Abstract
Context: There is evidence that leptin is involved in the etiology of obesity-related cardiovascular disease in adults. This raises the question of whether leptin levels in adolescence are indicative of adiposity-related cardiovascular risk.
Objective: This study investigated gender-specific patterns of plasma leptin during adolescence, assessed which adiposity measurements are most strongly associated with plasma leptin, and estimated to what degree leptin-adiposity associations are influenced by genetic factors.
Methods: Plasma leptin concentrations were determined using a sandwich immunoassay. Associations between plasma leptin and several adiposity measurements were examined using generalized estimating equations. Genetic contribution to leptin-adiposity association was estimated by Cholesky decomposition models using twin design.
Results: Plasma leptin levels were higher in females than males at all Tanner stages. In females, body mass index, waist circumference, fat mass index (FMI), and percentage body fat (%BF) had similar associations with leptin levels. In males, %BF and FMI were more strongly associated with leptin levels than body mass index and waist circumference. In both males and females, percentage trunk fat had the weakest association with leptin among the five adiposity measures. Shared genetic factors account for more than 80% of the phenotypic correlation between %BF and leptin.
Conclusions: We found gender differences in leptin levels and leptin-adiposity associations. In both genders, leptin levels were strongly associated with %BF and FMI, particularly in males. Shared genetic factors contributed largely to the phenotypic correlation between leptin and %BF. Our findings underscored the importance of further investigation of leptin as a biomarker of adiposity in adolescents.
Leptin levels were strongly associated with percentage body fat and fat mass index, and shared genetic factors contributed largely to the phenotypic correlation between leptin and adiposity measures.
Leptin, an adipokine encoded by the obese (ob) gene (1), is primarily produced by white adipose cells. It has a variety of important central and peripheral actions, related to regulation of food intake, energy balance, and metabolism (2,3). There is evidence that leptin may play an etiologic role in cardiovascular disease by stimulating vascular inflammation, oxidative stress, and vascular smooth muscle hypertrophy (4,5,6). Clinical research has supported leptin as a predictor of coronary events, myocardial infarction, and stroke independent of adiposity. Some studies reported that leptin was an independent predictor of diabetes (7,8).
To date, most studies of leptin and adiposity have been conducted in adults or obese children (9,10,11,12). More data on leptin and its relationship with adiposity in healthy adolescents are needed to explore whether leptin may be a marker of adiposity and/or cardiovascular risk in the young. There is reason to think that adult data cannot be applied directly to adolescents. Adolescence is a period of rapid physical growth and functional maturation, accompanied by growing gender differences in anthropometric measurements, body composition, and metabolism. Decreased insulin sensitivity in puberty (pubertal insulin resistance) is well documented (13). Puberty is also a critical period for development of obesity (14).
Previous studies examining patterns of plasma leptin concentrations and its relationship to adiposity in adolescents were conducted in Western populations (15,16,17,18). There has been one report on leptin during puberty in urban Chinese adolescents (19) but none in rural Chinese adolescents. Generalizability of the earlier studies is limited by sample size, less than 200 in three of the five previous studies (15,16,17) and less than 800 in the other two (18,19). Furthermore, it is not clear whether the patterns of plasma leptin concentrations and its relationship to adiposity reported from the relatively heavier Western and Chinese urban populations can be applied to relatively lean rural Chinese adolescents. Rural China, where rapid economic changes have resulted in a wide range of adiposity, offers an excellent setting in which to examine the role of leptin as a biomarker of excessive adiposity in a population during the nutritional transition. Such information will have global public health implications for countries that currently are or will be in a similar situation.
The contribution of genetic factors to leptin-adiposity associations in adolescents remains to be determined. Individually, leptin levels and body fat are known to be largely genetically determined (20,21). Strong genetic influence on leptin and body mass index (BMI) was reported in a study of old adults (22). The only such study in (Hispanic) children also found a high genetic influence on correlations between obesity and leptin (23). To our knowledge, no study has been conducted to examine to what degree genetic and environmental factors contribute to associations between adiposity and leptin in Chinese adolescents.
In this study we addressed three issues in a large sample of rural Chinese adolescents. First, we examined gender-specific patterns of plasma leptin concentrations during puberty. Second, we evaluated which adiposity measurements are most strongly associated with plasma leptin. Finally, we estimated to what degree genetic factors contribute to leptin levels and to correlations between leptin levels and adiposity.
