Abstract
There is substantial interest in the development of drugs that limit the extent of ischemia-induced cardiac damage caused by myocardial infarction or by certain surgical procedures. Here an unbiased proteomic search identified mitochondrial aldehyde dehydrogenase 2 (ALDH2) as an enzyme whose activation correlates with reduced ischemic heart damage in rodent models. A high-throughput screen yielded a small-molecule activator of ALDH2 (Alda-1) that, when administered to rats prior to an ischemic event, reduced infarct size by 60%, most likely through its inhibitory effect on the formation of cytotoxic aldehydes. In vitro, Alda-1 was a particularly effective activator of ALDH2*2, an inactive mutant form of the enzyme that is found in 40% of East Asian populations. Thus, pharmacologic enhancement of ALDH2 activity may be useful for patients with wildtype or mutant ALDH2 subjected to cardiac ischemia, such as during coronary bypass surgery. (140/140 words)
Cardiac ischemia is the leading cause of death. The discovery of a cardioprotective mechanism called preconditioning (induced by repetitive sublethal ischemic events) has triggered the search for pharmacological agents that mimic this effect (1, 2). Adenosine (3), ethanol (4), and selective activation of protein kinase Cε (εPKC) (4, 5) mimic ischemic preconditioning and reduce cardiac infarct size. Systematic searches for mediators of cardiac protection have identified a number of proteins whose levels or phosphorylation changes with cardioprotection (8, 9). However, whether the changes were critical for cardiac protection was not determined.
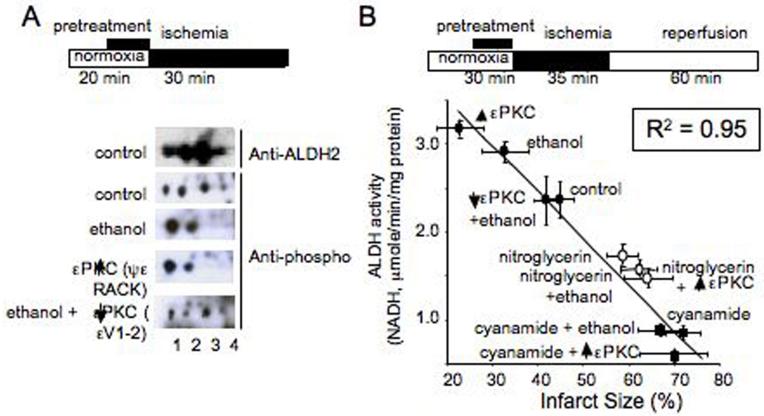
We used an unbiased proteomic approach in ischemic rat hearts treated with ethanol and a selective inhibitor and an activator of εPKC that we generated (4, 10, 11). We found that one protein whose phosphorylation status consistently correlated with cardioprotection was mitochondrial aldehyde dehydrogenase 2 [ALDH2; Fig. 1 and Fig. S1A-D (5)]. Under normoxic conditions, ALDH2 appeared as four phosphoproteins on 2-D IEF/SDS gel electrophoresis. After preconditioning by a brief exposure to ethanol (50mM, 10min) (6) or selective activation of εPKC by the isozyme-specific agonist peptide, ψεRACK (1μM, 10min), which causes cardioprotection (7), there were only two (the more acidic) ALDH2 spots (Fig. 1A). The ethanol-induced shift in ALDH2 mobility was inhibited in the presence of the εPKC-selective antagonist peptide, εV1-2 (Fig. 1A), a treatment that we previously found to inhibit ethanol-induced cardiac protection (6). Therefore, ethanol-induced ALDH2 phosphorylation, which correlates with cardiac protection from ischemia, is dependent on εPKC activation.
Figure 1. Ethanol and εPKC activation induce phosphorylation of mitochondrial ALDH2.
