Abstract
In this review, we consider the role played by eosinophilic leukocytes in the pathogenesis and pathophysiology of respiratory virus infection. The vast majority of the available information on this topic focuses on respiratory syncytial virus (RSV; Family Paramyxoviridae, genus Pneumovirus), an important pediatric pathogen that infects infants worldwide. There is no vaccine currently available for RSV. A formalin-inactivated RSV vaccine used in a trial in the 1960s elicited immunopathology in response to natural RSV infection; this has been modeled experimentally, primarily in inbred mice and cotton rats. Eosinophils are recruited to the lung tissue in response to formalin-inactivated RSV vaccine antigens in humans and in experimental models, but they may or may not be involved in promoting the severe clinical sequelae observed. Pulmonary eosinophilia elicited in response to primary RSV infection has also been explored; this response is particularly evident in the youngest human infants and in neonatal mouse models. Although pulmonary eosinophilia is nearly always perceived in a negative light, the specific role played by virus-elicited eosinophils – negative, positive or neutral bystander – remain unclear. Lastly, we consider the data that focus on the role of eosinophils in promoting virus clearance and antiviral host defense, and conclude with a recent study that explores the role of eosinophils themselves as targets of virus infection.
Keywords: Respiratory syncytial virus, Pneumoviruses, Cytokines, Hypersensitivity, Vaccine
1. Introduction
Eosinophils, granule-bearing leukocytes found in peripheral blood and tissues, are best known for their roles in asthma, allergy, and other disorders in which eosinophils are recruited in response to cytokines released by type-2 helper (Th2) T lymphocytes. Eosinophils do not ordinarily come to mind when one thinks generally of respiratory virus infection. However, at least for one important respiratory virus, the human respiratory syncytial virus (RSV), eosinophils and their unique secretory mediators have been detected in lung tissue in response to primary infection and as a feature of a characteristic hypersensitivity response to inactivated vaccines and vaccine components. Interestingly, eosinophil recruitment and accumulation are almost always perceived in a negative light, as it is assumed that these cells contribute to tissue damage, bronchoconstriction and respiratory dysfunction via degranulation of their cationic secretory proteins and enzymes. However, recent descriptions of antiviral activity both in vitro and in vivo suggest that eosinophil function may encompass both of these functions, and present more of a “double-edged sword.” Clearly, we do not have a complete understanding of the role of eosinophils in disease caused by RSV; this review highlights many of the questions that remain to be answered.
2. Human RSV disease
Respiratory syncytial virus (RSV) infection is a near universal affliction of infancy and childhood, accounting for approximately 50% of all pneumonia and up to 90% of the reported cases of bronchiolitis in infancy. Of those infants infected during the first year of life, one-third develop lower respiratory tract disease, and 2.5% are hospitalized, accounting for more than 90,000 children in the United States every year. In many previously healthy infants, RSV disease is a mild and self-limited infection involving the upper and lower respiratory tract, with varying degrees of peribronchiolar and interstitial inflammation. In others, disease progresses to severe bronchiolitis and pneumonia, including submucosal edema and bronchiolar obstruction requiring oxygen, and in the worst cases, mechanical ventilation. Infants at particularly high risk for severe disease include those born prematurely, infants and children with cardiac or pulmonary anomalies, and the immunocompromised, although a recent study by Hall et al. (2009) noted that a substantial portion of the children with serious illness had no pre-existing medical condition. Prophylactic monoclonal antibody therapy is available for high risk infants only, but no vaccine has been approved for use. RSV has also recently been recognized as an important pathogen in the institutionalized elderly. The clinical features and pathology of RSV disease have been reviewed extensively, and the reader is referred to these excellent sources of additional information (Melero, 2007, Breary and Smyth, 2007, Stevens et al., 2008, Miyairi and DeVincenzo, 2008, Collins and Crowe, 2007).
3. Basic biology of human and mouse eosinophils
Eosinophils are leukocytes of the granulocyte lineage, as are neutrophils and basophils. Eosinophils differentiate in the bone marrow from CD34 antigen-positive pluripotent progenitor cells and are released into the bloodstream in more or less completely mature state. Under normal, homeostatic conditions, very few eosinophils can be detected in peripheral blood (only ∼2–3% of total leukocytes), as the vast majority reside in the tissues, primarily the gastrointestinal tract. In response to as yet incompletely characterized stimuli, typically observed in allergic states, during infection with helminthic parasites, and in some idiopathic hypereosinophilic states, Th2 lymphocytes are activated, which results in the production of a specific subset of “Th2” cytokines, including interleukin-5 (IL-5). Interleukin-5 has a unique impact on the eosinophil lineage, as it induces the expansion of eosinophil progenitors in the bone marrow, it primes of eosinophils in the periphery, and it prolongs eosinophil survival in the tissues. Eosinophils are capable of responding to a wide variety of other stimuli, and can undergo chemotaxis in response to eotaxin-1 (CCL11), MIP-1α (CCL3), and RANTES (CCL5), which are chemoattractant cytokines that interact with eosinophils via specific cell surface receptors (CCR3, and CCR1/CCR5, respectively). Interestingly, despite years of research, there is still no absolute consensus on eosinophil physiology and function, even in well-characterized disease states. For example, while eosinophils and eosinophil secretory mediators can promote destruction of helminth eggs and larvae in experiments performed in vitro, experiments performed in vivo with cytokine-deficient and eosinophil-deficient mice have yielded complex and inconsistent results (reviewed in Klion and Nutman, 2004, Fabre et al., 2009). Similarly, although the weight of evidence suggests that eosinophils contribute to the pathophysiology of allergy and asthma, a chronic respiratory disease in which bronchoconstriction in response to environmental triggers is typically associated with production of Th2 cytokines and recruitment of eosinophils to the airways (reviewed in Lee et al., 2001, Foster et al., 2008, Nissim Ben Efraim and Levi-Schaffer, 2008), asthmatic responses are obviously negative sequelae of eosinophil function that alone cannot represent a direct evolutionary advantage to the host organism. Among the more recent hypotheses, several groups have focused on eosinophils as immunomodulatory mediators, as eosinophils can interact both directly and indirectly with T cells and mast cells, and can release a wide variety of preformed cytokines and other secretory mediators, primarily from cytoplasmic granules (Jacobsen et al., 2007a, Akuthota et al., 2008, Lacy and Moqbel, 2001). There are several recent and complete reviews of eosinophil biology that provide extensive coverage of these and related subjects (Rothenberg and Hogan, 2006, Hogan et al., 2008).
