Abstract
Objectives
Determine if cetuximab dose escalation to induce grade 2 rash correlates with anti-tumor activity and if sera-based markers could predict likelihood of response.
Methods
Patients with persistent/recurrent ovarian or primary peritoneal carcinoma received an initial dose of cetuximab 400 mg/m2, then 250 mg/m2 weekly for two 3-week cycles. Patients who had stable disease (SD) and <grade 2 rash were dose escalated in 75 mg/m2 increments every 3 weeks until grade 2 rash or to a maximum weekly dose of 400 mg/m2. Pre- and post-treatment serum samples were evaluated for potential predictive markers of response.
Results
One of 25 patients achieved partial remission (PR) and 9 patients had SD. The median progression free survival was 2.1 months; the 1-year survival rate was 54.8%. Rash (96%) was the most common drugrelated adverse event. At first response assessment, 4 patients remained at 250 mg/m2; 8 patients were dose-escalated to 325 mg/m2; of these, 4 ultimately were increased to 400 mg/m2. Patients with progressive disease (PD) were removed from the study. Ninety-two serologic markers were analyzed from 20 patients to identify markers associated with clinical activity and/or predictive of outcome. Pretreatment levels of twelve markers were significantly elevated in patients exhibiting PD versus SD or PR; however, changes in marker levels during the course of treatment were not significant indicators of response.
Conclusions
Single-agent cetuximab showed minimal activity in patients with recurrent ovarian cancer. Patients with elevated levels of 12 serologic markers at baseline were more likely to have earlier disease progression.
Keywords: Ovarian cancer, Clinical trial, Cetuximab, Serum proteomics, Phase II
Introduction
Ovarian cancer is the leading cause of death among women with gynecologic malignancies. Ovarian cancer accounted for an estimated 22,430 new cases and 15,280 deaths in 2007 [1]. Epithelial ovarian cancer accounts for nearly 90% of all ovarian malignancies [2]. The standard therapy for advanced ovarian carcinoma is maximal cytoreductive surgery followed by platinum-based chemotherapy. In women treated with platinum-containing combinations as primary therapy, the response rates are 60-90%, with complete responses being most common in women who have optimal surgical cytoreduction. Despite these high response rates, most patients eventually die of disease persistence or recurrence, and long-term survival remains approximately 15-30% [2]. The addition of a third cytotoxic drug has not thus far resulted in increased survival [3]. Research is now focused on the evaluation of combining biologic agents with primary chemotherapy.
The epidermal growth factor (EGF) receptor (EGFR) is a member of the ERBB receptor tyrosine kinase family that consists of four structurally similar but functionally distinct membrane glycoproteins [4]. EGFR activation stimulates multiple cellular processes including cell cycle progression, inhibition of apoptosis, angiogenesis, tumor cell motility and metastasis [5]. Growth factors, such as the EGF and transforming growth factor-α, are potent mitogens for several human epithelial cell types including ovarian and have been implicated in cancer development and resistance to cisplatin [6,7]. Thus, interruption of the mitogenic signaling pathways associated with EGFR tyrosine kinase is likely to inhibit cell proliferation of malignant tumors. EGFR expression in ovarian cancer is correlated with poor prognosis for patient survival making it a potential target in new regimens combining biologics and chemotherapy.
Cetuximab (Erbitux®, Bristol-Myers Squibb, New York, NY) is a chimeric monoclonal antibody which blocks the binding of EGF to its receptor, thus inhibiting ligand induced autophosphorylation of the receptor [8]. In addition, cetuximab leads to EGFR internalization effectively removing the receptor from the cell surface preventing further interaction with ligand. Cetuximab is now an integral part of the therapy in metastatic colorectal cancer (mCRC) and in advanced head and neck cancer [9-11].
The present study was conducted to determine the safety and efficacy of single agent cetuximab for the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma. An association between the development of an acneiform skin rash and response to cetuximab-containing therapy has been observed [12-15]. Thus, this trial was designed with dose escalation to titrate dose to a grade 2 skin rash to assure delivery of sufficient drug to obtain a biological effect.
Materials and methods
Patients
Women age 18 and older with a histologic diagnosis of persistent or recurrent epithelial ovarian carcinoma or primary peritoneal carcinoma were eligible for this trial. Patients' tumors had to demonstrate any degree of EGFR expression by immunohistochemistry using Dako kits (Dako North America, Carpinteria, CA) centrally performed by ImPATH Predictive Oncology (New York, NY). Patients were permitted to have up to two prior cytotoxic regimens and were required to have a platinum free interval of less than or equal to 12 months. Patients must have recovered from recent surgery, radiation therapy or chemotherapy.