(A). Homogenates of hearts subjected to ischemia ex vivo were separated by IEF/SDS 2-D gel and probed with a mixture of phospho-serine/threonine antibodies. Using a Langendorff apparatus, hearts were perfused with oxygenated Kreb-Henseleit buffer alone as control, with 50mM ethanol for 10 minutes, with 1μM εPKC agonist (ψεRACK) for 10 minutes (C), or with 1μM εPKC antagonist (εV1-2) for 5 minutes followed by 10 minutes of perfusion together with 50mM ethanol. The hearts were then subjected to a 30 minute period of no-flow ischemia before homogenization. Treatment with ethanol and ψεRACK induced a leftward shift of ALDH2 as compared to control, which was blocked with εV1-2 treatment. Blots were probed with anti-ALDH2 or anti-phospho Ser and Thr (5). (B). ALDH2 activity correlates with cardiac protection from ischemic injury (B). Measurements of ALDH activities in normoxic and ischemic hearts treated with ethanol (EtOH; 50mM), εPKC agonist (ψεRACK) or εPKC antagonist (εV1-2) in the presence of ethanol using the Langendorf apparatus (5). Ischemic hearts were also treated with the ALDH2 inhibitor, cyanamide (CYA) in the presence or absence of ethanol, εPKC agonist and antagonist, and the ALDH2 desensitizer, nitroglycerin (GTN). Shown is ALDH2 activity (μmoles of NADH/min/mg protein) as a function of infarct size, measured by TTC staining from corresponding heart samples derived from the same studies as in Table 1. Linear regression yielded a high inverse correlation of R2 = 0.95.
How this mitochondrial enzyme was regulated by the cytosolic εPKC was not obvious. We first demonstrated that εPKC phosphorylates ALDH2 in vitro and that this phosphorylation results in a 38±9% increase in ALDH2 catalytic activity [n=6; p<0.005; Fig. S2A; SOM note 3,4; (5)]. At least two phosphorylation sites were identified by mass spectroscopy, including Thr185 and Thr412 and possibly on Ser279 (5). This ALDH2 phosphorylation resulted in a 38±9% increase in ALDH2 activity (n=6; p<0.005). Further, co-immunoprecipitation of extracts from normoxic and ischemic hearts with anti-εPKC or with anti-ALDH2 antibodies confirmed the association of ALDH2 and εPKC in the mitochondrial fraction [Fig. S3; (5)]. Immuno-electron microscopy studies showed recently that following cardiac preconditioning, εPKC is transported from the cytosol into the inner membrane of mitochondria (8). It is therefore likely that εPKC can enter the mitochondria and phosphorylate ALDH2 directly.
We next determined whether ALDH2 is activated in the intact heart following εPKC activation or ethanol treatment and whether there is a correlation between the activity of ALDH2 and infarct size under various treatment conditions. Ischemia alone did not affect ALDH2 activity (Table 1). However, ethanol treatment caused a 20% increase in ALDH2 activity relative to control and a 27% reduction in infarct size (Table 1 and Fig. 1B; from 45% to 33%; p<0.05). Treatment with the selective εPKC activator, ψεRACK (7), increased ALDH2 activity by 33% with a concomitant 50% reduction in infarct size and inhibition of εPKC by the selective antagonist, εV1-2 (9), abolished both the ethanol-induced increase in ALDH2 activity and the ethanol-induced cardiac protection from ischemia (Table 1). Further, in the presence of the ALDH inhibitor, cyanamide [CYA, 5mM; (5, 10)], ALDH2 activity was inhibited by 63% and infarct size increased by 50%, without causing cardiac damage under normoxic conditions; cyanamide also abolished ethanol- or ψεRACK-induced protection and ALDH2 activation (Table 1, Fig. 1B).
