Abstract
Cationic gold(I) phosphite catalysts activate allenes for epoxide cascade reactions. The system is tolerant of numerous functional groups (sulfones, esters, ethers, sulfonamides) and proceeds at room temperature in dichloromethane. The cyclization pathway is sensitive to the substitution pattern of the epoxide, and the backbone structure of the A-ring. It is capable of producing medium-ring ethers, fused 6-5 bicyclic, and linked pyran-furan structures. The resulting cycloisomers are reminiscent of structures found in numerous polyether natural products.
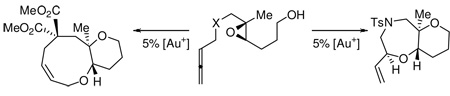
The use of cascade reactions1 to enhance the molecular complexity of readily prepared starting materials provides great opportunities for the development of novel catalytic protocols. The biosynthesis of molecules like glabrescol,2 the brevetoxins,3 and monensin4 have each been proposed to rely on polyepoxide cascades, a strategy that has also been adopted in natural product synthesis. For example the McDonald synthesis of durgamone / nakorone A5 and the Jamison assembly of “ladder” polyethers utilize a polyepoxide cascade as the critical step (Scheme 1).6
Scheme 1.
Synthesis of Durgamone (McDonald) and Ladder Polyethers (Jamison)
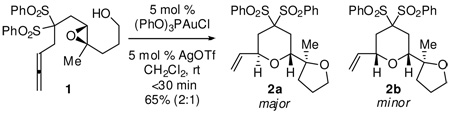
Gold(I)-phosphine and phosphite complexes have recently been shown to activate carbon-carbon multiple bonds for attack by oxygen nucleophiles.7–9 Most demonstrative of the exquisite chemoselectivity of Au(I) catalysts is the epoxide O-nucleophile class of reactions,10 which are themselves susceptible to Lewis acid activation. These observations led us to ask if C-C unsaturation could be chemoselectively activated towards epoxide nucleophiles to initiate a biomimetic cascade multi-ether ring forming reaction.11,12 Starting from our efforts on Au(I)-catalyzed allene activation,13,14 we designed experiments to determine the feasibility of activating an allene in the presence of a hydroxy-epoxide. To this end we investigated the cyclization of 1 using a variety of L-AuCl/AgX combinations. From this screen emerged an optimal catalyst formulation: (PhO)3PAuCl (5 mol %) + 5 mol % AgOTf in CH2Cl2.15 As shown in eq 1, this enabled the cyclization of 1 in <30 min at rt to a diastereomeric mixture of the pyrans 2a and 2b in 65% combined yield (2.2:1). No allene activation occurred in the absence of the gold catalyst.16
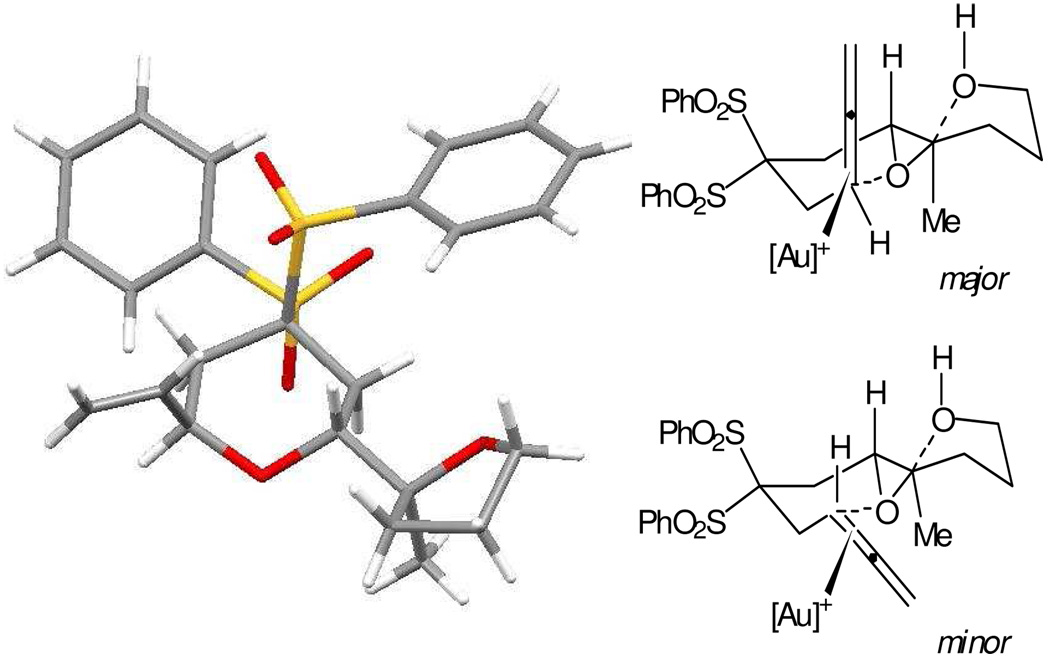
From a combination of NMR data on 2a and 2b and X-ray data on 2a (Figure 1), the two compounds were determined to be epimeric at the allyl-ether stereocenter. The relative configuration at the pyran-furan linkage was consistent with clean inversion of the original epoxide and adoption of a twist-boat solution conformer. The epimeric allylic ethers presumably stem from the competing cyclization geometries shown in Figure 1.17
 |
(1) |
Figure 1.