Mature eosinophils from all species are readily recognized by their eccentric bilobed nuclei and their characteristic red-staining cytoplasmic granules; examples of human and mouse eosinophils are shown in Fig. 1 . As noted, human eosinophil granules are storage sites for cationic secretory mediators, including a unique eosinophil peroxidase, the eosinophil major basic protein, ribonucleases eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN), and numerous enzymes and cytokines. Despite similar morphology, human and mouse eosinophils differ from one another, and cannot be presumed to function identically in all circumstances. For instance, while there are several reports describing high affinity IgE receptor (FcɛRI) in human eosinophils from parasite-infected and asthmatic subjects (Gounni et al., 1994, Barata et al., 1997, Rajakulasingam et al., 1998), this finding and its functional significance has been questioned (Kita et al., 1999), and FcɛRI has never been detected on eosinophils isolated from mice. Similarly, the sialic acid-binding Ig-like lectin Siglec 8, can be detected on the surface of human eosinophils, while mouse eosinophils express the highly divergent functional ortholog, Siglec F. The mouse eosinophil ribonucleases are highly divergent orthologs of human EDN and ECP (Larson et al., 1996) and Charcot-Leyden crystal protein, a major human eosinophil component, cannot even be identified in the mouse genome. Mouse eosinophils also display a profoundly reduced propensity to degranulate and undergo differential chemotaxis to known exogenous stimuli (Clark et al., 2004, Borchers et al., 2002, Lee and Lee, 2005). As such, eosinophils from humans and mice may look similar to one another, but they may not be formally identical to one another in their actions and in their capacity to cause, to ameliorate, or even to serve as biomarkers for disease.
Fig. 1.

Eosinophils. (A) Human eosinophils isolated from peripheral blood by negative selection and (B) mouse eosinophils detected in bone marrow.
A number of recent studies have associated eosinophils and eosinophil degranulation products with various aspects of RSV infection in both human disease and in parallel mouse models, which need to be understood with the aforementioned caveats in mind. Here, we review some of these findings with an eye toward understanding what is and what is not known regarding the role of eosinophils, their role in vaccine-induced pathology, their interactions with respiratory virus pathogens and the outcome of severe respiratory virus disease.
4. Hypersensitivity responses to formalin-inactivated RSV vaccine—are the eosinophils at fault?
In the early 1960s, a number of children were enrolled in a clinical trial of a formalin-inactivated RSV vaccine. The negative outcomes of this trial, including a record of the detailed responses of the vaccinated children who encountered a natural RSV infection sometime thereafter, have been documented and reviewed extensively (Castilow and Varga, 2008, Olson and Varga, 2008, Castilow et al., 2007, Openshaw and Tregoning, 2005). Briefly, it has been concluded that non-neutralizing, non-protective antibodies developed in children immunized with the formalin-inactivated virus, and, upon encountering a natural RSV challenge, the vaccinated children developed a hypersensitivity response to the virus antigens, characterized by bronchoconstriction and severe pneumonia. Lung histology from two children that ultimately died as a result of this trial revealed deposition of antibody-virus complexes and a pronounced tissue eosinophilia (Kim et al., 1969). One or more of these features, which have collectively been termed “enhanced disease” have been replicated and modeled with formalin-inactivated RSV in multiple species, including other primates, ferrets, cotton rats, and mice (Byrd and Prince, 1997) as well as with formalin-inactivated bovine RSV in cows (Antonis et al., 2003). Interestingly, hypersensitivity responses of this nature are not unique to formalin-inactivated RSV; there are a limited number of reports describing aberrant responses to formalin-inactivated measles vaccine (Griffin et al., 2008). This phenomenon has also been replicated experimentally to varying extents in cotton rats with formalin-inactivated versions of human metapneumovirus (de Swart et al., 2007) and parainfluenza virus (Ottolini et al., 2000), and in some reports, even to carrier antigens (Piedra et al., 1993). There is also a recent report of a severe hypersensitivity reaction, including Th2 cytokine-mediated eosinophil infiltration into the lung tissue, in BALB/c mice immunized with a vaccinia-virus construct expressing the SARS coronavirus (N) nucleocapsid protein (Yasui et al., 2008). Our group has demonstrated that immunization of mice with formalin-inactivated pneumonia virus of mice (PVM) followed by intranasal virus challenge likewise results in pulmonary hypereosinophilia in the absence of a serum-neutralizing antibody response (Percopo et al., 2009; Fig. 2 ). PVM is a natural rodent pneumovirus pathogen that is related to RSV; PVM replicates extensively in mouse bronchiolar epithelial cells tissue, eliciting a profound and potentially lethal inflammatory response, similar to the more severe forms of RSV disease (Rosenberg and Domachowske, 2008, Easton et al., 2004).
Fig. 2.
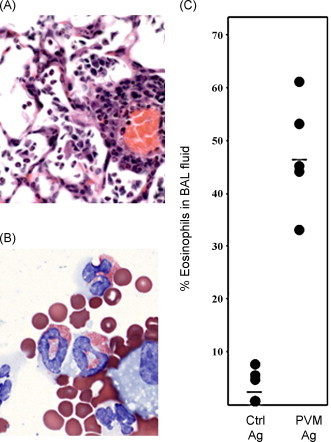
Hypersensitivity responses in mice vaccinated with formalin-fixed pneumovirus antigens. (A) Lung tissue from a mouse vaccinated with formalin-inactivated pneumonia virus of mice (PVM; a mouse pneumovirus related to human RSV) and then challenged intranasally with actively replicating virus (B) Eosinophils detected in bronchoalveolar lavage fluid from the mouse described in (A); (C) percentage eosinophils detected in bronchoalveolar lavage fluid in mice vaccinated with formalin-inactivated PVM vs. control antigen. Panels (A) and (B) reprinted with permission from Percopo et al. (2009).
Gene-deletion and cytokine depletion mouse model studies all point to Th2 cytokines (IL-4, IL-5 and IL-13) as crucial to eliciting pulmonary eosinophilia in response to formalin-inactivated RSV (Connors et al., 1994, Castilow et al., 2008a, Castilow et al., 2008b). A recent study by Moghaddam et al. (2006) suggested that the oxidation of RSV antigens resulting from formalin exposure elicits a Th2 response in vivo. Delgado et al. (2009) and Cyr et al. (2009) have both reported that independent toll-like receptor (TLR) stimulation (Kawai and Akira, 2007) in conjunction with RSV antigens results in a rebalancing of the Th1/Th2 cytokine responses, thereby reducing pathology. In initial studies aimed at exploring the molecular mechanism of Th2-mediated immunopathology, pulmonary eosinophilia was observed in mice immunized with recombinant vaccinia virus expressing RSV-G protein followed by live RSV challenge in most (Johnson et al., 1998, Johnson et al., 2003, Johnson et al., 2008) but not all (Olszewska et al., 2004) published trials, results which initially suggested that the pathology related to formalin-inactivation might be attributed mechanistically to aberrant reactivity to this one protein alone. However, although the endpoint – pulmonary eosinophilia – looks more or less the same, recent analysis indicates that the pulmonary eosinophilia that develops in response to RSV-G protein and eosinophilia that develops in response to formalin-inactivated RSV proceed via different molecular mechanisms (Castilow et al., 2007, Johnson et al., 2004, Johnson and Graham, 2004).