Table 1. ALDH2 activity and infarct size in hearts subjected to ischemia and reperfusion, ex vivo.
| Treatment | ALDH2 activity (μ molNADH/min/mg protein) | Infarct size (%) | n |
|---|---|---|---|
| Normoxia | 2.5±0.1 | 5±4 | 3 |
|
| |||
| Nor + Cya | 0.9±0.1# | 7±5 | 3 |
|
| |||
| Ischemia | 2.4±0.2 | 45±6 | 5 |
|
| |||
| Isc + EtOH | 2.9±0.1* | 33±10* | 5 |
|
| |||
| Isc + ψεR | 3.2±0.1* | 23±10** | 5 |
|
| |||
| Isc + εV1-2 + EtOH | 2.4±0.3 | 42±5 | 5 |
|
| |||
| Isc + Cya | 0.9±0.1** | 67±9** | 5 |
|
| |||
| Isc + Cya + EtOH | 0.9±0.1** | 73±8** | 5 |
|
| |||
| Isc + Cya + ψεR | 0.6±0.1** | 70±7** | 5 |
|
| |||
| Isc + GTN | 1.7±0.1** | 59±8* | 8 |
|
| |||
| Isc+ GTN + EtOH | 1.5±0.1** | 63±9* | 8 |
|
| |||
| Isc+ GTN + ψεR | 1.5±0.1** | 61±6* | 8 |
|
| |||
| Isc + SNP | 2.3±0.1 | 45±7 | 8 |
|
| |||
| Isc +GTN off | 2.1±0.2 | 33±8* | 5 |
|
| |||
| Isc + GTN on | 1.5±0.1* | 59±7* | 5 |
p<0.01 from normoxia
p<0.01
p<0.05 from ischemia
Because cyanamide inhibits several ALDHs, we used another means to inhibit ALDH2. ALDH2 metabolizes nitroglycerin, leading to generation of the vasodilator, nitric oxide. Yet, prolonged treatment with nitroglycerin decreases ALDH2 activity (5, 11). We reasoned that if ALDH2 activity is critical for cardioprotection from ischemic damage, prolonged treatment with nitroglycerin should inhibit εPKC-dependent preconditioning. As expected, a 30-minute treatment of nitroglycerin (GTN; 2μM) in the ex vivo myocardial infarction model in rodents greatly inhibited ALDH2 activity and abolished ethanol- and εPKC-induced activation of ALDH2 (Table 1, Fig. 1B), whereas the activity of another cardiac dehydrogenase remained unchanged [Fig. S4A, B; (5)], indicating that the changes in ALDH2 activity are likely specific. Concomitantly, GTN treatment increased ischemic cardiac damage from 45% in control to 59%, and to 63% or 61% in the presence of ethanol or the εPKC activator (Table 1, Fig. 1B). This effect was not due to nitric oxide generation; treatment with another nitric oxide generating vasodilator, sodium nitroprusside (SNP), did not affect ALDH2 activity nor did it result in an increase in infarct size (Table 1). Therefore, there is an inverse correlation between ALDH2 activity and cardiac damage (R2=0.95, Fig. 1A), strongly suggesting that ALDH2 plays a pivotal positive role in mediating cardiac protection against ischemic injury. Creatine phosphate kinase release from the heart as an indicator of cardiac damage (4) yielded similar results [Fig. S4C, D; R2= 0.97, (5)].
Nitroglycerin confers cardiac protection if the prolonged nitroglycerin treatment is terminated at least one hour prior to the ischemic event (12). Consistent with these findings, we found that 13 hours of nitroglycerin treatment (5μg/min/kg, delivered by a patch) that was terminated 3 hours prior to the ischemic event decreased cardiac infarct size from 45% to 33% (GTN-off; Table 1). However, similar to our ex vivo data, when the nitroglycerin patch was left on, infarct size increased from 45% to 59% (GTN-on; Table 1). Therefore, sustained nitroglycerin treatment increased ischemic damage, probably by inducing ALDH2 inactivation [SOM, Note 5; (5)].
