Abstract
Gap junctions are aggregates of intercellular channels that permit direct cell–cell transfer of ions and small molecules. Initially described as low-resistance ion pathways joining excitable cells (nerve and muscle), gap junctions are found joining virtually all cells in solid tissues. Their long evolutionary history has permitted adaptation of gap-junctional intercellular communication to a variety of functions, with multiple regulatory mechanisms. Gap-junctional channels are composed of hexamers of medium-sized families of integral proteins: connexins in chordates and innexins in precordates. The functions of gap junctions have been explored by studying mutations in flies, worms, and humans, and targeted gene disruption in mice. These studies have revealed a wide diversity of function in tissue and organ biology.
Gap junctions that allow material to move between cells are composed of connexins. The choice of connexin affects the permeability and other features of these junctions.
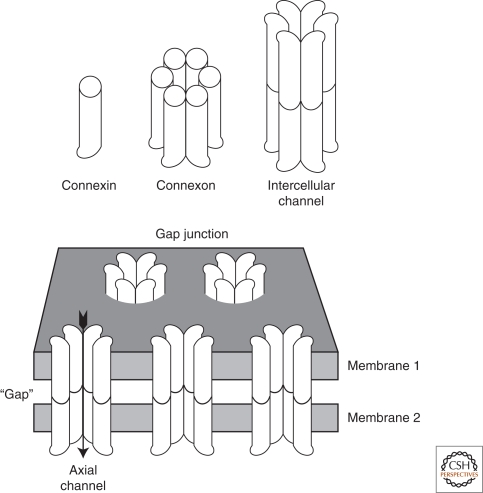
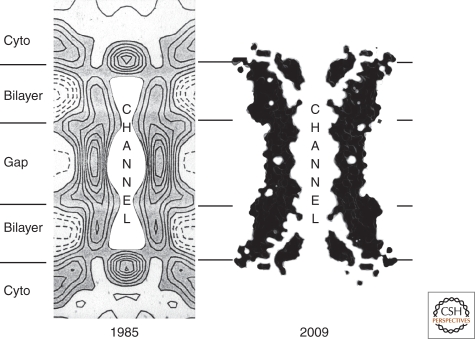
Gap junctions are clusters of intercellular channels that allow direct diffusion of ions and small molecules between adjacent cells. The intercellular channels are formed by head-to-head docking of hexameric assemblies (connexons) of tetraspan integral membrane proteins, the connexins (Cx) (Goodenough et al. 1996). These channels cluster into polymorphic maculae or plaques containing a few to thousands of units (Fig. 1). The close membrane apposition required to allow the docking between connexons sterically excludes most other membrane proteins, leaving a narrow ∼2 nm extracellular “gap” for which the junction is named (Fig. 2). Gap junctions in prechordates are composed of innexins (Phelan et al. 1998; Phelan 2005). In chordates, connexins arose by convergent evolution (Alexopoulos et al. 2004), to expand by gene duplication (Cruciani and Mikalsen 2007) into a 21-member gene family. Three innexin-related proteins, called pannexins, have persisted in vertebrates, although it is not clear if they form intercellular channels (Panchin et al. 2000; Bruzzone et al. 2003). 7Å-resolution electron crystallographic structures of intercellular channels composed of either a carboxy-terminal truncation of Cx43 (Unger et al. 1999; Yeager and Harris 2007) or an M34A mutant of Cx26 (Oshima et al. 2007) are available. The overall pore morphologies are similar with the exception of a “plug” in the Cx26 channel pore. The density of this plug is substantively decreased by deletion of amino acids 2–7, suggesting that the amino-terminus contributes to this structure (Oshima et al. 2008). A 3.5-Å X-ray crystallographic structure has visualized the amino-terminus of Cx26 folded into the mouth of the channel without forming a plug, thought to be an image of the open channel conformation (Maeda et al. 2009). The amino-terminus has been physiologically implicated in voltage-gating of the Cx26 and Cx32 channels (Purnick et al. 2000; Oh et al. 2004), lending support to a role for the amino-terminus as a gating structure. However, Cx43 also shows voltage-gating, and its lack of any structure resembling a plug remains unresolved. A comparison of a 1985 intercellular channel structure (Makowski 1985) with the 2009 3.5Å structure (Maeda et al. 2009) summarizes a quarter-century of X-ray progress (Fig. 3).
Figure 1.
A diagram showing the multiple levels of gap junction structure. Individual connexins assemble intracellularly into hexamers, called connexons, which then traffic to the cell surface. There, they dock with connexons in an adjacent cell, assembling an axial channel spanning two plasma membranes and a narrow extracellular “gap.”
Figure 2.
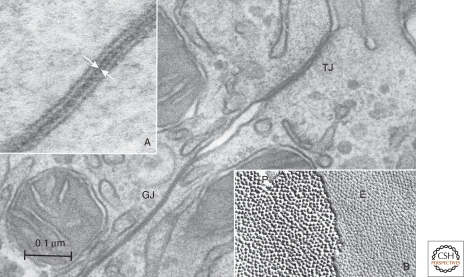
Electron microscopy of gap junctions joining adjacent hepatocytes in the mouse. The gap junction (GJ) is seen as an area of close plasma membrane apposition, clearly distinct from the tight junction (TJ) joining these cells. (Inset A) A high magnification view of the gap junction revealing the 2–3 nm “gap” (white arrows) separating the plasma membranes. (Inset B) A freeze-fracture replica of a gap junction showing the characteristic particles on the protoplasmic (P) fracture face and pits on the ectoplasmic (E) fracture face. The particles and pits show considerable disorder in their packing with an average 9-nm center-to-center spacing.
Figure 3.
A comparison of axial sections through gap-junction structures deduced from X-ray diffraction. The 1985 data (Makowski 1985) were acquired from gap junctions isolated biochemically from mouse liver containing mixtures of Cx32 and Cx26. The intercellular channel (CHANNEL) is blocked at the two cytoplasmic surfaces by electron density at the channel mouths along the sixfold symmetry axis. The 2009 data (Maeda et al. 2009), acquired from three-dimensional crystals of recombinant Cx26, resolve this density at the channel opening as the amino-termini of the connexin proteins, the 2009 model possibly showing an open channel structure.
Most cells express multiple connexins. These may co-oligomerize into the same (homomeric) or mixed (heteromeric) connexons, although only certain combinations are permitted (Falk et al. 1997; Segretain and Falk 2004). A connexon may dock with an identical connexon to form a homotypic intercellular channel or with a connexon containing different connexins to form a heterotypic channel (Dedek et al. 2006). Although only some assembly combinations are permitted (White et al. 1994), the number of possible different intercellular channels formed by this 21-member family is astonishingly large. This diversity has significance because intercellular channels composed of different connexins have different physiological properties, including single-channel conductances and multiple conductance states (Takens-Kwak and Jongsma 1992), as well as permeabilities to experimental tracers (Elfgang et al. 1995) and to biologically relevant permeants (Gaunt and Subak-Sharpe 1979; Veenstra et al. 1995; Bevans et al. 1998; Gong and Nicholson 2001; Goldberg et al. 2002; Ayad et al. 2006; Harris 2007).
Opening of extrajunctional connexons in the plasma membrane, described as “hemichannel” activity, can be experimentally induced in a variety of cell types. Because first observations of hemichannel activity were in an oocyte expression system (Paul et al. 1991) and dissociated retinal horizontal cells (DeVries and Schwartz 1992), the possible functions of hemichannels composed of connexins and pannexins has enjoyed vigorous investigation (Goodenough and Paul 2003; Bennett et al. 2003; Locovei et al. 2006; Evans et al. 2006; Srinivas et al. 2007; Schenk et al. 2008; Thompson and MacVicar 2008; Anselmi et al. 2008; Goodenough and Paul 2003). Hemichannels have been implicated in various forms of paracrine signaling, for example in providing a pathway for extracellular release of ATP (Cotrina et al. 1998; Kang et al. 2008), glutamate (Ye et al. 2003), NAD+ (Bruzzone et al. 2000), and prostaglandins (Jiang and Cherian 2003).