Crystal structure of trans-fused pyran 2a (Sulfur = yellow, oxygen = red, carbon = gray, hydrogen = white)
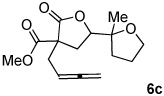
Treating these reactions conditions as optimal, a series of substrates were examined to establish the effect of epoxide stereochemistry, regiochemistry, and backbone functionality (Table 1). The ethereal and malonate-derived backbones, in contrast to 2a,b, stereoselectively provided the cis-pyran in good dr (1:8 by NMR).18 Worth noting in 5 was a competing (H+ and Ag+ catalyzed) ring opening of the epoxide by the alcohol and subsequent lactonization to 6c (~10% yield). Attempts to further enhance chemoselectivity for allene activation through base addition, lowered catalyst loading, solvent change, or lowered temperature were not fruitful.
Table 1.
Scope of the Allene Initiated Cascade Cyclization
| substrate | product(s) | yielda | selectivity | |
|---|---|---|---|---|
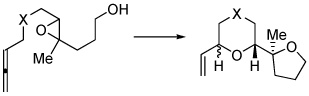 |
(trans cis)b | |||
| 1 | X = (PhO2S)2C | 2a,b | 65% | 2.2:1 |
| 3 | X = O | 4a,b | 60% | 1:8 |
| 5 | X = (MeO2C)2C | 6a,b | 65%c | 1:8 |
 |
||||
 |
||||
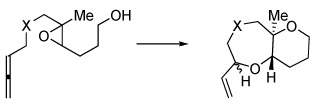
| 7 | X = (PhO2S)2C | 8a,b | 35% | 7:1 |
| 9 | X = NTs | 10a,b | 59%d | 4:1 |
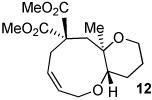
| 11 | X = (MeO2C)2C | |||
 |
40% | --e | ||
 |
45% | 2.5:1 | ||
General conditions: 5 mol % (PhO)3PAuCl / 5 mol % AgOTf, CH2Cl2, rt, 15–45 min.
Isolated yields after silica gel chromatography.
Relative stereochemistry of ethereal α and α’ stereocenters in the vinyl-bearing oxacycle.
~10% of 6c also generated.
<10% of the 9-endo product also formed.
Only one isomer detected by 1H, 13C NMR.
In the case of 7, 9, and 11, the alternative positioning of the methyl group on the epoxide led to fused ethers. As noted by Kozmin,8j McDonald,19 and Fürstner,8b the methyl group positioning on an epoxide influences the ring-opening regiochemistry by distorting the epoxonium intermediate to localize charge at the most substituted center.20,21
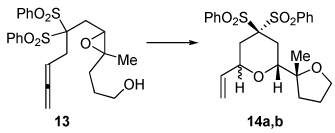
Unexpectedly, 5-Me diester 11 did not yield the expected [5.4.0] bicyclic ether, but instead provided the 9-endo product 12 as a single stereoisomer. Traces of this cyclization mode were detected for compound 9 (~10%), but its production could not be improved. Fused medium-ring ethers are, of course, ubiquitous in marine-derived natural products. Changing the epoxide stereochemistry in 13 led to stereoisomeric 14a,b (c.f. 2a,b), indicative of a cyclization that was stereospecific and invertive at the epoxide.