Other eligibility criteria included GOG Performance Status of 0-2 if the patient received one prior regimen; GOG Performance Status of 0 or 1 if the patient received 2 prior regimens; adequate bone marrow (absolute neutrophil count ≥1500/μl), renal (serum creatinine ≤1.5×upper limit of normal, and hepatic (bilirubin ≤1.5×upper limit of normal, hepatic transaminases and alkaline phosphatase ≤1.5×upper limit of normal) function. Patients provided written informed consent consistent with federal, state, and institutional requirements. The protocol was approved by the institutional review boards at each participating institution and done in accordance with assurances filed with and approved by the Department of Health and Human Services.
Treatment plan
Patients received single agent cetuximab in cycles consisting of 21 days. The initial dose of cetuximab was 400 mg/m2 IV administered over 120 min followed by weekly IV infusions of cetuximab 250 mg/m2 administered over 60 min.
At the end of the second cycle (week 6), response was evaluated radiographically by CT or MRI scans. Patients who achieved complete or partial remission (CR, PR) or stable disease (SD) and developed grade 2 rash, continued to receive weekly infusions of cetuximab 250 mg/m2 until disease progression or unacceptable toxicity. Patients with SD and <grade 2 rash had their weekly dose escalated by 75 mg/m2 (administered over 90 min) for one cycle (cycle 3) if there were no other drug-related grade 3 nonhematologic toxicities in prior cycles.
At the end of the third cycle (week 9), patients with CA-125 levels that had decreased by 10% or more from the week 6 CA-125 level continued to receive 325 mg/m2 weekly of cetuximab until disease progression or unacceptable toxicity. Patients with CA-125 levels that did not show response (increased or remained stable, i.e., decreased by <10% from the CA-125 value obtained at week 6), and who had <grade 2 rash, had their doses escalated by 75 mg/m2 to a dose of 400 mg/m2 weekly if there were no other drug-related grade 3 nonhematologic toxicities in prior cycles; treatment continued until disease progression or unacceptable toxicity. Patients with grade 2 rash, regardless of CA-125 level, continued to receive weekly infusions of 325 mg/m2 (or current dose) until the next radiographic response assessment. The maximum dose of cetuximab administered was 400 mg/m2 weekly since previously generated pharmacokinetic data showed EGFR saturation at this dose [16].
To prevent cetuximab-related hypersensitivity reactions, patients were premedicated with diphenhydramine hydrochloride 50 mg (or an equivalent antihistamine) intravenously before the first dose of cetuximab. Premedication was recommended before subsequent doses of cetuximab, but the premedication dose could be reduced at the discretion of the investigator.
Assessment of efficacy and safety
Patients needed to have measurable disease at the start of study treatment and were evaluated for response according to the RECIST [17]. In addition, progression-free survival (PFS) and overall survival were measured. All patients who received cetuximab were evaluated for safety. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 2.0.
Statistical analysis of clinical data
See Appendix (online only) for a description of the statistical analysis of the clinical data.
Proteomic assessments
See Appendix (online only) for a description of the immunoassays (fluorescent bead-based and traditional ELISAs) performed in this study.
Results
Patient characteristics
Twenty-five patients were enrolled onto this study (Table 1), all of whom were EGFR positive. All were treated and included in the efficacy and safety analyses. Reasons for early discontinuation of study treatment included, study drug toxicity (1 patient), clinical deterioration without documented progression (1 patient) and ileus unrelated to cetuximab treatment (1 patient). These three patients received only the loading dose. Thus, there were 22 patients evaluable for responsedose escalation assessment.
Table 1.
Patient characteristics
| Number of patients screened | 28 |
| Treated and evaluable | 25 |
| Age (years) | |
| Median | 58 |
| Range | 31-79 |
| Performance statusa | |
| 0 | 18 (72%) |
| 1 | 5 (20%) |
| 2 | 2 (8%) |
| Primary disease sitea | |
| Ovary | 18 (72%) |
| Peritoneum | 7 (28%) |
| Histological subtype | |
| Serous adenocarcinoma | 22 |
| Clear cell | 1 |
| Mixed | 1 |
| Undifferentiated | 1 |
| Number of prior cytotoxic chemotherapya | |
| 1 | 5 (20%) |
| 2 | 20 (80%) |
| Platinum resistantb (0-6 months) | 16 |
| Platinum sensitive (6-12 months) | 9 |
| Time since initial diagnosis (months) | |
| Median | 15.9 |
| Range | 5-52 |
Percentages are based on the total number of all treated subjects.
Platinum-free interval refers to time from initial therapy to first relapse.
Demographic and baseline characteristics are presented in Table 1. Sixteen patients had relapsed within 0-6 months of receiving firstline treatment (0-6 month group); 9 had relapsed within 6-12 months (6-12 month group). The median age was 58 years. Performance status was 0 (72%), 1 (20%), and 2 (8%). Seventy-two percent of patients had epithelial ovarian carcinoma, and the remaining 28% had primary peritoneal carcinoma. Twenty percent of patients had one prior regimen and 80% had two prior regimens.
