Abstract
It has been reported that endurance exercise-trained men have decreases in cardiac output with no change in systemic vascular conductance during post-exercise hypotension, which differs from sedentary and normally active populations. As inadequate hydration may explain these differences, we tested the hypothesis that fluid replacement prevents this post-exercise fall in cardiac output, and further, exercise in a warm environment would cause greater decreases in cardiac output. We studied 14 trained men ( 4.66 ± 0.62 l min−1) before and to 90 min after cycling at 60%
4.66 ± 0.62 l min−1) before and to 90 min after cycling at 60% for 60 min under three conditions: Control (no water was consumed during exercise in a thermoneutral environment), Fluid (water was consumed to match sweat loss during exercise in a thermoneutral environment) and Warm (no water was consumed during exercise in a warm environment). Arterial pressure and cardiac output were measured pre- and post-exercise in a thermoneutral environment. The fall in mean arterial pressure following exercise was not different between conditions (P= 0.453). Higher post-exercise cardiac output (Δ 0.41 ± 0.17 l min−1; P= 0.027), systemic vascular conductance (Δ 6.0 ± 2.2 ml min−1 mmHg−1; P= 0.001) and stroke volume (Δ 9.1 ± 2.1 ml beat−1; P < 0.001) were seen in Fluid compared to Control, but there was no difference between Fluid and Warm (all P > 0.05). These data suggest that fluid replacement mitigates the post-exercise decrease in cardiac output in endurance-exercise trained men. Surprisingly, exercise in a warm environment also mitigates the post-exercise fall in cardiac output.
for 60 min under three conditions: Control (no water was consumed during exercise in a thermoneutral environment), Fluid (water was consumed to match sweat loss during exercise in a thermoneutral environment) and Warm (no water was consumed during exercise in a warm environment). Arterial pressure and cardiac output were measured pre- and post-exercise in a thermoneutral environment. The fall in mean arterial pressure following exercise was not different between conditions (P= 0.453). Higher post-exercise cardiac output (Δ 0.41 ± 0.17 l min−1; P= 0.027), systemic vascular conductance (Δ 6.0 ± 2.2 ml min−1 mmHg−1; P= 0.001) and stroke volume (Δ 9.1 ± 2.1 ml beat−1; P < 0.001) were seen in Fluid compared to Control, but there was no difference between Fluid and Warm (all P > 0.05). These data suggest that fluid replacement mitigates the post-exercise decrease in cardiac output in endurance-exercise trained men. Surprisingly, exercise in a warm environment also mitigates the post-exercise fall in cardiac output.
Blood pressure is typically reduced ∼5 to 10 mmHg following a single bout of dynamic exercise in most individuals (Kenney & Seals, 1993; Halliwill, 2001; MacDonald, 2002). The response in the general population (healthy sedentary and normally active individuals) is characterized by a sustained increase in systemic vascular conductance that is not completely offset by ongoing elevations in cardiac output (Halliwill, 2001). Several mechanisms appear to underlie the sustained peripheral vasodilatation following exercise. Halliwill et al. (1996a) demonstrated that the baroreflex is reset to defend a lower pressure following exercise; thus, sympathetic vasoconstrictor outflow is reduced post-exercise in humans. In addition to this reduction in sympathetic nerve activity, there is reduced vascular responsiveness to a given level of sympathetic nerve activity (Halliwill et al. 1996a, 2003) and a sustained histamine-receptor-dependent vasodilatation (Lockwood et al. 2005; McCord et al. 2006; McCord & Halliwill, 2006).
The overall haemodynamic response seen in endurance exercise-trained men may be different than what is reported for the general population. In contrast to a sustained post-exercise peripheral vasodilatation, two reports in the literature suggest that post-exercise hypotension in endurance exercise-trained men is secondary to reduced cardiac output. First, Senitko et al. (2002) found that post-exercise cardiac output was reduced and systemic vascular conductance was unchanged in a group of endurance exercise-trained men following cycling at 60% of  . Second, Dujic et al. (2006) confirmed these results in elite male Croatian soccer players. The overall haemodynamic pattern observed during post-exercise hypotension in these two studies suggests that additional factors alter post-exercise haemodynamics in these populations, beyond vasodilatation of the previously exercised skeletal muscle.
. Second, Dujic et al. (2006) confirmed these results in elite male Croatian soccer players. The overall haemodynamic pattern observed during post-exercise hypotension in these two studies suggests that additional factors alter post-exercise haemodynamics in these populations, beyond vasodilatation of the previously exercised skeletal muscle.
The reasons for these haemodynamic differences remain unclear and may be due to greater plasma volume losses secondary to greater sweat rates (from higher absolute workloads) in trained men (Frye & Kamon, 1981; Myhre et al. 1985) that may ultimately reduce central blood volume and ventricular filling (i.e. preload) during recovery from exercise. However, these studies failed to document hydration status (i.e. pre- to post-exercise changes in body weight) and the amount of oral fluid consumed during exercise was ad libitum (Senitko et al. 2002) or fluid restricted (Dujic et al. 2006). It is known that ad libitum drinking fails to match sweat losses, resulting in incomplete fluid replacement (Bean & Eichna, 1943; Adolph, 1947; Wolf, 1958). In addition, trained men are at greater risk of dehydration due to greater sweat rates (Costill, 1977). Currently, limited studies exist on the role that fluid replacement plays in post-exercise haemodynamics in a group of endurance exercise-trained men.
Cardiovascular responses during exercise and heat stress have been studied extensively, but little is known about the influence of augmented thermal load or dehydration on post-exercise haemodynamics. During exercise, the rate of heat production increases above baseline with the production of heat being proportional to the rate of work (Saltin & Hermansen, 1966). As core temperature rises, heat loss responses are initiated which increase the rate of heat dissipation (via sweating and cutaneous vasodilatation). If heat loss responses are not effective at removing heat (e.g. due to environment conditions or cardiovascular constraints), heat will be stored and core temperature will continue to rise. For the purpose of this study, heat storage refers to an imbalance between heat production and heat dissipation resulting in a net rise in body temperature over the course of a bout of exercise, whereas the thermal load refers to the resultant elevated body temperature, which is the stimulus for physiological responses. As significant thermoregulatory and cardiovascular adjustments occur following dynamic exercise in the heat (Kilgour et al. 1993), it seems likely that the thermal load present at the end of exercise may alter these adjustments. Thus, if heat storage is augmented during exercise (e.g. exercising in a warm environment or at higher absolute workloads), thermal load would be greater and this could potentially alter post-exercise responses.
A few studies have reported elevated heart rates or altered cardiovascular regulation following the combination of exercise and heat stress (Brouha, 1960; Mathews et al. 1969; Kilgour et al. 1993; Charkoudian et al. 2003). As post-exercise hypotension is a highly regulated physiological phenomenon, the haemodynamics following exercise in the heat may remain well regulated but exhibit a different overall balance of cardiac output and vascular conductance compared to exercise in a thermoneutral environment. For example, a reduction in preload (i.e. central venous pressure) that has been previously observed in a trained population could be exacerbated when exercising in a warm environment due to a redistribution of cardiac output from less compliant to more compliant vascular beds, such as the skin (Rowell, 1986), in response to elevated core temperature (i.e. a greater thermal load at the end of exercise) due to elevated heat production during exercise.