GAP JUNCTIONAL INTERCELLULAR CHANNELS ARE DYNAMICALLY REGULATED
Communication via intercellular channels is regulated at multiple levels. The most rapid timescales involve changing the unitary conductance of single channels or altering their probability of opening. Slower regulation is achieved by altering the number of channels present in the membrane by changing rates of synthesis and assembly, posttranslational modification and/or protein degradation. The mechanisms of regulation can overlap between these different time frames, for example, phosphorylation is involved both in changing single channel conductance and in protein trafficking to the cell surface and degradation. The different timescales will be considered in turn.
Rapid Regulation
On the shortest time scale, it is known that gap-junction channels are gated by voltage and can display multiple voltage-dependent conductance states (Turin and Warner 1977; Spray et al. 1979; Neyton and Trautmann 1985; Chen and DeHaan 1992; Bukauskas and Weingart 1993). Voltage-gating is a common property of connexins, although they show substantive differences in their sensitivities. Voltage-gating could explain the rectifying neuronal synapses observed in crayfish (Furshpan and Potter 1959), Drosophila (Allen et al. 2006), and hatchetfish (Auerbach and Bennett 1969; Hall et al. 1985), in which action potentials are permitted to pass orthodromically but not antidromically. This behavior requires a structural asymmetry that could be most simply modeled by a heterotypic intercellular channel in which one connexon showed fast voltage-dependent closure whereas the other did not. Indeed, rectification was observed in heterotypic junctions formed between connexins expressed in paired Xenopus oocytes (Dahl et al. 1987), but the time scale was too slow to completely explain rectifying synapses (Swenson et al. 1989). In addition to rapid closure of a channel in response to postsynaptic depolarization, rectification at an electrical synapse could also be achieved by opening channels in response to presynaptic depolarization. However, it requires at least 9.5 ms to reopen a closed Cx40 channel in this manner, which is also too slow to account for synaptic rectification (Bukauskas et al. 1995). Although rectifying synapses require near-instantaneous rectification of current, somewhat slower voltage inactivation may be functional in other contexts. For example, Cx45/Cx43 heterotypic junctions may rectify fast enough to influence dendro–dendritic interactions in the central nervous system or may modulate re-entry circuits in myocardium (Bukauskas et al. 2002a).
Fast rectification has been shown using Cx32/Cx26 heterotypic channels (Oh et al. 1999). However, neither connexin displays particularly fast homotypic voltage-dependent gating and thus the rectification observed cannot be predicted from the properties of the individual channels. A model is that the asymmetry of the heterotypic channel results in a separation of fixed positive and negative charges across the two junctional membranes and that rectification of ionic currents occurs within the channel rather than resulting from voltage-induced connexin conformational changes. Regardless, Cx26 and Cx32 are not typically found in excitable cells and are unlikely to participate in rectifying synapses.
Recently, Phelan et al. 2008 have explored innexin composition and physiology of rectifying synapses in the Drosophila giant fiber system. These rectifying synapses were shown to be composed of heterotypic channels formed from two different products of the shaking-B innexin gene: Shaking-B (neural+16) and Shaking-B (lethal). The former innexin is expressed in the presynaptic neuron and the latter in postsynaptic cell. Although technical limitations did not permit direct electrophysiological measurements in vivo, the two innexins were expressed in the Xenopus paired-oocyte system that allowed the characterization of both homotypic and heterotypic innexin interactions. Homotypic intercellular channels composed of Shaking-B (lethal) were highly voltage-dependent compared with those composed of Shaking-B (neural+16). However, in neither case did homotypic channels display rectification. In contrast, Shaking-B (neural+16) and Shaking-B (lethal) assembled heterotypic junctions that rectified. Importantly, channel closure was complete within 5 ms of the application of a transjunctional voltage, and displayed the appropriate gating polarity seen in vivo. However, crayfish junctions in vivo show channel gating within 0.8–1 ms (Furshpan and Potter 1959; Giaume et al. 1987) fivefold faster than the values measured using innexin channels in paired oocytes. Because it is not known how fast channels rectify in the fly, it is not yet possible to conclude that innexin composition explains the entire phenomenon. Regardless, this study provides the first molecular in vivo model to explain part of this 40-year-old conundrum.
Other than rectification, voltage gating of gap-junction channels may not be an important mode of channel regulation in vivo (Harris 2002). However, experimental manipulation of transjunctional voltage reveals a range of conductance states that are likely stabilized by other forms of channel regulation. For example, phosphorylation may function to favor one conductance state more than another, and hence be of great importance in terms of channel selectivity. Although phosphorylation is observed in most members of the connexin family (Lampe and Lau 2000; Lampe and Lau 2004; Laird 2005), most studies have focused on Cx43, which contains 21 serine and two tyrosine residues that are targets of phosphorylation by protein kinase A (PKA), protein kinase C (PKC), p34(cdc2)/cyclin B kinase, casein kinase 1, mitogen-activated protein kinase (MAPK), and pp60 (src) kinase (review (Solan and Lampe 2005)). Phosphorylation of Cx43 changes the shape of the current voltage relationship (Moreno et al. 1994). In particular, phosphorylation of serine368 (Lampe et al. 2000) by PKC results in a ∼50% reduction in unitary conductance. The change in conductance state likely reflects significant changes in channel permeation. For example, driving intercellular channels into subconductance states with transjunctional voltage has been shown to produce a change in charge selectivity (Bukauskas et al. 2002b) or a block of intercellular cAMP and dye-transfer (Qu and Dahl 2002) with little effect on macroscopic electrical coupling. Phosphorylation effects on permeation have also been noted with Cx43 hemichannels where dephosphorylation was correlated with increased channel permeability in liposome reconstitution studies (Kim et al. 1999).
Cx45 has been shown to change its open probability in response to activation of cAMP-dependent protein kinases (van Veen et al. 2000). Activation of pp60v-src is correlated with tyrosine phosphorylation of Cx43 and concomitant channel inactivation (Swenson et al. 1990; Lampe et al. 2000; Lampe and Lau 2000; Lin et al. 2001; for a review see Pahujaa et al. 2007), although recent studies suggest this regulation may be complex, as src activation also led to phosphorylation of MAPK and PKC sites in Cx43 (Solan and Lampe 2008).
Although many studies have shown changes in channel conductance with phosphorylation in cell culture, there are also in vivo studies documenting this role. For example, during reinitiation of meiosis by luteinizing in developing mouse ovarian follicles, Cx43 is multiply serine phosphorylated via MAPK (Norris et al. 2008), resulting in closure of gap junctional channels between mural granulosa cells, and internalization of gap junctions (Gilula et al. 1978). Another example in which connexin phosphorylation has a clear physiological relevance is in light–dark adaptation, which is globally regulated in the retina by the extrasynaptic release of dopamine (Puopolo et al. 2001). Dopamine acts on most if not all retinal neurons to adjust the gain of neural networks so that sensitivity to contrast can be maintained as the intensity of background illumination changes. In the outer retina, dopamine release rapidly and reversibly leads to a decrease in junctional coupling between horizontal cells (Lasater and Dowling 1985; DeVries and Schwartz 1989; Xin and Bloomfield 1999), which among other actions decreases the size of their receptive field (i.e., restricts the response of a given horizontal cell to a smaller number of photoreceptors), with the overall effect being an improvement in contrast sensitivity. In the inner retina, dopamine has similar effects on junctional coupling between amacrine cells, particularly the AII amacrine, which expresses Cx36 and is a critical part of the rod photoreceptor signaling pathway. D1 dopamine receptor activation in mouse AII amacrine cells leads to a PKA-mediated phosphorylation of Cx36 correlating with a decrease of dye coupling in vivo (Urschel et al. 2006). In the teleost retina, it was shown using phospho-specific antibodies that the natural stimulus of dark-adaptation dramatically increased the levels of Cx35 (the teleost ortholog of Cx36) phosphorylation (Kothmann et al. 2007). Furthermore, these phosphorylation events occurred at sites shown to regulate Cx35 channel gating using in vitro expression studies (O’Brien et al. 2004).