Four patients in the 0-6 month group achieved a maximum cetuximab dose of 400 mg/m2. Among those who received 325 mg/m2 as their highest dose were 3 patients in the 6-12 month group and 1 in the 0-6 month group. Fifteen patients remained at a dose of 250 mg/m2 for the duration of the study, 9 in the 0-6 month groups and 6 in the 6-12 month group. Two patients in the 0-6 month group received only the loading dose of cetuximab. Figure S1 (Appendix, online only) shows a flow diagram describing the patients' initial treatment, dose escalation, and removal from the study.
Efficacy
Efficacy results are presented in Table 2. One patient in the 0-6 month group achieved a PR with a 3.5 month duration yielding an overall response rate of 4%. The trial was stopped after the first 25 patients due to futility since there was only one objective response observed (in a patient with a serous carcinoma). Another 36% achieved SD (31% for the 0-6 month group and 44% for the 6-12 months group). The median PFS was 1.8 months (95% CI 1.3-2.6). The median overall survival was 13 months (95% CI 5.6- not reached). PFS and overall survival at the other time points are also listed in Table 2.
Table 2.
Outcome statistics
| Relapse within 0-6 months (n = 16) | Relapse within 6-12 months (n = 9) | Total (n = 25) | |
|---|---|---|---|
| Overall response | 1 (6.3%) | 0 | 1 (4%) |
| Stable disease | 5 (31.3%) | 4 (44.4%) | 9 (36%) |
| Progression-free survival | |||
| Median, months | 1.6 (1.3-2.7)a | 2.1 (1.4-2.6)a | 1.8 (1.3-2.6)a |
| @ 4 months | 4 (25%) | 1 (11.1%) | 5 (20%) |
| @ 6 months | 3 (18.8%) | 0 | 3 (12%) |
| @ 12 months | 0 | 0 | 0 |
| Overall survival | |||
| Median, months | 8.2 (5.6-NRb)a | NRb (13.6-NRb)a | 13 (5.6-NRb)a |
| @ 4 months | 14 (87.5%) | 9 (100%) | 23 (92%) |
| @ 6 months | 10 (62.5%) | 9 (100%) | 19 (76%) |
| @ 12 months | 3 (37.5%) | 7 (77.8%) | 10 (40%) |
95% confidence interval.
NR = not reached.
Toxicity
Table 3 summarizes all of the reported grade 3 and 4 toxicities. The most common grade 3 toxicities were arthralgia, headache, and acneiform rash. One patient experienced a grade 4 infusion related reaction leading to the patient's discontinuation of study treatment. One other patient discontinued study treatment due to reclassification of the original tumor as being of gastrointestinal origin (this patient also had the aforementioned ileus). Overall, the most common toxicities for all grades were acneiform rash, headache, and asthenia/malaise.
Table 3.
Grade 3 or 4 toxicities- all treated patients
| n = 25 | Any grade | Grade 3 | Grade 4 |
|---|---|---|---|
| Acneiform rash | 24 (96%) | 2 (8%) | 0 |
| Arthalgias | 4 (16%) | 2 (8%) | 0 |
| Headache | 12 (48%) | 2 (8%) | 0 |
| Asthenia/Malaise | 11 (44%) | 1 (4%) | 0 |
| Chills | 9 (36%) | 0 | 0 |
| Nausea | 7 (28%) | 0 | 0 |
| Stomatitis | 6 (24%) | 0 | 0 |
| Constipation | 6 (24%) | 0 | 0 |
| Diarrhea | 5 (20%) | 0 | 0 |
| Infusion reaction | 1 (4%) | 0 | 1 (4%) |
The incidence and severity of the acneiform rash is summarized in Table 4 according to the worst CTC grade. The patient with the PR who received a maximum dose of 400 mg/m2 weekly of cetuximab had a grade 3 rash. There were three patients who received maximum cetuximab doses of 400 mg/m2 and achieved SD as their best response. Among the four patients who received a maximum dose of 325 mg/m2, 1 patient with SD experienced a grade 1 rash and 2 patients with SD experienced grade 2 rashes. Of the 14 patients administered 250 mg/m2, there were three patients whose maximum dose was 250 mg/m2 who achieved SD. In addition, there were 11 patients receiving 250 mg/m2 who had progressive disease (PD) and experienced rash including 7 with grade 1 rash, 3 with grade 2 rash, and 1 with grade 3 rash.
Table 4.
Tumor response and acneiform rash severity by maximum dose
| Dose | Number of patients | Responses at each dose and grade |
|---|---|---|
| 22a | ||
| 250 mg/m2 rash | ||
| Grade 0,1 | 8 | 1SDb, 7PDc |
| Grade 2 | 5 | 2 SD, 3PD |
| Grade 3 | 1 | 1PD |
| 325 mg/m2 rash | ||
| Grade 0,1 | 1 | 1SD |
| Grade 2 | 3 | 2SD, 1UDd |
| Grade 3 | 0 | |
| 400 mg/m2 rash | ||
| Grade 0,1 | 1 | 1SD |
| Grade 2 | 2 | 2SD |
| Grade 3 | 1 | 1PRe |
3 patients received only the loading dose (1 due to rapid progression; 1 due to severe hypersensitivity reaction; 1 due to reclassification of original histology.