Therefore the goal of this study was to assess the influence of fluid replacement on post-exercise hypotension as well as central (cardiac) and peripheral haemodynamics in a group of highly fit endurance exercise-trained men to determine if cardiac output is maintained following exercise with adequate fluid replacement. We hypothesized that, in a group of trained men, adequate fluid replacement during exercise would prevent the post-exercise fall in cardiac output whereas exercise in a warm environment would further decrease cardiac output due to exaggerated loss of plasma volume and enhanced peripheral vasodilatation secondary to increased thermal load.
Methods
This study was approved by the Institutional Review Board of the University of Oregon. Each subject gave written informed consent before participating in the study. All studies were performed according to the Declaration of Helsinki.
Subjects
A total of 14 men (between 20 and 40 years) participated in this study. On the basis of their exercise habits over the previous 12 months, subjects were classified as ‘endurance exercise trained’, defined as performing endurance-type exercises (running, cycling or swimming) on at least 4 days week−1 and averaging at least 1 h day−1 that they exercise. All subjects were primarily cyclists and runners performing between 7 and 12 h of endurance exercise per week.
Subject characteristics
Subject characteristics are presented in Table 1. 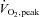 values (4.66 ± 0.62 l min−1) and maximal power output (406 ± 60 W) for men were reflective of a highly fit endurance exercise-trained population.
values (4.66 ± 0.62 l min−1) and maximal power output (406 ± 60 W) for men were reflective of a highly fit endurance exercise-trained population.
Table 1.
Subject characteristics of endurance-trained men
| Characteristic | |
|---|---|
| Age (years) | 26.5 ± 6.9 |
| Height (cm) | 178.5 ± 8.7 |
| Weight (kg) | 73.5 ± 9.9 |
| Body mass index (kg m−2) | 23.0 ± 1.9 |
 (l min−1) (l min−1) |
4.66 ± 0.62 |
| Maximum heart rate (beats min−1) | 194.5 ± 7.8 |
 (W) (W) |
206 ± 32 |
| Baecke sport index (arbitrary units) | 15.3 ± 1.6 |
| Index of physical activity (MET h−1 week−1) | 171 ± 56 |
Values are presented as means ±s.d., n= 14;  , peak oxygen consumption; MET, metabolic equivalents.
, peak oxygen consumption; MET, metabolic equivalents.
Subjects were healthy with no allergies, non-smoking, normotensive and taking no medications. The experimental design consisted of four separate testing days: a screening visit and three study visits. Subjects were scheduled for the first study visit within 7 to 10 days after the screening visit and the second and third visits were separated by 3 to 7 days. All data were collected in Eugene, Oregon, during the winter months (January and February) when subjects were naturally un-acclimatized to heat, but were maintaining their exercise training.
Screening visit
Subjects reported to the laboratory for a screening visit and cycle ergometer test at least 2 h post-prandial and abstained from caffeine, alcohol and exercise for 24 h prior to the screening visit. Subjects performed an incremental cycle exercise test on an electronically braked cycle ergometer (Lode Excaliber, Groningen, the Netherlands). Subjects were attached to a 5-lead electrocardiogram (Q710, Quinton Instruments, Bothell, WA, USA) in order to determine heart rate through the protocol. Seat height was adjusted and recorded and subjects were instructed to do a self-selected warm-up for approximately 5 min before the start of the test. The test consisted of 1 min workload increments to determine peak oxygen uptake ( ). Specifically, after a 5 min warm-up period of light cycling (∼100–150 W), workload increased at 25 or 30 W every minute until volitional fatigue. Selection of the workload increment was subjective based on the warm-up and the overall size of the subject, with the goal of producing exhaustion within 9–12 min. Whole body oxygen uptake (
). Specifically, after a 5 min warm-up period of light cycling (∼100–150 W), workload increased at 25 or 30 W every minute until volitional fatigue. Selection of the workload increment was subjective based on the warm-up and the overall size of the subject, with the goal of producing exhaustion within 9–12 min. Whole body oxygen uptake ( ) was measured with a mixing chamber (Parvomedics, Sandy, UT, USA) integrated with a mass spectrometry system (Marquette MGA 1100, MA Tech Services, St Louis, MO, USA). All subjects reached subjective exhaustion (Borg, 1970) (rating of perceived exertion = 19–20) within a 9 to 15 min period and maximal heart rate was determined just before termination of exercise. After the subjects rested for 15–20 min, they returned to the cycle ergometer for assessment of the workload corresponding to a steady-state
) was measured with a mixing chamber (Parvomedics, Sandy, UT, USA) integrated with a mass spectrometry system (Marquette MGA 1100, MA Tech Services, St Louis, MO, USA). All subjects reached subjective exhaustion (Borg, 1970) (rating of perceived exertion = 19–20) within a 9 to 15 min period and maximal heart rate was determined just before termination of exercise. After the subjects rested for 15–20 min, they returned to the cycle ergometer for assessment of the workload corresponding to a steady-state  of 60% of
of 60% of  . This workload was used on each of the study days for the 60 min exercise bout. Subjects self-reported activity levels on two questionnaires (Baecke et al. 1982; Kohl et al. 1988) during the 15–20 min rest period before the start of the 60% of
. This workload was used on each of the study days for the 60 min exercise bout. Subjects self-reported activity levels on two questionnaires (Baecke et al. 1982; Kohl et al. 1988) during the 15–20 min rest period before the start of the 60% of  verification test.
verification test.
Study visits and experimental protocol
Subjects reported to the laboratory on three separate study days at approximately the same time of day at least 2 h post-prandial and abstained from caffeine for 12 h and from exercise and all medications for 24 h prior to the study. Subjects pre-hydrated the night before each study day by drinking 7 ml (kg of body weight)−1 of water. Subjects recorded all food and drink consumed after 17.00 the night before and the morning of each of the study visits in a dietary log that was given to the subjects after the screening visit. Subjects ingested a temperature-sensing pill (HQInc, Palmetto, FL, USA) at least 5 h prior to the study visits. For subjects who reported to the lab in the morning, the pill was swallowed the night before. This time lapse allows for the temperature-sensing pill to move through the stomach and into the intestinal tract for more accurate core temperature measurements that are unaffected by oral fluid consumption.
Upon arrival, subjects voided their bladders into a urine collection container for a urine specific gravity measurement which was measured in duplicate in order to determine baseline hydration status. If results of urine specific gravity showed subjects were dehydrated, they were asked to rest and drink 500 ml of water and then 30 min later provide a urine sample for re-testing before continuing with the protocol. After providing a urine sample, subjects were instructed to undress to their underwear behind a privacy screen and were weighed to the nearest 5 g. The subjects were then laid in a supine position for instrumentation. A venous catheter was inserted into the right antecubital arm region to obtain blood samples throughout the protocol. All pre-exercise samples and measurements were taken in the supine position after 40 min of quiet supine rest.