Slow Regulation
A slower temporal level of regulation involves connexin biosynthesis and junctional plaque assembly and turnover (Segretain and Falk 2004). Connexins can show a remarkably rapid turnover rate for a membrane protein. For example, the in vivo half-life of Cx32 in gap junctional plaques from rodent hepatocytes is less than 5 hours (Fallon and Goodenough 1981) and turnover of Cx43 in tissue culture cells is even faster (Musil and Goodenough 1991; Laird et al. 1991). Gap junctions have been shown to turn over by addition of subunits at the edges and removal of subunits from the center of plaques (Gaietta et al. 2002; Lauf et al. 2002). Accretion of connexons at the edges of pre-existing plaques could require nothing more than lateral diffusion in the plasma membrane, but it is not at all clear how the selective removal of connexins/connexons/intercellular channels from the center of a plaque might be orchestrated. Gap junctions are also removed from the cell surface by gross internalization of the entire plaque, leaving large double-membrane vesicles in the cytoplasm (Albertini and Anderson 1975; Larsen et al. 1979; Jordan et al. 2001). Studies with cultured cells suggest that internalization is a clathrin-mediated process (Piehl et al. 2007; Nickel et al. 2008). The relationship between the removal of connexins from the center of pre-existing junctional plaques and the clathrin-dependent endocytosis of whole junctional plaques remains unclear.
Gap junction assembly is associated with multiple phosphorylation steps (Musil and Goodenough 1991). Cx43 is phosphorylated soon after synthesis, and trafficking of the protein through the Golgi to the plasma membrane is accompanied by phosphorylation of specific residues, suggesting a requirement for these modifications in protein transport (Solan and Lampe 2007). Consistent with this notion, chemical or temperature blockade of trafficking in the ER or Golgi results in incomplete Cx43 phosphorylation (Musil and Goodenough 1993). Phosphorylation is also used by different connexins to both block and enhance degradation (Laird et al. 1995). For example, it has been shown that phosphorylation protects Cx32 from calpain digestion (Elvira et al. 1993), while serine phosphorylation of Cx45.6, the chick lens counterpart of Cx50, stimulates protein turnover (Yin et al. 2008). Cx43 can be degraded by both the proteosomal and lysosomal pathways, although no ubiquitin ligase has been shown to specifically associate with a connexin (Laing and Beyer 1995; Berthoud et al. 2004). Proteosome inhibitors block connexin degradation and up-regulate both gap junction assembly and intercellular dye transfer, demonstrating control of gap-junctional intercellular communication (GJIC) via the degradation pathway (Musil et al. 2000). Cx43 dephosphorylation has been associated with disassembly of gap junctions in cells treated with the gap junction blocking agent 18 β-glycyrrhetinic acid (Guan et al. 1996).
Assembly is also affected by interaction with connexin binding partners. A Cx43-interacting protein, CIP85 can induce the turnover of Cx43 through the lysosomal pathway (Lan et al. 2005). Another important interactor is ZO-1, which colocalizes with Cx43 in myocardium and links this connexin to α-spectrin in HEK293 cells in culture (Toyofuku et al. 1998). Cx43 binds to the second PDZ domain of ZO-1 (Giepmans and Moolenaar 1998). Mutations in Cx43 that alter the consensus PDZ binding domain do not inhibit the formation of gap junctions or the activity of intercellular channels. However, there is a dramatic deregulation of plaque size and abnormally large gap junctions are observed (Falk 2000; Hunter et al. 2005). The size expansion results from increased accretion of cytoplasmic pools of Cx43 connexons to the edges of existing junctional plaques and not from de novo synthesis or inhibited degradation. ZO-1 is preferentially associated with the periphery of gap junctional plaques in cells expressing Cx43, suggesting that ZO-1 is a negative regulator of accretion. It has been proposed that accretion is suppressed by a ZO-1 mediated association with filamentous actin (Hunter and Gourdie 2008). In addition, Cx43 may directly associate with tubulin (Giepmans et al. 2001), possibly explaining the observed transport of Cx43 along microtubule tracks (Lauf et al. 2002) that in turn may influence the rate or location of plaque assembly.
The myriad forms of regulation of gap junction function seem surprisingly diverse in comparison to other membrane channels. As reviewed in the following section, the multiple cellular, tissue, and organ functions that have adapted gap-junctional communication as part of their mechanisms have developed a diverse set of regulatory strategies to provide the spatial and temporal controls required in different contexts. Indeed, in some cases, the evolution of multiple connexin genes may have occurred in part because of requirements for unique mechanisms of regulation. In other cases, for example with Cx43, which is used by many different cell types in specialized contexts, multiple regulatory mechanisms are needed to provide specialized control. It is clear from this diversity that the regulation of gap junctional intercellular communication must be experimentally determined on a case-by-case basis as different mechanisms have evolved to subserve this function in different cellular contexts.
UNIVERSAL FUNCTIONS OF GAP JUNCTIONS
The ability of adjacent cells to share ions through low-resistance pathways is fundamental to the function of electrically excitable cells, such as neurons, heart, and smooth muscle. Indeed, gap junctions (electrical synapses) were first discovered in myocardium and nerve because of their properties of electrical transmission between adjacent cells (Weidmann 1952; Furshpan and Potter 1957). In these contexts, connecting cells with gap junctions provides both increased speed in synaptic transmission and the ability to synchronize groups of cells for coordinated electrical and mechanical output.
In addition to electrically excitable cells, virtually all cells in solid tissues are joined by gap junctions. A core function of GJIC is to share metabolic demands across groups of cells and thereby buffer spatial gradients of nutrients or signaling molecules. For example, targeted deletion of Cx32 in mice has been shown to result in a loss of responsiveness to sympathetic stimulation, resulting in an impaired mobilization of glucose from glycogen stores. Postganglionic sympathetic axons terminate at the edges of the liver lobules and thus can only directly stimulate a fraction of the hepatocytes. Presumably, the remainder of the lobule is stimulated indirectly by diffusion of second messengers through gap junctions (Stümpel et al. 1998). Gap junctions may also function as suppressors of somatic cell mutations so that loss of a critical metabolic enzyme or ion channel in one cell might be compensated by its neighbors. For example, Lesch-Nyhan syndrome results from impaired activity of hypoxanthine phosphoribosyltransferase (HGPRTase), a key enzyme in the nucleotide salvage pathway. Impaired HGPRTase results in an elevated concentration of phosphoribosyl pyrophosphate, a marked increase in the rate of purine biosynthesis, and an overproduction of urate. Mutant fibroblasts from patients with Lesch-Nyhan syndrome can be metabolically rescued in cell culture by gap junction formation with normal cells (Cox et al. 1970), a process termed metabolic cooperation (Subak-Sharpe et al. 1969). Furthermore, metabolic cooperation likely accounts for the lack of symptoms in heterozygous female Lesch-Nyhan carriers. As HGPRTase is located on the X chromosome, random X-inactivation results in a mosaic of mutant and normal cells. Thus, individuals are asymptomatic because of metabolic rescue of mutant cells by adjacent nonmutant cells.
SPECIALIZED FUNCTIONS REVEALED BY CONNEXIN MUTATIONS
Human Mutations
Given the long phylogenetic history of gap junctions in metazoans (Fraser and Bode 1981; Potenza et al. 2002; Starich et al. 2003; Nogi and Levin 2005), it is not surprising that this method of cell–cell communication has been adapted to subserve a wide variety of physiological functions in different cell types. Many cell- and tissue-specific functions of GJIC have been brought to light by human mutations and targeted connexin gene deletion in mice (for reviews see Simon and Goodenough 1998; White and Paul 1999; Gerido and White 2004; Dobrowolski and Willecke 2008). In humans, mutations in Cx32 underlie X-linked Charcot-Marie-Tooth syndrome, a common peripheral demyelination neuropathy (Bergoffen et al. 1993), and mutations in Cx47 result in a central demyelinating condition called Pelizaeus-Merzbacher-Like-Disease (Uhlenberg et al. 2004). More than half of all profound hereditary deafness results from mutations in Cx26, which are often syndromic and involve skin disorders (Kelsell et al. 1997; Denoyelle et al. 1997). Similarly, although usually less severe, disorders of the skin and the auditory system accompany mutations in Cx31 and Cx30 (Common et al. 2002; Abrams et al. 2006; Yang et al. 2007; Apps et al. 2007; Yum et al. 2007). Familial cataracts are commonly associated with mutations in either Cx46 or Cx50, whose expression is largely restricted to the ocular lens (Gong et al. 2007; Richard 2005; van Steensel 2004; Vreeburg et al. 2007; Mese et al. 2007). Finally, mutations in Cx43 give rise to oculodentodigital dysplasia, a pleomorphic, syndromic condition affecting a large number of cell types (Paznekas et al. 2003).