SD = stable disease.
PD = progressive disease.
UD = undeterminable.
PR = partial remission.
Proteomic analysis
Proteomic assays were performed to evaluate serum proteins prior to and during the course of treatment to identify potential markers associated with clinical activity and/or predictive of outcome. Patient samples prior to cetuximab treatment were available from 10 of the 12 patients who showed PD, from all 9 patients who showed SD and the 1 patient who had a PR for serum-based proteomic analysis. For this analysis, the SD and PR patients were grouped together. Immunoassays for 92 markers including many cytokines and chemokines, growth and angiogenic factors, hormones, proteases, and apoptotic and cell adhesion molecules were performed on these 20 pretreatment samples. These markers were chosen because many have been reported to have an association with ovarian cancer [18-23] (A.E. Lokshin and A.K. Godwin, unpublished data) or in general are cancer antigens. Some are directly related to the cetuximab treatment itself, e.g., EGFR, TGF-α, amphiregulin, epiregulin. A complete list of the markers measured is provided in Table S1 (Appendix, online only).
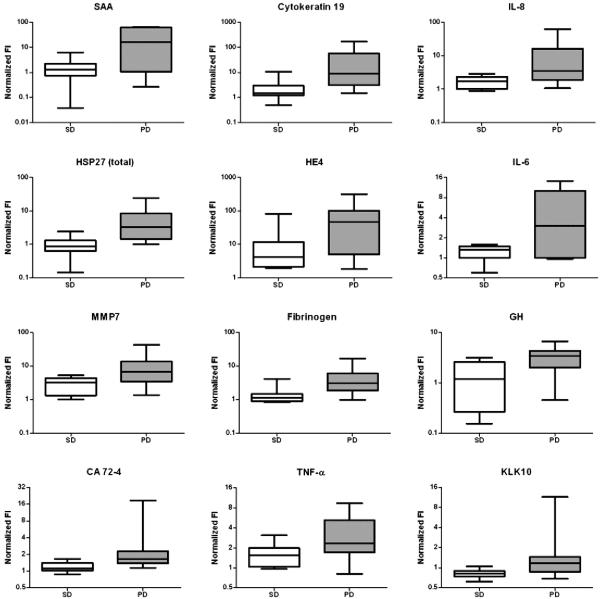
SAM (Significance Analysis of Microarrays) methodology [24] was employed to compare the log-transformed, normalized serum measurements for the PD and PR/SD groups. Serum levels of 12 of the proteins were found to be significantly different between the two groups (at least a two-fold change with a false discovery rate (FDR) of at most 10%) (Fig. 1 and Table S2 (Appendix, online only)). These 12 proteins (SAA, cytokeratin 19, IL-8, HSP27, HE4, IL-6, MMP7, fibrinogen, GH, CA 72-4, TNF-α and KLK10) were all elevated in the PD group relative to the PR/SD group at the time treatment was initiated.
Fig. 1.
Box plots show normalized fluorescent intensity values of protein markers in pretreatment serum samples from patients who displayed SD or a PR (white boxes) and patients who displayed PD (gray boxes). Only markers with a ≥ two-fold difference between the two groups and a ≤10% FDR are shown.
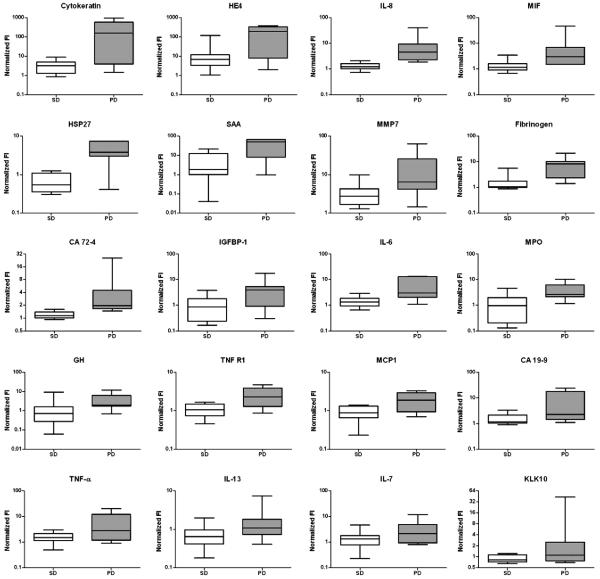
Sera from patients just prior to the start of cycle 3 (i.e. following 6 weeks of treatment) also were evaluated for levels of the same ninety-two markers measured in the pretreatment samples in order to answer the following questions: 1) What proteins are differentially expressed between PD and PR/SD groups at the start of cycle 3 and 2) what proteins show changes in expression relative to pretreatment levels following two cycles of treatment. For these measurements, serawere available from 7 out of the 12 patients who showed PD, 8 out of the 9 patients who showed SD and the one patient who had PR. A total of 20 proteins showed a significant difference between the two groups in these post-treatment serum samples (Fig. 2 and Table S3 (Appendix, online only)). Serum levels of all 20 proteins were elevated in the PD group relative to the PR/SD group.