Experimental design and conditions
The three study visits were identical in measurements and procedures, with the exception of temperature of the room during exercise and whether or not water was consumed during the exercise session. The exercise bout was conducted in an environmental chamber; this allowed for tight control of ambient temperature and relative humidity for the entire exercise period. Following pre-exercise measurement, subjects were taken directly into the environmental chamber to immediately undergo exercise under the specific conditions for the session. Once the 60 min exercise period was completed, the subjects were immediately taken back to the laboratory for recovery measurements in a thermoneutral environment. The three conditions included the following fluid and temperature manipulations: (1) Control – no water was consumed during exercise in a thermoneutral environment (22°C and 30% humidity); (2) Fluid – water was consumed to match sweat loss during exercise in a thermoneutral environment (22°C and 30% humidity); and (3) Warm – no water was consumed during exercise in a warm environment (30°C and 30% humidity). The ambient temperature of the laboratory for pre- and post-exercise measurements was controlled between 21 and 23°C.
Each of the study days were randomized prior to the screening visit and order was divided and assigned to each subject. Since Control condition was used to calculate the amount of water to be consumed during Fluid condition, Control condition always preceded Fluid condition in the order of study visits. Thus, the three possible orders of the conditions were: Control, Fluid, Warm (five subjects); Control, Warm, Fluid (four subjects); Warm, Control, Fluid (five subjects).
Timing of the measurements
All pre- and post-exercise measurements were taken in a supine position and subjects rested in this position between measurements. The post-exercise pressure reductions are present with supine, seated and standing postures; however, the magnitude of the decrease is more pronounced and prolonged with the orthostatic influence of upright posture (Kenney & Seals, 1993; Raine et al. 2001). Thus, pre-exercise and post-exercise measurements must be taken in the same posture independent of the mode and posture during exercise. Pre-exercise (baseline) measurements were obtained after 40 min of quiet supine rest. Post-exercise measurements were taken every 7 min for 90 min after completion of the exercise bout, with the exception of femoral blood flow which was taken pre-exercise and then every 30 min following exercise. Pre-exercise and post-exercise measurements included heart rate, arterial pressure, cardiac output, core body temperature, femoral blood flow and skin blood flow. Blood samples of haematocrit and haemoglobin were taken once pre-exercise, after 45 min of exercise and then every 30 min post-exercise. Nude body weights were obtained at baseline, before exercise, after exercise and at the end of the study. During exercise, blood pressure (measured by manual sphygmomanometer on the arm), core body temperature and heart rate were measured every 10 min. At the end of the protocol, maximum skin blood flow values were obtained through local heating to 43°C. All urine excreted during the study days was collected and weighed to the nearest 5 g.
Exercise
On each study day the exercise bout consisted of a 60 min period of seated upright cycling exercise at 60% of  , as determined during the screening visit which consistently produces a sustained (∼2 h) post-exercise hypotension in healthy normotensive subjects (Halliwill, 2001).
, as determined during the screening visit which consistently produces a sustained (∼2 h) post-exercise hypotension in healthy normotensive subjects (Halliwill, 2001).
Oral ingestion of fluid
To match water losses, the amount of water consumed during Fluid condition for each subject was based on sweat loss during Control condition for each subject. This was determined from differences in nude weight corrected for urine loss, from immediately before exercise to the end of the study, during the individual subject's Control condition. This amount of water was consumed during exercise for Fluid condition. No water was consumed during exercise for either Control or Warm condition.
Measurements
Heart rate and arterial pressure
Heart rate and arterial pressure were monitored throughout all experimental procedures. Heart rate was monitored using a 5-lead electrocardiogram (Q710, Quinton Instruments). Resting arterial pressure was measured in the arm by using an automated oscillometric device (Dinamap Pro100 vital signs monitor, Critikon, Inc., Tampa, FL, USA).
Cardiac output
Cardiac output was estimated using an open-circuit acetylene wash-in method as described previously (Johnson et al. 2000; Pricher et al. 2004). This method allows for the non-invasive estimation of cardiac output. We chose an open-circuit method because subjects are exposed to stable oxygen and carbon dioxide levels throughout the measurement in contrast to rebreathing techniques. In brief, subjects breathed 8 to 10 breaths of a gas mixture consisting of 0.6% acetylene–9.0% helium–20.9% oxygen, and balance nitrogen. During the wash-in phase, breath-by-breath acetylene and helium uptake were measured by a respiratory mass spectrometer (Marquette MGA 1100, MA Tech Services) and tidal volume was measured via a pneumotach (model 3700, Hans Rudolf, Kansas City, MO, USA) linearized by the technique of Yeh et al. (1982) and calibrated by using test gas before each study. The pneumotach and valve system had a combined dead space of 24 ml. Cardiac output calculations have been described previously (Johnson et al. 2000). Stroke volume was determined from cardiac output/heart rate. Systemic vascular conductance was calculated as cardiac output/mean arterial pressure and expressed as ml min−1 mmHg−1.
Core temperature
Internal body temperature was assessed by an ingestible pill telemetry system (HQInc).
Femoral blood flow
Femoral blood flow was determined through measurements of femoral artery diameter and velocity using an ultrasound probe (10 Mhz linear-array vascular probe, GE Vingmed System 5, Horton, Norway). The entire width of the femoral artery was insonated at an angle of 60 deg. Velocity measurements were recorded immediately before diameter measurements. Femoral blood flow was then calculated as artery cross-sectional area multiplied by femoral mean blood velocity, and reported in ml min−1. Femoral vascular conductance was calculated as flow for both legs/mean arterial pressure and expressed as ml min−1 mmHg−1.
Blood sampling
A 2.5 cm, 22-gauge flexible catheter was placed in the antecubital area in the right arm using sterile techniques at the beginning of the study. Samples were collected in 3 ml heparizined arterial blood gas syringes (Smiths Medical ASD, Inc., Keene, NH, USA). Haemoglobin and haematocrit were measured in quadruplicate. Haemoglobin was measured spectrophotometrically with a diode-array spectrophotometer (OSM3 hexoximeter, Radiometer, Copenhagen, Denmark). Haematocrit was measured with the microcapillary method after 5 min of centrifuging at 9500 g (Autocrit Ultra 3, Becton Dickson, USA). Throughout the study visit, changes in blood and plasma volume were calculated from haemoglobin concentration and haematocrit using the method of Dill & Costill (1974).
Index of skin blood flow
An index of skin blood flow was derived from measuring red blood cell flux values via laser-Doppler flowmetry (DRT4, Moor Instruments Ltd, Devon, UK). Laser-Doppler probes were placed on the skin of the right ventral forearm and the anterior right thigh. Skin blood flows were expressed as cutaneous vascular conductance, calculated as laser-Doppler flux/mean arterial pressure, and normalized to the maximal values achieved during local heating to 43°C at the end of the protocol (Minson et al. 2001).