Subjects and Methods
Study sample and recruitment
The study sample consists of 1021 subjects aged 13–21 yr from the Anqing twin cohort. The study subjects were first recruited from 1998 to 2000 and were followed up from 2005 to 2008 as part of an ongoing National Institutes of Health-funded prospective study. The study was carried out in the Anqing area of Anhui Province, China. Demographic and geographic characteristics of this rural area were described elsewhere (24). Briefly, for the original baseline recruitment, eligible subjects were those who met the following criteria: 1) aged 6–60 yr; 2) both twins were available for the survey; 3) both twins (or parents/guardians of children) agreed and consented to participate in the survey; 4) no previous history of stroke and cardiovascular, renal, hepatic, or malignant diseases; and 5) females were not nursing or pregnant. In the follow-up survey, eligible twins were those who met the following criteria: 1) both twins participated in the baseline survey; and 2) both twins agreed and consented to participate in the follow-up study and written consent was obtained from each participant. Eligible twins were invited to a central office to complete a questionnaire interview, blood drawing, and physical examination including Tanner stage determination, anthropometric measurements, and dual-energy x-ray absorptiometry (DEXA) scan. This report focused on those subjects aged 13–21 yr at the time of their follow-up survey and had complete measures of adiposity, plasma leptin concentration, and Tanner staging. The study protocol was approved by the Institutional Review Boards of Children’s Memorial Hospital and the Institute of Biomedicine, Anhui Medical University (Hefei, China).
Anthropometry and puberty
Weight, height, and waist circumference were collected using standard protocols. Height was measured to the nearest 0.1 cm on a portable stadiometer. Weight was measured to the nearest 0.1 kg with the subjects standing motionless on a scale and BMI was calculated as weight (kilograms)/height squared (square meters). A waist circumference (WC) measurement was taken at the level of the umbilicus to the nearest millimeter (millimeters). Each anthropometric measure was repeated three times and the mean computed for the subsequent analyses.
Tanner stages (I-V) were assessed by physical examination using established methods based on visual inspection of pubic hair and genitals for males and pubic hair and breasts for females (25).
DEXA
Whole-body scans were performed by DEXA (GE-Lunar Prodigy, Madison, WI) to measure body composition in three body regions, trunk (chest, abdomen, and pelvis), arms, and legs. A standard software calculation (26) was used to calculate total body fat measured with a Lunar DPXL instrument (Madison, WI) that was set up in Anqing. Percentage body fat (%BF) was measured as total body fat divided by body weight, percentage trunk fat (%TF) was calculated as trunk fat divided by total body fat, and fat body mass index (FMI) was calculated as total body fat mass divided by height squared.
Laboratory assays
Blood was drawn from the antecubital vein from each participant in the upright position in the morning (0700–0800 h) after an overnight fast. Plasma was separated from blood cells in the field within 30 min of collection and kept frozen at −80 C for later assay. Plasma leptin concentrations were determined using a sandwich immunoassay-based flowmetric xMAP technology (Millipore Corp., Bedford, MA) on a Luminex 200 machine (Luminex multianalyte profiling system, Luminex, Corp., Austin, TX), as described previously (27). The assay sensitivity for leptin was 138.8 pg/ml, and the intraassay coefficient of variation for leptin was 5.1%. Duplicate assays were performed for each plasma sample and the average values were used in all analyses. Twin zygosity [dizygotic (DZ) or monozygotic (MZ)] was determined using 10 autosomal polymorphic microsatellite markers as described by Wang et al. (28).
Statistical analysis
Due to the skewness of original values, plasma leptin concentrations and the ratios of plasma leptin concentrations to %BF were transformed by natural logarithm to normalize the distribution for all subsequent statistical analyses, including structural equation modeling.
We first assessed the patterns of plasma leptin concentrations across different Tanner stages and examined gender difference. To control the effects of adiposity, we repeated the analyses using the ratio of plasma leptin concentrations to %BF instead of plasma leptin concentrations.
The correlations between plasma leptin concentrations and adiposity measurements were examined using locally weighted regression smoothing plots, stratified by gender. We used gender- and age-specific Z-scores of adiposity measurements to ensure the comparability of scales across various adiposity measurements. We examined the strength of association of leptin with adiposity for quartiles of adiposity to assess dose-response associations. These analyses were done separately for males and females. To evaluate which adiposity measurements were most strongly associated with plasma leptin levels, we treated each adiposity measurement as a continuous variable and estimated its partial R2 for explaining variation in leptin levels, using a linear regression model adjusted for age and Tanner stages. To account for intrapair correlation in the twins, we applied generalized estimating equations. All analyses were performed using SAS software, version 9.0 (SAS Institute, Cary, NC).