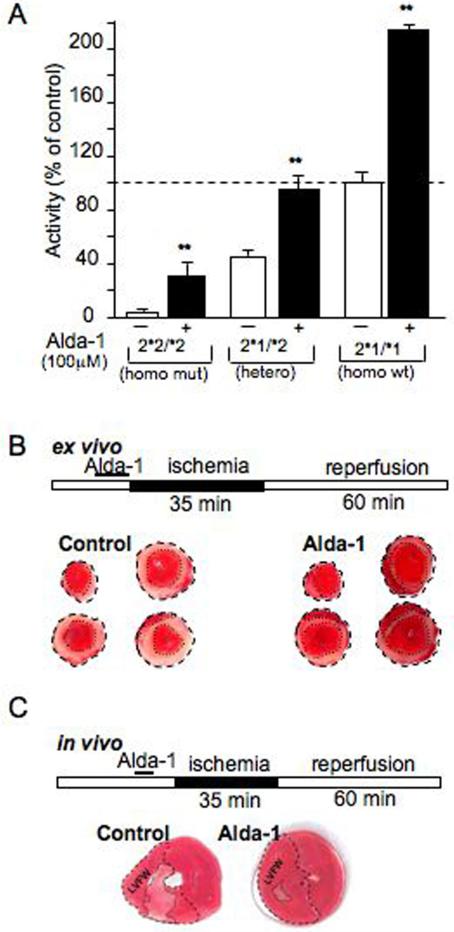
The inverse correlation between ALDH2 activity and cardioprotection against ischemic damage in rat (Fig. 1B; R2=0.95; Fig. S4D) does not prove that ALDH2 activation is sufficient to induce cardioprotection. We therefore searched for ALDH2 agonists using a high-throughput screen [SOM, Note 1; (5)] and identified N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide (Alda-1, MW=324) and similar analogs as ALDH2 activators (Fig. 2A, Fig. S5A). We next determined whether Alda-1 activates ALDH2*2, a common East Asian mutant form that has only 1-5% of the catalytic activity of the wildtype ALDH2*1 form. [This E487K mutation is at the interface of the tetramer (13).] Alda-1 (EC50 ∼20μM) increased the activity of the mutant, ALDH2*2, by 11 fold, the heterotetramer by 2.2 fold (similar to the base levels of wildtype ALDH2) and the wildtype ALDH2*1/*1 homotetramers by 2.1 fold (Fig. 2A, Fig. S5C, D). Alda-1 had no effect on the activity of alcohol dehydrogenase 1 (5, 14), the cytosolic aldehyde dehydrogenase, ALDH1 (15) or the mitochondrial enzyme ALDH5 [(5, 16); Fig. S5B].
Figure 2. Alda-1 increases ALDH2 activity.
(A). Activation of wild type, heterozygotes and homozygotes mutants of ALDH2 by Alda-1 (100 μM). Enzymatic activity of recombinant ALDH2 proteins (20μg each) are presented as percent of control [n=3, **p<0.01 vs. control; (5)]. Alda-1 reduces cardiac damage in an ex vivo model of ischemia and reperfusion injury (B). Top: Ex vivo cardiac ischemia model protocol. Myocardial infarct size, induced by 35 minutes ischemia followed by 60 minutes of reperfusion after 10 minutes pretreatment with Alda-1 (20 μM) or vehicle control using Langendorff apparatus, as in Fig. 1B, 2B [n=7, *p<0.01; (5)]. Representative cross-sectional slices derived from a single heart stained by TTC without (control) and with Alda-1 treatment (n=7). Infarct area is indicated by the light pink color and marked with dotted lines. Alda-1 reduces cardiac damage in an in vivo model of acute myocardial infarction (C). Top: In vivo cardiac ischemia model protocol. Reduction of infarct size by pretreatment of Alda-1 (8.5mg/kg) before LAD ligation was also determined in vivo (n=7, **p<0.01; SOM, Fig. S6A, B). Shown is TTC staining of representative cross-sectional slices (seven rats per group).