Urine specific gravity and body weight
Baseline hydration status was estimated in duplicate from urine specific gravity (hand refractometer, NSG Precision Cells, Inc., Farmingdale, NY, USA). Nude body weights were obtained four separate times throughout the study (Sartorius EB6CE-I, Precision Weighing Balances, Bradford, MA, USA). After exercise, subjects towelled dry before body weight was measured. The extent of dehydration was calculated as the change in body weight from directly before exercise to the end of exercise and to the end of the study (total body water loss). The overall sweat loss (ml) (from directly before exercise to the end of exercise and to the end of the study) was calculated with the following formula: [total body water loss − volume of urine loss + oral fluid replacement].
Statistics
The statistical analysis was generated using SAS/Stat software, Version 9.1.3 of the SAS system for Windows (Copyright 2004 SAS Institute Inc. Cary, NC, USA). Subject characteristics are reported as means ±s.d. and all additional data are reported as means ±s.e.m. Subject characteristics and baseline exercise and haemodynamic values were analysed across all conditions using one-way repeated-measures analysis of variance. When significance was detected least squared means were used to compare group means. Differences in the post-exercise haemodynamic variables [dependent variables] were analysed with a three condition level longitudinal analysis of variance with a quadratic fit mixed linear model with SAS PROC MIXED. Significance was set at a P value less than 0.05.
Results
Pre-exercise haemodynamics
Table 2 shows the resting (pre-exercise) haemodynamics for subjects across the three study visits. Supine resting heart rate (P= 0.881) and mean arterial pressure (P= 0.943) were not different across conditions.
Table 2.
Pre-exercise haemodynamics
| Control | Fluid | Warm | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 110.7 ± 2.5 | 111.0 ± 2.8 | 109.3 ± 2.0 |
| Diastolic blood pressure (mmHg) | 61.3 ± 2.1 | 60.2 ± 2.3 | 60.7 ± 1.9 |
| Body weight (kg) | 72.7 ± 10.1 | 72.8 ± 10.2 | 72.8 ± 10.3 |
| Haematocrit (%) | 40.6 ± 0.7 | 40.9 ± 0.5 | 40.7 ± 0.6 |
| Haemoglobin (g dl−1) | 14.2 ± 0.2 | 14.1 ± 0.1 | 14.0 ± 0.2 |
| Urine specific gravity | 1.019 ± 0.001 | 1.018 ± 0.001 | 1.019 ± 0.001 |
Values are presented as means ±s.e.m.; n= 14.
Exercise
Subjects exercised for 60 min at 60% of 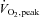 on all study days. Average workloads are shown in Table 1 and were the same for each condition. Exercise mean arterial pressure did not differ across conditions (Control: 82.3 ± 0.9 mmHg; Fluid: 80.7 ± 1.6 mmHg; Warm: 81.1 ± 1.3 mmHg; P= 0.646). Table 3 shows exercise haemodynamics across the three study visits.
on all study days. Average workloads are shown in Table 1 and were the same for each condition. Exercise mean arterial pressure did not differ across conditions (Control: 82.3 ± 0.9 mmHg; Fluid: 80.7 ± 1.6 mmHg; Warm: 81.1 ± 1.3 mmHg; P= 0.646). Table 3 shows exercise haemodynamics across the three study visits.
Table 3.
Exercise haemodynamics
| Time during exercise | Control | Fluid | Warm |
|---|---|---|---|
| Core temperature (°C) | |||
| 15 min | 37.6 ± 0.1 | 37.7 ± 0.1 | 37.7 ± 0.1 |
| 30 min | 38.0 ± 0.1 | 38.0 ± 0.1 | 38.4 ± 0.1*† |
| 45 min | 38.3 ± 0.1 | 38.0 ± 0.2 | 38.6 ± 0.1*† |
| 60 min | 38.3 ± 0.1 | 38.1 ± 0.1* | 38.6 ± 0.1*† |
| Heart rate (beats min−1) | |||
| 15 min | 151.6 ± 2.6 | 151.9 ± 2.5 | 161.1 ± 2.3*† |
| 30 min | 161.4 ± 2.7 | 158.6 ± 3.3 | 168.1 ± 2.5*† |
| 45 min | 164.3 ± 2.7 | 157.8 ± 3.2* | 173.0 ± 2.6*† |
| 60 min | 167.6 ± 3.1 | 157.5 ± 3.3* | 176.6 ± 2.8*† |
| Heart rate reserve (%) | |||
| 15 min | 68.9 ± 2.3 | 69.5 ± 2.2 | 75.8 ± 1.6*† |
| 30 min | 76.1 ± 2.1 | 74.3 ± 2.3 | 78.0 ± 1.2*† |
| 45 min | 78.3 ± 2.2 | 73.8 ± 2.4* | 84.5 ± 1.8*† |
| 60 min | 80.7 ± 2.3 | 73.6 ± 2.7* | 87.1 ± 2.1 *† |
Values are presented as means ±s.e.m.; n= 14;
P < 0.05 versus Control;
P < 0.05 versus Fluid.
Total body water, plasma volume and blood volume
The goal of the study was to either maintain (Fluid) or decrease total body water post-exercise (Control and Warm). Based on the fluid replacement calculation from overall sweat loss determined during Control, subjects were given 1022 ± 45 ml during Fluid to match the amount of sweat lost during the Control session.
Total body water loss
From before exercise to the end of exercise, total body water decreased by 852 ± 65 ml during Control (−1.2 ± 0.1% body mass; P < 0.001) and decreased by 1027 ± 46 ml during Warm (−1.4 ± 0.1% body mass; P < 0.001). In contrast, total body water was maintained during Fluid, as noted by a non-significant increase in total body water of 8 ± 102 ml (0.0 ± 0.1% body mass; P= 0.911). Due to continued sweat losses during the 90 min recovery period, total body water decreased by 1078 ± 63 ml during Control (−1.5 ± 0.1% body mass; P < 0.001) and decreased by 1352 ± 121 ml during Warm (−1.8 ± 0.2% body mass; P < 0.001) from before exercise to the end of the study. At the same time point, total body water decreased by 331 ± 90 ml during Fluid (−0.5 ± 0.1% body mass; P < 0.001). Total body water losses were greater in Warm than Control (P= 0.046) and greater in Control than in Fluid (P < 0.001).
Urinary loss
Urine losses from before exercise to the end of the study were 121 ± 27 ml in Control, 313 ± 39 ml in Fluid and 90 ± 28 ml in Warm. There were no differences in urine losses between Control and Warm (P= 0.483), but urine losses were higher in Fluid than in Control (P < 0.001) or Warm (P < 0.001). This confirms that urinary water loss is decreased during heat stress and conditions of inadequate fluid intake, which is consistent with prior research (Pivarnik et al. 1984).
Sweat loss
Total sweat losses from before exercise to the end of the study were 957 ± 44 ml in Control, 1004 ± 105 ml in Fluid and 1262 ± 101 ml in Warm. There were no differences in sweat rates between Control and Fluid (P= 0.706), but sweat rates were higher in Warm than in Control (P= 0.019) or Fluid (P= 0.044).