Targeted Mutations in Mice
In mice, targeted mutations of connexins have uncovered a wide variety of gap-junction functions in various organs. In many of these cases, a given connexin occupies a particular niche, supplying an essential function that is not compensated by another connexin. For example, Cx26 deletion is embryonic lethal because of a disruption of glucose transport between syncytiotrophoblast I and II in the labyrinth layer of the placenta, which are coupled by gap junctions (Gabriel et al. 1998). In contrast, the human placenta contains only one giant syncytiotrophoblast and so is not vulnerable to Cx26 mutations. Cx45 deletions are also embryonic lethal (Kruger et al. 2000; Willecke et al. 2002), in this case likely the result of myocardial arrhythmia shortly after the heart begins to beat (Nishii et al. 2003). Cx37 knockouts are female sterile from a failure of ovarian follicle development at the antral stage. Presumably, loss of communication between oocyte and cumulus cells leads to premature resumption of meiosis and luteinization (Simon et al. 1997). The loss of Cx40, prevalent in the His-Purkinje system, results in cardiac arrhythmias resembling right-bundle-branch block in humans (Simon et al. 1998; Kirchhoff et al. 1998).
Unique roles played by some connexins have been shown by knockin experiments. The Cx43 coding sequence was replaced in three separate mouse lines with Cx32, Cx40, or Cx26 coding regions. All three animal lines showed new functional defects unique to each connexin, revealing that the three connexins were not able to substitute for Cx43 in all contexts (Plum et al. 2000; Winterhager et al. 2007). Although none of the lines displayed the pulmonary outflow defects seen in the Cx43KO mouse (Reaume et al. 1995), a knockin of Cx31 into the Cx43 locus did show the defect (Zheng-Fischhofer et al. 2006). Thus, connexins may have both unique and redundant functions.
SURPRISING AND PUZZLING RESULTS FROM CONNEXIN MUTATIONS
Other functions that emerge from connexin deletions may result from the loss of a complex interplay of multiple connexin-family members in an incompletely defined network, producing unexpected and unexplained outcomes. Some of these examples are explored here in more detail.
Gap Junctions in the Vascular System
Arterioles are composed of a longitudinal layer of endothelial cells facing the blood, which is separated by a basal lamina from a layer of circular smooth muscle cells that control lumen diameter. There is a surprising complexity of connexin expression in the arteriolar layers. Smooth muscle cells express mainly Cx43 (Gabriels and Paul 1998) and endothelial cells mainly Cx40 (Little et al. 1995; van Kempen and Jongsma 1999), although both cell types express both connexins. Cx32 expression has been reported in endothelial cells (Okamoto et al. 2009). Smooth muscle cells uniquely express Cx45 (Kruger et al. 2000), whereas only the endothelium contains Cx37 (Gabriels and Paul 1998; van Kempen and Jongsma 1999). In addition, there can be significant regional variations in the relative abundance of these connexins in the vessel wall. As an example, endothelial Cx43 is dramatically up-regulated at the expense of the other connexins in areas that experience shear stresses such as vessel branch points (Gabriels and Paul 1998). Not only are gap junctions formed within arteriolar layers, but junctions are also formed between smooth muscle and endothelial cells. The connexin content of the myoendothelial junctions is not yet clear, although in vitro studies suggest that the endothelial side contains largely if not exclusively Cx40 (Isakson and Duling 2005).
Gap junctions have been strongly implicated in the conducted spread of vasodilation. Local endothelial stimulation initiates a rapidly propagated, bidirectional wave of relaxation along the vessel axis (Welsh and Segal 1998; Figueroa et al. 2003; de Wit et al. 2006). An intact endothelium is required for conducted vasodilation, which does not decay with distance and so must contain a self-regenerative component. The propagation of vasomotor activity is significantly depressed in Cx40 KO but not Cx37 KO animals (Figueroa et al. 2003; de Wit et al. 2000). While it was initially surprising that the loss of Cx37, which is co-expressed in endothelial cells, had no effect on propagation, this could be explained by the fact that loss of Cx40 causes a dramatic (>20-fold) reduction in the levels of endothelial Cx37, while loss of Cx37 results in only a mild (∼fourfold) reduction in the levels of Cx40 (Simon and McWhorter 2003).
A simple model for the role of gap junctions in propagation is that endothelial stimulation results in a change in membrane potential that is passively conducted along the endothelial layer through gap junctions, critically those containing Cx40. However, this model does not explain self-propagation. Even more problematic, knockin of Cx45 into the Cx40 locus does not rescue the Cx40 KO phenotype, suggesting that ionic spread of membrane potential changes through endothelial–endothelial gap junctions is not a critical factor (Wolfle et al. 2007). On the other hand, studies using connexin-mimetic peptides to selectively inhibit junctional communication in rabbit iliac arteries suggest that although Cx40 is required for endothelium-dependent smooth muscle hyperpolarization, Cx43 is required for spread of that hyperpolarization within the smooth muscle layer (Chaytor et al. 2005). Taken together, these observations suggest another model in which propagation requires both myoendothelial gap junctions as well as gap junctions joining smooth muscle cells. In the first phase, endothelial stimulation leads to release of an endothelium-derived hyperpolarizing factor (EDHF), causing hyperpolarization of immediately adjacent smooth muscle. It has been suggested that EDHF signaling requires myoendothelial junctions (Griffith 2007), which are permeable to inositol trisphosphate and Ca2+ (Isakson et al. 2007). A second phase might involve electrotonic spread of hyperpolarization within the smooth muscle layer through gap junctions composed of Cx43. The extent of this spread would be modest as electrical coupling in this layer is relatively weak. In the third phase, smooth muscle must restimulate endothelial cells distal to the site of initial stimulus, regenerating additional rounds of EDHF release. Relaxation of smooth muscle accompanies release of a second factor, endothelium-derived relaxation factor (likely nitric oxide), which can move from endothelium to smooth muscle in the absence of gap junctions. This model is consistent with the loss of conducted vasodilation in the Cx40 KO, but not Cx37 KO, and predicts a Cx40 KO phenocopy in a smooth muscle-specific Cx43 KO, which has not yet been evaluated.
In addition to vasomotor responses, connexin knockouts can dramatically impact systemic blood pressure. Conditional disruption of Cx43 in vascular endothelial cells results in hypotension and bradycardia (Liao et al. 2001), accompanied by elevated plasma levels of nitric oxide because of increased activity of endothelial nitric oxide synthase. These phenotypes are currently without explanation and are not seen in another model of vascular deletion of Cx43 (Theis et al. 2001). In contrast to the hypotension accompanying vascular loss of Cx43, constitutive deletion of Cx40 results in hypertension (de Wit et al. 2006). In this case, disregulation of angiotensin levels may be responsible. In these animals, renin-producing cells are anatomically displaced during development (Kurtz et al. 2007) and are also less responsive to feedback inhibition by plasma angiotensin, leading to increased plasma levels of renin (Wagner et al. 2007). Why the loss of Cx40 results in this cellular localization defect is not known. Interestingly, although knockin of Cx45 into the Cx40 locus is unable to rescue propagation of the vasomotor activity (Wolfle et al. 2007), it abrogates the hyperreninemia, partially attenuating the systemic hypertension and restoring angiotensin-suppression of renin release (Schweda et al. 2008). Parenthetically, Cx45 deletion from smooth muscle in the juxtaglomerular apparatus later in development also results in increased renin secretion and significant blood pressure elevation (Hanner et al. 2008; Yao et al. 2008).