For %BF, the adiposity measurement that was mostly associated with plasma leptin levels, we estimated genetic and environmental contribution to their phenotypic correlation. Using the twin design, we fitted the bivariate Cholesky decomposition model, using maximum likelihood estimation with structural equation modeling procedures (29). In the full model, additive genetic (a2), common environmental, and unique environmental (e2) components were structured, in addition to age and gender. We then fitted submodels by restricting certain components (A, C, or E) as zero. The χ2 goodness of fit and Akaike information criterion were used for model comparisons. For the best-fitted model (AE model in present study), we calculated the phenotypic correlations between %BF and plasma leptin levels as: rTP = 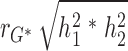 +
+ 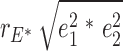 , where rG, and rE are the correlation estimates of additive genetic component and unique environmental components, separately, between %BF and plasma leptin levels, h12 and h22 are additive genetic heritability estimates, and e12 and e22 are the environmental component estimates for adiposity measurement and plasma leptin levels. The genetic and unique environmental contributions to the phenotypic correlations are
, where rG, and rE are the correlation estimates of additive genetic component and unique environmental components, separately, between %BF and plasma leptin levels, h12 and h22 are additive genetic heritability estimates, and e12 and e22 are the environmental component estimates for adiposity measurement and plasma leptin levels. The genetic and unique environmental contributions to the phenotypic correlations are 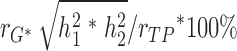 , and
, and 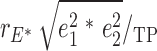 *100%, respectively.
*100%, respectively.
Results
Characteristics of the study subjects
A total of 497 males and 524 females aged 13–21 yr were included in this study. Their general characteristics are summarized in Table 1, grouped by gender. Females were older and farther along in puberty (1.5% Tanner I vs. 14.1% Tanner I in males). Adiposity measurements (BMI, FMI, WC, %BF, %TF), ln leptin, and ln (leptin/%BF) in females were significantly higher than those in males.
Table 1.
Characteristics of the study subjects from the Anqing twin cohort
| Variables | Mean± sd
|
||
|---|---|---|---|
| Boys (n = 497) | Girls (n = 524) | P value | |
| Age, yr | 16.0 ± 1.8 | 16.4 ± 1.9 | 0.006 |
| Height, cm | 159.6 ± 8.5 | 152.5 ± 5.3 | <0.001 |
| Weight, kg | 47.2 ± 8.2 | 46.0 ± 6.5 | 0.044 |
| BMI, kg/m2 | 18.4 ± 2.1 | 19.7 ± 2.3 | <0.001 |
| WC, cm | 64.0 ± 5.7 | 65.8 ± 6.3 | 0.010 |
| FMI, kg/m2 | 2.2 ± 1.3 | 5.5 ± 1.7 | <0.001 |
| %BF, % | 11.8 ± 5.3 | 27.2 ± 5.7 | <0.001 |
| %TF, % | 44.2 ± 6.4 | 48.3 ± 4.3 | <0.001 |
| Leptin, ng/mla | 1.99 ± 2.13 | 8.20 ± 5.48 | <0.001 |
| Leptin/%BFa | 16.3 ± 14.6 | 29.1 ± 15.9 | <0.001 |
| Tanner stages, n (%) | |||
| I | 70 (14.1) | 8 (1.5) | |
| II | 114 (22.9) | 92 (17.6) | |
| III | 130 (26.2) | 212 (41.5) | |
| IV | 146 (29.4) | 153 (29.2) | |
| V | 37 (7.4) | 59 (11.3) | <0.001 |
For all statistical analyses in this article, leptin values were natural logarithm transformed to better approximate normal distribution.
Gender difference in plasma leptin levels by Tanner stage
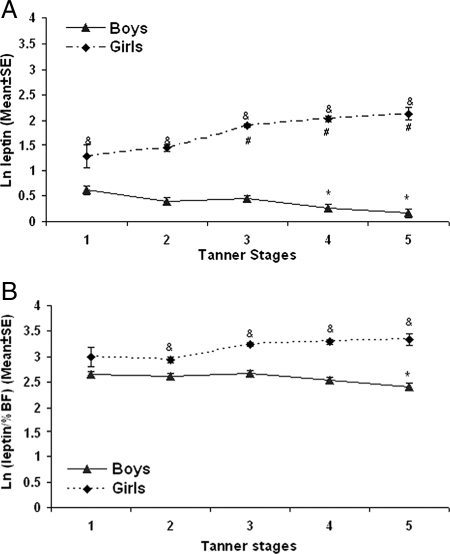
We examined plasma leptin concentrations by Tanner stages, stratified by gender. At each stage of puberty, including tanner I, females had significantly higher leptin concentrations than males. Among males, plasma leptin concentrations fell with maturation and were significantly lower in Tanner stages IV and V than those in Tanner stage I. In contrast, among females, plasma leptin concentrations rose with maturation and were significantly higher in Tanner stages III, IV, and V than Tanner stage I (Fig. 1A).
