Abstract
Objective
To estimate the association between maternal age and fetal death (spontaneous abortion, ectopic pregnancy, stillbirth), taking into account a woman's reproductive history.
Design
Prospective register linkage study.
Subjects
All women with a reproductive outcome (live birth, stillbirth, spontaneous abortion leading to admission to hospital, induced abortion, ectopic pregnancy, or hydatidiform mole) in Denmark from 1978 to 1992; a total of 634 272 women and 1 221 546 pregnancy outcomes.
Main outcome measures
Age related risk of fetal loss, ectopic pregnancy, and stillbirth, and age related risk of spontaneous abortion stratified according to parity and previous spontaneous abortions.
Results
Overall, 13.5% of the pregnancies intended to be carried to term ended with fetal loss. At age 42 years, more than half of such pregnancies resulted in fetal loss. The risk of a spontaneous abortion was 8.9% in women aged 20-24 years and 74.7% in those aged 45 years or more. High maternal age was a significant risk factor for spontaneous abortion irrespective of the number of previous miscarriages, parity, or calendar period. The risk of an ectopic pregnancy and stillbirth also increased with increasing maternal age.
Conclusions
Fetal loss is high in women in their late 30s or older, irrespective of reproductive history. This should be taken into consideration in pregnancy planning and counselling.
Introduction
An increasing risk of fetal death, and in particular spontaneous abortion, with increasing maternal age has been observed by several authors.1–7 Previous spontaneous abortions and multigravidity are also well known risk factors for spontaneous abortion in subsequent pregnancies.8–10 As these factors are highly correlated, it remains to be evaluated what the effect of maternal age is when taking into account a woman's reproductive history.11,12
As the association between age and spontaneous abortion reflects both biological mechanisms and forces of selection, the significance of the association is expected to change over time. Decades ago, older pregnant women were mainly those with low fecundity or high parity. At present, many women delay childbearing for social reasons.
To study the effects of maternal age on fetal loss we derived our data from the population based Danish health registries, which cover the population of Denmark. This allowed us to control for the confounding effects of reproductive history and calendar period.
Subjects and methods
Since 1 April 1968, the civil registration system in Denmark has assigned an individual, unique registration number to all citizens. This number permits accurate linkage of information from different registries. A research database of parity was established with data from this registration system, including information on all live births to women born between 1 April 1935 and 31 March 1978. This is described in detail elsewhere.13
For our study, we added mandatory reported information on fetal death (spontaneous abortion, hydatidiform mole, ectopic pregnancy, and stillbirth) and induced abortion from three national health registries. The national discharge registry, established in 1977, comprises diagnoses at discharge for all patients admitted to hospital. From this registry we obtained all cases of spontaneous abortion, hydatidiform mole, and ectopic pregnancy. The medical birth registry, established in 1973, contains information on all births in Denmark. From this registry we obtained information on stillbirths. Information on induced abortions was obtained from the national register of induced abortions. We identified all reproductive outcomes in Denmark in the period 1978-92. Reproductive outcome was defined as live birth, stillbirth, spontaneous abortion including hydatidiform mole, ectopic pregnancy, or induced abortion.
We restricted analyses concerning the combined effects of maternal age and reproductive history on outcome of pregnancy to pregnancy outcomes in the period 1988-92. In these analyses reproductive history was exact parity status (complete information on births) and complete information on other reproductive outcomes in the preceding 10 years.
Maternal age at conception was estimated by deducting gestational age at birth from maternal age at delivery. Gestational age was recorded for more than 90% of the live births and stillbirths, and for the remaining cases we applied the mean gestational age for the entire population. As gestational age was not recorded for spontaneous abortion, hydatidiform mole, and ectopic pregnancy, we set this at 9 weeks, 12 weeks, and 8 weeks respectively.
According to Danish standards, a stillbirth is defined as the birth of a child with a gestational age of 28 weeks or more who does not show any sign of life. Parity was defined in two groups: nulliparous women (no previous live births or stillbirths) and parous women.