The double knockout (dKO) of Cx37 and Cx40 displays an additional phenotype not seen in either individual knockout. dKO animals die perinatally with dramatic vascular abnormalities. By E18.5, numerous hemorrhages are visible through the skin and internally in the testes, lungs, and intestines. Vasculogenesis is aberrant in the testis and in the connective tissues of the small bowel, but seemingly unaffected in other organs (Simon and McWhorter 2002; Simon and McWhorter 2003). It is not known if these new pathologies result from a combination of the individual regulation and selectivities of the individual connexins, or if this is because of unique properties exhibited by heteromeric or heterotypic intercellular channels.
Gap Junctions in the Ocular Lens
During development, the optic vesicle induces the overlying ectoderm to invaginate and pinch off a hollow sphere of cells, the lens vesicle. The posterior cells of the vesicle then elongate anteriorly as lens fibers, which contact the anterior cells occluding the vesicle lumen. The lens thus becomes a solid cyst of cells, with an anterior epithelium and posterior fibers. The organ eventually loses an enveloping basket of blood vessels, becoming totally avascular and therefore dependent on the aqueous humor for all metabolic needs. The lens continues to grow in volume throughout the life of the organism by appositional growth, differentiating new lens fibers from a stem cell population at the equatorial surface. The older fibers do not turn over, remaining in the lens interior. To achieve a high refractive index and transparency, the differentiating fibers synthesize high concentrations of soluble proteins, the crystallins, and then undergo a limited apoptosis, destroying their nuclei and all light-scattering organelles. Thus, the lens fibers are metabolically dependent on the anterior epithelial cells that retain their organelles. The lens fibers are joined to each other and to the epithelial cells by large numbers of gap junctions (Goodenough 1992). The asymmetric location of the Na+K+ATPase in the epithelium results in a translenticular potential and a DC current flow (Candia et al. 1970), modeled as the circulatory system of the lens (Rae 1979; Mathias 1985; Mathias and Rae 1989). As the high concentration of the crystallins requires a tight control of ionic balance to remain in solution, the ionic syncytium created by the gap junctions is essential for lens transparency.
Cx43, 46, and 50 are expressed in the lens. Cx43 and 50 are found abundantly in the lens epithelium (Beyer et al. 1987; Jiang et al. 1995; Martinez-Wittinghan et al. 2003). Cx46 and 50 are found joining the lens fibers where they colocalize to the same junctional plaques (Paul et al. 1991) and have been shown to co-oligomerize into the same connexons and intercellular channels (Konig and Zampighi 1995; Jiang and Goodenough 1996). Indeed, immunofluorescence studies have shown colocalization of Cx46 and 50 in all junctional plaques joining the fibers. Given this anatomical overlap, it is surprising that targeted deletion of Cx46 and 50 result in distinctly different phenotypes (Gong et al. 1997; White et al. 1998). First, both cause cataracts but with differences in timing of onset and in morphology. Second, deletion of Cx50, but not Cx46, results in a slower postnatal growth rate with concomitant decrease in lens size and microphthalmia (White et al. 1998). Interestingly, the normal growth rate is uniquely dependent on Cx50 because replacing the coding region of Cx50 with that of Cx46 (Cx5046/46) does not fully rescue the lens mitotic rate (White 2002; Sellitto et al. 2004). The identity of the Cx50-dependent signal controlling mitosis is not known (White et al. 2007). The Cx46/Cx50 double knockout shows a phenotype more severe but predictable as the sum of the two individual connexin deletions (Xia et al. 2006).
Cx5046/46 animals are completely free of cataracts (White 2002), suggesting that this pathology could be prevented by simply restoring adequate numbers of junctional channels. Thus, it is surprising that mice heterozygous for Cx46 and Cx50 at the Cx50 locus (Cx50+/46) develop a cataract (Martinez-Wittinghan et al. 2003). Furthermore, this cataract is morphologically different from those in either Cx46KO or Cx50KO lenses. Although the latter two are primarily nuclear, the Cx50+/46 cataract is largely subepithelial. Additional crosses show that the Cx50+/46 cataract is insensitive to dosage of Cx46 at the Cx46 locus, proving that this unexpected phenotype is the result of changes in connexin stoichiometry in the epithelium, where Cx46 is not normally detected. Importantly, the phenotype only occurs when Cx50 and Cx46 are coexpressed in the epithelium, because no cataract is observed in the homozygous (Cx5046/46) knockin (White 2002). In addition to the cataract, Cx50+/46 lenses display impaired dye transfer both within the epithelial plane and between epithelium and underlying fibers (Martinez-Wittinghan et al. 2003). Why mixing of Cx46 and Cx50 in the epithelium should depress dye transfer and cause a novel cataract is completely without explanation because those connexins functionally interact in heterotypic and heteromeric configurations both in vivo and in expression systems (White et al. 1994; Jiang and Goodenough 1996; Hopperstad et al. 2000).
Demonstration of mechanisms underlying the specificity of connexin intercellular channels in these contexts is still missing. It was shown that fiber–fiber conductance was lower in the Cx5046/46 knockin than WT (Martinez-Wittinghan et al. 2004), thus the knockin approach may provide equal numbers of channels but does not provide equal levels of coupling. Regardless, the relationship between coupling level and differential mitotic rates remains obscure. We favor the notion that differential permeability of intercellular channels may play a more important role, as connexin-dependent differences in small molecule permeability have been observed in several studies (Harris 2007). For example, Cx43 channel permeability to cAMP is approximately three times higher than Cx26 and approximately five times higher than Cx40 (Kanaporis et al. 2008), providing a conceptual framework for the observed differences in knockin phenotypes (Harris 2008).
Gap Junctions in Myelin and the Central Nervous System
Mutations in Cx32 associated with the X-linked form of Charcot-Marie-Tooth syndrome result in a peripheral neuropathy associated with myelin failure in Schwann cells. Cx32 forms “reflexive” gap junctions that the Schwann cell makes with itself at the paranodal membranes and incisures of Schmidt-Lantermann. This anatomy suggests that the reflexive junctions in myelin are essential for communication between perinuclear and adaxonal Schwann cell cytoplasm. Measurements of the rate of diffusion between these two cytoplasmic compartments in individual Schwann cells support this notion (Balice-Gordon et al. 1998). However, there is no significant difference between diffusion rates in WT and Cx32 KO animals. To explain this discrepancy, it was hypothesized that Cx29, which is equally abundant although with a somewhat different intracellular distribution, might substitute for the loss of Cx32. However, Cx29 does not accumulate in gap junctional plaques in vivo in oligodendrocytes or Schwann cells (Altevogt et al. 2002; Nagy et al. 2003; Altevogt and Paul 2004) or form function gap junctions when expressed in tissue culture cells (Altevogt et al. 2002). On the other hand, the Cx29 KO does show a myelin defect but one that is restricted to cell bodies of the spiral ganglion neurons in the organ of Corti (Tang et al. 2006).
An additional surprising role for connexins has been shown in the developing neocortex (Elias et al. 2007). Cx26 and Cx43 protein expression was substantively knocked down by electroporation of shRNAs into E16 embryonic cortex. Connexin knockdown resulted in the stalling of migration of neurons along radial glia in the intermediate zone and a loss of cells arriving in the lower and upper cortical plates. Further experiments showed that normal migration was dependent on neuronal rather than glial expression of connexins (Elias et al. 2007). Connexin knockdown neurons showed normal timing of exit from mitosis and no detectable changes in apoptosis, which is unexpected because changes in cell–cell communication and hemichannel involvement in Ca2+ waves have been correlated with stages of the mitotic cycle (Bittman et al. 2007). Surprisingly, a channel-dead mutant (Beahm et al. 2006) rescued the migration defect, whereas mutations that resulted in both the loss of connexon pairing (but not hemichannel activity) and the loss of interaction with cytoplasmic partners (C-terminal truncations) were unable to rescue (Elias et al. 2007). These data led to the conclusion that the adhesive properties of connexins, rather than channel activity, were required for correct neuronal migration. In this context, it is of interest that Cx43 hemichannels can confer adhesivity between HeLa and C6 glioma cells in culture (Cotrina et al. 2008).