Figure 1.
A, Plasma ln (leptin levels) of the study subjects grouped by different tanner stages. *, P < 0.05 compared with boy subjects at tanner stage I; #, P < 0.05 compared with girl subjects at tanner stage I; &, P < 0.05 compared between males and females at the same tanner stage. X-axis, Tanner stages; y-axis:, ln leptin (mean ± se). B, Plasma ln (leptin levels) of the study subjects divided by %BF and grouped by different tanner stages. *, P < 0.05 compared with boy subjects at tanner stage I; &, P < 0.05 compared between boys and girls at the same tanner stage. X-axis, Tanner stages; y-axis, ln leptin (mean ± se).
Because fat in females rises with maturation and could account for the observed differences, we analyzed gender-specific ratio of leptin/%BF across Tanner stages (Fig. 1B). Although the gender gap narrowed compared with Fig. 1A, the ratio of leptin to %BF was still significantly higher in females than males in Tanner stages II-V. As shown in Fig. 1B, in males, this ratio behaved like leptin levels, falling with maturation; it was significantly lower in Tanner V than in prepuberty. In females, however, the ratio was relatively stable across all Tanner stages.
Association of plasma leptin with adiposity measurements
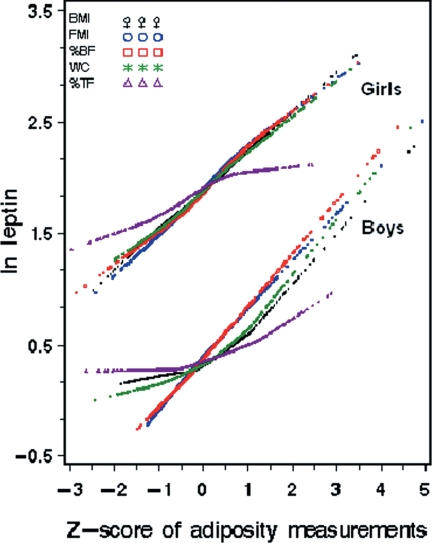
As shown in Fig. 2, plasma leptin concentrations were positively and approximately linearly associated with following adiposity measurements: BMI, WC, FMI, %BF and %TF in both males and females.
Figure 2.
Correlations between ln leptin concentrations and body composition measurements expressed in Z-scores, stratified by gender. X-axis, Z-score of adiposity measurements. Y-axis, ln leptin.
Table 2 shows the association of leptin level with age/gender-specific quartiles of each adiposity measure. There were statistically significant associations between plasma leptin concentrations and degrees of adiposity for all the five adiposity measures, but the pattern and strength of association varied by adiposity measure. For example, in males, the betas for the highest quartiles of adiposity measures ranged from 0.43 to 1.08 and in females from 0.38 to 0.88. Notably, the strongest associations were found for FMI and %BF, for which a dose-response association was seen across all quartiles for males and females.
Table 2.