We estimated the risk of fetal loss according to maternal age as a proportion of all pregnancies intended to be carried to term—that is, live births, stillbirths, spontaneous abortions, and ectopic pregnancies. For the risk of stillbirth according to maternal age, only pregnancies at risk of becoming a stillbirth were taken into consideration, and consequently the risk constitutes the proportion of stillbirths among all births.
The number of pregnancies intended to be carried to term might be slightly biased because some fetal losses occurred before an intended induced abortion. Such cases would wrongly be counted as intended pregnancies. To evaluate this bias we estimated an adjusted number of fetal losses by deducting the expected number of pregnancies that ended as a fetal loss before an intended induced abortion from the total number of fetal losses. The adjusted risk of fetal loss was calculated as the adjusted number of fetal losses divided by the number of live births plus adjusted number of fetal losses.
Results
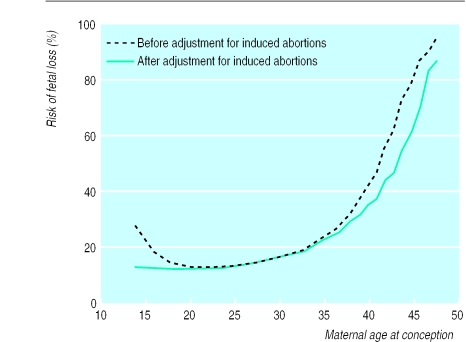
From 1978-92, a total of 634 272 women had 1 221 546 pregnancies, of which 126 673 ended in fetal loss, 285 022 in an induced abortion, and 809 762 in a live birth (table). The overall risk of fetal loss was 13.5%. The risk of fetal loss according to maternal age at conception followed a J-shaped curve, with a steep increase after 35 years of age (fig 1). More than one fifth of all pregnancies in 35 year old women resulted in fetal loss, and at 42 years of age more than half of the intended pregnancies (54.5%) resulted in fetal loss. After adjustment for induced abortions, the increased risk of fetal loss disappeared in women aged less than 20 years and the increased risk in women aged more than 35 years was at a slightly lower level (fig 1).
Figure 1.
Risk of fetal loss from spontaneous abortion, ectopic pregnancy, and stillbirth according to maternal age at conception
Spontaneous abortion
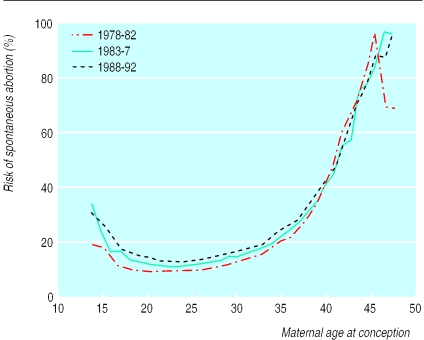
Spontaneous abortion accounted for 80% of fetal losses. The overall risk of spontaneous abortion was 10.9%. A curve was observed for the association between maternal age at conception and spontaneous abortion, which was similar to that for all fetal losses (fig 2). The risk of spontaneous abortion varied from a minimum of 8.7% by the age of 22 years to 84.1% by the age of 48 years or more. When the figures were adjusted for the effect of induced abortion, the risk in women in their teens was similar to those in their early 20s.
Figure 2.
Risk of spontaneous abortion according to maternal age at conception, stratified according to calendar period
The overall risk of spontaneous abortion leading to admission to hospital increased from 9.3% in 1978-82 to 11.1% in 1983-87 and 12.5% in 1988-92. However, the association between maternal age and risk of spontaneous abortion was not confounded by calendar period or maternal birth cohort.