In summary, connexins and innexins are universally used to promote intercellular interactions between cells in solid tissues and circulating elements of the blood (Wong et al. 2006). They show multiple levels of regulation from instantaneous to hours. Genetic studies have shown that gap junctions are involved in a wide variety of functions in homeostasis, regulation, regeneration, and development. Given that a complex spectrum of small molecules within a cell can potentially diffuse through gap-junctional channels into neighbors, the identification of the relevant small molecules subserving each function has been difficult. Connexons, the hexameric precursor to the gap-junction channel, can function as a hemichannel in nonjunctional membranes promoting paracrine signaling. Even without channel function, the adhesivity of connexons can provide critical migratory cues. Unraveling the multiple functions of connexins and innexins and the contributions to these functions controlled by channel selectivity and regulation, is fundamental to understanding many aspects of collective cellular behavior.
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from grants EY02430 (DAG) and GM37751 (DLP).
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Abrams CK, Freidin MM, Verselis VK, Bargiello TA, Kelsell DP, Richard G, Bennett MV, Bukauskas FF 2006. Properties of human connexin 31, which is implicated in hereditary dermatological disease and deafness. Proc Natl Acad Sci 103:5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini DF, Anderson E 1975. Structural modifications of lutein cell gap junctions during pregnancy in the rat and the mouse. Anat Rec 181:171–194 [DOI] [PubMed] [Google Scholar]
- Alexopoulos H, Bottger A, Fischer S, Levin A, Wolf A, Fujisawa T, Hayakawa S, Gojobori T, Davies JA, David CN, et al. 2004. Evolution of gap junctions: The missing link? Curr Biol 14:R879–R880 [DOI] [PubMed] [Google Scholar]
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P 2006. Making an escape: Development and function of the Drosophila giant fibre system. Semin Cell Dev Biol 17:31–41 [DOI] [PubMed] [Google Scholar]
- Altevogt BM, Paul DL 2004. Four classes of intercellular channels between glial cells in the CNS. J Neurosci 24:4313–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL 2002. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci 22:6458–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, et al. 2008. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci 105:18770–18775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps SA, Rankin WA, Kurmis AP 2007. Connexin 26 mutations in autosomal recessive deafness disorders: A review. Int J Audiol 46:75–81 [DOI] [PubMed] [Google Scholar]
- Auerbach AA, Bennett MV 1969. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol 53:211–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad WA, Locke D, Koreen IV, Harris AL 2006. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem 281:16727–16739 [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Bone LJ, Scherer SS 1998. Functional gap junctions in the Schwann cell myelin sheath. J Cell Biol 142:1095–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue M, Chandrasekhar A, Nicholson BJ, Sosinsky GE 2006. Mutation of a conserved threonine in the third transmembrane helix of α‐ and β‐connexins creates a dominant negative closed gap junction channel. J Biol Chem 281:7994–8009 [DOI] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC 2003. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH 1993. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262:2039–2042 [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC 2004. Pathways for degradation of connexins and gap junctions. Cardiovasc Res 62:256–267 [DOI] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL 1998. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273:2808–2816 [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA 1987. Connexin43: A protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105:2621–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman K, Owens DF, Kriegstein AR, LoTurco JJ 1997. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci 17:7037–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A 2000. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 15:10–12 [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H 2003. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100:13644–13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Weingart R 1993. Multiple conductance states of newly formed single gap junction channels between insect cells. Pflügers Arch 423:152–154 [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R 1995. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J 68:2289–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Angele AB, Verselis VK, Bennett MV 2002a. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: Properties of fast and slow gating mechanisms. Proc Natl Acad Sci 99:7113–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Verselis VK 2002b. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol 119:171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia OA, Bentley PJ, Mills CD, Toyofuku H 1970. Asymmetrical distribution of the potential difference in the toad lens. Nature 227:852–853 [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Bakker LM, Edwards DH, Griffith TM 2005. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol 144:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, DeHaan RL 1992. Multiple-channel conductance states and voltage regulation of embryonic chick cardiac gap junctions. J Membr Biol 127:95–111 [DOI] [PubMed] [Google Scholar]
- Common JE, Becker D, Di WL, Leigh IM, O’Toole EA, Kelsell DP 2002. Functional studies of human skin disease- and deafness-associated connexin 30 mutations. Biochem Biophys Res Commun 298:651–656 [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M 2008. Adhesive properties of connexin hemichannels. Glia 56:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JHC, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M 1998. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci 95:15735–15740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RP, Krauss ME, Balis ME, Dancis J 1970. Evidence for transfer of enzyme product as the basis of metabolic cooperation between tissue culture fibroblasts of Lesch- Nyhan disease and normal cells. Proc Natl Acad Sci 67:1573–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO 2007. Evolutionary selection pressure and family relationships among connexin genes. Biol Chem 388:253–264 [DOI] [PubMed] [Google Scholar]
- Dahl G, Miller T, Paul D, Voellmy R, Werner R 1987. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science 236:1290–1293 [DOI] [PubMed] [Google Scholar]
- Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U 2006. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci 24:1675–1686 [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, et al. 1997. Prelingual deafness: High prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet 6:2173–2177 [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA 1989. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol 414:351–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA 1992. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol 445:201–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Wolfle SE, Hopfl B 2006. Connexin-dependent communication within the vascular wall: Contribution to the control of arteriolar diameter. Adv Cardiol 42:268–283 [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U 2000. Impaired conduction of vasodilation along arterioles in connexin40- deficient mice. Circ Res 86:649–655 [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Willecke K 2008. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Signal 11:283–295 [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, Hülser DF, Willecke K 1995. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 129:805–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR 2007. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448:901–907 [DOI] [PubMed] [Google Scholar]
- Elvira M, Diez JA, Wang KKW, Villalobo A 1993. Phosphorylation of connexin-32 by protein kinase C prevents its proteolysis by mu-calpain and m-calpain. J Biol Chem 268:14294–14300 [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L 2006. The gap junction cellular internet: Connexin hemichannels enter the signalling limelight. Biochem J 397:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM 2000. Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci 113:4109–4120 [DOI] [PubMed] [Google Scholar]
- Falk MM, Buehler LK, Kumar NM, Gilula NB 1997. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J 16:2703–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon RF, Goodenough DA 1981. Five hour half-life of mouse liver gap-junction protein. J Cell Biol 90:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR 2003. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res 92:793–800 [DOI] [PubMed] [Google Scholar]
- Fraser SE, Bode HR 1981. Epithelial cells of Hydra are dye-coupled. Nature 294:356–358 [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD 1957. Mechanism of nerve-impulse transmission at a crayfish synapse. Nature 180:342–343 [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD 1959. Transmission at the giant motor synapses of the crayfish. J Physiol 145:289–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel H-D, Jung D, Bützler C, Temme A, Traub O, Winterhager E, Willecke K 1998. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol 140:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels JE, Paul DL 1998. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res 83:636–643 [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky G, Tsien RY, Ellisman MH 2002. Multicolor and electron microscopic imaging of connexin trafficking. Science 296:503–507 [DOI] [PubMed] [Google Scholar]
- Gaunt SJ, Subak-Sharpe JH 1979. Selectivity in metabolic cooperation between cultured mammalian cells. Exp Cell Res 120:307–320 [DOI] [PubMed] [Google Scholar]
- Gerido DA, White TW 2004. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta 1662:159–170 [DOI] [PubMed] [Google Scholar]
- Giaume C, Kado RT, Korn H 1987. Voltage-clamp analysis of a crayfish rectifying synapse. J Physiol 386:91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH 1998. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8:931–934 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH 2001. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11:1364–1368 [DOI] [PubMed] [Google Scholar]
- Gilula NB, Epstein ML, Beers WH 1978. Cell-to-cell communication and ovulation. A study of the cumulus- oocyte complex. J Cell Biol 78:58–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD 2002. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 277:36725–36730 [DOI] [PubMed] [Google Scholar]