Association of adiposity measurements with ln leptin levels in the study subjects, stratified by gender
| Variables | Plasma ln leptin concentrations
|
|||||
|---|---|---|---|---|---|---|
| Males
|
Females
|
|||||
| Mean ± sd | β (se) | P value | Mean ± sd | β (se) | P value | |
| BMI quartiles | ||||||
| Q1 | 0.22 ± 0.58 | Reference | 1.44 ± 0.67 | Reference | ||
| Q2 | 0.28 ± 0.68 | 0.10 (0.08) | NS | 1.80 ± 0.57 | 0.33 (0.08) | <0.001 |
| Q3 | 0.29 ± 0.70 | 0.16 (0.09) | NS | 1.89 ± 0.82 | 0.40 (0.10) | <0.001 |
| Q4 | 0.75 ± 0.82 | 0.63 (0.11) | <0.001 | 2.39 ± 0.53 | 0.85 (0.09) | <0.001 |
| FMI quartiles | ||||||
| Q1 | −0.01 ± 0.61 | Reference | 1.43 ± 0.67 | Reference | ||
| Q2 | 0.20 ± 0.60 | 0.21 (0,08) | 0.012 | 1.68 ± 0.63 | 0.24 (0.08) | 0.003 |
| Q3 | 0.35 ± 0.58 | 0.35 (0,08) | <0.001 | 2.00 ± 0.60 | 0.50 (0.09) | <0.001 |
| Q4 | 1.02 ± 0.69 | 1.02 (0,09) | <0.001 | 2.40 ± 0.68 | 0.88 (0.09) | <0.001 |
| %BF quartiles | ||||||
| Q1 | −0.05 ± 0.65 | Reference | 1.44 ± 0.61 | Reference | ||
| Q2 | 0.18 ± 0.60 | 0.23 (0.08) | 0.006 | 1.72 ± 0.70 | 0.25 (0.08) | 0.003 |
| Q3 | 0.37 ± 0.52 | 0.41 (0.08) | <0.001 | 1.98 ± 0.62 | 0.48 (0.08) | <0.001 |
| Q4 | 1.04 ± 0.66 | 1.08 (0.09) | <0.001 | 2.38 ± 0.68 | 0.84 (0.09) | <0.001 |
| WC quartiles | ||||||
| Q1 | 0.15 ± 0.67 | Reference | 1.49 ± 0.64 | Reference | ||
| Q2 | 0.23 ± 0.64 | 0.14 (0.09) | NS | 1.72 ± 0.62 | 0.19 (0.09) | 0.024 |
| Q3 | 0.38 ± 0.59 | 0.30 (0.09) | <0.001 | 1.94 ± 0.77 | 0.40 (0.10) | <0.001 |
| Q4 | 0.79 ± 0.83 | 0.64 (0.10) | <0.001 | 2.37 ± 0.62 | 0.77 (0.09) | <0.001 |
| %TF quartiles | ||||||
| Q1 | 0.23 ± 0.61 | Reference | 1.60 ± 0.65 | Reference | ||
| Q2 | 0.35 ± 0.69 | 0.13 (0.08) | NS | 1.86 ± 0.70 | 0.19 (0.08) | 0.016 |
| Q3 | 0.37 ± 0.75 | 0.18 (0.09) | 0.049 | 1.95 ± 0.76 | 0.25 (0.08) | 0.003 |
| Q4 | 0.60 ± 0.81 | 0.43 (0.11) | <0.001 | 2.10 ± 0.75 | 0.38 (0.10) | <0.001 |
| Continuous variables | Partial R2 | β (se) | P value | Partial R2 | β (se) | P value |
| BMI | 0.178 | 0.16 (0.02) | <0.001 | 0.194 | 0.17 (0.01) | <0.001 |
| FMI | 0.374 | 0.41 (0.02) | <0.001 | 0.235 | 0.25 (0.02) | <0.001 |
| %BF | 0.397 | 0.09 (0.01) | <0.001 | 0.214 | 0.07 (0.01) | <0.001 |
| WC | 0.223 | 0.07 (0.01) | <0.001 | 0.176 | 0.06 (0.01) | <0.001 |
| %TF | 0.039 | 0.03 (0.01) | <0.001 | 0.030 | 0.03 (0.01) | <0.001 |
First quartile (Q1), second quartile (Q2), third quartile (Q3), and fourth quartile (Q4) were classified based on each 1-yr age- and gender-specific quartiles of body composition parameters. Generalized estimating equations were applied to compare the ln leptin differences between first quartile and other quartiles of adiposity variables. Partial R2s were calculated by comparing the multiple linear regression models with or without respective adiposity variables; the variables age and Tanner stages were included in the background model (without adiposity variables). NS, Non significant (P < 0.05).
To further assess which adiposity measurement was most strongly associated with plasma leptin, we treated each adiposity measurement as a continuous variable and estimated its partial R2 (independent contribution to plasma leptin levels) after adjustment for age and Tanner stage. Consistent with the results above, the highest R2 was seen for FMI and %BF in males (0.374 and 0.397, respectively) and females (0.235 and 0.214); the association was stronger in males than females.