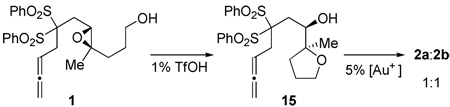
One mechanistic quandary revolved around whether the reaction initiated at the allene as envisioned in Figure 1, or proceeded by epoxide opening and allene hydroalkoxylation.22 This alternative pathway was investigated by pre-cyclizing 1 with 1% TfOH to 15, and then carrying out a hydro-alkoxylation to 2a,b (eq 2). Unlike the direct cyclization of 1, 2a,b was obtained in a 1:1 ratio, suggesting an allene first mechanism for 1.
 |
(2) |
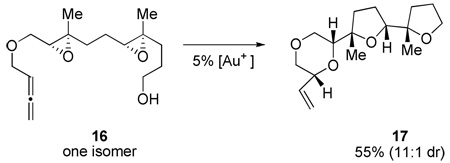
To investigate the feasibility of a tricyclization cascade, the isomerically pure di-epoxide 16 was prepared using the asymmetric epoxidation protocols of Sharpless23 and Shi24 (eq 3). Upon exposure to the standard reaction conditions, isomerization ensued to form tricyclic ether 17. Notably, 17 was formed in analogy to product 4 (Table 1) without significant loss in yield or diastereoselectivity, lending further support to the feasibility of the current methodology for multicyclization events in complex molecule synthesis.
 |
(3) |
In conclusion, a method to generate polyether skeletons from allene-epoxide cascades has been developed. The method provides access to several ring structures commonly encountered in natural products and promises to yield catalyst control over cyclization regiochemistry. Studies are underway to utilize other heteroatomic traps and to elucidate the cyclization mechanism.
Supplementary Material
Experimental procedures, spectra of new compounds and CIF data for 2a. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank Prof. Michael Crimmins and Prof. Jeff Johnson (UNC) for helpful discussions. The authors acknowledge NIH Institutes of General Medicine (GM-60578) for financial support. J.L.Z. thanks the National Research Council for a Postdoctoral Fellowship, and S.J.L. thanks the ARO for Staff Research Funding.
Contributor Information
Stephen J. Lee, Email: Stephen.Lee2@us.army.mil.
Michel R. Gagné, Email: mgagne@unc.edu.
References
- 1.Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134–7185. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 2.(a) Xiong Z, Corey EJ. J. Am. Chem. Soc. 2000;122:9328–9329. [Google Scholar]; (b) Xiong Z, Corey EJ. J. Am. Chem. Soc. 2000;122:4831–4832. [Google Scholar]
- 3.Nakata T. Chem. Rev. 2005;105:4314–4347. doi: 10.1021/cr040627q. [DOI] [PubMed] [Google Scholar]
- 4.(a) Fukuyama T, Akasaka K, Karanewsky DS, Wang C-LJ, Schmid G, Kishi Y. J. Am. Chem. Soc. 1979;101:262–263. [Google Scholar]; (b) Collum DB, McDonald JH, III, Still WC. J. Am. Chem. Soc. 1980;102:2120–2121. [Google Scholar]
- 5.Tong R, Valentine JC, McDonald FE, Cao R, Fang X, Hardcastle KI. J. Am. Chem. Soc. 2007;129:1050–1051. doi: 10.1021/ja068826+. [DOI] [PubMed] [Google Scholar]
- 6.(a) Morten CJ, Jamison TF. J. Am. Chem. Soc. 2009;131:6678–6679. doi: 10.1021/ja9025243. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Byers JA, Jamison TF. J. Am. Chem. Soc. 2009;131:6383–6385. doi: 10.1021/ja9004909. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vilotijevic I, Jamison T. Science. 2007;317:1189–1192. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For relevant reviews, see: Shcn HC. Tetrahedron. 2008;64:3885–3903. Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351–3378. doi: 10.1021/cr068430g. Jimenez-Nunez E, Echavarren AM. Chem. Rev. 2008;108:3326–3350. doi: 10.1021/cr0684319. Arcadi A. Chem. Rev. 2008;108:3266–3325. doi: 10.1021/cr068435d. Li Z, Brouwer C, He C. Chem. Rev. 2008;108:3239–3265. doi: 10.1021/cr068434l. Widenhoefer RA. Chem. Eur. J. 2008;14:5382–5391. doi: 10.1002/chem.200800219. Hashmi ASK. Chem. Rev. 2007;107:3180–3211. doi: 10.1021/cr000436x. Fürstner A, Davies PW. Angew. Chem. Int. Ed. 2007;46:3410–3449. doi: 10.1002/anie.200604335.