Fig. 2.
Box plots show normalized fluorescent intensity values of protein markers in serum samples post treatment from patients who displayed SD or a PR (white boxes) and patients who displayed PD (gray boxes). Only markers with a ≥ two-fold difference between the two groups and a ≤10% FDR are shown.
When cycle 3 data were analyzed for changes in unique markers relative to pretreatment levels which would distinguish the PD and PR/SD groups, no such markers with significant differences from baseline levels unique to one group were observed. However, when we analyzed these data for changes in marker levels from baseline levels within a particular group (either the PD or PR/SD), we did observe increased levels of 12 markers in the PD group and 2 markers in the PR/SD group. The PR/SD group also showed 2 markers with decreased levels relative to baseline values. The results of these analyses are reported in Table S4 (Appendix, online only).
Discussion
Cetuximab has become part of the standard of care in the treatment of mCRC and locally advanced and metastatic head and neck cancer [9-11]. Monoclonal antibodies against EGFR have a different spectrum of activity compared with small molecule tyrosine kinase inhibitors (TKIs) [25]. For example, monoclonal antibodies against EGFR are active in colon cancer while small molecule TKIs are not. We and others have shown that gefitinib and erlotinib were inactive in recurrent ovarian carcinoma [26,27]. The low response rate correlated with a low activation mutation rate of EGFR [26,27]. More recently, EMD72000 (matuzumab) has been reported as inactive but well tolerated in patients with recurrent ovarian cancer [28]. Only 5% of the patients had PFS greater than 6 months.
There is a known correlation between clinical outcome and the intensity of skin rash in patients with mCRC treated with cetuximab [14]. Similar results were reported in patients with ovarian cancer treated with erlotinib and gefitinib [26,27]. In light of these findings, the current trial was designed to evaluate if cetuximab, dose escalated to induce grade 2 acneiform rash, to ensure delivery of a biologically active dose, would increase the response rate in patients with recurrent ovarian carcinoma. The low level of clinical efficacy and small number of patients in this trial precludes identifying a relationship between dose and response. In a similar but larger trial involving 166 evaluable patients with mCRC, dose escalation of single agent cetuximab to grade 2 rash improved its response rate [14].
Cetuximab has been combined with carboplatin and paclitaxel as front-line treatment in a feasibility trial [29]. The data from the first 17 accrued patients showed that combination was well tolerated. Median PFS of 14.4 months though was not better than that observed in large frontline trials such as GOG 182 (approximately 16 months) [3] although clearly, comparisons with historical controls are not conclusive. EGFR targeted therapy seems to have minimal activity as a single agent treatment and offers little improvement when combined with chemotherapy in the treatment of epithelial ovarian carcinoma.
While the development of cetuximab has explored clinical utility by evaluating its use in nearly all subjects, efforts are needed to better identify patients more likely to benefit from this therapy. A targeted approach to patient selection could potentially improve survival, spare patients' needless toxicity and reduce expenses associated with futile therapy. It may also be possible to accelerate the development of cetuximab in front-line indications by enrichment of clinical trial populations with those patients that are more likely to respond. For example, we and others have shown that mCRC patients with a mutation in K-RAS are less likely to respond to cetuximab [30-33].Other studies have since discovered that the K-RAS mutation also predicts response to a related drug, panitumumab [34,35]. Although K-RAS mutations have been reported in ovarian cancers, especially the mucinous subtype [36], it is unlikely that lack of activity observed in our trial can be attributed to RAS mutations. In addition, we have reported previously that expression and/or mutation of the EGFR was associated with response to another EGFR inhibitor, gefitinib [27]; however we have no evidence that either were a factor in the clinical responses observed in this trial. Therefore, additional progress is needed to identify biomarkers that may prove useful for selecting ovarian cancer patients with an improved chance of responding to therapy [37].
In this aspect, a goal of this study was to assess the utility of screening the serum of patients for proteins potentially associated with drug activity and/or clinical outcome. Many clinical trials incorporate correlative components including serum proteomic analysis to identify predictors of response to therapy [38-45]. However, most of these studies are limited in the scope of markers evaluated. Tumors have systemic effects that are best evaluated by monitoring the numerous proteins that represent a variety of pathways. The predictive capabilities of multi-marker combinations tend to be superior to any single marker [19,20,23,46-48]. The current study incorporated multiplexed immunoassays to measure nearly 100 serum proteins as part of this multi-center Phase II clinical trial. Unfortunately, our study was limited due to the number of patients showing clinical response, thus precluding enrollment of patients in this two-stage study design. Nevertheless, we identify 12 markers whose baseline levels were significantly elevated in patients who had PD relative to PR/SD (Fig. 1 and Table S2). These proteins remained elevated following two cycles of cetuximab treatment (Fig. 2 and Table S3) suggesting these may be important prognostic markers rather than predictors of response to therapy.