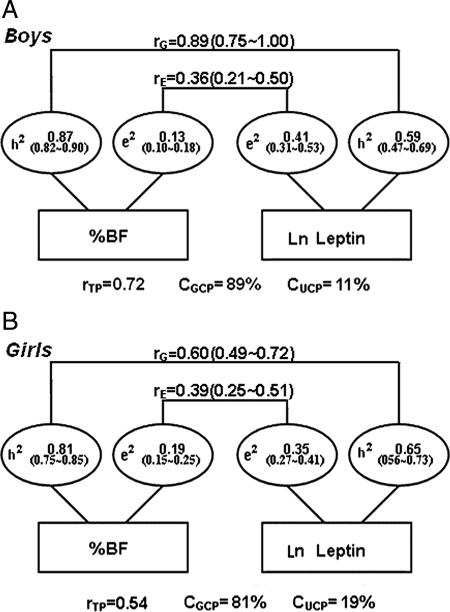
Genetic correlations between percentage body fat and plasma leptin
Figure 3 shows heritability estimates of genetic and environmental contributions to %BF, plasma leptin level, and leptin-%BF associations in 218 male twin pairs (MZ: 135 pairs; DZ: 83 pairs) (Fig. 3A) and 243 female twin pairs (MZ: 167 pairs; DZ: 76 pairs) (Fig. 3B). Based on the statistically best-fitted models (AE model), the heritability estimate for %BF is 0.87 [95% confidence interval (CI) 0.82–0.90] for males and 0.81 (95% CI 0.75–0.85) for females. The heritability estimate for ln leptin is 0.59 (95% CI 0.47–0.69) for males and 0.65 (95% CI 0.56–0.73) for females. Unique environmental factors explained the remaining phenotype variance in %BF and ln leptin. There were significant genetic and unique environmental correlations between %BF and ln leptin in males [rG = 0.89 (0.75–1.00), rE = 0.36 (0.21–0.50)] and females [rG = 0.60 (0.49–0.72), rE = 0.39 (0.25–0.51)].
Figure 3.
A, Genetic and environmental contributions to leptin-%BF association in 218 male adolescent twin pairs adjusted with age and Tanner stages. AE model was the best-fitting model for the bivariate analyses of %BF and leptin in males; rG, and rE denoted genetic and unique environmental correlations, respectively; a2 and e2 denoted percentage of total phenotypic variance accounted for genetic factors and unique environmental factors; rTP, total phenotypic correlation; CGCP, genetic contribution to total phenotypic correlation; CUCP, unique environmental contribution to total phenotypic correlation. B, Genetic and environmental contributions to leptin-%BF association in 243 female adolescent twin pairs adjusted with age and Tanner stages. AE model was the best-fitting model for the bivariate analyses of %BF and leptin in females; rG, and rE denoted genetic and unique environmental correlations, respectively; a2 and e2 denoted percentage of total phenotypic variance accounted for genetic factors and unique environmental factors; rTP, total phenotypic correlation; CGCP, Genetic contribution to total phenotypic correlation; CUCP, unique environmental contribution to total phenotypic correlation.
Discussion
To our knowledge, this study is the first of this kind in rural Chinese adolescents to investigate gender-specific patterns of plasma leptin and its associations with an array of adiposity measurements and estimate the genetic contribution to the phenotypic correlations between leptin and adiposity. The study results provide new information to the field regarding leptin patterns during puberty, varying associations of leptin with different adiposity measures, and to what degree genetics and environment affect leptin and leptin-adiposity correlations. In the following, we further elaborated on the consistency of our findings compared with previous publications, the scientific plausibility of our findings, and clinical and public health implications of our findings.
Gender difference in leptin levels and patterns during adolescence
Previous studies in adults have consistently shown that females have higher leptin than males. However, there are conflicting data as to when the gender difference arises. Several studies reported no significant gender difference during pre- and early puberty (16,17,18). For example, Horlick et al. (16) found significant gender differences in ratios of leptin to fat mass only during Tanner stages IV and V. Others reported significant gender difference during prepuberty, puberty, and postpuberty adolescence (15). The inconsistency remains unexplained and could be due to differences in sample sizes, demography, or study methods.
Our data showed that plasma leptin concentrations were higher in females than males at every Tanner stage and that the gender gap widened as maturation progressed. Our results support those showing differences across pre- and pubertal development. Because our sample size was larger (except for Tanner stage I in females), it provides the statistical power need to clarify the patterns of interest.
Furthermore, in our study, correction for body fat eliminates maturational trends in leptin in females, which indicates that adiposity is the main determinant of rising leptin in adolescent females.
Our results were consistent with the early observations in urban Chinese adolescents (19), in which increased trend of leptin in girls and falling trend in boys with age were reported. In contrast, our study focused on rural Chinese adolescents and investigated gender differences across the Tanner stages, a more reliable puberty index rather than age. Furthermore, we demonstrated that gender differences were persistent, even after correcting for percentage body fat.
The fact that the falling trend in leptin during male maturation persists after correction for body fat indicates that something other than adiposity controls that pattern. One possibility is that testosterone may have an independent effect on leptin. Because there is evidence that testosterone administration can lower leptin (30), the role of testosterone in regulating leptin levels in adolescent males merits further study along with related factors, such as lean body mass.
