- 257.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 258.Spitzer NC, Olson E, Gu X. Spontaneous calcium transients regulate neuronal plasticity in developing neurons. J Neurobiol. 1995;26:316–324. doi: 10.1002/neu.480260304. [DOI] [PubMed] [Google Scholar]
- 259.Zirpel L, Janowiak MA, Taylor DA, Parks TN. Developmental changes in metabotropic glutamate receptor-mediated calcium homeostasis. J Comp Neurol. 2000;421:95–106. [PubMed] [Google Scholar]
- 260.Endoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 2004;1024:212–224. doi: 10.1016/j.brainres.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 261.Haak LL. Metabotropic glutamate receptor modulation of glutamate responses in the suprachiasmatic nucleus. J Neurophysiol. 1999;81:1308–1317. doi: 10.1152/jn.1999.81.3.1308. [DOI] [PubMed] [Google Scholar]
- 262.Zur Nieden R, Deitmer JW. The Role of Metabotropic Glutamate Receptors for the Generation of Calcium Oscillations in Rat Hippocampal Astrocytes In Situ. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj013. [DOI] [PubMed] [Google Scholar]
- 263.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lin SH, Chong ZZ, Maiese K. The metabotropic glutamate receptor system: G-Protein mediated pathways that modulate neuronal and vascular cellular injury. Curr Med Chem-Central Nervous System Agents. 2002;2:17–28. [Google Scholar]
- 265.Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 266.Vincent AM, Maiese K. The metabotropic glutamate system promotes neuronal survival through distinct pathways of programmed cell death. Exp Neurol. 2000;166:65–82. doi: 10.1006/exnr.2000.7487. [DOI] [PubMed] [Google Scholar]
- 267.Luyt K, Varadi A, Durant CF, Molnar E. Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J Neurochem. 2006;99:641–656. doi: 10.1111/j.1471-4159.2006.04103.x. [DOI] [PubMed] [Google Scholar]
- 268.Kermer P, Klocker N, Bahr M. Modulation of metabotropic glutamate receptors fails to prevent the loss of adult rat retinal ganglion cells following axotomy or N-methyl-D-aspartate lesion in vivo. Neurosci Lett. 2001;315:117–120. doi: 10.1016/s0304-3940(01)02318-7. [DOI] [PubMed] [Google Scholar]
- 269.Pshenichkin S, Dolinska M, Klauzinska M, Luchenko V, Grajkowska E, Wroblewski JT. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivation - Possible role as a dependence receptor. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Abraham KE, McGinty JF, Brewer KL. The role of kainic acid/AMPA and metabotropic glutamate receptors in the regulation of opioid mRNA expression and the onset of pain-related behavior following excitotoxic spinal cord injury. Neuroscience. 2001;104:863–874. doi: 10.1016/s0306-4522(01)00134-8. [DOI] [PubMed] [Google Scholar]
- 272.Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pellegrini-Giampietro DE, Peruginelli F, Meli E, Cozzi A, Albani-Torregrossa S, Pellicciari R, Moroni F. Protection with metabotropic glutamate 1 receptor antagonists in models of ischemic neuronal death: time-course and mechanisms. Neuropharmacology. 1999;38:1607–1619. doi: 10.1016/s0028-3908(99)00097-0. [DOI] [PubMed] [Google Scholar]
- 274.Shuaib A, Kanthan R. Amplification of inhibitory mechanisms in cerebral ischemia: an alternative approach to neuronal protection. Histol Histopathol. 1997;12:185–194. [PubMed] [Google Scholar]
- 275.Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–5678. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]