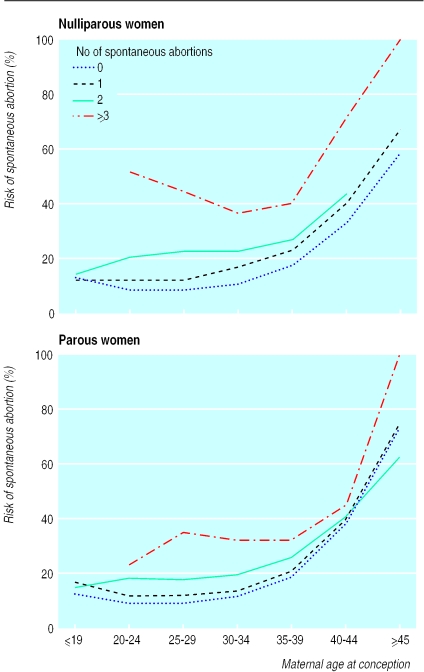
Figure 3 shows the age related risk of spontaneous abortion stratified by parity status and number of previous spontaneous abortions. The association between spontaneous abortion and age was similar in all strata, although the level increased with increasing number of previous spontaneous abortions. The incidence of spontaneous abortion varied according to a woman's parity and number of spontaneous abortions in the preceding 10 years; among women aged 25-29 years spontaneous abortion occurred in 8.9% of nulliparous women and 9.3% of parous women without a history of spontaneous abortion, in 12.4% and 11.8% of those with a history of one spontaneous abortion, and in 22.7% and 17.7% of those with a history of two spontaneous abortions. After three or more spontaneous abortions, the proportion of pregnancies ending in spontaneous abortion increased to 44.6% in nulliparous women and 35.4% in parous women.
Figure 3.
Risk of spontaneous abortion in nulliparous and parous women according to maternal age at conception and number of spontaneous abortions in preceding 10 years
In women with no history of spontaneous abortions we found a slightly lower overall risk of spontaneous abortion among nulliparous women than parous women (10.0% v 11.6%). This tendency was found in all strata of age except for women aged 40-44 years. Among women with a history of spontaneous abortion, the reverse tendency was observed; in general, nulliparous women had a higher age specific risk than did parous women (fig 3).
Under the assumption that only 80% of women with abortions in recognised pregnancies were hospitalised the risk of spontaneous abortion would be: 12-19 years, 13.3%; 20-24, 11.1%; 25-29, 11.9%; 30-34, 15.0%; 35-39, 24.6%; 40-44, 51.0%; and 45 or more, 93.4%.
Ectopic pregnancy
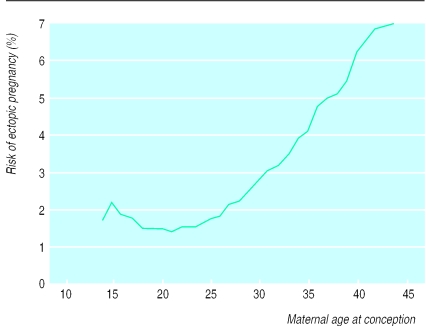
The overall risk of ectopic pregnancy was 2.3%. The incidence of ectopic pregnancy showed a steady increase with increasing maternal age at conception from 1.4% of all pregnancies at the age of 21 years to 6.9% of pregnancies in women aged 44 years or more (fig 4). This association was not confounded by calendar period.
Figure 4.
Risk of ectopic pregnancy according to maternal age at conception
Stillbirth
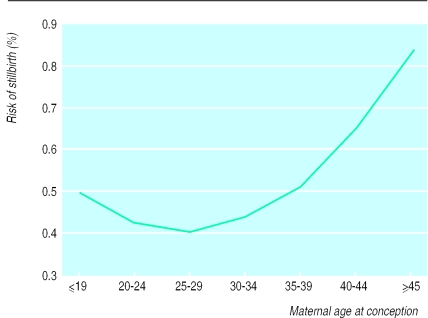
The overall risk of stillbirth was 4.3 per thousand women. The association between maternal age and stillbirth showed a J-shaped curve, but the effect of age was less than for spontaneous abortions and ectopic pregnancies (fig 5). When restricting the analysis to nulliparous women, we found an identical pattern, although the level was slightly higher. The proportion of stillbirths was substantially increased in teenage pregnancies and was at the same level as for the 35-39 year age group. The incidence of stillbirth was unchanged during the study period.
Figure 5.