- 8.For examples of Au(I)-alkyne-initiated cascade cyclizations, see: Toullec PY, Blarre T, Michelet V. Org. Lett. 2009 doi: 10.1021/ol900864n. Fürstner A, Morency L. Angew. Chem. Int. Ed. 2008;47:5030–5033. doi: 10.1002/anie.200800934. Zriba R, Gandon V, Aubert C, Fensterbank L, Malacria M. Chem. Eur. J. 2008;14:1482–1491. doi: 10.1002/chem.200701522. Yang T, Campbell L, Dixon DJ. J. Am. Chem. Soc. 2007;129:12070–12071. doi: 10.1021/ja074550+. Yang T, Campbell L, Dixon DJ. J. Am. Chem. Soc. 2007;129:12070–12071. doi: 10.1021/ja074550+. Sherry BD, Maus L, Laforteza BN, Toste DF. J. Am. Chem. Soc. 2006;128:8132–8133. doi: 10.1021/ja061344d. Nieto-Oberhuber C, Muñoz MP, Lopez S, Jimenez-Nuñez E, Nevado C, Herrero-Gomez E, Raducan M, Echavarren AM. Chem. Eur. J. 2006;12:1677–1693. doi: 10.1002/chem.200501088. Couty S, Meyer C, Cossy J. Angew. Chem. Int. Ed. 2006;45:6726–6730. doi: 10.1002/anie.200602270. Antoniotti S, Genin E, Michelet V, Genêt J-P. J. Am. Chem. Soc. 2005;127:9976–9977. doi: 10.1021/ja0530671. Zhang L, Kozmin SA. J. Am. Chem. Soc. 2005;127:6962–6963. doi: 10.1021/ja051110e. Mamane V, Gress T, Krause H, Fürstner A. J. Am. Chem. Soc. 2004;126:8654–8655. doi: 10.1021/ja048094q. Nieto-Oberhuber C, Muñoz MP, Buñuel E, Nevado C, Cardenas DJ, Echavarren AM. Angew. Chem. Int. Ed. 2004;43:2402–2406. doi: 10.1002/anie.200353207.
- 9.For a related Hg(II)-catalyzed variant, see: Imagawa H, Iyenaga T, Nishizawa M. Org. Lett. 2005;7:451–453. doi: 10.1021/ol047472t. (b) For Pt(II), see: Nelsen DL, Gagné MR. Organometallics. 2009;28:950–952. doi: 10.1021/om801069s.
- 10.(a) Dai L-Z, Shi M. Chem. Eur. J. 2008;14:7011–7018. doi: 10.1002/chem.200701954. [DOI] [PubMed] [Google Scholar]; (b) Dai L-Z, Shi M. Tetrahedron Lett. 2008;49:6437–6439. [Google Scholar]; (c) Lin G-Y, Li C-W, Hung S-H, Liu R-S. Org. Lett. 2008;21:5059–5062. doi: 10.1021/ol802047g. [DOI] [PubMed] [Google Scholar]; (d) Cordonnier MC, Blanc A, Pale P. Org. Lett. 2008;10:1569–1572. doi: 10.1021/ol800219k. [DOI] [PubMed] [Google Scholar]; (e) Dai L-Z, Qi M-J, Shi Y-L, Liu X-G, Shi M. Org. Lett. 2007;9:3191–3194. doi: 10.1021/ol0713640. [DOI] [PubMed] [Google Scholar]
- 11.Previous (unpublished) efforts in our laboratories with “carbophilic” Lewis acids always resulted in epoxide activation over alkene, which has also been observed by Yang and He under gold(III) / silver activation catalysis. See for example: Liu Y, Li X, Lin G, Xiang Z, Xiang J, Zhao M, Chen J, Yang Z. J. Org. Chem. 2008;73:4625–4629. doi: 10.1021/jo8005649. Shi Z, He C. J. Am. Chem. Soc. 2004;126:5964–5965. doi: 10.1021/ja031953a.