4.1. Are eosinophils contributing to the pathophysiology?
Much of the focus of the enhanced disease/hypersensitivity studies has been on the presence of eosinophils and mechanisms of eosinophil recruitment to the lung tissue, yet we really do not know if eosinophils are directly responsible for the pathophysiologic responses bronchoconstruction seen in the human subjects, whether they play an as yet unexplored role in altering subsequent immune responses (Akuthota et al., 2008, Jacobsen et al., 2007a), or whether they are perhaps simply innocent bystanders. In other words, we still do not know whether the vaccinated children became ill because of pulmonary eosinophilia, whether the eosinophils were engaged in altering future responses to virus infection, or whether eosinophilia is a neutral secondary finding. These questions have been explored to some extent in mouse models using wild type and gene-deleted mice immunized with vaccinia-virus vectors expressing RSV-G and RSV-F proteins (Castilow et al., 2008a, Castilow et al., 2008b, Johnson et al., 2008, Castilow and Varga, 2008), but, as noted above, these experimental systems are now recognized as mechanistically unrelated to the pathology induced by formalin-inactivated RSV antigens. As such, although findings address the role of eosinophils, and suggest that eosinophils may not be contributing to systemic disease (Castilow et al., 2007, Johnson et al., 2004, Johnson and Graham, 2004), these conclusions may not be directly relevant to the way in which eosinophils contribute to pathology in the setting of formalin-inactivated RSV antigens. The role of eosinophils in modulating pathology induced by formalin-inactivated vaccine antigens has not been explored, and might be addressed in mouse models of explicit eosinophil deficiency, i.e.…the ΔdblGATA (Yu et al., 2002) or TgPHIL mice (Lee et al., 2004).
Thus, although our longstanding prejudices might make it easy to conclude that eosinophils contributed directly to the lung and systemic pathology observed in the initial vaccine trials and in the subsequent mouse modeling experiments, it is important to recognize that the presence of eosinophils in the lung may or may not result in wheezing and bronchoconstriction. The data from mouse models are inconclusive on this point. Furthermore, the presence of eosinophils alone, even in human conditions, does not necessarily imply severe respiratory pathology. For example, in eosinophilic bronchiolitis, patients complain of only minimal respiratory symptomatology despite pronounced pulmonary eosinophilia (Scott and Wardlaw, 2006). Thus, at current writing, while eosinophils may serve as important biomarkers for aberrant hypersensitivity reactions, we can reach no conclusions regarding their contributions to pathophysiology from the published experimental data.
5. Eosinophil recruitment in response to primary RSV infection—a cause for alarm?
Although respiratory virus infection is not among the diseases typically associated with Th2 lymphocyte activation and profound pulmonary eosinophilia, eosinophils and/or eosinophil granule secretory proteins have been detected in lung washings or systemically in infants in need of supplemental oxygen via mechanical ventilation secondary to severe RSV infection (Kristjánsson et al., 2006, Harrison et al., 1999, Garofalo et al., 1992) (Fig. 3 ). As mentioned earlier, it is not at all certain whether pulmonary eosinophilia is uniquely related to the RSV pathogen, or whether one observes eosinophilia in response to RSV because it is the predominant severe respiratory pathogen among very young infants. Although not reported as frequently, the eosinophil granule protein ECP has been detected in nasopharyngeal secretions in response to other respiratory virus infections, including influenza and parainfluenza (Colocho Zelaya et al., 1994, Kristjansson et al., 2005).
Fig. 3.

Detection of eosinophil granule proteins in lung washings from infants diagnosed with RSV. Concentrates of lung washings from infants undergoing ventilatory support for ELISA-confirmed severe RSV disease (lanes 1–10) probed with antibodies against eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP); lanes 11–12, concentrates of lung washings from infants ventilated secondary to other causes, +C, positive control. Reprinted with permission from Harrison et al. (1999).
A number of recent studies have suggested that the age at which the individual experiences a first RSV infection has a profound impact on the nature of the primary response. In general, Th2 cytokines (IL-4, IL-5) and evidence of eosinophilia (cells and/or degranulation) are detected more readily in younger infants, although results are not completely consistent in all studies. For example, Kristjansson et al. (2005) examined the responses of infants diagnosed with RSV and found that those who were less than 3 months of age at the time of first infection had higher levels of IL-4 in their nasphopharyngeal secretions than children who were older, although no differences were observed in nasopharyngeal levels of ECP. Likewise, Sung et al. (2001) documented elevated levels of both IL-4 and IL-5 in serum samples of RSV-infected infants who were less than 18 months old at the time of primary infection than in older infants. Similarly, Kim et al. (2003) examined eosinophils in bronchoalveolar lavage (BAL) fluid in RSV infected infants (ages 0.4–1.8 years) and found that the number of eosinophils detected in BAL fluid correlated closely with IL-5 concentration, although interestingly, the age range of the group in which eosinophils were detected was not significantly different from the age range of the group in which eosinophils were absent.
Nasal eosinophilia has been detected in response to respiratory viruses (rhinoviruses, coronaviruses) other than RSV, although the circumstances tend to be limited and highly specific, such as in patients with pre-existing respiratory allergies (van Benten et al., 2001). Of particular interest, several groups have reported that influenza infection stimulates the production of the eosinophil chemoattractants eotaxin (CCL11) and RANTES (CCL5) in normal nasal and airway epithelial cells in culture, which suggests the possibility of eosinophil recruitment (Kawaguchi et al., 2000, Kawaguchi et al., 2001, Matsukura et al., 1996, Matsukura et al., 1998). The role of eosinophils in acute SARS-CoV remains completely unexplored, but the eosinophil secretory ribonuclease, EDN, was among the 52 signature genes that discriminated between individuals recovering from severe SARS-CoV infection and healthy controls (Lee et al., 2005).
Several large clinical studies have led to the consistent conclusion that infants who have recovered from severe RSV bronchiolitis are at significantly increased risk for both recurrent wheezing and childhood asthma (Mohapatra and Boyapalle, 2008, Pérez-Yarza et al., 2007, Dakhama et al., 2005a, Dakhama et al., 2005b). Given the presumed role of eosinophils in pathogenesis of acute allergic asthma, it seems reasonable to ask whether the eosinophils recruited to the lungs during severe primary RSV bronchiolitis might cause, or at least predict progression to wheezing. Causation is of course difficult to ascertain in human subjects, however, a prospective study by Pifferi et al. (2001) demonstrated that infants who were less than 1 year old and who had elevated serum ECP levels during a primary RSV infection were nearly 10 times more likely to have developed symptoms of wheezing in later childhood than older children, and children without elevated serum ECP. However, Sigurs et al. (1994), following much the same methodology found that serum ECP was not predictive of progression to wheezing. In a more recent prospective study, Castro et al. (2008) examined the outcomes of RSV-infected, <1 year old infants; in this study, 48% of those enrolled went on to develop allergic symptomatology by age 6, with a statistically significant higher prevalence of asthma developing among the children who were infected with RSV at a younger age (below 6 months). Among those developing asthma, there were no differences in peripheral blood eosinophil counts at the time of acute RSV infection, nor were the cytokine profiles (as determined by phorbol-myristate stimulation of isolated mononuclear cells) of children who developed allergic disease different from those who did not.