The markers identified include several cytokines (IL-6, IL-8, TNF-α) that have immunomodulatory properties, serum levels of which have all been well documented to be associated with ovarian cancer [49-60]. Proteases (MMP7 and KLK10) that may facilitate metastasis have also been reported to be associated with ovarian cancer [21,61-65]. HE4 is a relatively novel serum marker that has been recently reported as well [66-68]. Most of the remaining markers have also been reported in serologic evaluations related to ovarian cancer [69-72]. These reports and the current data warrant further validation of these markers as being predictive of disease progression. The promise of personalized medicine is gaining ground; however, a quick and easy test to direct an entire course of treatment is not yet reality. The ability to scan a patient's tumor tissue and/or blood for proteins, genes, or other traces of molecular information that will delineate the specific nature of that individual's disease is technically possible. The type of exploratory studies we report are necessary and timely to advance personalized care for ovarian cancer patients, but will clearly benefit greatly from larger trials with more favorable responses. Nevertheless, personalized care, once the appropriate markers are discovered, will have profound implications for patients, their physicians, and the entire pharmaceutical industry.
Supplementary Material
Acknowledgments
We thank Adele M. Marrangoni, Brian Nolen, Liudmila Velikokhatnaya, Matthew T. Winans, Denise Prosser, Dandan Chen for technical assistance and Dr. Kathleen Alpaugh for protocol support of the clinical trial.
The study was supported in part by Bristol-Myers Squibb Company, New York, New York, the Ovarian Cancer Research Fund, and the Ovarian Cancer SPORE at FCCC (P50 CA083638).
Footnotes
Conflict of interest statement Russell J. Schilder-No conflict of interest.
Harsh B. Pathak-No conflict of interest.
Anna E. Lokshin-No conflict of interest.
Robert W. Holloway-No conflict of interest.
Ronald D. Alvarez-No conflict of interest.
Carol Aghajanian-No conflict of interest.
Hua Min-No conflict of interest.
Karthik Devarajan-No conflict of interest.
Eric Ross-No conflict of interest.
Charles W. Drescher-No conflict of interest.
Andrew K. Godwin-ImClone (research funding).
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygyno.2008.12.003.
References
- [1].Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- [2].Ozols RFRS, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJPC, Young RC, Barakat RR, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 895–987. [Google Scholar]
- [3].Bookman MA, for the Gynecologic Cancer InterGroup through the Gynecologic Oncology G GOG0182-ICON5: 5-arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. ASCO Meet Abstr. 2006;24:5002. [Google Scholar]
- [4].Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- [5].Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–65. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [6].Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–80. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- [7].Strawn LM, Shawver LK. Tyrosine kinases in disease: overview of kinase inhibitors as therapeutic agents and current drugs in clinical trials. Expert Opin Investig Drugs. 1998;7:553–73. doi: 10.1517/13543784.7.4.553. [DOI] [PubMed] [Google Scholar]
- [8].Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983;80:1337–41. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- [10].Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an eastern cooperative oncology group study. J Clin Oncol. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- [11].Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- [12].Saltz L, Kies M, Abbruzzese J, Azarnia N, Needle M. The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies. 2003 ASCO Annual Meeting. 2003 [Google Scholar]
- [13].Susman E. Rash correlates with tumour response after cetuximab. Lancet Oncol. 2004;5:647. doi: 10.1016/s1470-2045(04)01627-4. [DOI] [PubMed] [Google Scholar]
- [14].Tejpar S, Peeters M, Humblet Y, Gelderblom H, Vermorken J, Viret F, et al. Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): pharmacokinetic (PK), pharmacodynamic (PD) and efficacy data. ASCO Meeting Abstracts. 2007;25:4037. [Google Scholar]
- [15].Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–46. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- [16].Fracasso PM, Burris H, 3rd, Arquette MA, Govindan R, Gao F, Wright LP, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–93. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]
- [17].Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States, National cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [18].Bast RC, Jr, Brewer M, Zou C, Hernandez MA, Daley M, Ozols R, et al. Prevention and early detection of ovarian cancer: mission impossible? Recent Results Cancer Res. 2007;174:91–100. doi: 10.1007/978-3-540-37696-5_9. [DOI] [PubMed] [Google Scholar]