- 12.For an example of exclusive Au(I)-catalyzed epoxide O-activation, see: Li Y, Tang PP, Chen YX, Yu B. J. Org. Chem. 2008;73:4323–4325. doi: 10.1021/jo8003875.
- 13.(a) Tarselli MA, Chianese AR, Lee SJ, Gagné MR. Angew. Chem. Int. Ed. 2007;46:6670–6673. doi: 10.1002/anie.200701959. [DOI] [PubMed] [Google Scholar]; (b) Tarselli MA, Gagné MR. J. Org. Chem. 2008;73:2439–2441. doi: 10.1021/jo7024948. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tarselli MA, Liu A, Gagné MR. Tetrahedron. 2009;65:1785–1789. doi: 10.1016/j.tet.2008.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold-allene activations were early to appear: Hashmi ASK, Schwarz L, Choi J-H, Frost TM. Angew. Chem. Int. Ed. 2000;39:2285–2288. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f.
- 15.Other silver additives (AgSbF6, AgBF4, AgNTf2, AgClO4, AgOTs) or phosphine ligands (EtPh2P, Cy3P, (4-Cl-Ph)3P, (4-MeO-Ph) 3P) led to lower yields of bicyclic product. Other solvents (PhMe, THF) led to complex mixtures.
- 16.Exposure to AgOTf in the absence of Au(I) did not produce any discernable allene activation. In preliminary (unpublished) reactivity studies for related transition metal-catalyzed cyclization of allenyl esters, Yb(III) and Pd(II) showed negligible levels of desired reactivity.
- 17.A pseudo-axial preference has been noted in previous electrophilic cyclizations, see for example: Johnson WS, Telfer SJ, Cheng S, Schubert U. J. Am. Chem. Soc. 1987;109:2517–2518. Koh JH, Gagné MR. Angew. Chem. Int. Ed. 2004;43:3459–3461. doi: 10.1002/anie.200453913. Wan S, Gunayadin H, Houk KN, Floreancig PE. J. Am. Chem. Soc. 2007;129:7915–7923. doi: 10.1021/ja0709674.
- 18.The role of the A-ring substituents on cyclization dr is not yet understood.
- 19.McDonald FE, Wang X, Do B, Hardcastle KI. Org. Lett. 2000;2:2917–2919. doi: 10.1021/ol0064009. [DOI] [PubMed] [Google Scholar]
- 20.(a) Stork G, Burgstahler AW. J. Am. Chem. Soc. 1955;77:5068–5077. [Google Scholar]; (b) Gamboni G, Schniz H, Eschenmoser A. Helv. Chim. Acta. 1954;37:964–971. [Google Scholar]
- 21.Jamison recently showed that water can overturn this propensity, see footnote 6a for details.
- 22.(a) Paton RS, Maseras F. Org. Lett. 2009;11:2237–2240. doi: 10.1021/ol9004646. [DOI] [PubMed] [Google Scholar]; (b) Zhang Z, Widenhoefer RA. Org. Lett. 2008;10:2079–2081. doi: 10.1021/ol800646h. [DOI] [PubMed] [Google Scholar]; (c) Nishina N, Yamamoto Y. Tetrahedron Lett. 2008;49:4908–4911. [Google Scholar]; (d) Zhang Z, Widenhoefer RA. Angew. Chem. Int. Ed. 2007;46:283–285. doi: 10.1002/anie.200603260. [DOI] [PubMed] [Google Scholar]; (e) Zhang Z, Liu C, Kinder RE, Han X, Qian H, Widenhoefer RA. J. Am. Chem. Soc. 2006;128:9066–9075. doi: 10.1021/ja062045r. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765. [Google Scholar]
- 24.Wang Z-X, Tu Y, Frohn M, Zhang J-R, Shi Y. J. Am. Chem. Soc. 1997;119:11224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, spectra of new compounds and CIF data for 2a. This material is available free of charge via the Internet at http://pubs.acs.org.