- Gong XQ, Nicholson BJ 2001. Size selectivity between gap junction channels composed of different connexins. Cell Adhes Commun 8:187–192 [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar N, Horwitz J, Gilula NB 1997. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91:833–843 [DOI] [PubMed] [Google Scholar]
- Gong X, Cheng C, Xia CH 2007. Connexins in lens development and cataractogenesis. J Membr Biol 218:9–12 [DOI] [PubMed] [Google Scholar]
- Goodenough DA 1992. The crystalline lens: A system networked by gap junctional intercellular communication. Semin Cell Biol 3:49–58 [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL 2003. Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4:285–295 [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL 1996. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65:475–502 [DOI] [PubMed] [Google Scholar]
- Griffith TM 2007. Which connexins connect? Circ Res 101:1219–1221 [DOI] [PubMed] [Google Scholar]
- Guan XJ, Wilson S, Schlender KK, Ruch RJ 1996. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18-β-glycyrrhetinic acid. Mol Carcinogenesis 16:157–164 [DOI] [PubMed] [Google Scholar]
- Hall DH, Gilat E, Bennett MV 1985. Ultrastructure of the rectifying electrotonic synapses between giant fibres and pectoral fin adductor motor neurons in the hatchetfish. J Neurocytol 14:825–834 [DOI] [PubMed] [Google Scholar]
- Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Kruger O, Willecke K, Peti-Peterdi J 2008. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 295:R371–R380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL 2002. Voltage-sensing and substate rectification: Moving parts of connexin channels. J Gen Physiol 119:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL 2007. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94:120–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL 2008. Connexin specificity of second messenger permeation: Real numbers at last. J Gen Physiol 131:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopperstad MG, Srinivas M, Spray DC 2000. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J 79:1954–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AW, Gourdie RG 2008. The second PDZ domain of zonula occludens-1 is dispensable for targeting to connexin 43 gap junctions. Cell Commun Adhes 15:55–63 [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG 2005. ZO-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16:5686–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Duling BR 2005. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97:44–51 [DOI] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR 2007. Ca2+ and 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100:246–254 [DOI] [PubMed] [Google Scholar]
- Jiang JX, Cherian PP 2003. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin e(2) by osteocytes in response to mechanical strain. Cell Commun Adhes 10:259–264 [DOI] [PubMed] [Google Scholar]
- Jiang JX, Goodenough DA 1996. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci 93:1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, White TW, Goodenough DA 1995. Changes in connexin expression and distribution during chick lens development. Dev Biol 168:649–661 [DOI] [PubMed] [Google Scholar]
- Jordan K, Chodock R, Hand AR, Laird DW 2001. The origin of annular junctions: A mechanism of gap junction internalization. J Cell Sci 114:763–773 [DOI] [PubMed] [Google Scholar]
- Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V 2008. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 131:293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M 2008. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28:4702–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM 1997. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387:80–83 [DOI] [PubMed] [Google Scholar]
- Kim DY, Kam Y, Koo SK, Joe CO 1999. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J Biol Chem 274:5581–5587 [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Nelles E, Hagendorff A, Krüger O, Traub O, Willecke K 1998. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Current Biol 8:299–302 [DOI] [PubMed] [Google Scholar]
- Konig N, Zampighi G 1995. Purification of bovine lens cell-to-cell channels composed of connexin44 and connexin50. J Cell Sci 108:3091–3098 [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Li X, Burr GS, O’Brien J 2007. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci 24:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger O, Plum A, Kim J, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K 2000. Defective vascular development in connexin 45-deficient mice. Development 127:4179–4193 [DOI] [PubMed] [Google Scholar]
- Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C 2007. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18:1103–1111 [DOI] [PubMed] [Google Scholar]
- Laing JG, Beyer EC 1995. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem 270:26399–26403 [DOI] [PubMed] [Google Scholar]
- Laird DW 2005. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 1711:172–182 [DOI] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP 1991. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 273:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Castillo M, Kasprzak L 1995. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol 131:1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF 2000. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384:205–215 [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF 2004. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Tenbroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF 2000. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149:1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Z, Kurata WE, Martyn KD, Jin C, Lau AF 2005. Novel Rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry 44:2385–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen WJ, Tung H-N, Murray S, Swenson CA 1979. Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol 83:576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE 1985. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci 82:3025–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM 2002. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci 99:10446–10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Day KH, Damon DN, Duling BR 2001. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci 98:9989–9994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Warn-Cramer BJ, Kurata WE, Lau AF 2001. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol 154:815–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR 1995. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol 268:H729–H739 [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G 2006. Pannexin 1 in erythrocytes: Function without a gap. Proc Natl Acad Sci 103:7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T 2009. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458:597–602 [DOI] [PubMed] [Google Scholar]
- Makowski L 1985. Structural domains in gap junctions: Implications for the control of intercellular communication. In Gap junction (ed. Bennett M.V.L., et al. ), pp. 5–12 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Martinez-Wittinghan FJ, Sellitto C, Li L, Gong X, Brink PR, Mathias RT, White TW 2003. Dominant cataracts result from incongruous mixing of wild-type lens connexins. J Cell Biol 161:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Wittinghan FJ, Sellitto C, White TW, Mathias RT, Paul D, Goodenough DA 2004. Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression. Invest Ophthalmol Vis Sci 45:3629–3637 [DOI] [PubMed] [Google Scholar]
- Mathias RT 1985. Steady-state voltages, ion fluxes and volume regulation in syncytial tissues. Biophys J 48:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Rae JL 1989. Cell to cell communication in the lens. In Cell interactions and gap junctions (ed. Sperelakis N., et al. ), pp. 29–50 CRC Press, Boca Raton, FL [Google Scholar]
- Mese G, Richard G, White TW 2007. Gap junctions: Basic structure and function. J Invest Dermatol 127:2516–2524 [DOI] [PubMed] [Google Scholar]
- Moreno AP, Saez JC, Fishman GI, Spray DC 1994. Human connexin43 gap junction channels - regulation of unitary conductances by phosphorylation. Circ Res 74:1050–1057 [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA 1991. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115:1357–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA 1993. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74:1065–1077 [DOI] [PubMed] [Google Scholar]
- Musil LS, Le AC, VanSlyke JK, Roberts LM 2000. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J Biol Chem 275:25207–25215 [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE 2003. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 44:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Trautmann A 1985. Single-channel currents of an intercellular junction. Nature 317:331–335 [DOI] [PubMed] [Google Scholar]
- Nickel BM, Defranco BH, Gay VL, Murray SA 2008. Clathrin and Cx43 gap junction plaque endoexocytosis. Biochem Biophys Res Commun 374:679–682 [DOI] [PubMed] [Google Scholar]
- Nishii K, Kumai M, Egashira K, Miwa T, Hashizume K, Miyano Y, Shibata Y 2003. Mice lacking connexin45 conditionally in cardiac myocytes display embryonic lethality similar to that of germline knockout mice without endocardial cushion defect. Cell Commun Adhes 10:365–369 [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M 2005. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol 287:314–335 [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA 2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Nguyen HB, Mills SL 2004. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. J Neurosci 24:5632–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA 1999. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol 114:339–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA 2004. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J 87:912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Akiyama M, Takeda M, Gabazza EC, Hayashi T, Suzuki K 2009. Connexin32 is expressed in vascular endothelial cells and participates in gap junction intercellular communication. Biochem Biophys Res Commun 382:264–268 [DOI] [PubMed] [Google Scholar]
- Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE 2007. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci 104:10034–10039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE 2008. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes 15:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahujaa M, Anikin M, Goldberg GS 2007. Phosphorylation of connexin43 induced by Src: Regulation of gap junctional communication between transformed cells. Exp Cell Res 313:4083–4090 [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S 2000. A ubiquitous family of putative gap junction molecules. Curr Biol 10:R473–R474 [DOI] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA 1991. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115:1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, et al. 2003. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 72:408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P 2005. Innexins: Members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711:225–245 [DOI] [PubMed] [Google Scholar]
- Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, Barnes TM, Ford C, Hekimi S, et al. 1998. Innexins: A family of invertebrate gap-junction proteins. Trends Genet 14:348–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Goulding LA, Tam JL, Allen MJ, Dawber RJ, Davies JA, Bacon JP 2008. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol 18:1955–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM 2007. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell 18:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum A, Hallas G, Magin TM, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J-S, Lamers WH, Evert M, Meda P, et al. 2000. Unique and shared functions of different connexins in mice. Curr Biol 10:1083–1091 [DOI] [PubMed] [Google Scholar]
- Potenza N, del Gaudio R, Rivieccio L, Russo GM, Geraci G 2002. Cloning and molecular characterization of the first innexin of the phylum annelida-expression of the gene during development. J Mol Evol 54:312–321 [DOI] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E 2001. Extrasynaptic release of dopamine in a retinal neuron: Activity dependence and transmitter modulation. Neuron 30:211–225 [DOI] [PubMed] [Google Scholar]
- Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA 2000. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J 79:2403–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Dahl G 2002. Function of the voltage gate of gap junction channels: Selective exclusion of molecules. Proc Natl Acad Sci 99:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JL 1979. The electrophysiology of the crystalline lens. Curr Topics in Eye Research 1:37–90 [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J 1995. Cardiac malformation in neonatal mice lacking connexin43. Science 267:1831–1834 [DOI] [PubMed] [Google Scholar]
- Richard G 2005. Connexin disorders of the skin. Clin Dermatol 23:23–32 [DOI] [PubMed] [Google Scholar]
- Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F 2008. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1:ra6. [DOI] [PubMed] [Google Scholar]
- Schweda F, Kurtz L, de Wit C, Janssen-Bienhold U, Kurtz A, Wagner C 2008. Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int 75:482–490 [DOI] [PubMed] [Google Scholar]
- Segretain D, Falk MM 2004. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta 1662:3–21 [DOI] [PubMed] [Google Scholar]
- Sellitto C, Li L, White TW 2004. Connexin50 is essential for normal postnatal lens cell proliferation. Invest Ophthalmol Vis Sci 45:3196–3202 [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA 1998. Diverse functions of vertebrate gap junctions. Trends Cell Biol 8:477–482 [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR 2002. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251:206–220 [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR 2003. Role of connexin37 and connexin40 in vascular development. Cell Commun Adhes 10:379–385 [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL 1997. Female infertility in mice lacking connexin 37. Nature 385:525–529 [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL 1998. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Current Biol 8:295–298 [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD 2005. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711:154–163 [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD 2007. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol 217:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD 2008. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes 15:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MVL 1979. Voltage dependence of junctional conductance in early amphibian embryos. Science 204:432–434 [DOI] [PubMed] [Google Scholar]
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK 2007. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 88:1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Miller A, Nguyen RL, Hall DH, Shaw JE 2003. The caenorhabditis elegans innexin INX-3 is localized to gap junctions and is essential for embryonic development. Dev Biol 256:403–417 [DOI] [PubMed] [Google Scholar]
- Stümpel F, Ott T, Willecke K, Jungermann K 1998. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology 28:1616–1620 [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H, Burk RR, Pitts JD 1969. Metabolic cooperation between biochemically marked mammalian cells in tissue culture. J Cell Sci 4:353–367 [DOI] [PubMed] [Google Scholar]
- Swenson KI, Jordan JR, Beyer EC, Paul DL 1989. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell 57:145–155 [DOI] [PubMed] [Google Scholar]
- Swenson KI, Piwnica-Worms H, McNamee H, Paul DL 1990. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul 1:989–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takens-Kwak BR, Jongsma HJ 1992. Cardiac gap junctions: Three distinct single channel conductances and their modulation by phosphorylating treatments. Pflügers Arch 422:198–200 [DOI] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, Chen P, Paul DL, Lin X 2006. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci 26:1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K 2001. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 29:1–13 [DOI] [PubMed] [Google Scholar]
- Thompson RJ, MacVicar BA 2008. Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin) 2. (epub ahead of print) [DOI] [PubMed]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M 1998. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731 [DOI] [PubMed] [Google Scholar]
- Turin L, Warner A 1977. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryos. Nature 270:56–57 [DOI] [PubMed] [Google Scholar]
- Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, et al. 2004. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M 1999. Three-dimensional structure of a recombinant gap junction membrane channel. Science 283:1176–1180 [DOI] [PubMed] [Google Scholar]
- Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K 2006. Protein kinase A mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem 281:33163–33171 [DOI] [PubMed] [Google Scholar]
- van Kempen MJ, Jongsma HJ 1999. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol 112:479–486 [DOI] [PubMed] [Google Scholar]
- van Steensel MAM 2004. Gap junction diseases of the skin. Am J Med Genet C Semin Med Genet Genet 131C:12–19 [DOI] [PubMed] [Google Scholar]
- van Veen TA, Van Rijen HV, Jongsma HJ 2000. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc Res 46:496–510 [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink PR 1995. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res 77:1156–1165 [DOI] [PubMed] [Google Scholar]
- Vreeburg M, Zwart-Storm EA, Schouten MI, Nellen RG, Marcus-Soekarman D, Devies M, van Geel M, van Steensel MA 2007. Skin changes in oculo-dento-digital dysplasia are correlated with C-terminal truncations of connexin 43. Am J Med Genet A 143A:360–363 [DOI] [PubMed] [Google Scholar]
- Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F 2007. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100:556–563 [DOI] [PubMed] [Google Scholar]
- Weidmann S 1952. The electrical constants of Purkinje fibres. J Physiol 118:348–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR 2004. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43:647–661 [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS 1998. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol 274:H178–H186 [DOI] [PubMed] [Google Scholar]
- White TW 2002. Unique and redundant connexin contributions to lens development. Science 295:319–320 [DOI] [PubMed] [Google Scholar]
- White TW, Paul DL 1999. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol 61:283–310 [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA 1994. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: The second extracellular domain is a determinant of compatibility between connexins. J Cell Biol 125:879–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Goodenough DA, Paul DL 1998. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Gao Y, Li L, Sellitto C, Srinivas M 2007. Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest Ophthalmol Vis Sci 48:5630–5637 [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G 2002. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737 [DOI] [PubMed] [Google Scholar]
- Winterhager E, Pielensticker N, Freyer J, Ghanem A, Schrickel JW, Kim JS, Behr R, Grummer R, Maass K, Urschel S, et al. 2007. Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev Biol 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C 2007. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res 101:1292–1299 [DOI] [PubMed] [Google Scholar]
- Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR 2006. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12:950–954 [DOI] [PubMed] [Google Scholar]
- Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X 2006. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res 83:688–696 [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA 1999. Dark- and light-induced changes in coupling between horizontal cells in mammalian retina. J Comp Neurol 405:75–87 [DOI] [PubMed] [Google Scholar]
- Yang JJ, Huang SH, Chou KH, Liao PJ, Su CC, Li SY 2007. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol 12:198–208 [DOI] [PubMed] [Google Scholar]
- Yao J, Oite T, Kitamura M 2008. Gap junctional intercellular communication in the juxtaglomerular apparatus. Am J Physiol Renal Physiol 296:F939–F946 [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR 2003. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J Neurosci 23:3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Harris AL 2007. Gap junction channel structure in the early 21st century: Facts and fantasies. Curr Opin Cell Biol 19:521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Liu J, Jiang JX 2008. Lens fiber connexin turnover and caspase-3-mediated cleavage are regulated alternately by phosphorylation. Cell Commun Adhes 15:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer S 2007. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol 293:C1032–C1048 [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Ghanem A, Kim JS, Kibschull M, Schwarz G, Schwab JO, Nagy J, Winterhager E, Tiemann K, Willecke K 2006. Connexin31 cannot functionally replace connexin43 during cardiac morphogenesis in mice. J Cell Sci 119:693–701 [DOI] [PubMed] [Google Scholar]