The finding that gender differences in leptin persist after correction for body fat indicates that factors other than adiposity contribute to gender differences in leptin during adolescence. Again, testosterone is a likely factor.
Associations between leptin and adiposity
Several studies examined the relationships between leptin and adiposity, with varying results (9,16,30,31,32). Leptin has been shown to be positively associated with BMI in adults (31,32). Peltz et al. (9) suggest that %BF is the only significant predictor of leptin in men, whereas both WC and %BF predict leptin in women. To date, few studies have examined the associations of anthropometric adiposity indexes and DEXA adiposity indexes with plasma leptin concentrations in adolescents (16).
Our study shows that associations between leptin and adiposity are not the same for all measures of adiposity. The measures with the strongest correlation in our sample were %BF and FMI, as determined by DEXA. These, then, are the preferred adiposity measures for future leptin research during adolescents. The measures that had an intermediate relationship with leptin were BMI and WC, whereas leptin was most weakly associated with %TF.
Given the other findings, we were surprised to learn that in this study, leptin is more strongly associated with %BF in males than females. More research is needed to clarify the interactions among adiposity, leptin, and testosterone in relation to future development of cardiovascular disease.
Genetic and environmental contributions to leptin levels, adiposity, and the correlations among them
This study has demonstrated that both leptin and %BF are highly heritable in this sample of rural Chinese adolescents. High heritability also was found for other adiposity measures (FMI, BMI, WC, and %TF) except for %TF in males (supplemental Figs. 1A to 4B). Furthermore, the substantial correlations between leptin and adiposity measures may be explained by a combination of shared genetic factors and shared unique environmental factors.
Our results are consistent with a previous study (23), which reported that the genetic correlation between fat mass and leptin was 0.88 (similarly, our values of 0.89 in males and 0.60 in females). However, in that study, the unique environmental correlation estimate of 0.75 was higher than those in our study. This discrepancy may be due to the differences of age groups (previous study: 4–19 yr, our study:13–21 yr), different degree of obesity (previous study: mean BMI in males, 25.9 kg/m2, in females, 24.3 kg/m2; our study both males and females, <20 kg/m2), different study designs (previous study: family study, our study: twin study), and different ethnic groups (previous study: Hispanic, our study: Chinese). Data from other samples will be needed to clarify the range of environmental correlations.
Summary
In this large sample of rural Chinese adolescents, we found gender differences in plasma leptin concentrations throughout all Tanner stages, which cannot be totally explained by gender difference in body fat. Plasma leptin concentrations were most strongly associated with FMI and %BF; the magnitude of the associations was greater in males than females. A common set of genetic factors appear to account for the majority of phenotypic correlation between leptin and %BF.
Further investigation is warranted to examine the role of testosterone and other factors in regulating male leptin levels during adolescence and to identify specific genetic loci that underlie the phenotypic correlation between leptin and %BF. A continued follow-up of this study sample will help evaluate the utility of leptin as a biomarker of adiposity and as an early precursor of cardiovascular diseases in adolescents.
Supplementary Material
Acknowledgments
We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and we thank all study participants for their support.
Footnotes
This work was supported by Grant R01 HD049059 from the National Institute of Child Health and Human Development; Grant R01 HL0864619 from the National Heart, Lung, and Blood Institute; Grant R01 AG032227 from the National Institute of Aging; and the Food Allergy Project.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 7, 2009
Abbreviations: a2, Additive genetic; %BF, percentage body fat; BMI, body mass index; DEXA, dual-energy x-ray absorptiometry; DZ, dizygotic; e2, environmental; FMI, fat body mass index; MZ, monozygotic; %TF, percentage trunk fat; WC, waist circumference.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA 2002 Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26:1407–1433 [DOI] [PubMed] [Google Scholar]
- Seufert J 2004 Leptin effects on pancreatic β-cell gene expression and function. Diabetes 53(Suppl 1):S152–S158 [DOI] [PubMed] [Google Scholar]
- Correia ML, Haynes WG 2004 Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens 13:215–223 [DOI] [PubMed] [Google Scholar]
- Beltowski J 2006 Leptin and atherosclerosis. Atherosclerosis 189:47–60 [DOI] [PubMed] [Google Scholar]