Plasma and blood volumes
Figure 1A shows the percentage changes in plasma volume from pre-exercise to end of exercise and to 90 min post-exercise. There was an initial reduction in plasma volume at the end of exercise followed by a substantial restoration of plasma volume by 30 min post-exercise. The loss in plasma volume at the end of exercise was not different between conditions (all P > 0.05), but across recovery, plasma volume in Fluid averaged 3.9 ± 1.9% higher compared to the Control (P= 0.051) and 4.1 ± 1.9% higher compared to Warm (P= 0.042). There were no differences in plasma volume between Control and Warm (P= 0.892).
Figure 1. The percentage change in plasma volume (A) and blood volume (B) from pre-exercise to end of exercise (time 0) and to 90 min post-exercise.
○, Control (no water during exercise in a thermoneutral environment); •, Fluid (water to match sweat loss during exercise in a thermoneutral environment); ▿, Warm (no water during exercise in a warm environment). *P < 0.05 Fluid versus Control; ‡P < 0.05 Warm versus Fluid. Horizontal lines above the data indicate the time points at which the measured variable diverged from pre-exercise values under each condition. Specifically, for the horizontal line labelled ‘a’, P < 0.05 Control versus pre-exercise; for ‘b’, P < 0.05 Fluid versus pre-exercise; for ‘c’, P < 0.05 Warm versus pre-exercise.
Figure 1B shows the percentage changes in blood volume from pre-exercise to end of exercise and to 90 min post-exercise. Blood volume followed an identical pattern to plasma volume, and blood volume in Fluid averaged 2.0 ± 1.0% higher compared to the Control (P= 0.052) and 2.6 ± 1.0% higher compared to Warm (P= 0.020). There were no differences in blood volume between Control and Warm (P= 0.623).
Post-exercise haemodynamics
Post-exercise haemodynamic responses for each condition are presented in Figs 2–5.
Figure 2. Mean arterial pressure (A), cardiac output (B) and systemic vascular conductance (C) from pre-exercise to 90 min post-exercise.
○, Control; •, Fluid; ▿, Warm. For this and subsequent figures, values are presented as means ±s.e.m., n= 14 for each group. *P < 0.05 Fluid versus Control; †P < 0.05 Warm versus Control. Horizontal lines above the data indicate the time points at which the measured variable diverged from pre-exercise values under each condition. Specifically, for the horizontal line labelled ‘a’, P < 0.05 Control versus pre-exercise; for ‘b’, P < 0.05 Fluid versus pre-exercise; for ‘c’, P < 0.05 Warm versus pre-exercise.
Figure 5. Core temperature (A), thigh cutaneous vascular conductance (B) and forearm cutaneous vascular conductance (C) from pre-exercise to 90 min post-exercise.
○, Control; •, Fluid; ▿, Warm. Horizontal lines above the data indicate the time points at which the measured variable diverged from pre-exercise values under each condition. Specifically, for the horizontal line labelled ‘a’, P < 0.05 Control versus pre-exercise; for ‘b’, P < 0.05 Fluid versus pre-exercise; for ‘c’, P < 0.05 Warm versus pre-exercise.
Arterial pressure
Figure 2A shows mean arterial pressure from pre-exercise to 90 min post-exercise. Mean arterial pressure was not different across conditions throughout 90 min of recovery (P= 0.453). For mean arterial pressure and subsequent variables, the figures illustrate the time-course of changes from pre-exercise under each of the three conditions. While mean arterial pressure remained elevated at 7 min post-exercise (P < 0.001 versus pre-exercise), this was followed by an extended period of reduced pressure (P= 0.001 versus pre-exercise).
Systemic haemodynamics
Figure 2B shows cardiac output from pre-exercise to 90 min post-exercise. There was an initial elevation in cardiac output at 7 min post-exercise (P < 0.001 versus pre-exercise) in all conditions. Cardiac output in Control was subsequently reduced below pre-exercise values (P= 0.001). There were no reductions in cardiac output in Fluid or Warm during recovery from exercise (P > 0.05). Cardiac output was different across conditions (P= 0.027). Fluid had cardiac outputs that averaged 0.414 l min−1 higher than Control (P= 0.017) across recovery. Warm had cardiac output that averaged 0.392 l min−1 higher than Control (P= 0.023) across recovery. There was no difference in Fluid compared to Warm (P= 0.896).
Figure 2C shows systemic vascular conductance from pre-exercise to 90 min post-exercise. There was an initial elevation in systemic vascular conductance at 7 min post-exercise (P < 0.001 versus pre-exercise) in all conditions. Subsequently, systemic vascular conductance in Control was reduced below pre-exercise values (P= 0.001). There were no reductions in systemic vascular conductance in Fluid or Warm during recovery (P > 0.05). Systemic vascular conductance was different across conditions (P= 0.001). Fluid had systemic vascular conductances that averaged 5.97 ml min−1 mmHg−1 higher than Control (P= 0.001) across recovery. Warm had systemic vascular conductances that averaged 5.92 ml min−1 mmHg−1 higher than Control (P= 0.008) across recovery. There was no difference in Fluid compared to Warm (P= 0.896).
Figure 3A shows heart rate from pre-exercise to 90 min post-exercise. Heart rate was elevated throughout post-exercise recovery (P= 0.001 versus pre-exercise) in all conditions. Heart rate did not differ between Fluid and Control during recovery (P= 0.101). Warm had heart rates that averaged 7.1 beats min−1 higher compared to Fluid and 5.4 beats min−1 higher compared to Control (both P < 0.001) across recovery.
Figure 3. Heart rate (A) and stroke volume (B) from pre-exercise to 90 min post-exercise.
○, Control; •, Fluid; ▿, Warm. *P < 0.05 Fluid versus Control; †P < 0.05 Warm versus Control; ‡P < 0.05 Warm versus Fluid. Horizontal lines above the data indicate the time points at which the measured variable diverged from pre-exercise values under each condition. Specifically, for the horizontal line labelled ‘a’, P < 0.05 Control versus pre-exercise; for ‘b’, P < 0.05 Fluid versus pre-exercise; for ‘c’, P < 0.05 Warm versus pre-exercise.
Figure 3B shows stroke volume from pre-exercise to 90 min post-exercise. Stroke volume was reduced throughout post-exercise recovery (P > 0.001) in all conditions. Stroke volume did not differ between Warm and Control during recovery (P= 0.753). Fluid had stroke volumes that averaged 9.1 ml beat−1 higher compared to Control (P < 0.001) and 8.4 ml beat−1 higher compared to Warm (P < 0.001) across recovery.
Leg haemodynamics
Figure 4A shows femoral blood flow from pre-exercise to 90 min post-exercise. Femoral blood flow was elevated throughout the entire post-exercise recovery period (P= 0.001 versus pre-exercise). Femoral blood flow was not different across conditions (P= 0.608).
Figure 4. Femoral blood flow (A) and femoral vascular conductance (B) from pre-exercise to 90 min post-exercise.
○, Control; •, Fluid; ▿, Warm. Horizontal lines above the data indicate the time points at which the measured variable diverged from pre-exercise values under each condition. Specifically, for the horizontal line labelled ‘a’, P < 0.05 Control versus pre-exercise; for ‘b’, P < 0.05 Fluid versus pre-exercise; for ‘c’, P < 0.05 Warm versus pre-exercise.