Risk of stillbirth according to maternal age at conception
Discussion
Our study shows an increasing risk of fetal loss with increasing maternal age in women aged more than 30 years. At 42 years of age, more than half of all pregnancies resulted in a spontaneous abortion, ectopic pregnancy, or stillbirth. The increasing risk of spontaneous abortion was observed in both nulliparous and parous women, regardless of parity, number of previous spontaneous abortions, or calendar period. Thus, although maternal age is highly correlated with parity and reproductive history, our data clearly show a strong and independent effect of maternal age on the risk of spontaneous abortion.
Age—or bias from selection?
The observed association could be the result of age related changes such as an increase in conceptions that are chromosomally abnormal or decreasing uterine and hormonal function. Also, age might be a surrogate measure of cumulative exposure to unknown factors.15,16
It could be argued that women at a particularly high risk of spontaneous abortion are overrepresented among pregnant women of high maternal age because of the tendency to carry on in an attempt to achieve the desired number of children. However, we found the same increasing risk of spontaneous abortion with increasing maternal age when restricting the analysis to women with no history of spontaneous abortion in the previous 10 years (women with an average low propensity of spontaneous abortion) and women with a history of live births (women with an average higher degree of achievement of the desired number of live births).
Alternatively, the increased risk of spontaneous abortion in older age groups could in part be explained by subfertile women reaching a higher average maternal age before achieving a pregnancy. Thus, subfertile women have a higher risk of spontaneous abortion than fertile women.17 Following this argument, however, the effect of maternal age in nulliparous women with no history of spontaneous abortions should be stronger than in women with a history of only live births, which was not observed. In women without a history of spontaneous abortion, we observed a slightly higher risk of spontaneous abortion in parous than nulliparous women.
Methodological issues
The estimated risk of fetal loss in pregnancies intended to be carried to term might be slightly biased by the fact that several pregnancies ended in fetal loss before an intended induced abortion. Such cases would wrongly be counted as an intended pregnancy. This bias was found to be particularly relevant to risk estimates in women aged less than 20 years but had only a minor influence on risk estimates in other ages.
Regan et al and others stated that “as the most predictive factor for spontaneous abortion is a previous spontaneous abortion, the outcome of a woman's first pregnancy has profound consequences for all subsequent pregnancies.”18 Our study does not agree with this statement. We found a substantially lower risk of spontaneous abortion among women under 30 years of age with a history of spontaneous abortion in their first pregnancy than among women aged 35 years or more with a history including only live births.
Our study was based on data from population based registers, with complete information on all pregnancy outcomes leading to hospital admission over a period of 15 years and complete live birth history for all women in the cohort. This rules out biases such as selection related to non-responders and differential misclassification of a woman's reproductive history.
Our results were restricted to spontaneous abortions leading to admission to hospital. If the tendency to be admitted was dependent on maternal age, this might confound the association. It is, however, unlikely that a differential referral to hospital could explain more than a minor part of the association. In the hypothetical scenario where all women aged 45 years or more were admitted to hospital and only 80% of women aged 20-24 years were admitted, the risk of spontaneous abortion would be 74.7% and 11.1% respectively instead of 74.7% and 8.9%.
Another potential problem could be that some induced abortions were recorded as spontaneous abortions. Our study takes advantage of the fact that both conditions are mandatorily reported and that induced abortion before 12 weeks of gestation is legal, available, free of charge, and a widely accepted practice in Denmark. Thus, we assumed that misclassification of induced abortions was minimal, which is supported by other studies.19
Stillbirths and ectopic pregnancies
As with spontaneous abortion, we also found an increasing risk of ectopic pregnancy and stillbirth with increasing maternal age. The increase in risk of ectopic pregnancies in teenage women is most likely caused by pelvic inflammatory disease. We observed the same pattern regardless of calendar period, which indicates that the association between age and ectopic pregnancy cannot be ascribed to secular changes in the incidence of ectopic pregnancies, in contrast to previous results from Finland.20 We also found a higher incidence of ectopic pregnancies than those reported from other Scandinavian countries.20,21 The difference could be due to a higher incidence of infection with genital Chlamydia trachomatis in Denmark than in other Scandinavian countries, especially during the 1980s.22,23
The risk of stillbirth was found to be high among teenagers, as previously reported.24 This may be a result of unfavourable social and behavioural conditions among pregnant teenagers, although a biological explanation cannot be excluded. The risk of stillbirth among women aged more than 35 years was increased but to a lesser extent than that observed for spontaneous abortion and ectopic pregnancy. The increase in risk among nulliparous women indicates that selective fertility is probably not the explanation for the association between age and stillbirth.