5.1. What can we learn from mouse models of primary virus challenge?
Given the complexities of natural disease, and the fact that there is no human condition in which an individual is uniquely devoid of eosinophils, it is helpful (if not crucial) to have appropriate animal models to explore questions of association and causation. Inbred mice have been employed extensively to study responses to RSV, although it is important to recognize that RSV inoculation of mice is formally a challenge-clearance model rather than an infection model, as RSV undergoes little if any replication in mouse lung tissue.
Schwarze et al., 1999, Schwarze et al., 2000 have explored RSV challenge in mouse models; in these studies, the authors describe Th2-cytokine dependent recruitment of eosinophils and associated airways hyperreactivity, a finding that has implicated eosinophils in the pathophysiologic mechanism. Eosinophilia was also noted in a mouse model of secondary RSV challenge; Culley et al. (2002) detected eosinophil recruitment to the airways upon secondary RSV challenge among mice undergoing primary challenge at one day of age; eosinophil recruitment declined dramatically if primary challenge was delayed until mice were 1 week old, although the authors found no statistically significant difference in systemic disease, measured as weight loss, between these two sets of challenged mice. Dakhama et al., 2005a, Dakhama et al., 2005b likewise found that the extent of airway hyperresponsiveness induced by a secondary challenge was directly dependent on the age of first virus challenge, similarly associated with augmented eosinophil recruitment when the primary challenge occurred in mice <1 week of age. Tasker et al. (2008) performed a similar study, and identified Th2 cytokine responses in neonatally primed mice that were associated with diminished virus replication in lung tissue. Finally, also noteworthy is the study of Harker et al. (2007) in which mice were primed with recombinant RSVs expressing Th1 (IFN-gamma) or Th2 (IL-4) cytokines prior to RSV challenge. In contrast to mice primed with rRSV/IFN-gamma, mice primed with rRSV/IL-4 sustained airway eosinophilia in response to subsequent RSV challenge. Although IL-4 clearly functions to suppress antiviral CD8+ T cell function in many other experimental settings (Wasik et al., 1997, Villacres and Bergmann, 1999, Bot et al., 2000), challenge with RSV/IL-4 had no impact on the number of CD8+ T cells recruited to the lung nor on the fraction producing IFN-gamma when compared to mice challenged with wild-type RSV alone. The RSV/IL-4 primed mice had reduced lung virus titer and were protected against weight loss, a finding that correlated with the recruitment of eosinophils. As is clear from these findings, the precise role of eosinophils remains uncertain, but one thing that is clear is the fact that eosinophils do not universally provoke lung pathology and systemic disease.
The role of RSV and PVM in enhancing asthmatic type responses via an interplay with known allergens has also been explored (Schwarze et al., 1997, Barends et al., 2004a, Barends et al., 2004b, Mäkelä et al., 2003); while the weight of evidence suggests that eosinophils play crucial roles in mouse models of asthma, what that precise role might be (promoting acute airways hyperreactivity vs. more chronic remodeling) is a complex and controversial issue that has been considered extensively by others (Jacobsen et al., 2007b, Lee et al., 2004, Humbles et al., 2004) and is beyond the scope of this review.
6. Do eosinophils promote antiviral host defense?
One of the more curious aspects of eosinophil biology is, as discussed thus far in this review, once they are detected, particularly in lung tissue, eosinophils are almost always considered as contributing in some negative way to the pathophysiology of disease. This is most intriguing, given our understanding of the role of their sister cell, the neutrophil, and the concept of the double-edged sword (Smith, 1994). In other words, we know that neutrophils are recruited in response to bacterial and fungal infection, and serve to promote host defense against these invasive pathogens. However, if signals go awry, if neutrophil clearance does not proceed, and/or if neutrophil activation persists, pathology ensues. We were among the first groups to suggest that eosinophil function might encompass a positive, host-defense aspect as part of perhaps a more subtle double-edged sword (Rosenberg and Domachowske, 1999, Rosenberg and Domachowske, 2001), and to consider the possibility that eosinophils may be recruited in part to promote primary antiviral host defense, perhaps in situations in which acquired immune responses are less than immediately effective (Milner et al., 2007). Interestingly, eosinophilia has been reported in association with T cell dysfunction in human immunodeficiency virus (HIV) infection (Tietz et al., 1997, Cohen and Steigbigel, 1996, Drabick et al., 1994); this finding may in turn be related to the propensity for hypersensitivity reactions observed among HIV-infected patients (Phillips and Mallal, 2007). However, correlations between eosinophilia and disease pathogenesis are often difficult to ascertain. In a primary study, Tietz et al. (1997) found that elevated eosinophil counts among HIV-infected patients correlated with progression of disease and declining CD4+ T cell counts, while Chorba et al. (2002) found no correlation between HIV viral loads, CD4+ T cell and eosinophil counts among more than 600 HIV-infected patients in sub-Saharan Africa, although concurrent helminthic parasite infection was clearly a confounding variable.
The first indication that eosinophils might have the means to function in promoting antiviral host defense came from a series of studies we performed in the late 1990s. In this work, we determined that eosinophils, acting at least in part via their secretory mediators, could reduce the infectivity of respiratory syncytial virus for target epithelial cells in vitro (Domachowske et al., 1998); Soukup and Becker (2003) likewise demonstrated that eosinophils inhibit RSV infection in tissue culture. Shortly thereafter, Adamko et al. (1999) demonstrated that eosinophils elicited by allergen sensitization served to limit virus replication and/or promote virus clearance in guinea pigs challenged with Sendai virus. In a more recent study, Phipps et al. (2007) demonstrated accelerated clearance of RSV from the lungs of the eosinophil-enriched IL-5 transgenic mice, and furthermore found that full antiviral activity was dependent on intact TLR signaling in eosinophils introduced exogenously (Fig. 4 ).
Fig. 4.
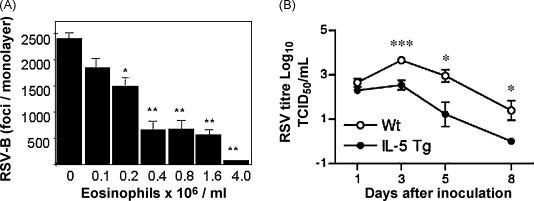
Eosinophils promote antiviral host defense. (A) Eosinophils reduce the infectivity of RSV for target epithelial cells in vitro; (B) eosinophil-enriched interleukin-5 transgenic mice promote accelerated RSV clearance compared to wild type mice, with reduced virus titers detected at all time points examined. Panels (A) and (B) reprinted with permission from Domachowske et al. (1998) and Phipps et al. (2007), respectively.