Figure 4B shows femoral vascular conductance from pre-exercise to 90 min post-exercise. Femoral vascular conductance was elevated throughout the entire post-exercise recovery period (P= 0.001 versus pre-exercise). Femoral vascular conductance was not different across conditions (P= 0.424).
Core temperature and skin blood flow
Figure 5A shows core temperature from pre-exercise to 90 min post-exercise. Core temperature was elevated post-exercise (P= 0.001 versus pre-exercise) in all conditions. Core temperature tended to be elevated in Warm compared to Fluid and Control (P= 0.065).
Figure 5B shows thigh cutaneous vascular conductance (scaled as a percentage of maximal cutaneous vascular conductance, or CVCmax) from pre-exercise to 90 min post-exercise. Thigh cutaneous vascular conductance was elevated post-exercise (P < 0.001 versus pre-exercise) in all conditions. Thigh cutaneous vascular conductance was not different across conditions (P= 0.274).
Figure 5C shows forearm cutaneous vascular conductance (scaled as a percentage of maximal cutaneous vascular conductance, or CVCmax) from pre-exercise to 90 min post-exercise. Forearm cutaneous vascular conductance was elevated post-exercise (P < 0.001 versus pre-exercise) in all conditions. Forearm cutaneous vascular conductance was not different across conditions (P= 0.121).
Discussion
We studied the independent influences of oral fluid replacement and exercise in a warm environment on post-exercise central and peripheral haemodynamics in a group of endurance exercise-trained men. First, we tested the hypothesis that fluid replacement during exercise would prevent the post-exercise decreases in cardiac output compared to no fluid replacement. In other words, we expected fluid replacement to reverse the previously reported post-exercise fall in cardiac output in endurance exercise-trained men. Our results suggest that fluid replacement partially mitigates the post-exercise decrease in cardiac output in trained men as cardiac output was ∼0.4 l min−1 higher compared to the no fluid replacement condition.
Second, we hypothesized that there would be greater decreases in cardiac output with greater plasma volume losses (due to increased sweat loss) and enhanced peripheral vasodilatation secondary to increased thermal load (demonstrated by elevated core body temperature) in the condition of no fluid replacement and exercise in a warm environment. Contrary to this hypothesis, cardiac output was not lowest following exercise in a warm environment, but rather it was similar to cardiac output in the fluid replacement condition.
Magnitude of post-exercise hypotension
Other studies have demonstrated the presence of post-exercise hypotension in endurance-trained men (Senitko et al. 2002; Dujic et al. 2006; McCord & Halliwill, 2006). Mean arterial pressure during exercise recovery and the magnitude of post-exercise hypotension were not different between conditions. This supports our belief that post-exercise hypotension is a highly regulated physiological response, in that manipulation of cardiac output is counteracted by vascular changes such that pressure remains reduced compared to pre-exercise. In contrast, two studies have suggested that fluid replacement might attenuate post-exercise hypotension. Using oral fluid replacement, Davis & Fortney (1997) showed some increases in post-exercise pressures, but only when moderate levels of lower body negative pressure were applied (i.e. post-exercise hypotension was unaffected by oral fluid replacement when subjects were supine). Charkoudian et al. (2003) used an intravenous infusion to replace fluids during recovery from exercise that was associated with moderate dehydration, but this approach expanded plasma volume by 7% above pre-exercise volumes, whereas oral fluid replacement in the current study did not result in a relative hypervolaemia. Thus, from the current study we can state that, in the absence of either superimposed orthostatic stress or hypervolaemia, oral fluid replacement that restores normal plasma volume does not attenuate post-exercise hypotension. Likewise, exercise in a moderately warm environment does not exacerbate post-exercise hypotension in the absence of orthostatic stress.
Post-exercise haemodynamics in trained men
In the general population, post-exercise hypotension is characterized by a sustained increase in systemic vascular conductance that is not completely offset by ongoing increases in cardiac output (Halliwill et al. 1996a,b; Halliwill, 2001). After exercise, the reduction in arterial pressure is sustained due to a rise in systemic vascular conductance and this causes a rise in blood flow through the vasodilated regions. The inactive muscle pump causes an increase in venous pooling and in combination with plasma volume loss from sweating during exercise, preload is reduced. However, stroke volume is maintained due to reduction in afterload and an increase in cardiac contractility, heart rate is increased, and the result is in an elevation in cardiac output. This is the current model that is used to describe the haemodynamic response in the general population (Halliwill, 2001); however, this appears to be an oversimplification of the response in trained subjects, as trained men have been shown to mediate post-exercise hypotension through a different haemodynamic balance as demonstrated by Senitko et al. (2002), Dujic et al. (2006) and the current study. In endurance exercise-trained men, systemic vascular conductance remains unchanged or decreases relative to pre-exercise and cardiac output falls during recovery from exercise.
In the present study, we report that when hydration is maintained (i.e. maintenance of total body water in the fluid replacement condition), the post-exercise decrease in cardiac output and stroke volume is attenuated. Oral fluids (∼1020 ml) were given to match sweat loss and were consumed during exercise and completely finished 15 min prior to the end of exercise, which maximizes time for gastric emptying and intestinal absorption (Gisolfi et al. 1998). Despite maintaining hydration in the fluid replacement condition, we did not consistently observe an elevation in cardiac output post-exercise as is typically seen in the general population (4 of the 14 subjects did show a rise in cardiac output following exercise with oral fluid replacement). This was probably not due to the small overall loss in total body water from the end of exercise to the end of the study (loss of ∼0.330 kg) as individual data show that cardiac output was not better maintained when there was no change in total body water from before exercise to the end of the study during the fluid replacement condition. Thus, the decrease in central (cardiac) haemodynamics in trained men, or failure to see an increased cardiac output when plasma volume was restored, may also be dependent on: (1) other factors that affect preload, (2) alterations in heart structure and filling capacity, and/or (3) alterations in thermal load during recovery from exercise.
First, preload can be altered independent of blood and plasma volumes by a redistribution of cardiac output (Rowell, 1986). Thus, cutaneous vasodilatation during recovery from exercise could result in reductions in stroke volume and cardiac output due to a greater amount of blood redistributed to the compliant cutaneous vasculature. Along these lines, Franklin et al. (1993) assessed post-exercise hypotension under three different environmental conditions during recovery from upright cycling exercise. Recovery in the warm condition caused elevations in core and skin temperature at 60 min post-exercise which worsened post-exercise hypotension. Franklin et al. (1993) determined that the persistence of vasodilatation during exercise recovery and magnitude of post-exercise hypotension was dependent on elevations in skin and core temperature, and concluded that thermoregulatory mechanisms might play a role in post-exercise hypotension. However, the timing of heat loss responses and post-exercise hypotension are not well correlated, as Wilkins et al. (2004) showed that during exercise recovery mean arterial pressure was reduced after cutaneous blood flow has returned to pre-exercise values in the general population. Also, in the present study, stroke volume and cardiac output remain reduced long after cutaneous blood flow has returned to pre-exercise levels in trained men. Lastly, exercise in the heat, which we anticipated would increase thermal load and increase post-exercise skin blood flow resulted in increased stroke volume and cardiac output relative to the control condition. Thus, we suggest that the short-lived redistribution of blood to the skin during recovery from exercise seems an unlikely source of a more significant drop in preload in trained men than in the general population, and seems unlikely to explain the post-exercise reductions in stroke volume and cardiac output in trained men.