We next used Alda-1 to determine whether direct ALDH2 activation was sufficient to induce cardioprotection. Rat hearts treated ex vivo with 20μM Alda-1 prior to 35 minutes of ischemia followed by 60 minutes of reperfusion (as in Fig. 2B) had a 26±6% smaller infarct (Fig. 2B) and 24±7% less phosphokinase (CPK) release (n=6; p<0.05). Alda-1 also reduced infarct size in an in vivo rat model of acute myocardial infarction. After 35 minutes of ischemia and 60 min of reperfusion, infarct size of the left ventricular free wall was 43±4% (n=7); (5). Administration of 8.5 mg/kg Alda-1 into the left ventricle five minutes before ischemia decreased the myocardial infarction by 60±4% (Fig. 2C; n=7; p<0.01; Fig. S6A, B). Importantly, although low levels of noxious stimuli trigger cardioprotection (1, 2), Alda-1-induced cardioprotection was not associated with such a stress. JNK, a sensitive marker of cell stress, was not activated by Alda-1 treatment [Fig. S7; (5)]. Therefore, activation of ALDH2 is sufficient to protect the heart from ischemia damage, in vivo.
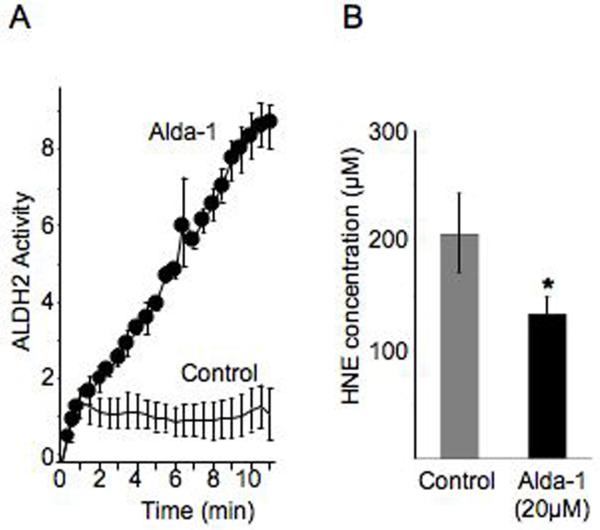
Since 4HNE is a toxic aldehyde that accumulates during cardiac ischemia (17), its removal by ALDH2 may be, at least in part, the mechanism by which ALDH2 activation is cardioprotective from ischemic damage. Further, 4HNE itself can inactivate ALDH2, thus limiting its own removal (18). We confirmed that 4HNE induced a quick inactivation of ALDH2 in vitro and found that HNE-induced inactivation was blocked by Alda-1 (Fig. 3A) thereby increasing the detoxification of 4HNE (Fig. 3B). The molecular basis for Alda-1-induced ALDH2 protection is under investigation, but is likely due to protection from 4HNE oxidation of ALDH2 (18).
Figure 3. Alda-1 effect on 4HNE metabolism by ALDH2.
(A).In vitro metabolism of 4HNE (200μM) by ALDH2 (arbitrary units) is lost within one minute of incubation with the substrate; (5), presumably due to 4HNE-induced ALDH2 inactivation (18). HNE-induced ALDH2 inactivation is blocked by Alda-1 (20μM; n=3) as compared with vehicle control (n=3). (B) The protection of ALDH2 from HNE-induced inactivation by Alda-1 correlates with a 34% reduction in 4HNE levels (n=4; p<0.05).
Together, we showed that activating ALDH2 prior to ischemic event reduces cardiac damage. Although some of the pharmacological tools that we used to regulate ALDH2 are relatively non-specific, twelve different conditions demonstrate the highly correlative relationship between ALDH2 activity and infarct size (R2=0.95; Fig. 1B). Therefore, our data strongly suggest that ALDH2 activity is critical for cardioprotection from ischemia. In addition, ALDH2 contributes to ethanol metabolism and ethanol was used to activate ALDH2. However, ethanol metabolism is unlikely to play a role in the ALDH2-mediated protection; ALDH2 activation occurred also in the absence of ethanol, when using the εPKC-selective activator or when using Alda-1. Finally, the importance of cytotoxic aldehydes such as HNE to overall ischemic injury has been previously suggested [SOM, Note 6; (17, 19, 20)]. It is possible that the major benefit of Alda-1 is in preventing the inactivation of cytoprotective ALDH2 by HNE, thus enabling continual detoxification of oxidative stress-induced cytotoxic aldehydes.