- [19].Yurkovetsky ZR, Linkov FY, D EM, Lokshin AE. Multiple biomarker panels for early detection of ovarian cancer. Future Oncol. 2006;2:733–41. doi: 10.2217/14796694.2.6.733. [DOI] [PubMed] [Google Scholar]
- [20].Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:7677–82. doi: 10.1073/pnas.0502178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–77. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- [22].Bast RC., Jr Status of tumor markers in ovarian cancer screening. J Clin Oncol. 2003;21:200s–5s. doi: 10.1200/JCO.2003.01.068. [DOI] [PubMed] [Google Scholar]
- [23].Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–72. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- [24].Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diasio RB, Fourie J. Targeting the epidermal growth factor receptor in the treatment of colorectal cancer: state of the art. Drugs. 2006;66:1441–63. doi: 10.2165/00003495-200666110-00003. [DOI] [PubMed] [Google Scholar]
- [26].Gordon AN, Finkler N, Edwards RP, Garcia AA, Crozier M, Irwin DH, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–92. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- [27].Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a gynecologic oncology group study. Clin Cancer Res. 2005;11:5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- [28].Seiden MV, Burris HA, Matulonis U, Hall JB, Armstrong DK, Speyer J, et al. A phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol Oncol. 2007;104:727–31. doi: 10.1016/j.ygyno.2006.10.019. [DOI] [PubMed] [Google Scholar]
- [29].Konner J, Schilder RJ, DeRosa FA, Gerst SR, Tew WP, Sabbatini PJ, et al. A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol Oncol. 2008;110:140–5. doi: 10.1016/j.ygyno.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [30].Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- [31].Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- [32].Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97:1139–45. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- [34].Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- [35].Freeman DJ, Juan T, Reiner M, Hecht JR, Meropol NJ, Berlin J, et al. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer. 2008;7:184–90. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- [36].Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- [37].Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–52. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- [38].Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–72. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- [39].Lipton A, Ali SM, Leitzel K, Demers L, Evans DB, Hamer P, et al. Elevated plasma tissue inhibitor of metalloproteinase-1 level predicts decreased response and survival in metastatic breast cancer. Cancer. 2007;109:1933–9. doi: 10.1002/cncr.22637. [DOI] [PubMed] [Google Scholar]
- [40].McMeekin DS, Sill MW, Darcy KM, Stearns-Kurosawa DJ, Webster K, Waggoner S, et al. A phase II trial of thalidomide in patients with refractory leiomyosarcoma of the uterus and correlation with biomarkers of angiogenesis: a gynecologic oncology group study. Gynecol Oncol. 2007;106:596–603. doi: 10.1016/j.ygyno.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [41].Sharma R, Rivory L, Beale P, Ong S, Horvath L, Clarke SJ. A phase II study of fixed-dose capecitabine and assessment of predictors of toxicity in patients with advanced/metastatic colorectal cancer. Br J Cancer. 2006;94:964–8. doi: 10.1038/sj.bjc.6603049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Souder C, Leitzel K, Ali SM, Demers L, Evans DB, Chaudri-Ross HA, et al. Serum epidermal growth factor receptor/HER-2 predicts poor survival in patients with metastatic breast cancer. Cancer. 2006;107:2337–45. doi: 10.1002/cncr.22255. [DOI] [PubMed] [Google Scholar]
- [43].Burger RA, Darcy KM, DiSaia PJ, Monk BJ, Grosen EA, Gatanaga T, et al. Association between serum levels of soluble tumor necrosis factor receptors/CA 125 and disease progression in patients with epithelial ovarian malignancy: a gynecologic oncology group study. Cancer. 2004;101:106–15. doi: 10.1002/cncr.20314. [DOI] [PubMed] [Google Scholar]
- [44].Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002;20:1467–72. doi: 10.1200/JCO.2002.20.6.1467. [DOI] [PubMed] [Google Scholar]
- [45].Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, et al. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. J Clin Oncol. 2008;26:3418–25. doi: 10.1200/JCO.2007.14.3420. [DOI] [PubMed] [Google Scholar]
- [46].Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- [47].Linkov F, Lisovich A, Yurkovetsky Z, Marrangoni A, Velikokhatnaya L, Nolen B, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiol Biomark Prev. 2007;16:102–7. doi: 10.1158/1055-9965.EPI-06-0602. [DOI] [PubMed] [Google Scholar]