As discussed earlier, we have explored the responses of mice immunized with formalin-inactivated PVM followed by live virus challenge. In experiments using eosinophil-deficient ΔdblGATA mice, we found that eosinophils were not a crucial component of the small degree of protection resulting from this immunization strategy (Percopo et al., 2009), a finding perhaps related to the virulence of this pathogen in inbred strains of mice, as well as its ability to infect eosinophils (Dyer et al., 2008).
7. Are eosinophils among the direct targets of respiratory virus infection?
Our studies with PVM (Bonville et al., 2006) and examination of pathology specimens from RSV patients (Welliver et al., 2007, Johnson et al., 2007) demonstrate that pneumovirus replication in vivo takes place primarily in respiratory epithelial cells. However, it is clear that other cells, including human monocytes, support replication of RSV and PVM in culture, and release proinflammatory cytokines in response to virus infection (Barr et al., 2000, Dyer et al., 2007, Krilov et al., 2000). Given the questions regarding the role of eosinophils and their interactions with respiratory viruses, we set out to determine whether pneumoviruses could infect and replicate within eosinophils, and to determine what the outcome of this infection might be. Kimpen et al., 1996, Kimpen et al., 1992 originally demonstrated that RSV could be taken up by purified human eosinophils, and virions were identified in phagolysosomal compartments, but virus replication was not examined. To explore PVM replication in mouse eosinophils, we utilized our recently described method for generating sustained cultures of >95% pure mature eosinophils from unselected bone marrow progenitors (Dyer et al., 2008). With eosinophils generated by this culture method, we demonstrated greater than a fourfold increase in PVM titer, associated with the replication-dependent release of the cytokine interleukin-6 (Fig. 5 ). Others have explored interactions of pneumoviruses with eosinophils, including Davoine et al. (2008) who determined that human eosinophils were unable to release granule proteins in response to RSV challenge without coincident exposure of virus to CD4+ T cells and antigen presenting cells. This differential response might be explained by the work of Melo et al. (2008), who documented that granule proteins and cytokines are released from eosinophils via unique and distinct secretory pathways. Among the questions left to be explored: How often are infected eosinophils detected in vivo, at what point during an acute infection are they detected and under what specific circumstances? Does virus infection and intracellular replication induce eosinophil apoptosis or disable eosinophils in some other, more subtle way, and thereby reduce their ability to promote virus clearance and antiviral host defense? Answers to these questions may shed some light on differential responses observed in the aforementioned experiments.
Fig. 5.
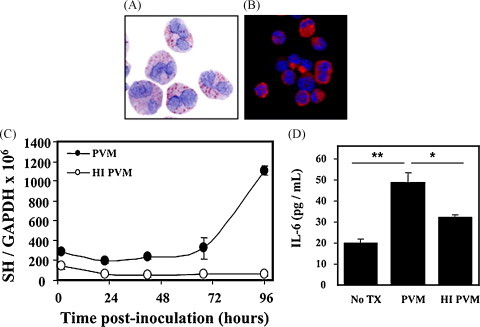
Pneumovirus replication in eosinophils. Cultured eosinophils derived from mouse bone marrow (A) stained with modified Giemsa and (B) stained with anti-mouse MBP antibody. (C) Replication of PVM in cultured mouse eosinophils; filled symbols, replication competent PVM; open symbols, heat-inactivated PVM. PVM is detected by quantitative RT-PCR targeting the virus SH gene. (D) Replication of PVM in cultured mouse eosinophils is accompanied by the replication-dependent release of interleukin-6; reprinted with permission from Dyer et al. (2008).
8. Conclusions and future research
The manner in which eosinophils respond to and participate in respiratory virus infection is very far from clear. While pulmonary eosinophilia is a hallmark, or biomarker of the aberrant hypersensitivity response to formalin-inactivated RSV, there is no clear indication that eosinophils actually contribute to the negative sequelae of disease. Likewise, while severe primary RSV is associated with pulmonary eosinophilia and progression to asthma, these two features have not been linked clearly to one another mechanistically or pathophysiologically. Finally, several groups have shown that eosinophils can promote virus clearance, but this interesting and positive feature of eosinophil function is not observed in all circumstances or in all situations. Among the possibilities that have yet to be explored, eosinophil function may be less dependent on numbers elicited, and related more closely to the quality and extent of activation, to the unique nature of the cytokines eliciting recruitment, and/or to the strength of the signals sustaining viability in situ. These are all issues that are worthy of consideration, as we attempt to improve our understanding of the true nature of the eosinophilic leukocyte, and strive to achieve some clarification and sense of balance between their perceived negative and their incompletely characterized positive contributions to homeostasis and host defense.
Acknowledgements
Research in Dr. Rosenberg's laboratory is supported by the Division of Intramural Research (DIR) of the National Institute of Allergy and Infectious Diseases. Research in Dr. Domachowske's laboratory is supported by the Children's Miracle Network of Greater New York.