- Beltowski J 2006 Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens 24:789–801 [DOI] [PubMed] [Google Scholar]
- Soderberg S, Zimmet P, Tuomilehto J, Chitson P, Gareeboo H, Alberti KG, Shaw JE 2007 Leptin predicts the development of diabetes in Mauritian men, but not women: a population-based study. Int J Obes (Lond) 31:1126–1133 [DOI] [PubMed] [Google Scholar]
- Welsh P, Murray HM, Buckley BM, de Craen AJ, Ford I, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Sattar N 2009 Leptin predicts diabetes but not CVD: results from a large prospective study in the elderly. Diabetes Care 32:308–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz G, Sanderson M, Perez A, Sexton K, Ochoa Casares D, Fadden MK 2007 Serum leptin concentration, adiposity, and body fat distribution in Mexican-Americans. Arch Med Res 38:563–570 [DOI] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE 2001 Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr 74:295–301 [DOI] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE, Ding J, Goodpaster BH, Kanaya AM, Simonsick EM, Tylavsky FA, Harris TB 2004 Serum leptin concentrations and body adipose measures in older black and white adults. Am J Clin Nutr 80:576–583 [DOI] [PubMed] [Google Scholar]
- Venner AA, Lyon ME, Doyle-Baker PK 2006 Leptin: a potential biomarker for childhood obesity? Clin Biochem 39:1047–1056 [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA 2001 Longitudinal study on pubertal insulin resistance. Diabetes 50:2444–2450 [DOI] [PubMed] [Google Scholar]
- Jasik CB, Lustig RH 2008 Adolescent obesity and puberty: the “perfect storm.” Ann NY Acad Sci 1135:265–279 [DOI] [PubMed] [Google Scholar]
- Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM 1999 Serum leptin concentration, body composition, and gonadal hormones during puberty. Int J Obes Relat Metab Disord 23:678–685 [DOI] [PubMed] [Google Scholar]
- Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, Pierson Jr J, leibel RL 2000 Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab 85:2509–2518 [DOI] [PubMed] [Google Scholar]
- Nagy TR, Gower BA, Trowbridge CA, Dezenberg C, Shewchuk RM, Goran MI 1997 Effects of gender, ethnicity, body composition, and fat distribution on serum leptin concentrations in children. J Clin Endocrinol Metab 82:2148–2152 [DOI] [PubMed] [Google Scholar]
- Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W 1997 Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab 82:2904–2910 [DOI] [PubMed] [Google Scholar]
- Sun C, Yu C, Wang S 2001 Study on the effects of leptin on puberty development in children. Zhonghua Yu Fang Yi Xue Za Zhi 35:293–296 [PubMed] [Google Scholar]
- Narkiewicz K, Szczech R, Winnicki M, Chrostowska M, Pawlowski R, Lysiak-Szydlowska W, Choe I, Kato M, Sivitz WI, Krupa-Wojciechowska B, Somers VK 1999 Heritability of plasma leptin levels: a twin study. J Hypertens 17:27–31 [DOI] [PubMed] [Google Scholar]
- Luke A, Guo X, Adeyemo AA, Wilks R, Forrester T, Lowe Jr W, Comuzzie AG, Martin LJ, Zhu X, Rotimi CN, Cooper RS 2001 Heritability of obesity-related traits among Nigerians, Jamaicans and U.S. black people. Int J Obes Relat Metab Disord 25:1034–1041 [DOI] [PubMed] [Google Scholar]
- Kaprio J, Eriksson J, Lehtovirta M, Koskenvuo M, Tuomilehto J 2001 Heritability of leptin levels and the shared genetic effects on body mass index and leptin in adult Finnish twins. Int J Obes Relat Metab Disord 25:132–137 [DOI] [PubMed] [Google Scholar]
- Cai G, Cole SA, Butte NF, Smith CW, Mehta NR, Voruganti VS, Proffitt JM, Comuzzie AG 2008 A genetic contribution to circulating cytokines and obesity in children. Cytokine 44:242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Kumar R, Venners S, Pongracic J, Wang B, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Xu X, Wang X 2007 Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol 42:631–639 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1970 Variations in pattern of pubertal changes in boys. In: Arch Dis Child 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobelli A, Formica C, Wang Z, Heymsfield SB 1996 Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 271:E941–E951 [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM 2005 Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem 51:1854–1866 [DOI] [PubMed] [Google Scholar]
- Wang B, Necheles J, Ouyang F, Ma W, Li Z, Liu X, Yang J, Xing H, Xu X, Wang X 2007 Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med 45:358–365 [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR 1992 Methodology for genetic studies of twins and families. Dordrecht, The Netherland: Kluwer Academic Publishers [Google Scholar]
- Adan L, Bussieres L, Trivin C, Souberbielle JC, Brauner R 1999 Effect of short-term testosterone treatment on leptin concentrations in boys with pubertal delay. Horm Res 52:109–112 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS 1996 Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab 81:4406–4413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.