Conclusion
Our study shows an important increase in the risk of spontaneous abortion and other types of fetal loss among women aged more than 40 years and that the increase is already considerable among those in their 30s. This increase is observed irrespective of a woman's reproductive history. For society, such findings would indicate that tendencies to postpone pregnancy increase the overall incidence of fetal loss and possibly the costs of health care. On the individual level, information about the increased risk of spontaneous abortion with high maternal age should be part of medical counselling so that it can be taken into consideration in decisions about reproduction.
What is already known on this topic
Maternal age at conception and history of fetal loss are risk factors for fetal death; these factors are highly correlated
What this study adds
Maternal age at conception is a strong and independent risk factor for fetal death, irrespective of previous reproductive outcome, as is a history of fetal loss
The chance of a successful pregnancy in women aged 40 years or more is poor
Patients could be counselled more fully about their chance of a successful pregnancy if their age and reproductive history were taken into account
Table.
Pregnancy outcomes by maternal age at conception in 634 272 women in Denmark, 1978-92
| Maternal age | Live births | Spontaneous abortions* | Induced abortions | Ectopic pregnancies | Stillbirths | All pregnancy outcomes |
|---|---|---|---|---|---|---|
| 12-19 | 44 674 | 5 427 | 49 884 | 808 | 223 | 101 016 |
| 20-24 | 246 038 | 24 465 | 74 683 | 4 163 | 1 046 | 350 395 |
| 25-29 | 312 904 | 33 728 | 59 014 | 7 233 | 1 270 | 414 149 |
| 30-34 | 157 457 | 22 391 | 48 641 | 5 861 | 699 | 235 049 |
| 35-39 | 43 471 | 11 369 | 36 195 | 2 679 | 226 | 93 940 |
| 40-44 | 5 101 | 3 962 | 15 421 | 614 | 34 | 25 132 |
| ⩾45 | 117 | 509 | 1 184 | 54 | 1 | 1 865 |
| Total No of events | 809 762 | 101 851 | 285 022 | 21 412 | 3 499 | 1 221 546 |
| Total No of women | 509 867 | 85 838 | 223 426 | 18 968 | 3 457 | 634 272 |
Includes 523 cases of hydatidiform mole.
Acknowledgments
This study was approved by the Danish Data Protection Board.
Editorial by Stein and Susser
Footnotes
Funding: This work was supported by a grant from the Danish National Research Foundation and the Danish Medical Research Council.
Competing interests: None declared.
References
- 1.Fretts RC, Schmittdiel J, McLean FH, Usher RH, Goldman MB. Increased maternal age and the risk of foetal death. N Engl J Med. 1995;333:953–957. doi: 10.1056/NEJM199510123331501. [DOI] [PubMed] [Google Scholar]
- 2.Petitti DB. The epidemiology of foetal death. Clin Obstet Gynecol. 1987;30:253–258. doi: 10.1097/00003081-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz GS, Skovron ML, Lapinski PH, Berkowitz RL. Delayed childbearing and the outcome of pregnancy. N Engl J Med. 1990;322:659–664. doi: 10.1056/NEJM199003083221004. [DOI] [PubMed] [Google Scholar]
- 4.Harlap S, Shiono PH, Ramcharan S. A life table of spontaneous abortions and the effect of age, parity and other variables. In: Porter IH, Hook EB, editors. Human embryonic and foetal death. New York: Academic Press; 1980. [Google Scholar]
- 5.Risch HA, Weiss NS, Clarke AE, Miller AB. Risk factors for spontaneous abortion and its recurrence. Am J Epidemiol. 1988;128:420–430. doi: 10.1093/oxfordjournals.aje.a114982. [DOI] [PubMed] [Google Scholar]
- 6.Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod. 1991;6:1332–1337. doi: 10.1093/oxfordjournals.humrep.a137535. [DOI] [PubMed] [Google Scholar]