References
- Adamko D.J., Yost B.L., Gleich G.J., Fryer A.D., Jacoby D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuthota P., Wang H.B., Spencer L.A., Weller P.F. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin. Exp. Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonis A.F., Schrijver R.S., Daus F., Steverink P.J., Stockhofe N., Hensen E.J., Langedijk J.P., van der Most R.G. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J. Virol. 2003;77:12067–12073. doi: 10.1128/JVI.77.22.12067-12073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barata L., Ying S., Humbert M., Barkans J., Meng Q., Durham S.R., Kay A.B. Allergen-induced recruitment of FcɛRI+ eosinophils in human atopic skin. Eur. J. Immunol. 1997;27:1236–1241. doi: 10.1002/eji.1830270527. [DOI] [PubMed] [Google Scholar]
- Barends M., de Rond L.G., Dormans J., van Oosten M., Boelen A., Neijens H.J., Osterhaus A.D., Kimman T.G. Respiratory syncytial virus, pneumonia virus of mice, and influenza A virus differently affect respiratory allergy in mice. Clin. Exp. Allergy. 2004;34:488–496. doi: 10.1111/j.1365-2222.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- Barends M., Van Oosten M., De Rond C.G., Dormans J.A., Osterhaus A.D., Neijens H.J., Kimman T.G. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J. Infect. Dis. 2004;189:1866–1872. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- Barr F.E., Pedigo H., Johnson T.R., Shepherd V.L. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am. J. Respir. Cell Mol. Biol. 2000;23:586–592. doi: 10.1165/ajrcmb.23.5.3771. [DOI] [PubMed] [Google Scholar]
- Bonville C.A., Bennett N.J., Koehnlein M., Haines D.M., Ellis J.A., DelVecchio A.M., Rosenberg H.F., Domachowske J.B. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Borchers M.T., Ansay T., DeSalle R., Daugherty B.L., Shen H., Metzger M., Lee N.A., Lee J.J. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J. Leukoc. Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- Bot A., Holz A., Christen U., Wolfe T., Temann A., Flavell R., von Herrath M. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000;269:66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- Breary S.P., Smyth R.L. Pathogenesis of RSV in children. In: Cane P.A., editor. Elsevier Publishers; Amsterdam: 2007. pp. 141–162. (Respiratory Syncytial Virus, Perspectives in Medical Virology, vol. 14). [Google Scholar]
- Byrd L.G., Prince G.A. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 1997;25:1363–1368. doi: 10.1086/516152. [DOI] [PubMed] [Google Scholar]
- Castilow E.M., Varga S.M. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008;3:445–454. doi: 10.2217/17460794.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilow E.M., Olson M.R., Varga S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- Castilow E.M., Legge K.L., Varga S.M. Cutting edge: eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J. Immunol. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilow E.M., Meyerholz D.K., Varga S.M. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J. Immunol. 2008;180:2376–2384. doi: 10.4049/jimmunol.180.4.2376. [DOI] [PubMed] [Google Scholar]
- Castro M., Schweiger T., Yin-Declue H., Ramkumar T.P., Christie C., Zheng J., Cohen R., Schechtman K.B., Strunk R., Bacharier L.B. Cytokine response after severe respiratory syncytial virus bronchiolitis in early life. J. Allergy Clin. Immunol. 2008;122:726–733. doi: 10.1016/j.jaci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorba T.L., Nkengasong J., Roels T.H., Monga B., Maurice C., Maran M., Djomand G. Assessing eosinophil count as a marker of immune activation among human immunodeficiency virus-infected persons in sub-Saharan Africa. Clin. Infect. Dis. 2002;34:1264–1266. doi: 10.1086/339940. [DOI] [PubMed] [Google Scholar]
- Clark K., Simson L., Newcombe N., Koskinen A.M., Mattes J., Lee N.A., Lee J.J., Dent L.A., Matthaei K.I., Foster P.S. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J. Leukoc. Biol. 2004;75:1001–1009. doi: 10.1189/jlb.0803391. [DOI] [PubMed] [Google Scholar]
- Cohen A.J., Steigbigel R.T. Eosinophilia in patients infected with human immunodeficiency virus. J. Infect. Dis. 1996;174:615–618. doi: 10.1093/infdis/174.3.615. [DOI] [PubMed] [Google Scholar]
- Collins P.L., Crowe J.E., Jr. Chapter 46: respiratory syncytial virus and metapneumovirus. In: Knipe D.M., Howley P.M., editors. Field's Virology. 5th ed. Lippincott, Williams & Wilkins Publishers; Philadelphia: 2007. [Google Scholar]
- Colocho Zelaya E.A., Orvell C., Strannegård O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr. Allergy Immunol. 1994;5:100–106. doi: 10.1111/j.1399-3038.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Connors M., Giese N.A., Kulkarni A.B., Firestone C.Y., Morse H.C.3rd, Murphy B.R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley F.J., Pollott J., Openshaw P.J. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr S.L., Angers I., Guillot L., Stoica-Popescu I., Lussier M., Qureshi S., Burt D.S., Ward B.J. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine. 2009;27:421–430. doi: 10.1016/j.vaccine.2008.10.073. [DOI] [PubMed] [Google Scholar]
- Dakhama A., Lee Y.M., Gelfand E.W. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr. Infect. Dis. J. 2005;24(11 Suppl.):S159–S169. doi: 10.1097/01.inf.0000188155.46381.15. [DOI] [PubMed] [Google Scholar]
- Dakhama A., Park J.W., Taube C., Joetham A., Balhorn A., Miyahara N., Takeda K., Gelfand E.W. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 2005;175:1876–1883. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- Davoine F., Cao M., Wu Y., Ajamian F., Ilarraza R., Kokaji A.I., Moqbel R., Adamko D.J. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J. Allergy Clin. Immunol. 2008;122:69–77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- de Swart R.L., van den Hoogen B.G., Kuiken T., Herfst S., van Amerongen G., Yüksel S., Sprong L., Osterhaus A.D. Immunization of macaques with formalin-inactivated human metapneumovirus induces hypersensitivity to hMPV infection. Vaccine. 2007;25:8518–8528. doi: 10.1016/j.vaccine.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P., Diaz L., Trento A., Chang H.Y., Mitzner W., Ravetch J., Melero J.A., Irusta P.M., Polack F.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domachowske J.B., Dyer K.D., Bonville C.A., Rosenberg H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- Drabick J.J., Magill A.J., Smith K.J., Nutman T.B., Benson P.M. Hypereosinophilic syndrome associated with HIV infection Military Medical Consortium for applied retroviral research. South Med. J. 1994;87:525–529. doi: 10.1097/00007611-199404000-00021. [DOI] [PubMed] [Google Scholar]