- [48].Yurkovetsky Z, Ta'asan S, Skates S, Rand A, Lomakin A, Linkov F, et al. Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin. Gynecol Oncol. 2007;107:58–65. doi: 10.1016/j.ygyno.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F, et al. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol. 2006;102:244–51. doi: 10.1016/j.ygyno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- [50].Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomark Prev. 2005;14:981–7. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- [51].Uslu R, Sanli UA, Dikmen Y, Karabulut B, Ozsaran A, Sezgin C, et al. Predictive value of serum interleukin-8 levels in ovarian cancer patients treated with paclitaxel-containing regimens. Int J Gynecol Cancer. 2005;15:240–5. doi: 10.1111/j.1525-1438.2005.15210.x. [DOI] [PubMed] [Google Scholar]
- [52].Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–5. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- [53].Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- [54].Foti E, Ferrandina G, Martucci R, Romanini ME, Benedetti Panici P, Testa U, et al. IL-6, M-CSF and IAP cytokines in ovarian cancer: simultaneous assessment of serum levels. Oncology. 1999;57:211–5. doi: 10.1159/000012033. [DOI] [PubMed] [Google Scholar]
- [55].Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- [56].Chopra V, Dinh TV, Hannigan EV. Angiogenin, interleukins, and growth-factor levels in serum of patients with ovarian cancer: correlation with angiogenesis. Cancer J Sci Am. 1996;2:279–85. [PubMed] [Google Scholar]
- [57].Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R, Gadducci A, et al. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer. 1995;71:354–6. doi: 10.1038/bjc.1995.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schroder W, Ruppert C, Bender HG. Concomitant measurements of interleukin-6 (IL-6) in serum and peritoneal fluid of patients with benign and malignant ovarian tumors. Eur J Obstet Gynecol Reprod Biol. 1994;56:43–6. doi: 10.1016/0028-2243(94)90152-x. [DOI] [PubMed] [Google Scholar]
- [59].Moradi MM, Carson LF, Weinberg B, Haney AF, Twiggs LB, Ramakrishnan S. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer. 1993;72:2433–40. doi: 10.1002/1097-0142(19931015)72:8<2433::aid-cncr2820720822>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [60].Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martinez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164:1038–42. doi: 10.1016/0002-9378(91)90582-c. [DOI] [PubMed] [Google Scholar]
- [61].Acar A, Onan A, Coskun U, Uner A, Bagriacik U, Atalay F, et al. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol. 2008;25:279–83. doi: 10.1007/s12032-007-9031-1. [DOI] [PubMed] [Google Scholar]
- [62].Oikonomopoulou K, Scorilas A, Michael IP, Grass L, Soosaipillai A, Rosen B, et al. Kallikreins as markers of disseminated tumour cells in ovarian cancer— a pilot study. Tumour Biol. 2006;27:104–14. doi: 10.1159/000092325. [DOI] [PubMed] [Google Scholar]
- [63].Shvartsman HS, Lu KH, Lee J, Lillie J, Deavers MT, Clifford S, et al. Overexpression of kallikrein 10 in epithelial ovarian carcinomas. Gynecol Oncol. 2003;90:44–50. doi: 10.1016/s0090-8258(03)00257-9. [DOI] [PubMed] [Google Scholar]
- [64].Luo LY, Katsaros D, Scorilas A, Fracchioli S, Bellino R, van Gramberen M, et al. The serum concentration of human kallikrein 10 represents a novel biomarker for ovarian cancer diagnosis and prognosis. Cancer Res. 2003;63:807–11. [PubMed] [Google Scholar]
- [65].Luo LY, Bunting P, Scorilas A, Diamandis EP. Human kallikrein 10: a novel tumor marker for ovarian carcinoma? Clin Chim Acta. 2001;306:111–8. doi: 10.1016/s0009-8981(01)00401-6. [DOI] [PubMed] [Google Scholar]
- [66].Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [67].Scholler N, Crawford M, Sato A, Drescher CW, O'Briant KC, Kiviat N, et al. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006;12:2117–24. doi: 10.1158/1078-0432.CCR-05-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–700. [PubMed] [Google Scholar]
- [69].Ogata Y, Heppelmann CJ, Charlesworth MC, Madden BJ, Miller MN, Kalli KR, et al. Elevated levels of phosphorylated fibrinogen-alpha-isoforms and differential expression of other post-translationally modified proteins in the plasma of ovarian cancer patients. J Proteome Res. 2006;5:3318–25. doi: 10.1021/pr060344+. [DOI] [PubMed] [Google Scholar]
- [70].Moshkovskii SA, Serebryakova MV, Kuteykin-Teplyakov KB, Tikhonova OV, Goufman EI, Zgoda VG, et al. Ovarian cancer marker of 11.7 kDa detected by proteomics is a serum amyloid A1. Proteomics. 2005;5:3790–7. doi: 10.1002/pmic.200401205. [DOI] [PubMed] [Google Scholar]
- [71].Skates SJ, Horick N, Yu Y, Xu FJ, Berchuck A, Havrilesky LJ, et al. Preoperative sensitivity and specificity for early-stage ovarian cancer when combining cancer antigen CA-125II, CA 15-3, CA 72-4, and macrophage colony-stimulating factor using mixtures of multivariate normal distributions. J Clin Oncol. 2004;22:4059–66. doi: 10.1200/JCO.2004.03.091. [DOI] [PubMed] [Google Scholar]
- [72].Gadducci A, Ferdeghini M, Cosio S, Fanucchi A, Cristofani R, Genazzani AR. The clinical relevance of serum CYFRA 21-1 assay in patients with ovarian cancer. Int J Gynecol Cancer. 2001;11:277–82. doi: 10.1046/j.1525-1438.2001.011004277.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.