Second, it is possible that the same fall in preload (between the general population and trained men) produces a greater fall in stroke volume due to cardiac hypertrophy present in trained men. Levine et al. (1991) proposed orthostatic intolerance among athletes was due to structural and mechanical adaptations of the heart due to chronic training and showed that endurance exercise-trained men had increased ventricular compliance, making stroke volume and cardiac output more sensitive to reductions in preload than the general population.
Third, alterations in thermal load during recovery from exercise may alter cardiac output. We have long presumed that excess heat and the associated dehydration would worsen post-exercise hypotension and potentially reduce orthostatic tolerance (Halliwill, 2001). In contrast, we observed higher cardiac outputs during recovery from exercise in a warm environment relative to exercise in a thermoneutral environment, probably linked to the increase in heart rate (see Fig. 2). We believe this is attributable to an inotropic affect (e.g. increased contractility) related to elevations in core temperature (Johnson & Proppe, 1996). Heart rate increases ∼30 beats min−1 for every degree rise in core temperature (el-Sherif et al. 1970) due to direct effects on the sinoatrial node, elevated sympathetic activity and parasympathetic withdrawal (Johnson & Proppe, 1996). Myocardial contractility is likewise augmented by some of these mechanisms and may be responsible for the overall elevation in cardiac output during heat stress (Dimsdale et al. 1984). Along these lines, Brothers et al. (2009) recently demonstrated that whole body heat stress improves indexes of systolic function, while diastolic function is maintained. In the present study, core temperature was highest at the end of exercise in the warm condition and a trend toward higher core temperature was evident throughout recovery (∼0.2°C). As we did not also include measurements of skin temperature in the present study, it is possible that this trend for greater body core temperature underestimates differences in whole body thermal load during recovery from exercise. Regardless, we believe this points toward a thermal influence on heart rate and cardiac output post-exercise.
Perspective
Why is cardiac output increased in the general population during recovery from exercise? Plasma volume in sedentary and recreationally active individuals is known to recover quickly following exercise even in the absence of fluid replacement (Gillen et al. 1991; Hayes et al. 2000), which may explain in part why cardiac output can be increased during post-exercise hypotension in the general population and not in trained men. However, we would postulate that thermal load may be a more important difference underlying the increased cardiac output in the general population. The amount of total heat produced is a function of the absolute metabolic rate; thus, the highly trained athletes will produce more heat compared to the sedentary individual when exercising at the same relative intensity but higher absolute intensity (MacDougall et al. 1974). Yet, in general, there are no striking differences in heat storage which would suggest that the ability to dissipate heat is also directly related to training status (Saltin & Hermansen, 1966; MacDougall et al. 1974). It may be that subtle mismatches between heat loss and heat production (i.e. due to the environment or changes in fluid balance) generate additional thermal load and cause elevations in cardiac output following exercise in some groups. Although speculative, we propose that less effective heat loss mechanisms (i.e. skin blood flow and sweating) in the general population may explain the commonly observed elevations in post-exercise cardiac output (Senitko et al. 2002). In contrast, trained men may show no rise in cardiac output post-exercise due to better heat loss ability (increased sweating and cutaneous blood flow) during and after exercise. However, the present study does not directly test this possibility. It may be germane to this notion that recovery from exercise is associated with changes in thermoregulation that favour heat storage over heat loss mechanisms (e.g. Kenny et al. 2003).
Is there an impact of not increasing post-exercise cardiac output in the endurance-trained population? To compensate for the fall in cardiac output, it is plausible that trained men have increased sympathetic vasoconstrictor outflow during recovery from exercise that would oppose the net peripheral vasodilatation observed in other groups. While the previously exercised muscle in endurance exercise-trained men shows vasodilatation post-exercise (McCord & Halliwill, 2006), there is no net rise (or even a reduction) in net systemic vascular conductance. These data suggest that there may be vasoconstriction in other areas; specifically, vasoconstriction in the splanchnic or renal vascular beds may offset the vasodilatation in the skeletal muscle. Pricher et al. (2004) found that there was no change in renal and splanchnic blood flow following exercise in a group of normally active subjects. It is possible that these vascular beds undergo baroreflex-mediated sympathetic vasoconstriction in response to leg vasodilatation in trained men; however, blood flow to these vascular beds was not measured in our study.
Considerations
In the current study, group comparisons (e.g. untrained men, untrained women and trained women) were not made. Thus, the influence of heat stress and fluid replacement on post-exercise haemodynamics across sex and/or training status was not tested. Clearly this is a logical next step in trying to understand how differences in heat storage and thermal load might lead to alterations in hemodynamic control, increased orthostatic intolerance, and heat-related syncope in the general and trained populations.
An additional limitation of the present study is that skin temperature was not measured. This would have provided a more robust measure of thermal load at the end of exercise and during post-exercise recovery. Instead, we have relied on body core temperature as an index of thermal load. However, this index appears to be consistent with the observed physiological responses such as changes in cutaneous vascular conductance and overall sweat loss.
Conclusion
We studied the independent influences of oral fluid replacement and exercise in a warm environment on post-exercise central and peripheral haemodynamics in a group of highly fit endurance exercise-trained men. We found that fluid replacement mitigates the decrease in cardiac output in endurance-trained men after exercise, as cardiac output was higher with fluid replacement than without. However, endurance-trained men may have cardiac structural alterations that contribute further to the decreases in cardiac output following exercise. Surprisingly, exercise in a warm environment also mitigates the decrease in cardiac output in endurance-trained men after exercise, suggesting an interesting role for thermal load on altering post-exercise haemodynamics.
Acknowledgments
This research project was conducted by Brenna M. Lynn in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Oregon. We sincerely thank the subjects involved in this study for their participation. The authors gratefully acknowledge the technical assistance of Zachary Barrett-O’Keefe. This research was supported by a grant from the American Heart Association, Grant-in-Aid 0555623Z, the Evonuk Memorial Grant, and the Department of Defense (DURIP 45938-LS-RIP).