Our basic research, aimed at identifying the molecular basis of cardiac protection, lead to the identification of a potential new treatment in which ALDH2 activity is directly enhanced pharmacologically for patients subjected to cardiac ischemia (e.g., during coronary by-pass surgery). The ability of Alda-1 to partially complement or restore mutant ALDH2*2 activity is should be noted as it is rare to find a small molecule that can specifically rescue a mutation in humans. Finally, our data in rodent models suggest that the prolonged use of nitroglycerin in carriers of Aldh2*2 that experience an ischemic event may need to be reconsidered and that these patients may benefit even more than carriers of the wildtype enzyme if treated with ALDH2 activators.
Acknowledgments
Daria Mochly-Rosen is the founder and a shareholder of KAI Pharmaceuticals. However, noneof the work was done in collaboration or with support from the company. This work is supported by NIH grant AA11147 to DMR. We also acknowledge support from the Stanford’s SPARK Program to C-HC and are grateful to Drs. Koichi Inagaki, Christopher L. Murriel for initial studies, to Dr. David Solow-Cordero for the high-throughput screen and to Federico Conti, Daniel Cheng and Amir Masoud Sadaghiani for the biochemical characterization of Alda-1.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. Their manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS
References
- 1.Bolli R. Am J Physiol Heart Circ Physiol. 2007;292:H19. doi: 10.1152/ajpheart.00712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downey JM, Davis AM, Cohen MV. Heart Fail Rev. 2007;12:181. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 3.Nakano A, Cohen MV, Downey JM. Pharmacol Ther. 2000;86:263. doi: 10.1016/s0163-7258(00)00058-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Gray MO, Mochly-Rosen D. Proc Natl Acad Sci U S A. 1999;96:12784. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Further information on data and methods and additional discussion are available on Science Online.
- 6.Chen C, Mochly-Rosen D. J Mol Cell Cardiol. 2001;33:581. doi: 10.1006/jmcc.2000.1330. [DOI] [PubMed] [Google Scholar]
- 7.Dorn GW, 2nd, et al. Proc Natl Acad Sci U S A. 1999;96:12798. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. Cardiovasc Res. 2007 doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 9.Gray MO, Karliner JS, Mochly-Rosen D. J Biol Chem. 1997;272:30945. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 10.Deitrich RA, Troxell PA, Worth WS. Biochem Pharmacol. 1976;25:2733. doi: 10.1016/0006-2952(76)90265-3. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, et al. Proc Natl Acad Sci U S A. 2005;102:12159. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill M, et al. Circulation. 2001;104:694. doi: 10.1161/hc3201.092218. [DOI] [PubMed] [Google Scholar]
- 13.Larson HN, Weiner H, Hurley TD. J Biol Chem. 2005;280:30550. doi: 10.1074/jbc.M502345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duester G, et al. Proc Natl Acad Sci U S A. 1984;81:4055. doi: 10.1073/pnas.81.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A. Proc Natl Acad Sci U S A. 1985;82:3771. doi: 10.1073/pnas.82.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasi P, et al. Mol Genet Metab. 2002;76:348. doi: 10.1016/s1096-7192(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 17.Eaton P, Li JM, Hearse DJ, Shattock MJ. Am J Physiol. 1999;276:H935. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 18.Doorn JA, Hurley TD, Petersen DR. Chem Res Toxicol. 2006;19:102. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 19.Lucas DT, Szweda LI. Proc Natl Acad Sci U S A. 1998;95:510. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Henderson GI, Freeman GL. J Mol Cell Cardiol. 2001;33:1919. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]