- Dyer K.D., Schellens I.M., Bonville C.A., Martin B.V., Domachowske J.B., Rosenberg H.F. Efficient replication of pneumonia virus of mice (PVM) in a mouse macrophage cell line. Virol. J. 2007;4:48. doi: 10.1186/1743-422X-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer K.D., Moser J.M., Czapiga M., Siegel S.J., Percopo C.M., Rosenberg H.F. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A.J., Domachowske J.B., Rosenberg H.F. Animal pneumoviruses: molecular genetics and pathogenesis. Clin. Microbiol. Rev. 2004;17:390–412. doi: 10.1128/CMR.17.2.390-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V., Beiting D.P., Bliss S.K., Gebreselassie N.G., Gagliardo L.F., Lee N.A., Lee J.J., Appleton J.A. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P.S., Rosenberg H.F., Asquith K.L., Kumar R.K. Targeting eosinophils in asthma. Curr. Mol. Med. 2008;8:585–590. doi: 10.2174/156652408785748013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R., Kimpen J.L., Welliver R.C., Ogra P.L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J. Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- Gounni A.S., Lamkhioued B., Ochiai K., Tanaka Y., Delaporte E., Capron A., Kinet J.P., Capron M. High affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- Griffin D.E., Pan C.H., Moss W.J. Measles vaccines. Front. Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A., Auinger P., Griffin M.R., Poehling K.A., Erdman D., Grijalva C.G., Zhu Y., Szilagyi P. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker J., Bukreyev A., Collins P.L., Wang B., Openshaw P.J., Tregoning J.S. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 2007;81:13105–131011. doi: 10.1128/JVI.01544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A.M., Bonville C.A., Rosenberg H.F., Domachowske J.B. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am. J. Respir. Crit. Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- Hogan S.P., Rosenberg H.F., Moqbel R., Phipps S., Foster P.S., Lacy P., Kay A.B., Rothenberg M.E. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Humbles A.A., Lloyd C.M., McMillan S.J., Friend D.S., Xanthou G., McKenna E.E., Ghiran S., Gerard N.P., Yu C., Orkin S.H., Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Jacobsen E.A., Taranova A.G., Lee N.A., Lee J.J. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J. Allergy Clin. Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Jacobsen E.A., Ochkur S.I., Lee N.A., Lee J.J. Eosinophils and asthma. Curr. Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- Johnson T.R., Graham B.S. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2004;23(1 Suppl.):S46–57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- Johnson T.R., Johnson J.E., Roberts S.R., Wertz G.W., Parker R.A., Graham B.S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.R., Parker R.A., Johnson J.E., Graham B.S. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 2003;170:2037–2045. doi: 10.4049/jimmunol.170.4.2037. [DOI] [PubMed] [Google Scholar]
- Johnson T.R., Varga S.M., Braciale T.J., Graham B.S. Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J. Virol. 2004;78:8753–8760. doi: 10.1128/JVI.78.16.8753-8760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.E., Gonzales R.A., Olson S.J., Wright P.F., Graham B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Johnson T.R., Rothenberg M.E., Graham B.S. Pulmonary eosinophilia requires interleukin-5, eotaxin-1, and CD4+ T cells in mice immunized with respiratory syncytial virus G glycoprotein. J. Leukoc. Biol. 2008;84:748–759. doi: 10.1189/jlb.0907621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M., Kokubu F., Kuga H., Tomita T., Matsukura S., Kadokura M., Adachi M. Expression of eotaxin by normal airway epithelial cells after influenza virus A infection. Int. Arch. Allergy Immunol. 2000;122(Suppl. 1):44–49. doi: 10.1159/000053632. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M., Kokubu F., Kuga H., Tomita T., Matsukura S., Suzaki H., Huang S.K., Adachi M. Influenza virus A stimulates expression of eotaxin by nasal epithelial cells. Clin. Exp. Allergy. 2001;31:873–880. doi: 10.1046/j.1365-2222.2001.01103.x. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Antiviral signaling through pattern recognition receptors. J. Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K., Parrott R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim C.K., Kim S.W., Park C.S., Kim B.I., Kang H., Koh Y.Y. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J. Allergy Clin. Immunol. 2003;112:64–71. doi: 10.1067/mai.2003.1618. [DOI] [PubMed] [Google Scholar]
- Kimpen J.L., Garofalo R., Welliver R.C., Ogra P.L. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr. Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- Kimpen J.L., Garofalo R., Welliver R.C., Fujihara K., Ogra P.L. An ultrastructural study of the interaction of human eosinophils with respiratory syncytial virus. Pediatr. Allergy Immunol. 1996;7:48–53. doi: 10.1111/j.1399-3038.1996.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Kita H., Kaneko M., Bartemes K.R., Weiler D.A., Schimming A.W., Reed C.E., Gleich G.J. Does IgE bind to and activate eosinophils from patients with allergy? J. Immunol. 1999;162:6901–6911. [PubMed] [Google Scholar]
- Klion A.D., Nutman T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Krilov L.R., McCloskey T.W., Harkness S.H., Pontrelli L., Pahwa S. Alterations in apoptosis of cord and adult peripheral blood mononuclear cells induced by in vitro infection with respiratory syncytial virus. J. Infect. Dis. 2000;181:349–353. doi: 10.1086/315203. [DOI] [PubMed] [Google Scholar]
- Kristjansson S., Bjarnarson S.P., Wennergren G., Palsdottir A.H., Arnadottir T., Haraldsson A., Jonsdottir I. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J. Allergy Clin. Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Kristjánsson S., Wennergren D., Eriksson B., Thórarinsdóttir H., Wennergren G. U-EPX levels and wheezing in infants and young children with and without RSV bronchiolitis. Respir. Med. 2006;100:878–883. doi: 10.1016/j.rmed.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Lacy P., Moqbel R. Immune effector functions of eosinophils in allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2001;1:79–84. doi: 10.1097/01.all.0000010989.39379.49. [DOI] [PubMed] [Google Scholar]
- Larson K.A., Olson E.V., Madden B.J., Gleich G.J., Lee N.A., Lee J.J. Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12370–12375. doi: 10.1073/pnas.93.22.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Lee N.A. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- Lee N.A., Gelfand E.W., Lee J.J. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J. Allergy Clin. Immunol. 2001;107:945–957. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Dimina D., Macias M.P., Ochkur S.I., McGarr y M.P., O’Neill K.R., Protheroe C., Pero R., Nguyen T., Cormier S.A., Lenkiewicz E., Colbert D., Rinaldi L., Ackerman S.J., Irvin C.G., Lee N.A. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Chen C.H., Chao A., Chen E.S., Wei M.L., Chen L.K., Yang K.D., Lin M.C., Wang Y.H., Liu J.W., Eng H.L., Chiang P.C., Wu T.S., Tsao K.C., Huang C.G., Tien Y.J., Wang T.H., Wang H.S., Lee Y.S. Molecular signature of clinical severity in recovering patients with severe acute respiratory syndrome coronavirus (SARS-CoV) BMC Genomics. 2005;6:132. doi: 10.1186/1471-2164-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä M.J., Tripp R., Dakhama A., Park J.W., Ikemura T., Joetham A., Waris M., Anderson L.J., Gelfand E.W. Prior airway exposure to allergen increases virus-induced airway hyperresponsiveness. J. Allergy Clin. Immunol. 2003;112:861–869. doi: 10.1016/s0091-6749(03)02020-7. [DOI] [PubMed] [Google Scholar]
- Matsukura S., Kokubu F., Noda H., Tokunaga H., Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J. Allergy Clin. Immunol. 1996;98(6 Pt 1):1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- Matsukura S., Kokubu F., Kubo H., Tomita T., Tokunaga H., Kadokura M., Yamamoto T., Kuroiwa Y., Ohno T., Suzaki H., Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am. J. Respir. Cell Mol. Biol. 1998;18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- Melero J.A. Molecular biology of human respiratory syncytial virus. In: Cane P.A., editor. Elsevier Publishers; Amsterdam: 2007. (Respiratory Syncytial Virus, Perspectives in Medical Virology, vol. 14). pp. 1–42. [Google Scholar]