References
- Adolph EF. Physiology of Man in the Desert. New York: Intersciences, I; 1947. [Google Scholar]
- Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bean WB, Eichna LW. Performance in relation to environmental temperature. Reactions of normal young me to simulated desert environment. Fed Proc. 1943;2:144–158. [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009;4:H1150–H1156. doi: 10.1152/ajpheart.01069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouha L. Effects of muscular work and heat on the cardiovascular system. Ind Med Surg. 1960;29:114–120. [PubMed] [Google Scholar]
- Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol. 2003;552:635–644. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costill DL. Sweating: its composition and effects on body fluids. Ann N Y Acad Sci. 1977;301:160–174. doi: 10.1111/j.1749-6632.1977.tb38195.x. [DOI] [PubMed] [Google Scholar]
- Davis JE, Fortney SM. Effect of fluid ingestion on orthostatic responses following acute exercise. Int J Sports Med. 1997;18:174–178. doi: 10.1055/s-2007-972615. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Hartley LH, Guiney T, Ruskin JN, Greenblatt D. Postexercise peril. Plasma catecholamines and exercise. JAMA. 1984;251:630–632. doi: 10.1001/jama.251.5.630. [DOI] [PubMed] [Google Scholar]
- Dujic Z, Ivancev V, Valic Z, Bakovic D, Marinovic-Terzic I, Eterovic D, Wisloff U. Postexercise hypotension in moderately trained athletes after maximal exercise. Med Sci Sports Exerc. 2006;38:318–322. doi: 10.1249/01.mss.0000187460.73235.3b. [DOI] [PubMed] [Google Scholar]
- el-Sherif N, Shahwan L, Sorour AH. The effect of acute thermal stress on general and pulmonary hemodynamics in the cardiac patient. Am Heart J. 1970;79:305–317. doi: 10.1016/0002-8703(70)90418-7. [DOI] [PubMed] [Google Scholar]
- Franklin PJ, Green DJ, Cable NT. The influence of thermoregulatory mechanisms on post-exercise hypotension in humans. J Physiol. 1993;470:231–241. doi: 10.1113/jphysiol.1993.sp019856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol. 1981;50:65–70. doi: 10.1152/jappl.1981.50.1.65. [DOI] [PubMed] [Google Scholar]
- Gillen CM, Lee R, Mack GW, Tomaselli CM, Nishiyasu T, Nadel ER. Plasma volume expansion in humans after a single intense exercise protocol. J Appl Physiol. 1991;71:1914–1920. doi: 10.1152/jappl.1991.71.5.1914. [DOI] [PubMed] [Google Scholar]
- Gisolfi CV, Summers RW, Lambert GP, Xia T. Effect of beverage osmolality on intestinal fluid absorption during exercise. J Appl Physiol. 1998;85:1941–1948. doi: 10.1152/jappl.1998.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29:65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Dinenno FA, Dietz NM. α-Adrenergic vascular responsiveness during postexercise hypotension in humans. J Physiol. 2003;550:279–286. doi: 10.1113/jphysiol.2003.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996a;495:279–288. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA, Hartwig TD, Eckberg DL. Augmented baroreflex heart rate gain after moderate-intensity, dynamic exercise. Am J Physiol Regul Integr Comp Physiol. 1996b;270:R420–R426. doi: 10.1152/ajpregu.1996.270.2.R420. [DOI] [PubMed] [Google Scholar]
- Hayes PM, Lucas JC, Shi X. Importance of postexercise hypotension in plasma volume restoration. Acta Physiol Scand. 2000;169:115–124. doi: 10.1046/j.1365-201x.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4: Environmental Physiology, vol I, Adaptive Responses to Heat. Bethseda, MD: American Physiological Society; 1996. [Google Scholar]
- Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension. 1993;22:653–664. doi: 10.1161/01.hyp.22.5.653. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Periard J, Journeay WS, Sigal RJ, Reardon FD. Effect of exercise intensity on the postexercise sweating threshold. J Appl Physiol. 2003;95:2355–2360. doi: 10.1152/japplphysiol.00651.2003. [DOI] [PubMed] [Google Scholar]
- Kilgour RD, Gariepy P, Rehel R. Cardiovascular responses during recovery from exercise and thermal stress. Aviat Space Environ Med. 1993;64:224–229. [PubMed] [Google Scholar]
- Kohl HW, Blair SN, Paffenbarger RS, Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol. 2005;563:633–642. doi: 10.1113/jphysiol.2004.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Beasley JM, Halliwill JR. H2-receptor-mediated vasodilatation contributes to postexercise hypotension. J Appl Physiol. 2006;100:67–75. doi: 10.1152/japplphysiol.00959.2005. [DOI] [PubMed] [Google Scholar]
- McCord JL, Halliwill JR. H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J Appl Physiol. 2006;101:1693–1701. doi: 10.1152/japplphysiol.00441.2006. [DOI] [PubMed] [Google Scholar]
- MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16:225–236. doi: 10.1038/sj.jhh.1001377. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Reddan WG, Layton CR, Dempsey JA. Effects of metabolic hyperthermia on performance during heavy prolonged exercise. J Appl Physiol. 1974;36:538–544. doi: 10.1152/jappl.1974.36.5.538. [DOI] [PubMed] [Google Scholar]
- Mathews DK, Fox EL, Tanzi D. Physiological responses during exercise and recovery in a football uniform. J Appl Physiol. 1969;26:611–615. doi: 10.1152/jappl.1969.26.5.611. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Myhre LG, Hartung GH, Nunneley SA, Tucker DM. Plasma volume changes in middle-aged male and female subjects during marathon running. J Appl Physiol. 1985;59:559–563. doi: 10.1152/jappl.1985.59.2.559. [DOI] [PubMed] [Google Scholar]
- Pivarnik JM, Leeds EM, Wilkerson JE. Effects of endurance exercise on metabolic water production and plasma volume. J Appl Physiol. 1984;56:613–618. doi: 10.1152/jappl.1984.56.3.613. [DOI] [PubMed] [Google Scholar]
- Pricher MP, Holowatz LA, Williams JT, Lockwood JM, Halliwill JR. Regional hemodynamics during postexercise hypotension. I. Splanchnic and renal circulations. J Appl Physiol. 2004;97:2065–2070. doi: 10.1152/japplphysiol.00465.2004. [DOI] [PubMed] [Google Scholar]
- Raine NM, Cable NT, George KP, Campbell IG. The influence of recovery posture on post-exercise hypotension in normotensive men. Med Sci Sports Exerc. 2001;33:404–412. doi: 10.1097/00005768-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Regulation During Physical Stress. New York: Press OU; 1986. Human circulation. [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21:1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Senitko AN, Charkoudian N, Halliwill JR. Influence of endurance exercise training status and gender on postexercise hypotension. J Appl Physiol. 2002;92:2368–2374. doi: 10.1152/japplphysiol.00020.2002. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Minson CT, Halliwill JR. Regional hemodynamics during postexercise hypotension. II. Cutaneous circulation. J Appl Physiol. 2004;97:2071–2076. doi: 10.1152/japplphysiol.00466.2004. [DOI] [PubMed] [Google Scholar]
- Wolf AV. Physiology of the Urge to Drink and Problems of Water Lack. Springfield, IL: Thomas; 1958. Thirst. [Google Scholar]
- Yeh MP, Gardner RM, Adams TD, Yanowitz FG. Computerized determination of pneumotachometer characteristics using a calibrated syringe. J Appl Physiol. 1982;53:280–285. doi: 10.1152/jappl.1982.53.1.280. [DOI] [PubMed] [Google Scholar]