- 7.Kline J, Stein Z. Spontaneous abortion. In: Bracken M, editor. Perinatal epidemiology. New York: Oxford University Press; 1984. [Google Scholar]
- 8.Christiansen OB. Epidemiological, immunogenetic and immunotherapeutic aspects of unexplained recurrent miscarriage. Dan Med Bull. 1997;44:396–424. [PubMed] [Google Scholar]
- 9.Warburton D, Fraser FC. Spontaneous abortion risks in man: data from reproductive histories collected in a medical genetic unit. Am J Hum Genet. 1964;16:1–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Naylor AF, Warburton D. Sequential analysis of spontaneous abortion. II: collaborative study data show that gravidity determines a very substantial rise in risk. Fertil Steril. 1979;31:282–286. [PubMed] [Google Scholar]
- 11.Wilcox AJ, Gladen BC. Spontaneous abortion: the role of heterogeneous risk and selective fertility. Early Hum Dev. 1982;7:165–178. doi: 10.1016/0378-3782(82)90135-9. [DOI] [PubMed] [Google Scholar]
- 12.Alberman E. Maternal age and spontaneous abortion. In: Bennett MJ, Edmonds DK, editors. Spontaneous and recurrent abortion. Oxford: Blackwell Scientific; 1987. [Google Scholar]
- 13.Westergaard T, Wohlfahrt J, Aaby P, Melbye M. Population based study of rates of multiple pregnancies in Denmark 1980-94. BMJ. 1997;314:775–779. doi: 10.1136/bmj.314.7083.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline J, Stein Z, Susser M. Conception to birth. New York: Oxford University Press; 1989. pp. 43–68. [Google Scholar]
- 15.Hassold T, Chiu D. Maternal age specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 16.Cano F, Simon C, Remohi J, Pellicer A. Effect of ageing on the female reproductive system: evidence for a role of uterine senescence in the decline in female fecundity. Fertil Steril. 1995;64:584–589. doi: 10.1016/s0015-0282(16)57797-8. [DOI] [PubMed] [Google Scholar]
- 17.Hakim RB, Gray RH, Zacur H. Infertility and early pregnancy loss. Am J Obstet Gynecol. 1995;172:1510–1517. doi: 10.1016/0002-9378(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 18.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidam LZ, Olsen JO. Self-reported data on spontaneous abortions compared with data obtained by computer linkage with the hospital registry. Scand J Soc Med. 1985;13:159–163. doi: 10.1177/140349488501300406. [DOI] [PubMed] [Google Scholar]
- 20.Mäkinen J, Rabtala M, Vanha-Kämmpä O. A link between the epidemic of ectopic pregnancy and the ‘baby-boom’-cohort. Am J Epidemiol. 1998;148:369–374. doi: 10.1093/oxfordjournals.aje.a009655. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Low N, Smith GD, Lindblom B, Herrmann B. Screening for chlamydial infections in the risk of ectopic pregnancy in a county in Sweden: ecological analysis. BMJ. 1998;316:1776–1780. doi: 10.1136/bmj.316.7147.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soerensen JL, Thranov IR, Hoff GE. Presence of genital chlamydia trachomatis in abortion seeker. Correlates with young age and nulliparity but not with previous genital infection. [In Danish.] Ugeskr Laeger. 1992;154:3053–3056. [PubMed] [Google Scholar]
- 23.Soegaard P, Moeller BR, Thorsen P, Nissen LR, Pedersen S, Kargo JC, et al. Prevalence of Chlamydia trachomatis among conscripts. A comparative study of urine samples and urethral swaps [In Danish.] Ugeskr Laeger. 1996;158:759–763. [PubMed] [Google Scholar]
- 24.Raymond EG, Cnattingius S, Kiely JL. Effects of maternal age, parity and smoking on the risk of stillbirth. Br J Obstet Gynaecol. 1994;101:301–306. doi: 10.1111/j.1471-0528.1994.tb13614.x. [DOI] [PubMed] [Google Scholar]