- Melo R.C., Spencer L.A., Dvorak A.M., Weller P.F. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J. Leukoc. Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J.D., Ward J.M., Keane-Myers A., Paul W.E. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. U.S.A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyairi I., DeVincenzo J.P. Human genetic factors and respiratory syncytial virus disease severity. Clin. Microbiol. Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Olszewska W., Wang B., Tregoning J.S., Helson R., Sattentau Q.J., Openshaw P.J. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- Mohapatra S.S., Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin. Microbiol. Rev. 2008;21:495–504. doi: 10.1128/CMR.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim Ben Efraim A.H., Levi-Schaffer F. Tissue remodeling and angiogenesis in asthma: the role of the eosinophil. Ther. Adv. Respir. Dis. 2008;2:163–171. doi: 10.1177/1753465808092281. [DOI] [PubMed] [Google Scholar]
- Olson M.R., Varga S.M. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines. 2008;7:1239–1255. doi: 10.1586/14760584.7.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska W., Suezer Y., Sutter G., Openshaw P.J. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine. 2004;23:215–221. doi: 10.1016/j.vaccine.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Openshaw P.J., Tregoning J.S. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 2005;18:541–555. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M.G., Porter D.D., Hemming V.G., Prince G.A. Enhanced pulmonary pathology in cotton rats upon challenge after immunization with inactivated parainfluenza virus 3 vaccines. Viral. Immunol. 2000;13:231–236. doi: 10.1089/vim.2000.13.231. [DOI] [PubMed] [Google Scholar]
- Percopo, C.M., Phipps, S., Foster, P.S., Domachowske, J.B., Rosenberg, H.F., 2009. Pulmonary eosinophils and their role in immunopathology associated with formalin-inactivated pneumovirus vaccination, in press. [DOI] [PMC free article] [PubMed]
- Pérez-Yarza E.G., Moreno A., Lázaro P., Mejías A., Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr. Infect. Dis. J. 2007;26:733–739. doi: 10.1097/INF.0b013e3180618c42. [DOI] [PubMed] [Google Scholar]
- Phillips E., Mallal S. Drug hypersensitivity in HIV. Curr. Opin. Allergy Clin. Immunol. 2007;7:324–330. doi: 10.1097/ACI.0b013e32825ea68a. [DOI] [PubMed] [Google Scholar]
- Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F., Foster P.S., Matthaei K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- Piedra P.A., Wyde P.R., Castleman W.L., Ambrose M.W., Jewell A.M., Speelman D.J., Hildreth S.W. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine. 1993;11:1415–1423. doi: 10.1016/0264-410x(93)90170-3. [DOI] [PubMed] [Google Scholar]
- Pifferi M., Ragazzo V., Caramella D., Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr. Pulmonol. 2001;31:419–424. doi: 10.1002/ppul.1069. [DOI] [PubMed] [Google Scholar]
- Rajakulasingam K., Till S., Ying S., Humbert M., Barkans J., Sullivan M., Meng Q., Corrigan C.J., Bungre J., Grant J.A., Kay A.B., Durham S.R. Increased expression of high affinity IgE (FcepsilonRI) receptor-alpha chain mRNA and protein-bearing eosinophils in human allergen-induced atopic asthma. Am. J. Respir. Crit. Care Med. 1998;158:233–240. doi: 10.1164/ajrccm.158.1.9708106. [DOI] [PubMed] [Google Scholar]
- Rosenberg H.F., Domachowske J.B. Eosinophils, ribonucleases and host defense: solving the puzzle. Immunol. Res. 1999;20:261–274. doi: 10.1007/BF02790409. [DOI] [PubMed] [Google Scholar]
- Rosenberg H.F., Domachowske J.B. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- Rosenberg H.F., Domachowske J.B. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol. Lett. 2008;118:6–12. doi: 10.1016/j.imlet.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M.E., Hogan S.P. The eosinophil. Annu. Rev. Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Schwarze J., Hamelmann E., Bradley K.L., Takeda K., Gelfand E.W. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze J., Cieslewicz G., Hamelmann E., Joetham A., Shultz L.D., Lamers M.C., Gelfand E.W. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J. Immunol. 1999;162:2997–3004. [PubMed] [Google Scholar]
- Schwarze J., Cieslewicz G., Joetham A., Ikemura T., Mäkelä M.J., Dakhama A., Shultz L.D., Lamers M.C., Gelfand E.W. Critical roles for interleukin-4 and interleukin-5 during respiratory syncytial virus infection in the development of airway hyperresponsiveness after airway sensitization. Am. J. Respir. Crit. Care Med. 2000;162(2 Pt 1):380–386. doi: 10.1164/ajrccm.162.2.9903057. [DOI] [PubMed] [Google Scholar]
- Scott K.A., Wardlaw A.J. Eosinophilic airway disorders. Semin. Respir. Crit. Care Med. 2006;27:128–133. doi: 10.1055/s-2006-939515. [DOI] [PubMed] [Google Scholar]
- Sigurs N., Bjarnason R., Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- Smith J.A. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Soukup J.M., Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin. Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Stevens W.W., Falsey A.R., Braciale T.J. RSV 2007 recent advances in respiratory syncytial virus research. Viral Immunol. 2008;21:133–140. doi: 10.1089/vim.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung R.Y., Hui S.H., Wong C.K., Lam C.W., Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur. J. Pediatr. 2001;160:117–122. doi: 10.1007/s004310000676. [DOI] [PubMed] [Google Scholar]
- Tasker L., Lindsay R.W., Clarke B.T., Cochrane D.W., Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin. Exp. Immunol. 2008;153:277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz A., Sponagel L., Erb P., Bucher H., Battegay M., Zimmerli W. Eosinophilia in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:675–677. doi: 10.1007/BF01708558. [DOI] [PubMed] [Google Scholar]
- van Benten I.J., KleinJan A., Neijens H.J., Osterhaus A.D., Fokkens W.J. Prolonged nasal eosinophilia in allergic patients after common cold. Allergy. 2001;56:949–956. doi: 10.1034/j.1398-9995.2001.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacres M.C., Bergmann C.C. Enhanced cytotoxic T cell activity in IL-4-deficient mice. J. Immunol. 1999;162:2663–2670. [PubMed] [Google Scholar]
- Wasik T.J., Jagodzinski P.P., Hyjek E.M., Wustner J., Trinchieri G., Lischner H.W., Kozbor D. Diminished HIV-specific CTL activity is associated with lower type 1 and enhanced type 2 responses to HIV-specific peptides during perinatal HIV infection. J. Immunol. 1997;158:6029–6036. [PubMed] [Google Scholar]
- Welliver T.P., Garofalo R.P., Hosakote Y., Hintz K.H., Avendano L., Sanchez K., Velozo L., Jafri H., Chavez-Bueno S., Ogra P.L., McKinney L., Reed J.L., Welliver R.C., Sr. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S., Kase R., Sekiguchi S., Morita K., Hishima T., Suzuki H., Karamatsu K., Yasutomi Y., Shida H., Kidokoro M., Mizuno K., Matsushima K., Kohara M. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- Yu C., Cantor A.B., Yang H., Browne C., Wells R.A., Fujiwara Y., Orkin S.H. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]


